Abstract
Background:
Definite etiology of amyotrophic lateral sclerosis (ALS) is still a matter of debate.
Aims:
The study was designed to evaluate the role of environmental, occupational, and familial risk factors in development of ALS.
Materials and Methods:
This was a case control study of 110 cases of definite ALS with 240 age and sex matched controls. Investigations were done on the following aspects- family history, occupation, living place, source of drinking water, exposure to industrial, chemical, agricultural toxins and heavy metals, physical and electrical injury, working under magnetic field for more than 10 years in both the groups. Clinical examinations, electrophysiological, and neuroimaging studies were done in every patient. Chi square test, logistic regression analysis, and calculation of odds ratio were used to analyze the data.
Results:
Rural livings (odds ratio = 1.99), smoking (odds ratio = 1.88), insecticides, and pesticides exposures (odds ratio = 1.61), electrical injury (odds ratio = 6.2) were detected as the associated factors in development amyotrophic lateral sclerosis.
Conclusions:
The study expressed the need of extensive research globally in molecular and genetic levels to detect the associated factors in etiopathogenesis of ALS for better understanding the etiology and for remedial aspects.
Keywords: Amyotropic lateral sclerosis, Environmental, Familial, Occupational risk factors
Introduction
Amyotrophic lateral sclerosis (ALS) is though uncommon but not a rare disease and seen in all the ethnic and socioeconomic groups of people in the world. Despite extensive researches in the last two decades, the definite etiology of ALS has not been detected. Genetic, environmental, occupational, toxins, high level physical activity, electrical injury, physical trauma, medical illness, and high magnetic field exposed activity; all have been suggested by different investigators at different times but not proved yet with certainty.[1] It has been speculated that there is slow ongoing process of selective motor neuron destruction by a complex chain of injurious events after exposure to certain factors and ultimately involving exicitotoxins, oxidative stress, altered calcium homeostasis, mitochondrial dysfunction, and enhanced motor neuron destruction.[2]
The treatment options of ALS are of very much limited and mostly ineffective, observed in different trials. So, associated factors in development ALS should be investigated from every corner of the world for better understanding of the etiopathogenesis of ALS for future effective remedy. In India there is no epidemiological study in recent times. This Case Control study tried to evaluate the role of familial, environmental, and occupational factors in development of ALS in the Indian context which may help the future study or researches on the etiopathogenesis and etiology of ALS.
Materials and Methods
This case control study was carried out from January 2008 to January 2011 in the Burdwan Medical College and Hospital, situated in the eastern part of India. The institute is the only neurological referral center in the rural background in the district of Burdwan, West Bengal, India. This study was approved by institution ethics committee. Total 110 definite cases of ALS (male = 95, female = 15) and 240 age (±2 years) and sex matched controls (male = 199, female = 41) were included in the study. All cases and controls were signed in informed consent. Detailed history was taken from every case and control on the basis of open ended structural interview. All the patients (ALS) were selected from the Neurology department and confirmed clinically by single Neurologist on the basis of diagnostic criteria of Escorial world Federation of Neurology having evidence of upper and lower motor neuron disease in the three of the four regions (bulbar, cervical, thoracic, and lumbar).[3] In every case, MRI of brain and spinal cord were done to exclude structural lesion and EMG, NCV study were done for documentation of electromyographic pattern of ALS. Exclusion of the medical diseases mimicking the ALS were done by appropriate investigations and examinations, e.g., hyperthyroidism, hyperparathyroidism, extrinsic, and intrinsic spinal cord disease, brain stem disease, past history of poliomyelitis, post polio syndrome, myeloradiculopathy, multifocal motor neuropathy, and inflammatory myopathy. Only the definite cases of ALS were included in the study.
For one case (ALS), one or more age (±2 years) and sex matched control were selected from the accompanying persons if available, and from those attending the neurology department. Control subjects had neither significant neurological nor medical illness mentioned above. No family member of the cases (ALS) was included as a control in the study. Residence of every control subject was more than 15 km away from the residence of any case and they were permanent resident of that place for more than 10 years. No control subject refused to participate.
Exposure to environmental risk factors was investigated for more than 10 years before the onset of disease in every patient and same duration in the control subjects, along with basic demographic information on: education, gender, living places for more than 10 years, occupation, economic status, and duration of disease. Exposure to toxin was taken positive when each subject spent more than 3 h a day for more than 3 months in a year for the last 10 years.
To detect ALS cases in the family, first and second degree relatives for every patient and control were investigated including the medical document and treatment history issued by neurologist or physician if present. No familial ALS case was detected in the familial tree among the cases and controls. Smoking factor was considered positive when the subject smoked at least five cigarettes or bidi (home made tobacco products in India for smoking) in a day for more than 1 year during the last 10 years. Source of drinking water was investigated for more than 10 years before the onset of the disease in every case and the same duration for the control subjects. Electrical shock or injury was taken positive when medical documents from physician or surgeon including treatment history and duration of hospital stay was available. None in the both group had the history of working under electromagnetic field.
Statistical analysis
Statistical tests were carried out using the normal deviate tests and chi-square tests using SPSS software for windows (version 17.0). To detect the significance of every associated or risk factors, conditional logistic regression analysis was used to analyze the data with calculation of odds ratio (OR) with 95% confidence interval.
Results
Among the 110 cases of definite ALS, 15 (13.6 %) were female, 95 (86.4%) were male and in the control group 41 (17.1%) were female and 199 (82.9 %) were male [Table 1]. Separate analysis for male and female were done and shown in Tables 2 and 3. Maximum number of cases were found in the 5th and 6th decade, but a significant number of cases have been detected at earlier age group in the study. Most of the cases and control subjects had rural domicile [98 among the cases (89.1%) and 193 among the controls (80.4%)]. Rural dwellings have been detected as an associated factor in the development of ALS (P = 0.037, OR = 1.98, 95% CI of 1.01-3.88).
Table 1.
Age group and Sex distribution
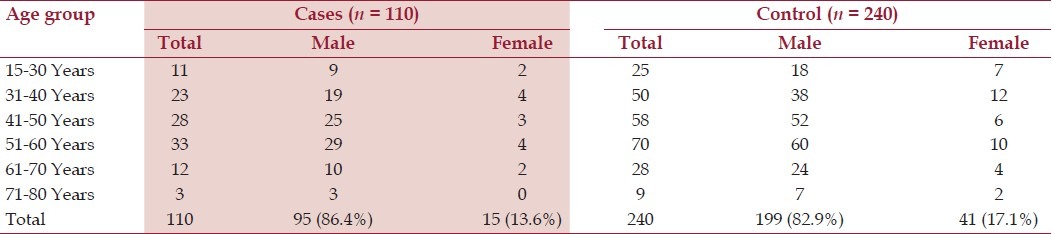
Table 2.
Cases, Controls and the risk factors
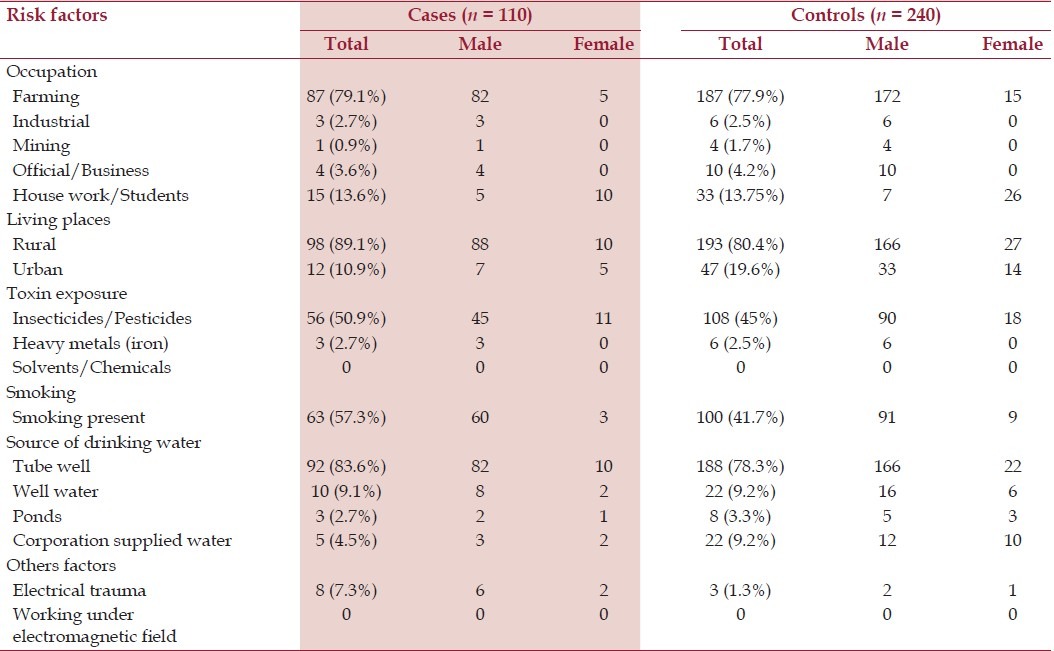
Table 3.
Significance level of the risk factors
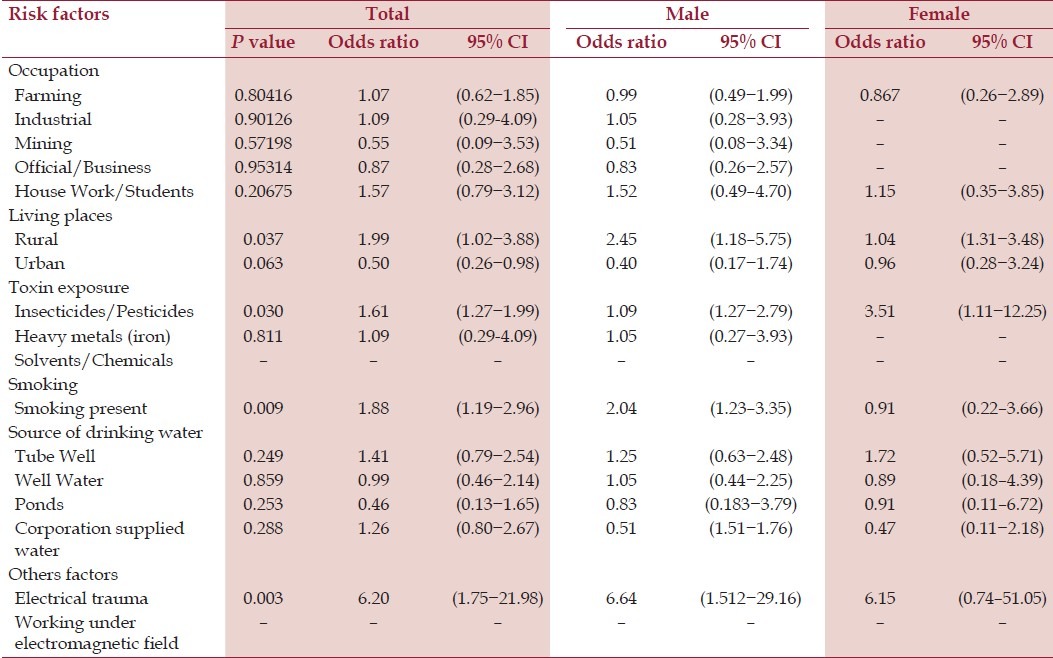
Among the cases, 87 (79.1%) were farmers whereas 187 (77.9%) in the control group. Neither farming nor any particular type of occupation was associated with development of ALS [Table 2]. Exposure to insecticides and pesticides was present in 56 (50.9%) cases and 108 (45%) controls and this has been found as an associated factor in the development of ALS (P = 0.03, OR = 1.61, 95% CI of 1.27-1.99). Other toxin like heavy metal exposures has no significant association in development of ALS.
Among the cases, 63 (57.3%) patients had the history of smoking whereas it was present in 100 (41.7%) persons in the control group. Smoking has been detected as an important associated factor in development of ALS (P = 0.009, OR = 1.88, 95% CI of 1.19-2.96).
History of electric shock or injury was present in eight (7.3%) cases and in three (1.3%) controls. A strong association has been detected between the electric shock or injury and development of ALS (P = 0.003, OR = 6.20, 95% CI = 1.75-21.98).
Indian people use drinking water from four sources- (1) Tube well, (2) Well water, (3) Corporation supplied water, and (4) Pond water. Among the cases, 92 (83.6%) used tube well water, 10 (9.0%) used well water, 3 (2.7%) used pond water, and 5 (4.5%) used corporation supplied water for drinking purposes for more than 10 years. On the other hand, in the control group, 188 (78.3%) used tube well water, 22 (9.2%) used well water, 8 (3.3%) used pond water, and 22 (9.2%) used corporation supplied water for drinking purposes for more than 10 years. The source of drinking water has been found not to be associated significantly in development of ALS [Table 2].
In the study, no case has been found with history of working under electromagnetic field, exposure to chemicals, and solvents during their occupational work for last 10 years. So, analyses of these factors have not been done.
Discussion
ALS is a chronic neurodegenerative disorder of unknown etiology seen in all the ethnic and socio economic groups of people in the world. Though a few numbers of ALS susceptibility genes have been discovered by different investigators, but till today how the selective destruction of motor neuron occurs has not been revealed out definitely. Role of exicitotoxins, free radicals, immune activation, neurofilament, and microtubule dysfunction have been suggested by different authors at different time.[3] Most of the ALS cases are sporadic in the world detected in different studies. It has been observed in different genetic studies across the world that 25-35% cases are familial ALS.[4] This study has not found any familial case of ALS, so role of familial or genetic factor in development of ALS could not be analyzed.
Men were predominantly affected than the female observed in the study which may not reflect the actual picture of male to female ratio as in the developed countries. As it was a hospital based study, attendance of female ALS patients in the hospital was less due to traditional lack of care for the female patients in many parts of India particularly in the rural areas.
Peak incidence of ALS has been detected in 65-74 years of age group observed in multiple studies particularly in the Western countries.[5] But in this study maximum numbers of ALS cases were detected in the 51-60 years age group. This study has detected a significant number of ALS patients in the second and third decades, indicating that ALS may occur at earlier age groups in fair numbers also.
A significant number of the ALS patients were the rural domicile people and the rural dwelling has been detected as a risk factor of ALS. Rural people are exposed to several chemicals used in the agricultural fields like fertilizers, insecticides, and pesticides and may have repeated physical trauma arising from their physical nature of jobs. On the other hand, these rural people were exposed to insecticides and pesticides during their occupational work in the agricultural field and also by the use of water for drinking purposes which sometimes may be contaminated with insecticides and pesticides. Role of insecticides and pesticides in development of ALS have not been studied well previously in India or elsewhere in the world though this study has found a significant association. A study by Weisskof et al. found no relation of pesticide exposure with development of ALS,[6] But an Australian case control study found exposure to pesticide as a risk factor of ALS.[7] In our study a significant association has been found between insecticide and pesticide exposure in development of ALS.
To detect the relationship between ALS and the occupational factors, this study has not found any significant association of any particular type of jobs like farming, industrial work, mining work, business profession, student, or house working in the development of ALS. Previously, occupation in the agriculture field was detected as an associated factor in development of ALS, but this study failed to detect any significant association of agricultural work or farming with ALS. It may be due to the fact that all the agricultural workers were not directly exposed to insecticides, pesticides. Besides this, association between agricultural works and ALS till today is a controversial issue. A case control study conducted in Brittany demonstrated a strong association between agricultural activity and ALS.[8] But at the same time, another study did in the northern part of Italy failed to demonstrate any significant association between agricultural work or farming and ALS.[9]
A few number of ALS patients and a few persons in the control group had the history of significant electrical shock or injury and a strong association has been detected between electrical injury and development of ALS. Role of electrical shock and injury in the development of ALS were evaluated previously in a few case control studies. There are some case reports in supporting development of ALS after high field electrical exposure.[10,11] A study by Noonan et al. demonstrated association of between magnetic field exposure and Parkinson's disease, but association with ALS was inconsistent in their study.[12] Again, Feychting et al. found increase risk of Alzheimer's disease with electromagnetic field exposure, but failed to show any association with ALS.[13] A systemic review of literature on ALS does not support causal relationship between ALS and electric shock.[14]
There is great controversy about smoking and development of ALS. Few studies found no association between smoking and ALS.[15–17] But analysis of EPIC cohort strongly supports the hypothesis that smoking causes ALS.[18] This is supported by a pooled analysis done by Hao Wang et al.[19] Our study has detected the smoking, present and past, as a significantly associated factor in development of ALS. Indian people have the habit of bidi smoking (homemade tobacco product) among the men particularly in rural dwellers and agricultural workers. Role of gender and smoking factors in development of ALS was interesting and conflicting issue. A prospective observational study has suggested that smoking may increase the likelihood of ALS in women but not in men and may even be protective.[20] But in this study a large number of ALS patients and the people in the control group had the habit or addiction of smoking (both current and past for more than 10 years) and all smokers were male. In the Indian culture women are usually nonsmoker. Probably a myriad of toxins associated with smoking may play a role in causing or triggering ALS by inducing oxidative stress.
This study has failed to show any significant association between the source of drinking water and development of ALS. None had the history of working under electromagnetic field, so role of this factor could not be evaluated in the development of ALS.
Conclusion
Though the case control study can only identify the association with the disease and do not exactly prove the casual relationship but still Case Control study may evaluate the putative risk factors for the disease. It is very difficult to conduct case control study with large number of cases of ALS even in the multicenter due to low incidence and prevalence rate. This study has observed the significant role of rural livings, smoking, insecticides, and pesticides exposure, electrical injury, or shock in development of ALS. But still today, many aspects of etiopathogenesis and risk factors of ALS are in the dark. So, there is need of further researches on the risk factors and etiopathogenesis of ALS for its effective remedy.
Acknowledgement
We are grateful to the Principal and Vice-Principal of Burdwan Medical College and Hospital, Burdwan, India, and Mr. Manoj Kr. Mondal, and Mr. Tapan Kr. Ghosh, Sr. Rehabilitation Specialist, for continuous active assistance with the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Wicklund MP. Amyotrophic lateral sclerosis: Possible role of environmental influences. Neurol Clin. 2005;23:461–84. doi: 10.1016/j.ncl.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig.Deciphering selective motor euron death in ALS. Nat Rev Neurosci. 2001;2:806–19. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 3.Brooks BR, Miller RG, Swash M. World Federation of Neurology Research group on Motor neuron disease.EI Escorial revisited: Revised criteria for diagnosis of Amyotrophic Lateral Sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 4.Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: What do we really know? Nat Rev Neurol. 2011;7:603–15. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 5.McGuire V, Nelson LM. Epidemiology of ALS. In: Mitsumoto H, Prezedborski S, Gordon PH, editors. Amyotrophic Lateral Sclerosis. New York: Taylor and Fraccis group; 2006. pp. 17–21. [Google Scholar]
- 6.Weisskopf MG, Morozova N, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:558–61. doi: 10.1136/jnnp.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: An Australian case-control study. Neuroepidemiology. 2006;27:130–5. doi: 10.1159/000095552. [DOI] [PubMed] [Google Scholar]
- 8.Furby A, Beauvais K, Kolev I, Rivain JG, Sebille V. Rural environmental and risk factors of amyotrophic lataral sclerosis: A case control study. J Neurol. 2010;257:792–8. doi: 10.1007/s00415-009-5419-5. [DOI] [PubMed] [Google Scholar]
- 9.Arman C, Kurland LT, Daube JR, O’Brien PC. Epidemiologic correlates of sporadic Amyotrophic Lateral Sclerosis. Neurology. 1991;41:1077–84. doi: 10.1212/wnl.41.7.1077. [DOI] [PubMed] [Google Scholar]
- 10.Huynh W, Lam A, Vucic S, Cheah BC, Clouston P, Kiernan MC. Corticospinal tract dysfunction and development of amyotrophic lateral sclerosis following electrical injury. Muscle Nerve. 2010;42:288–92. doi: 10.1002/mus.21681. [DOI] [PubMed] [Google Scholar]
- 11.Al-Ajmi A, Rousseff RT, Khuraibet AJ. Clinically definite ALS presenting weeks after mild electric injury: Causality or coincidence? Neurol Sci. 2012 doi: 10.1007/s10072-011-0918-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Noonan CW, Reif JS, Yost M, Touchstone J. Occupational exposure to magnetic fields in case referent studies of neurodegenerative disease. Scand J work Environ Health. 2002;28:42–8. doi: 10.5271/sjweh.645. [DOI] [PubMed] [Google Scholar]
- 13.Feychting M, Jonsson F, Pedersen NL, Ahlbom A. Occupational magnetic field exposure and neurodegenerative disease. Epidemiology. 2003;14:413–9. doi: 10.1097/01.EDE.0000071409.23291.7b. [DOI] [PubMed] [Google Scholar]
- 14.Abhinav K, Al-Chalabi A, Hortobagyi T, Leigh PN. Electrical injury and amyotrophic lateral sclerosis: A systematic review of the literature. J Neurol Neurosurg Psychiatry. 2007;78:450–3. doi: 10.1136/jnnp.2006.104414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt S, Kwee LC, Allen KD, Oddone EZ. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci. 2010;291:22–9. doi: 10.1016/j.jns.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pamphlett R, Ward EC. Smoking Is Not a Risk Factor for Sporadic Amyotrophic Lateral Sclerosis in an Australian Population. Neuroepidemiology. 2012;38:106–13. doi: 10.1159/000336013. [DOI] [PubMed] [Google Scholar]
- 17.Fang F, Bellocco R, Hernán MA, Ye W. Smoking, snuff dipping and the risk of amyotrophic lateral sclerosis – A prospective cohort study. Neuroepidemiology. 2006;27:217–21. doi: 10.1159/000096956. [DOI] [PubMed] [Google Scholar]
- 18.Gallo V, Bueno-De-Mesquita HB, Vermeulen R, Andersen PM, Kyrozis A, Linseisen J, et al. Smoking and risk for amyotrophic lateral sclerosis: Analysis of the EPIC cohort. Ann Neurol. 2009;65:378–85. doi: 10.1002/ana.21653. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, O’Reilly EJ, Weisskopf MG, Logroscino G, McCullough ML, Thun MJ, et al. Smoking and risk of amyotrophic lateral sclerosis: A pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68:207–13. doi: 10.1001/archneurol.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisskopf MG, McCullough ML, Calle EE, Thun MJ, Cudkowich M, Ascherio A. Prospective study of cigarette smoking and Amyotrophic Lateral Sclerosis. Am J Epidemiol. 2004;160:26–33. doi: 10.1093/aje/kwh179. [DOI] [PubMed] [Google Scholar]


