Abstract
Higher cyclooxygenase 2 (COX-2) expression is often observed in aggressive colorectal cancers (CRCs). Here, we attempt to examine the association between COX-2 expression in therapy-refractory CRC, how it affects chemosensitivity, and whether, in primary tumors, it is predictive of clinical outcomes. Our results revealed higher COX-2 expression in chemoresistant CRC cells and tumor xenografts. In vitro, the combination of either aspirin or celecoxib with 5-fluorouracil (5-FU) was capable of improving chemosensitivity in chemorefractory CRC cells, but a synergistic effect with 5-FU could only be demonstrated with celecoxib. To examine the potential clinical significance of these observations, in vivo studies were undertaken, which also showed that the greatest tumor regression was achieved in chemoresistant xenografts after chemotherapy in combination with celecoxib, but not aspirin. We also noted that these chemoresistant tumors with higher COX-2 expression had a more aggressive growth rate. Given the dramatic response to a combination of celecoxib + 5-FU, the possibility that celecoxib may modulate chemosensitivity as a result of its ability to inhibit MDR-1 was examined. In addition, assessment of a tissue microarray consisting of 130 cases of CRCs revealed that, in humans, higher COX-2 expression was associated with poorer survival with a 68% increased risk of mortality, indicating that COX-2 expression is a marker of poor clinical outcome. The findings of this study point to a potential benefit of combining COX-2 inhibitors with current regimens to achieve better response in the treatment of therapy-refractory CRC and in using COX-2 expression as a prognostic marker to help identify individuals who would benefit the greatest from closer follow-up and more aggressive therapy.
Introduction
Aspirin and related products are commonly used to treat inflammatory conditions, and epidemiological studies have also suggested that long-term treatment with aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), or selective cyclooxygenase 2 (COX-2) inhibitors may reduce the risk of colorectal cancers (CRCs) [1,2] and the development of its precursor lesions in sporadic CRC [3,4] and in those with familial adenomatous polyposis [5]. They decrease prostaglandin synthesis, which are modulators of cell growth [6], by inhibiting rate-limiting COX-1 and COX-2 enzymes that catalyze the conversion of arachidonic acid to prostaglandins and other eicosanoids. COX-1 is constitutively expressed in many cell types, whereas COX-2 is a primary response gene that is induced by growth factors and cytokines. In CRC, increased expression of COX-2 is found in up to 85% of cases but not in normal colonic epithelium [7,8]. There is also a progressive overexpression of COX-2 from early adenomas to carcinomas [9]. Animal studies support a key role of COX-2 in the initiation of CRC, as treatment of APCΔ716(+/-) mice with a COX-2 inhibitor reduces the number of intestinal polyps [10]. Its selective inhibition results in apoptosis in CRC cells [11,12]. Higher levels of COX-2 expression have also been seen in other types of cancers. For example, it promotes resistance to apoptosis in non-small cell lung cancer [13], and its inhibition can diminish growth and induce cell cycle arrest in hepatocellullar carcinoma cell lines [14]. These observations have led to studies assessing their cytotoxic effect when used in combination with conventional chemotherapies, such as 5-fluorouracil (5-FU) or irinotecan (CPT-11) in CRC cells [15].
Despite convincing in vitro evidence demonstrating an inhibitory effect of aspirin and related products on CRC cells, clinical studies assessing the efficacy of COX inhibitors in the treatment of CRC have met with variable results. Here we examine the efficacy of COX inhibition on a specific subset of tumors: chemorefractory CRC. CRC cells resistant to 5-FU or CPT-11 [16,17] were used to assess the effect of aspirin, a nonselective COX inhibitor, or celecoxib, a selective COX-2 inhibitor, in improving chemosensitivity when CRC cells were exposed in combination with 5-FU or CPT-11 in vitro and in vivo. Our findings provide a potential biologic explanation for the negative results of recent clinical trials showing lack of efficacy of COX inhibitors in CRC. In addition, we also demonstrate that COX-2 expression is clinically relevant and identifies those individuals with shorter overall survival.
Materials and Methods
Cell Lines
Human CRC cells MIP101 and RKO were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 1% penicillin-streptomycin, 1% kanamycin (Invitrogen, Carlsbad, CA) and 10% newborn calf serum (NCS) at 37°C and 5% CO2. For resistant cell lines, media were also supplemented as follows: MIP101 cells resistant to 5-FU (MIP/5-FU), 500 µM 5-FU; CPT-11 (MIP/CPT), 100 µM CPT-11; RKO cells resistant to 5-FU (RKO/5-FU), 25 µM5-FU; CPT-11 (RKO/CPT), 18 µM CPT-11 [16,17]. Chemoresistant cell lines were generated after incremental exposure to each of the specific agents (5-FU 10-500 µM, CPT-11 1-50 µM) during a 3-month period, as previously described, resulting in IC50 at least eight-fold higher than their sensitive counterparts [16,17]. All cell lines used in these studies had fewer than 20 passages.
Reverse Transcription-Polymerase Chain Reaction
RNA from CRC cells was extracted using TRIzol reagent (Invitrogen) 24 hours after seeding [16]. Three hundred nanograms of total RNA was used to generate cDNA (SuperScript III; Invitrogen). Specific primers for COX-1 (forward, 5′GTTTGGCATGGTGAGTGTTG-3′; reverse, 5′-AGGCACAGATTCAGGGAATG-3′), COX-2 (forward, 5′CTGTTGCGGAGAAAGGAGTC-3′; reverse, 5′-TCAAACAAGCTTTTACAGGTGA-3′), and β-actin [18] were used. Polymerase chain reaction products were separated on 3.0% agarose gel electrophoresis followed by ethidium bromide staining.
Aspirin and Celecoxib—In Vitro Studies
MIP101, MIP/5-FU, MIP/CPT, RKO, RKO/5-FU, and RKO/ CPT cells were treated with aspirin 1.8 mM (in PBS; Sigma, St Louis, MO) [19,20], celecoxib 30 µM (in 0.1% dimethyl sulfoxide; Pfizer, New York, NY) [21], or control vehicles. Aspirin concentrations were based on similar concentrations used in other in vitro studies [19,20,22] and at pharmacological levels relevant to clinical practice [23]. The concentration of celecoxib used was also based on previously published reports [21,24], and these concentrations were clinically relevant given that serum concentrations of COX-2 inhibitors in patients range from 20 to 100 µM[24,25].
Treated cells were then assessed for the following:
Cell viability. At 24 hours after seeding (∼50% confluence), cells were treated with 1.8 mM aspirin or 30 µM celecoxib for 48 hours followed by the addition of 5-µM 5-FU for an additional 48 hours. Cell viability was assessed by MTT assay. Synergistic effect between aspirin and celecoxib in combination with chemotherapy was assessed by using the following formula: RI (index) = [(cell survival observed with drug A alone) x (cell survival observed with drug B alone)] / (cell survival observed with the combination of drug A and drug B), where an RI ≤ 1.0 represented the absence of synergism or antagonism [26,27].
Clonogenic assay. At 24 hours after seeding, MIP/5-FU cells were treated with 1.8 mM aspirin or 30 µM celecoxib for 4 hours before the addition of 250 µM 5-FU (this higher concentration was used because the IC50 is 10-fold higher in MIP/5-FU cells than the chemosensitive MIP101 cells). Cells were incubated for a total of 7 days and then stained with Crystal violet as previously described [16] (n = 4 independent experiments).
Cell proliferation. Cells were seeded at equal numbers and 24 hours later treated with 1.8 to 3.6 mM aspirin or with 25 to 50 µM celecoxib. Cells were collected and counted for three consecutive days with a hematocytometer. Each group was counted thrice; averaged readings were based on three independent experiments.
Caspase 3/7 assay. At 24 hours after seeding, cells were treated with 1.8 mM aspirin or 30 µM celecoxib for 48 hours followed by incubation with 5 µM 5-FU for 24 hours. Total cell lysates were prepared using Chaps cell extract buffer (Cell Signaling Technology, Danvers, MA), and 20 µg of total protein/sample was used in a Caspase-Glo 3/7 Assay (Promega, Fitchburg, WI), as previously described [18]. Relative luminescence units were quantified using Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT).
Immunoblot analysis. Forty micrograms of total protein/sample was loaded from cell lysates prepared from cells that were treated with 1.8 mM aspirin or 30 µM celecoxib for 48 hours followed by exposure to 5 µM 5-FU. Samples were separated on a 10% SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA) [28]. Immunodetection was performed using antibodies against COX-2 (1:1000; LabVision, Fremont, CA) and MDR-1 (1:1000; kindly provided by Dr Victor Ling, British Columbia Cancer Agency) followed by incubation with the appropriate secondary antibody. All immunoblots were also probed with antibodies to β-actin (0.32 µg/ml; Abcam, Cambridge, MA) as a loading control. Proteins were detected with SuperSignal West Dura (Pierce, Rockford, IL).
Cell cycle analysis. MIP101, MIP/5-FU, and MIP/CPT cells seeded at 200,000 cells/well in a six-well plate in DMEM (10%, NCS) were subjected to cell cycle synchronization with double thymidine block (thymidine 2 mM [Sigma] in DMEM supplemented with 2% NCS) as previously described [16,28]. After an initial 16 hours of thymidine block, cells were released in DMEM (10% NCS) for 12 hours, which was then followed by a second 14-hour thymidine block, and incubated with 1.8 mM aspirin or 50 µM celecoxib. Cells released from this block were collected at timed intervals and processed for cell cycle analysis as previously described [28] and analyzed with Becton Dickinson FACSCalibur (Franklin Lakes, NJ) (n = 3 independent experiments).
Animal Studies
Tumor xenograft animal models were used to assess the efficacy of the combination of aspirin or celecoxib and chemotherapy (5-FU or CPT-1) on tumor progression in vivo. Tumor xenografts of nude mice (6 weeks old; Taconic, Hudson, NY) were implanted with 1 x 106 (MIP101, MIP/5-FU, and MIP/CPT) cells into the flanks of each animal. Treatment groups included aspirin ± 5-FU (or CPT-11), celecoxib ± 5-FU (or CPT-11), 5-FU or CPT-11 alone, and saline (n = 4 to 8 mice per group). Treatment regimens were initiated once the average tumor size reached 75 to 100 mm3 as previously described [16]. All studies were approved by the Animal Care Committee at the University of British Columbia. Tumors were measured using a handheld caliper (VWR, Radnor, PA) with concurrent body weight measurements until the completion of the study. Chemotherapy with 5-FU was provided using a 3-week cycle regimen (six cycles) as previously described [16]. Aspirin was administered intraperitoneally at 37.9 µg/g of mouse twice a week (based on an equivalent daily dose of 650 mg of aspirin for a 60-kg person), whereas celecoxib was given at 46.7 µg/g mouse twice weekly (equivalent to 800 mg daily for a 60-kg person). Control animals received saline. All mice received care according to standard animal care protocol and guidelines.
Tissue Microarray of Human CRCs
Paraffin-embedded blocks from 130 clinical samples of CRCs were constructed (each sample was represented by two cores). Samples were selected from cases with at least a 5-year follow-up period and linked to clinicopathologic data. This study was approved by the ethics board at the University of British Columbia (Canada) and Ajou School of Medicine (Korea). Immunohistochemical staining was performed using a COX-2 rabbit polyclonal antibody (LabVision). Briefly, after deparaffinization, the sections were rehydrated, washed, and subjected to microwave antigen retrieval in 10 mM citrate buffer, pH 6.0. The sections then immersed in 3% H2O2 for 15 minutes to block the endogenous peroxidase activity and next incubated for 30 minutes at room temperature with the anti-COX-2 antibody at 1:100 dilution, followed by detection with Cap-Plus Detection kit and 3,3′-diaminobenzidine (Zymed, South San Francisco, CA), and counterstained with Harris hematoxylin. The proportion of tumor cells showing positive staining was semiquantitatively evaluated as grade 0 (<5% staining), grade 1 (5%–30%), grade 2 (30% to 60%), and grade 3 (>60% staining) by two independent pathologists without having any knowledge of the clinical outcome.
Statistical Analysis
Statistical difference between groups was calculated and analyzed using Student's t test, 2-tailed; significance was defined as P <.05, using Smith's Statistical Package. Associations between various factors were assessed using Fisher exact test. Time to death was used in the analysis of overall survival. Significance levels, estimates of relative risk, and 95% confidence intervals were calculated using a proportional hazard regression model. Survival curves were estimated by the Kaplan-Meier method [29].
Results
Expression of COX-2 in Sensitive and Resistant CRC Cells
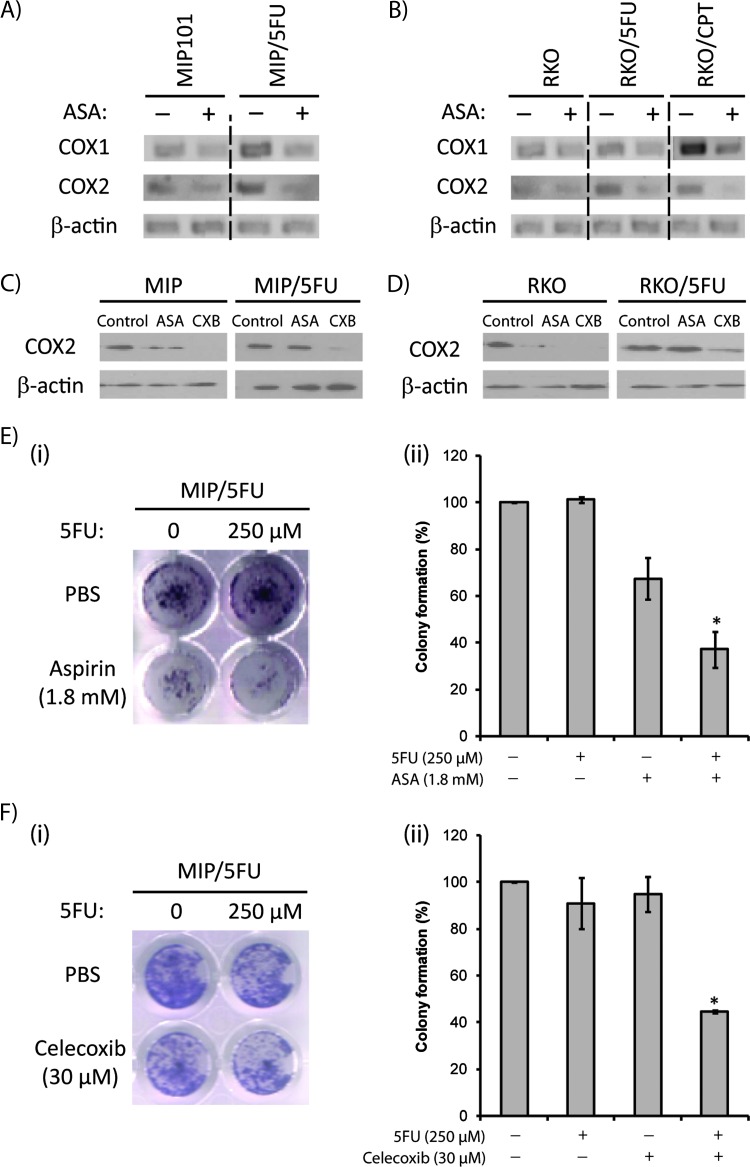
We assessed levels of COX-1 and COX-2 in sensitive MIP101 and RKO CRC cells, and their chemoresistant counterparts (5-FU chemotherapy-resistant MIP/5-FU and RKO/5-FU cells; and CPT-11-resistant RKO/CPT cells). We observed higher basal levels of COX-1 and COX-2 gene expression in chemoresistant cell lines compared with the sensitive cells (Figure 1, A and B). In all cases, COX-2 expression levels decreased after a 48-hour exposure to 1.8 mM aspirin or 30 µM of celecoxib in MIP101-(Figure 1C) and RKO-(Figure 1D) chemosensitive cell lines; however, only celecoxib was effective in decreasing COX-2 levels in the resistant MIP/5-FU and RKO/5-FU cells.
Figure 1.
Higher levels of COX-2 expression are seen in CRC cells. Levels of COX-1 and COX-2 were assessed in sensitive MIP101 and resistant MIP/5-FU cells (A) or sensitive RKO and resistant RKO/5-FU cells (B) by reverse transcription-polymerase chain reaction. The effects of aspirin or celecoxib exposures on COX-2 protein expression were also examined in MIP101 and MIP/5-FU (C) and RKO and RKO/5-FU cells (D) by immunoblot analysis. Clonogenic assays showing MIP/5-FU cells incubated with 1.8 mM aspirin (E) or 30 µM celecoxib (F) and exposed to 5-FU for 7 days. All results represent mean ± SE (n > 3 independent studies). ASA indicates aspirin; Cxb, celecoxib.
Aspirin and Celecoxib Reduce Cell Viability and Resensitize Therapy-Resistant CRC Cells to Chemotherapy In Vitro
An effect of aspirin or celecoxib alone and in combination with 5-FU in resistant MIP/5-FU cells on cell viability was initially assessed by clonogenic assays, which demonstrated significantly fewer colonies of therapy-resistant cells exposed to a combination of 1.8 mM aspirin or 30 µM celecoxib with 5-FU (Figure 1, E and F). Specifically, only 37.1% ± 7.6% and 44.6% ± 0.7% of colonies remained after treatment with aspirin (Figure 1Eii) or celecoxib (Figure 1Fii) in combination with 250 µM5-FU (P < .01), whereas no significant change was observed after exposure to either agents alone.
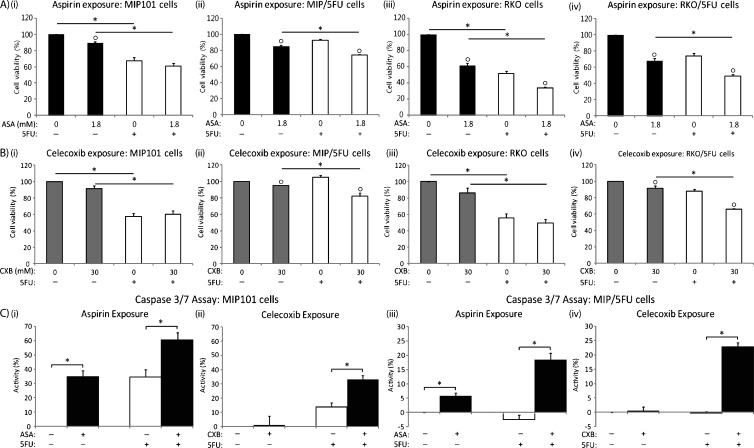
Cell viability, as assessed by MTT assays, demonstrated a differential response between sensitive and chemorefractory CRC cells to either aspirin or celecoxib: consistently, greater reductions in cell viability was observed in chemorefractory cells to either aspirin (Figure 2, Aii and Aiv) or celecoxib (Figure 2, Bii and Biv) in combination with 5-FU over single 5-FU treatments. Specifically, after a combination of aspirin with 5-FU, a further decrease in cell viability of 18.2% and 24.7% was observed in resistant MIP/5-FU (Figure 2Aii) and RKO/5-FU cells (Figure 2Aiv), respectively. Similarly with celecoxib (in combination with 5-FU, over 5-FU alone), cell viability decreased by an additional 22.0% in the resistant cells (MIP/5-FU — from 104.9% ± 5.8% viable cells [5-FU only] to 82.0% ± 9.4% [5-FU + celecoxib]; RKO/5-FU — from 87.9% ± 6.1% [5-FU only] to 65.9% ± 1.7% [5-FU + celecoxib]; P <.001).
Figure 2.
Exposure to aspirin and celecoxib decreases cell viability and enhances apoptosis in sensitive and chemoresistant CRC cells in vitro. (A) MIP101 (i), MIP/5-FU (ii), RKO (iii), and RKO/5-FU (iv) cells were treated with 1.8 mM aspirin (ASA) for 48 hours followed by exposure to 5 µM 5-FU for an additional 24 hours. (B) MIP101 (i), MIP/5-FU (ii), RKO (iii), and RKO/5-FU (iv) cells were similarly treated with 30 µM celecoxib (CXB). The percentage of viable cells was calculated based on the OD490 reading of nontreated control cells after an MTT assay as described in the Materials and Methods section. (C) Caspase 3/7 assay was performed after treatment of MIP101 (i and ii) and MIP/5-FU (iii and iv) with either 1.8 mM aspirin or 30 µM celecoxib for 48 hours (±5 µM 5-FU for 24 hours). All results represent mean ± SE (n = 3 independent studies). Statistical significance (“*” = P < .01 between the groups indicated by lines; or “○” = P < .01 in comparison to the group not treated with either ASA or CXB) is based on 2-tailed pairwise Student's t test.
In contrast, in sensitive MIP101 CRC cells, neither aspirin nor celecoxib (in combination with 5-FU) provided additional reductions over those seen with single treatments of 5-FU; whereas in RKO cells, a further decrease in cell viability of 17.7% was only observed after treatment with aspirin (with 5-FU) but not in combination with celecoxib.
To evaluate whether aspirin (or celecoxib) works in a synergistic manner with 5-FU to influence cell viability, the MTT assay results were analyzed using RI index, where values greater than 1.0 denote synergism. The results revealed that a synergistic effect was only noted following treatment with celecoxib in combination with 5-FU in the resistant MIP/5-FU and RKO/5-FU cells (Table 1) and not in the sensitive cells. Interestingly, despite the ability of aspirin (+5-FU) to potentiate further reductions in cell viability in resistant cells, the effects of the drugs were not synergistic.
Table 1.
Assessment of Synergism between COX-2 Inhibitors and Chemotherapy.
| Aspirin (mM) | 5-FU (5 µM) RI | Celecoxib (µM) | 5-FU (5 µM) RI | |
| MIP101 cells | 1.8 | 0.99 | 30 | 0.87 |
| MIP/5-FU cells | 1.8 | 1.05 | 30 | 1.21 |
| RKO cells | 1.8 | 0.93 | 30 | 0.97 |
| RKO/5-FU cells | 1.8 | 1.02 | 30 | 1.22 |
The Combination of Chemotherapy with a COX Inhibitor Enhances Apoptosis in Chemotherapy-Resistant CRC Cells
The possibility that the effect of either aspirin or celecoxib, together with 5-FU, in decreasing cell viability in sensitive and resistant cells could be due, in part, to apoptosis was also examined. MIP101 cells responded to 5-FU by increasing caspase 3/7 activity by 34.5% ± 5.23% (5-FU alone), and this increased even further by 60.3% ± 5.08% (P < .05) after co-incubation with aspirin (Figure 2Ci). Celecoxib showed a less, yet significant, increase in caspase 3/7 activity after the combination therapy in these sensitive cells (Figure 2Cii). In the case of resistant MIP/5-FU cells, caspase activity did not change significantly after incubation with 5-FU alone when compared with control untreated cells; but in combination with aspirin or celecoxib, there was an 18.4% ± 2.3% (P < .05) and 22.8% ± 1.5% (celecoxib + 5-FU) increase in caspase 3/7 activity (Figure 2, Ciii and Civ).
Reduction in Cell Viability Results in Part from Decreased Cell Proliferation after Aspirin Exposure
We noted that the aspirin (or celecoxib)-mediated reduction in cell viability in both sensitive MIP101 and resistant MIP/5-FU cells were due in part to a slower rate of cell proliferation, which translated to an increase in cell doubling time from 37.3 ± 1.3 to 42.6 ± 4.9 hours (P < .05) and from 37.3 ± 1.3 to 73.1 ± 7.8 hours (P < .05) for MIP101 cells exposed to 1.8 and 3.6 mM aspirin, respectively (Figure W1 A). Similarly, cell doubling time increased from 58.8 ± 4.4 to 76.3 ± 2.5 hours (P < .05) and 58.8 ± 4.4 to 74.9 ± 1.6 hours (P <.05) for MIP/5-FU cells in the presence of 1.8 and 3.6 mM aspirin, respectively. The effect of celecoxib in diminishing the rate of cell proliferation was most apparent after incubation with 50 µM of celecoxib, with the average cell doubling time increasing from 26.8 ± 0.35 hours in control MIP101 cells to 33.1 ± 1.87 hours (P < .05) in celecoxib-exposed cells and similarly from 47.9 ± 5.3 hours in control MIP/5-FU to 61.8 ± 4.1 hours (P < .05) after exposure to 50 µM of celecoxib (Figure W1B).
Effect of Aspirin and Celecoxib on Cell Cycle Progression in Chemotherapy-Resistant Cells
These results demonstrated that the rate of cell proliferation was diminished after incubation of sensitive MIP101 and resistant MIP/5-FU cells to aspirin or celecoxib, and it was associated with an increase in cell doubling times. Next, we examined the possibility of whether a delay in cell cycle progression could be a contributing factor. Indeed, a delay to progress through the G1/S phase was observed because a significantly greater percentage of cells remained in the G1 phase of the cell cycle after exposure to 1.8 mM of aspirin in the sensitive MIP101 and resistant MIP/5-FU or MIP/CPT cells (Figure W2). In MIP101 cells, of the 73.8% of cells that were initially in the G1 phase of the cell cycle, only 29.4% remained after 6 hours. In the presence of aspirin, 37.6% of cells remained in G1 phase. This delay is even more dramatic in resistant MIP/ 5-FU and MIP/CPT cells where, in the presence of aspirin, 60.2% and 40.4% of cells failed to progress to the next phase, respectively.
A similar delay in cell cycle progression was observed with celecoxib. In control MIP 101 cells, 34.5% of the initial 60.3% of MIP101 cells progressed to the S-phase of the cell cycle, but after exposure to celecoxib, only 14.8% of the cells entered the S phase, whereas the majority remained in the G1 phase. This delay was also observed in MIP/CPT cells incubated with celecoxib (Figure W2).
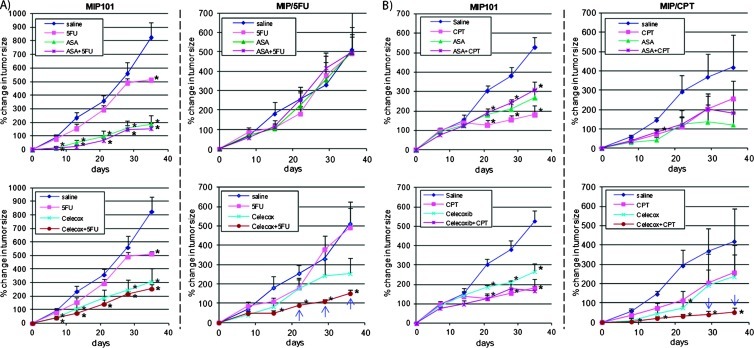
Chemoresistant Tumor Xenografts Treated with a Combination of Celecoxib with 5-FU Have Greater Tumor Regression
In vitro, the combination of celecoxib (but not aspirin) with 5-FU effectively improved sensitivity to 5-FU in 5-FU-chemorefractory CRC cells in a synergistic manner. To examine the potential clinical significance of these observations, in vivo studies were undertaken, which demonstrated that the greatest tumor regression was also achieved in resistant MIP/5-FU and CPT-resistant MIP/CPT xenografts, but not in the sensitive MIP101 xenografts, after a combination of chemotherapy with celecoxib (Figure 3). In chemosensitive xenografts of MIP101 cells, 5-FU or CPT-11 in combination with either aspirin or celecoxib did not influence tumor growth in comparison to either chemotherapies or COX inhibitors alone (Figure 3, A and B, left panels). Specifically, in xenografts of sensitive MIP101 cells, the percentage change in tumor size was less than 300% of the original tumor by day 36 of treatment after a combination of chemotherapy with either aspirin or celecoxib, which were not significantly different in comparison to any of the agents alone (P >.05).
Figure 3.
Celecoxib in combination with chemotherapy induces tumor regression of chemoresistant CRCs in vivo. Mice with MIP101, MIP/5-FU, and MIP/CPT tumor xenografts were treated with saline (control) or with different drug combinations followed by measurement of tumor size twice per week for a total of 36 days. Percent change in tumor size was calculated with respect to the size of the tumor before treatment. Results represent data averaged from data obtained from four to eight mice per group. Statistical significance: *P < .05 in comparison to saline-treated controls; blue arrow, P < .05 in comparison to single treatments (celecoxib, 5-FU or CPT-11).
In xenografts of resistant MIP/5-FU and MIP/CPT cells, however, treatment with celecoxib in combination with either 5-FU or CPT11 resulted in a significantly slower rate of growth by day 21 (in MIP/ 5-FU xenografts) and day 28 (in MIP/CPT xenografts) in comparison to either agents alone. In xenografts of MIP/5-FU cells, tumors remained less than 100% of the original size after treatment with celecoxib in combination with 5-FU, whereas tumors treated with either agents alone had already increased by more than 200% by 21 days of initiating treatment, P < .05 (Figure 3A, lower right panel). Celecoxib in combination with CPT-11 seemed to be even more efficacious because tumor xenografts of MIP/CPT cells treated with this combination had less than 40% change in tumor size by day 28 days, whereas all other treatment groups had greater than 200% change in tumor size (P < .05; Figure 3B, lower right panel).
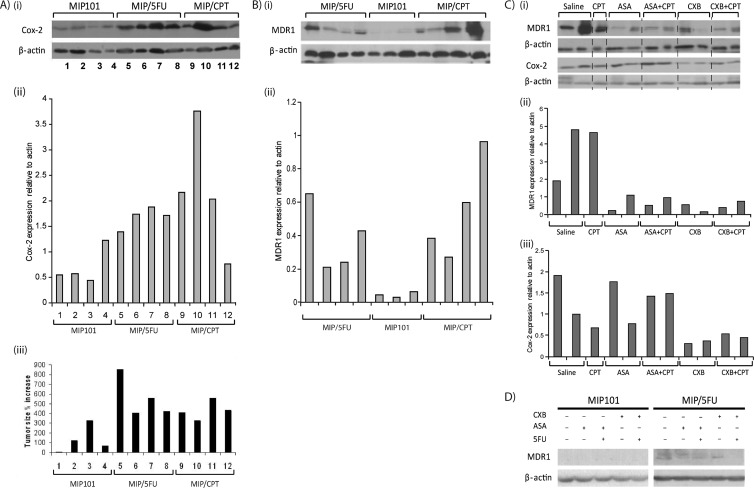
We also confirmed that resistant MIP/5-FU and MIP/CPT tumor xenografts had higher COX-2 expression in comparison to xenografts of sensitive MIP101 cells (Figure 4, Ai and Aii). Interestingly, tumors with a higher COX-2 expression also experienced a more rapid increase in tumor size (Figure 4Aiii, tumor samples 5–12), in comparison to sensitive tumors with a lower COX-2 expression (Figure 4Aiii, tumor samples 1-4).
Figure 4.
Expression of COX-2 and MDR-1 in tumor xenografts of sensitive and resistant CRC cells. (A) Basal levels of COX-2 expression in MIP101 (samples 1–4), MIP/5-FU (samples 5–8), and MIP/CPT (samples 9–12) tumor xenografts treated with saline by immunoblot analysis (i) and relative levels of COX-2 (ii) normalized to β-actin (densitometry); (iii) bar diagram shows percent increase in tumor size of these same tumors. (B) MDR-1 expression of MIP101, MIP/5-FU, and MIP/CPT tumor xenografts assessed by immunoblot analysis (i) and relative levels of MDR-1 (ii) normalized to β-actin. (C) MDR-1 and COX-2 expression in MIP/CPT tumor xenografts after treatment with indicated drugs, (i) by immunoblot analysis, with relative levels of MDR-1 (ii) and COX-2 (iii) normalized to β-actin. (D) MIP101 and MIP/5-FU cells were assessed for MDR-1 expression after treatment with different drug combinations in vitro by immunoblot analysis. In all cases, β-actin was used as a loading control.
Reduction in MDR-1 Expression in Tumor Xenografts after Exposure to COX inhibitors
Given the dramatic tumor regression observed in therapy-refractory tumor xenografts to a combination of celecoxib and 5-FU, the possibility that celecoxib may be modulating chemosensitivity as a result of its ability to influence multidrug-resistant efflux pumps was examined. In vivo, lower MDR-1 levels were detected in tumor xenografts of sensitive MIP101 cells, in comparison to xenografts of resistant MIP/5-FU or MIP/CPT cells (Figure 4B). In addition, in xenografts of MIP/CPT cells, a reduction in MDR-1 levels was noted after exposure to celecoxib alone or in combination with 5-FU (Figure 4, Ci and Cii). Aspirin (alone or in combination with 5-FU) was also effective in reducing MDR-1 expression. However, in these same tumors, COX-2 levels were most effectively reduced after exposure to celecoxib, whereas no significant decrease in COX-2 could be observed after treatment with aspirin (Figure 4Ciii).
Interestingly, in vitro, celecoxib in combination with 5-FU seemed to be more effective in reducing MDR-1 expression in MIP/5-FU cells, whereas no significant changes were detected after exposure to a combination that included aspirin (Figure 4D).
COX-2 Expression and Clinical Outcomes in CRCs
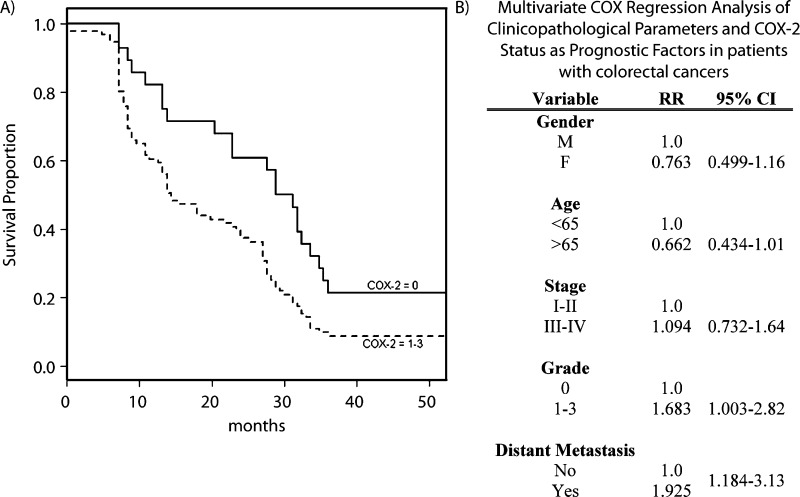
To assess the clinical significance of higher COX-2 expression in CRC, we evaluated tissues from 130 individuals and assessed their clinical outcomes during a 50-month period. On the basis of clinicopathologic characteristics, COX-2 expression was significantly higher in patients older than 65 years (P < .05; Table 2). There did not seem to be an association between the age of the patient and disease stage (P = .68), although there was a trend toward a greater likelihood of developing distant metastasis in these older individuals (P =.053). Differences in COX-2 expression were independent of disease stage (P = .59). However, based on multivariate Cox regression analysis, the mortality risk was significantly higher in individuals with higher COX-2 expression (grades 1–3) in comparison to those with a low expression (grade 0; P < .05; Figures 5A and W3), representing a 68% increased risk in mortality (Figure 5B), adjusting for age, sex, disease stage, and status of distant metastasis.
Table 2.
Clinicopathologic Characteristics of Cases of CRCs.
| Characteristics | No. Patients | % | COX-2-Positive Cases | |
| n | % | |||
| All cases | 131 | 100.0 | 92 | 70.2 |
| Age (years) | ||||
| <50 | 22 | 16.8 | 13 | 59.1 |
| >50 | 98 | 74.8 | 79 | 80.6 |
| Sex | ||||
| M | 65 | 49.6 | 37 | 56.9 |
| F | 55 | 42.0 | 47 | 85.5 |
| Stage | ||||
| I | 1 | 0.8 | 1 | 100.0 |
| II | 47 | 35.9 | 30 | 63.8 |
| III | 72 | 55.0 | 52 | 72.2 |
| IV | 8 | 6.1 | 8 | 100.0 |
| Recurrence | ||||
| None | 118 | 90.1 | 61 | 51.7 |
| Local | 13 | 9.9 | 8 | 61.5 |
| Distant | 38 | 29.0 | 28 | 73.7 |
| Overall survival (years) | ||||
| >5 | 4 | 3.1 | 2 | 50.0 |
| <5 | 126 | 96.2 | 87 | 70.6 |
| Disease-free survival (years) | ||||
| >5 | 4 | 3.1 | 2 | 50.0 |
| <5 | 126 | 96.2 | 87 | 70.6 |
Figure 5.
Association between COX-2 expression in human CRC and prognosis. (A) Kaplan-Meier survival curve based on COX-2 expression in human CRC. COX-2 = 0 represents grade 0 expression; COX-2 = 1–3, represents grade 1–3 expression. (B) Multivariate COX regression analysis of clinicopathologic parameters and COX-2 status as prognostic factors in patients with CRCs.
Discussion
Accumulating evidence from animal and in vitro studies has demonstrated antitumor properties of aspirin and related NSAIDs against CRC [30,31]. However, clinical studies have been equivocal in showing a therapeutic advantage of including a COX-2 inhibitor to a treatment regimen in CRC. In this study, we show that CRC cells that have become resistant to chemotherapy have significantly higher levels of COX-2 gene and protein expression than chemosensitive cells. In addition, we demonstrate the potential therapeutic efficacy of introducing celecoxib in combination with chemotherapy to overcome resistance in these therapy-refractory cancer cells in vitro and in vivo.
Previous studies using “chemotherapy-naive” sensitive cells support our findings with sensitive CRC cells in that aspirin, and other nonselective COX inhibitors, suppress tumor growth [32,33]. For example, using HT-29 cells, the combination of aspirin and 5-FU in vitro was effective in inhibiting cell proliferation, and increasing apoptosis by upregulating Bax. Another nonselective NSAID, ibuprofen, has also been shown to improve the effects of CPT-11, by modulating tumor angiogenesis [32]. Several studies have also demonstrated a positive effect of combining specific COX-2 inhibitors with chemotherapy 5-FU and/or CPT-11 to promote tumor regression [34,35]. In vitro studies by Chen et al. [15] using HT-29 and SW620 cells showed a reduction in cell viability only after sequential administration of a COX-2 inhibitor (etodolac) followed by either 5-FU or SN-38. In preclinical experimental models, celecoxib decreased the growth of HT-29 tumor xenografts in a dose-dependent manner, and when administered in combination with CPT-11, tumor growth was inhibited by ∼92% [36]. Another COX-2 inhibitor, rofecoxib, also reduced the growth of primary MC-26 colon cancer intrasplenic tumors and liver metastasis when used in combination with either 5-FU or CPT-11 [37]. They also showed that tumors exposed to COX-2 inhibitor had lower levels of COX-2, cyclin D1, β-catenin, matrix metalloproteinases 1 and 9, and vascular endothelial growth factor expression. Whereas most preclinical in vitro and in vivo studies have been more consistent in showing an improvement in chemotherapy response after a combination of either a nonselective or a selective COX-2 inhibitor with chemotherapy, clinical studies have unfortunately demonstrated variable efficacy [32,36–39]. However, this study helps shed some light and provides a potential explanation for the inconsistent findings of recent clinical trials assessing the efficacy of combining selective COX-2 inhibitors with various chemotherapies [34,38–41]. We demonstrate that: (1) in tumors that have become refractory to therapy, the use of a specific COX-2 inhibitor (celecoxib) dramatically enhances therapeutic response in vivo, in comparison to a nonselective inhibitor (aspirin); and (2) that celecoxib resensitizes therapy-refractory CRC cells to chemotherapy (either 5-FU or CPT-11), by enhancing apoptosis in addition to suppressing MDR-1 expression and tumor growth. These observations suggest that a selective COX-2 inhibitor is more efficacious in promoting tumor regression in therapy-resistant cells not only by augmenting apoptosis and inhibiting tumor growth via its down-regulation of COX-2 expression but also by improving drug availability by lowering MDR-1 levels. Our findings correlate well with the results of clinical trials where only patients with advanced CRC who had previously failed chemotherapy were included in the study [38]. Gasparini et al. [38] were able to demonstrate a positive therapeutic response to a COX-2 inhibitor when used in combination with chemotherapy. Similarly, in line with our in vivo results that showed no additional benefit of treating chemosensitive tumors with COX-2 inhibitors, clinical trials that mostly included chemonaive patients also failed to demonstrate an objective response with the inclusion of a COX-2 inhibitor in their chemotherapy regimens [39,40,42,43]. It must be noted that patients who had prior adjuvant chemotherapy at least 6 months before enrollment were included in some of these studies with negative results, but the number of these patient was either not reported [40] or was too small (16% in the phase 2 study by El-Rayes et al. [39]) to allow for a subgroup analysis to determine whether COX-2 affected therapeutic efficacy in this particular subset of patients.
We also observed, not unexpectedly, an up-regulation in multidrug-resistant efflux pump MDR-1 in tumor xenografts of resistant MIP/5-FU and MIP/CPT. But interestingly, MDR-1 expression was significantly lower in tumor xenografts after treatment with a COX inhibitor, and in vitro, its inhibitory effects were most pronounced with celecoxib in combination with chemotherapy. As noted above, these observations help explain the greater efficacy of celecoxib in therapy-resistant CRCs in that it not only suppresses tumor growth but also downregulates the expression of the multidrug-resistant efflux pump, thereby resulting in resensitization of cells to chemotherapy. Recent studies in breast cancer have also indicated that celecoxib can reduce multidrug-resistant pump activity [44], with the greatest effect in a mitoxantrone-resistant breast cancer cell line, MCF7-MX [45] where reductions in mitoxantrone efflux was observed after treatment with celecoxib. More recently, MDR-1 expression was shown to be induced by prostaglandin E2 (PGE2), a metabolite of COX-2, in hepatocellular carcinoma [46], which also helps explain the observation that COX-2 inhibition diminishes MDR-1 mRNA in thyroid cancer cells [47], CRCs cells [48], and our own observations.
In this study, exposure to a dose equivalent to taking celecoxib at 400 mg twice daily effectively reduced COX-2 expression in tumor xenografts. A similar dose has also been shown to inhibit PGE2 synthesis in tumors of patients with pancreatic cancers [49], thereby supporting the pharmacodynamic effects of this dose. It must be noted, however, that although inhibition of PGE2 was achieved in surgically resected human pancreatic tumors after only 5 to 15 days of celecoxib administration in the study by Jimeno et al. [49], there was no evidence of tumor regression in tumor xenografts in mice treated for 28 days with celecoxib alone. The absence of a demonstrable response to a single agent alone is not surprising and suggests that an approach that includes a combination chemotherapy cocktail in patients known to have high intratumoral COX-2 expression may be necessary to improve response in pancreatic cancers.
In addition to providing evidence that chemotherapy-resistant CRCs have significantly higher levels of COX-2 expression than chemosensitive tumors do, we also demonstrated a strong clinical association between high levels of COX-2 expression in the primary tumor and subsequent risk of mortality from colon cancer. In support of our findings, the association between high COX-2 gene expression with poor survival and response to chemotherapy had also been previously demonstrated in a smaller study examining 40 patients [50]. These observations suggest that COX-2 expression may be a useful prognostic marker and can be used to risk stratify patients into a group that would benefit not only from more aggressive follow-up and treatment but also from a combination treatment that includes a COX-2 inhibitor, such as celecoxib, to help improve therapeutic response by downregulating MDR-1 and thereby facilitate tumor regression.
The results of the current study demonstrate that CRC cells that are refractory to either 5-FU or CPT-11 undergo greater apoptosis and have a better response to growth inhibition as a result of exposure to specific COX-2 inhibitors in vivo. Our findings have important clinical implications because they suggest that a personalized treatment cocktail that includes a COX-2 inhibitor in selected patients (those with therapy-refractory tumors with high COX-2 and MDR-1 expression) is more likely to achieve greater tumor regression and an overall improvement in therapeutic response.
Supplementary Material
Acknowledgments
The authors thank M. J. Tang for technical assistance.
Abbreviations
- 5-FU
5-fluorouracil
- COX
cyclooxygenase
- CPT-11
irinotecan
- CRC
colorectal cancer
- MIP/5-FU
MIP101 cells resistant to 5-fluoruracil
- MIP/CPT
MIP101 cells resistant to irinotecan
- RKO/5-FU
RKO cells resistant to 5-fluorouracil
- RKO/CPT
RKO cells resistant to irinotecan
Footnotes
This work was supported by grants from the Canadian Association of Gastroenterology, Canadian Institutes of Health Research (CIHR, CST-85477), and the Michael Smith Foundation for Health Research (MSFHR). I.T. Tai is an MSFHR scholar and a CIHR new investigator.
This article refers to supplementary materials, which are designated by Figures W1 to W3 and are available online at www.neoplasia.com.
References
- 1.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 3.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 4.Arber N, Spicak J, Racz I, Zavoral M, Breazna A, Gerletti P, Lechuga MJ, Collins N, Rosenstein RB, Eagle CJ, et al. Five-year analysis of the prevention of colorectal sporadic adenomatous polyps trial. Am J Gastroenterol. 2011;106:1135–1146. doi: 10.1038/ajg.2011.116. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 6.DeWitt D, Smith WL. Yes, but do they still get headaches? Cell. 1995;83:345–348. doi: 10.1016/0092-8674(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 7.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 8.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part II) J Natl Cancer Inst. 1998;90:1609–1620. doi: 10.1093/jnci/90.21.1609. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 10.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 11.Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. JBiol Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 12.Chen WS, Liu JH, Wei SJ, Liu JM, Hong CY, Yang WK. Colon cancer cells with high invasive potential are susceptible to induction of apoptosis by a selective COX-2 inhibitor. Cancer Sci. 2003;94:253–258. doi: 10.1111/j.1349-7006.2003.tb01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krysan K, Merchant FH, Zhu L, Dohadwala M, Luo J, Lin Y, Heuze-Vourc'h N, Pold M, Seligson D, Chia D, et al. COX-2-dependent stabilization of survivin in non-small cell lung cancer. Faseb J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J, Imanishi H, Amuro Y, Hada T. NS-398, a selective cyclooxygenase 2 inhibitor, inhibited cell growth and induced cell cycle arrest in human hepatocellular carcinoma cell lines. Int J Cancer. 2002;99:755–761. doi: 10.1002/ijc.10409. [DOI] [PubMed] [Google Scholar]
- 15.Chen WS, Liu JH, Liu JM, Lin JK. Sequence-dependent effect of a cyclooxygenase-2 inhibitor on topoisomerase I inhibitor and 5-fluorouracil- induced cytotoxicity of colon cancer cells. Anticancer Drugs. 2004;15:287–294. doi: 10.1097/00001813-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Tai IT, Dai M, Owen DA, Chen LB. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest. 2005;115:1492–1502. doi: 10.1172/JCI23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong JC, Chan SK, Schaeffer DF, Sagaert X, Lim HJ, Kennecke H, Owen DA, uh KW, Kim YB, Tai IT. Absence of MMP2 expression correlates with poor clinical outcomes in rectal cancer, and is distinct from MMP1-related outcomes in colon cancer. Clin Cancer Res. 2011;17:4167–4176. doi: 10.1158/1078-0432.CCR-10-1224. [DOI] [PubMed] [Google Scholar]
- 18.Tang MJ, Tai IT. A novel interaction between procaspase 8 and SPARC enhances apoptosis and potentiates chemotherapy sensitivity in colorectal cancers. J Biol Chem. 2007;282:34457–34467. doi: 10.1074/jbc.M704459200. [DOI] [PubMed] [Google Scholar]
- 19.Weiss H, Amberger A, Widschwendter M, Margreiter R, Ofner D, Dietl P. Inhibition of store-operated calcium entry contributes to the anti-proliferative effect of non-steroidal anti-inflammatory drugs in human colon cancer cells. Int J Cancer. 2001;2:877–882. doi: 10.1002/ijc.1280. [DOI] [PubMed] [Google Scholar]
- 20.Shao J, Fujiwara T, Kadowaki Y, Fukazawa T, Waku T, Itoshima T, Yamatsuji T, Nishizaki M, Roth JA, Tanaka N. Overexpression of the wild-type p53 gene inhibits NF-κB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000;19:726–736. doi: 10.1038/sj.onc.1203383. [DOI] [PubMed] [Google Scholar]
- 21.Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P. Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res. 2005;7:R422–R435. doi: 10.1186/bcr1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Din FV, Dunlop MG, Stark LA. Evidence for colorectal cancer cell specificity of aspirin effects on NFκB signalling and apoptosis. Br J Cancer. 2004;91:381–388. doi: 10.1038/sj.bjc.6601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Insel P. Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. In: Hardman JG, Limberd LE, Molinoff PB, Rudden RW, Gilman AG, editors. The Pharmacological Basis of Therapeutics. McGraw-Hill: New York, NY; 1996. pp. 617–659. [Google Scholar]
- 24.Munkarah AR, Genhai Z, Morris R, Baker VV, Deppe G, Diamond MP, Saed GM. Inhibition of paclitaxel-induced apoptosis by the specific COX-2 inhibitor, NS398, in epithelial ovarian cancer cells. Gynecol Oncol. 2003;88:429–433. doi: 10.1016/s0090-8258(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 25.Rose MJ, Woolf EJ, Matuszewski BK. Determination of celecoxib in human plasma by normal-phase high-performance liquid chromatography with column switching and ultraviolet absorbance detection. J Chromatogr B Biomed Sci Appl. 2000;738:377–385. doi: 10.1016/s0378-4347(99)00552-6. [DOI] [PubMed] [Google Scholar]
- 26.Kern DH, Morgan CR, Hildebrand-Zanki SU. In vitro pharmacodynamics of 1-β-d-arabinofuranosylcytosine: synergy of antitumor activity with cis-diamminedichloroplatinum(II) Cancer Res. 1988;48:117–121. [PubMed] [Google Scholar]
- 27.Romanelli S, Perego P, Pratesi G, Carenini N, Tortoreto M, Zunino F. In vitro and in vivo interaction between cisplatin and topotecan in ovarian carcinoma systems. Cancer Chemother Pharmacol. 1998;41:385–390. doi: 10.1007/s002800050755. [DOI] [PubMed] [Google Scholar]
- 28.Taghizadeh F, Tang MJ, Tai IT. Synergism between vitamin D and secreted protein acidic and rich in cysteine-induced apoptosis and growth inhibition results in increased susceptibility of therapy-resistant colorectal cancer cells to chemotherapy. Mol Cancer Ther. 2007;6:309–317. doi: 10.1158/1535-7163.MCT-06-0517. [DOI] [PubMed] [Google Scholar]
- 29.Fisher LD, van Belle G. Biostatistics: A Methodology for the Health Sciences. New York, NY: John Wiley & Sons, Inc; 1993. [Google Scholar]
- 30.Ricchi P, Pignata S, Di Popolo A, Memoli A, Apicella A, Zarrilli R, Acquaviva AM. Effect of aspirin on cell proliferation and differentiation of colon adenocarcinoma Caco-2 cells. Int J Cancer. 1997;73:880–884. doi: 10.1002/(sici)1097-0215(19971210)73:6<880::aid-ijc20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Leonetti C, Scarsella M, Zupi G, Zoli W, Amadori D, Medri L, Fabbri F, Rosetti M, Ulivi P, Cecconetto L, et al. Efficacy of a nitric oxide-releasing nonsteroidal anti-inflammatory drug and cytotoxic drugs in human colon cancer cell lines in vitro and xenografts. Mol Cancer Ther. 2006;5:919–926. doi: 10.1158/1535-7163.MCT-05-0536. [DOI] [PubMed] [Google Scholar]
- 32.Yao M, Zhou W, Sangha S, Albert A, Chang AJ, Liu TC, Wolfe MM. Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res. 2005;11:1618–1628. doi: 10.1158/1078-0432.CCR-04-1696. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Horvath CM, Waxman S. Regrowth of 5-fluorouracil-treated human colon cancer cells is prevented by the combination of interferon gamma, indomethacin, and phenylbutyrate. Cancer Res. 2000;60:3200–3206. [PubMed] [Google Scholar]
- 34.Becerra CR, Frenkel EP, Ashfaq R, Gaynor RB. Increased toxicity and lack of efficacy of rofecoxib in combination with chemotherapy for treatment of metastatic colorectal cancer: a phase II study. Int J Cancer. 2003;105:868–872. doi: 10.1002/ijc.11164. [DOI] [PubMed] [Google Scholar]
- 35.Mizutani Y, Kamoi K, Ukimura O, Kawauchi A, Miki T. Synergistic cytotoxicity and apoptosis of JTE-522, a selective cyclooxygenase-2 inhibitor, and 5-fluorouracil against bladder cancer. J Urol. 2002;168:2650–2654. doi: 10.1016/S0022-5347(05)64237-1. [DOI] [PubMed] [Google Scholar]
- 36.Trifan OC, Durham WF, Salazar VS, Horton J, Levine BD, Zweifel BS, Davis TW, Masferrer JL. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 2002;62:5778–5784. [PubMed] [Google Scholar]
- 37.Yao M, Kargman S, Lam EC, Kelly CR, Zheng Y, Luk P, Kwong E, Evans JF, Wolfe MM. Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res. 2003;63:586–592. [PubMed] [Google Scholar]
- 38.Gasparini G, Gattuso D, Morabito A, Longo R, Torino F, Sarmiento R, Vitale S, Gamucci T, Mariani L. Combined therapy with weekly irinotecan, infusional 5-fluorouracil and the selective COX-2 inhibitor rofecoxib is a safe and effective second-line treatment in metastatic colorectal cancer. Oncologist. 2005;10:710–717. doi: 10.1634/theoncologist.10-9-710. [DOI] [PubMed] [Google Scholar]
- 39.El-Rayes BF, Zalupski MM, Manza SG, Rusin B, Ferris AM, Vaishampayan U, Heilbrun LK, Venkatramanamoorthy R, Shields AF, Philip PA. Phase-II study of dose attenuated schedule of irinotecan, capecitabine, and celecoxib in advanced colorectal cancer. Cancer Chemother Pharmacol. 2008;61:283–289. doi: 10.1007/s00280-007-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maiello E, Giuliani F, Gebbia V, Di Renzo N, Pezzella G, Romito S, Mallamaci R, Lopez M, Colucci G. FOLFIRI with or without celecoxib in advanced colorectal cancer: a randomized phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM) Ann Oncol. 2006;17(suppl 7):vii55–vii59. doi: 10.1093/annonc/mdl952. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 42.Kohne CH, De Greve J, Hartmann JT, Lang I, Vergauwe P, Becker K, Braumann D, Joosens E, Muller L, Janssens J, et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann Oncol. 2008;19:920–926. doi: 10.1093/annonc/mdm544. [DOI] [PubMed] [Google Scholar]
- 43.Andre T, Tournigand C, Mineur L, Fellague-Chebra R, Flesch M, Mabro M, Hebbar M, Postel Vinay S, Bidard FC, Louvet C, et al. Phase II study of an optimized 5-fluorouracil-oxaliplatin strategy (OPTIMOX2) with celecoxib in metastatic colorectal cancer: a GERCOR study. Ann Oncol. 2007;18:77–81. doi: 10.1093/annonc/mdl336. [DOI] [PubMed] [Google Scholar]
- 44.Chen C, Shen HL, Yang J, Chen QY, Xu WL. Preventing chemoresistance of human breast cancer cell line, MCF-7 with celecoxib. J Cancer Res Clin Oncol. 2011;37:9–17. doi: 10.1007/s00432-010-0854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalalinia F, Elahian F, Behravan J. Potential role of cyclooxygenase-2 on the regulation of the drug efflux transporter ABCG2 in breast cancer cell lines. J Cancer Res Clin Oncol. 2011;137:321–330. doi: 10.1007/s00432-010-0893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achari C, Reddy GV, Reddy TC, Reddanna P. Chebulagic acid synergizes the cytotoxicity of doxorubicin in human hepatocellular carcinoma through COX-2 dependant modulation of MDR-1. Med Chem. 2011;7:432–442. doi: 10.2174/157340611796799087. [DOI] [PubMed] [Google Scholar]
- 47.Vivaldi A, Ciampi R, Tacito A, Molinaro E, Agate L, Bottici V, Pinchera A, Collecchi P, Elisei R. Celecoxib, a cyclooxygenase-2 inhibitor, potentiates the chemotherapic effect of vinorelbine in the medullary thyroid cancer TT cell line. Mol Cell Endocrinol. 2012;355:41–48. doi: 10.1016/j.mce.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Sui H, Zhou S, Wang Y, Liu X, Zhou L, Yin P, Fan Z, Li Q. COX-2 contributes to P-glycoprotein-mediated multidrug resistance via phosphorylation of c-Jun at Ser63/73 in colorectal cancer. Carcinogenesis. 2011;32:667–675. doi: 10.1093/carcin/bgr016. [DOI] [PubMed] [Google Scholar]
- 49.Jimeno A, Amador ML, Kulesza P, Wang X, Rubio-Viqueira B, Zhang X, Chan A, Wheelhouse J, Kuramochi H, Tanaka K, et al. Assessment of celecoxib pharmacodynamics in pancreatic cancer. Mol Cancer Ther. 2006;5:3240–3247. doi: 10.1158/1535-7163.MCT-06-0565. [DOI] [PubMed] [Google Scholar]
- 50.Uchida K, Schneider S, Yochim JM, Kuramochi H, Hayashi K, Takasaki K, Yang D, Danenberg KD, Danenberg PV. Intratumoral COX-2 gene expression is a predictive factor for colorectal cancer response to fluoropyrimidinebased chemotherapy. Clin Cancer Res. 2005;11:3363–3368. doi: 10.1158/1078-0432.CCR-04-1650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.