Abstract
Nitric oxide (NO) plays a role in defence against hemibiotrophic pathogens mediated by salicylate (SA) and also necrotrophic pathogens influenced by jasmonate/ethylene (JA/Et). This study examined how NO-oxidizing haemoglobins (Hb) encoded by GLB1, GLB2, and GLB3 in Arabidopsis could influence both defence pathways. The impact of Hb on responses to the hemibiotrophic Pseudomonas syringae pathovar tomato (Pst) AvrRpm1 and the necrotrophic Botrytis cinerea were investigated using glb1, glb2, and glb3 mutant lines and also CaMV 35S GLB1 and GLB2 overexpression lines. In glb1, but not glb2 and glb3, increased resistance was observed to both pathogens but was compromised in the 35S-GLB1. A quantum cascade laser-based sensor measured elevated NO production in glb1 infected with Pst AvrRpm1 and B. cinerea, which was reduced in 35S-GLB1 compared to Col-0. SA accumulation was increased in glb1 and reduced in 35S-GLB1 compared to controls following attack by Pst AvrRpm1. Similarly, JA and Et levels were increased in glb1 but decreased in 35S-GLB1 in response to attack by B. cinerea. Quantitative PCR assays indicated reduced GLB1 expression during challenge with either pathogen, thus this may elevate NO concentration and promote a wide-ranging defence against pathogens.
Key words: Botrytis cinerea, haemoglobin, hypersensitive response, nitric oxide, Pseudomomas syringae, salicylic acid.
This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details)
Introduction
Induced resistance to plant pathogens is based on recognition events involving pathogen (microbial)-associated molecular patterns (PAMPs/MAMPs), pathogen-delivered effectors (Thomma et al., 2011), or plant cell-wall fragment defences (Denoux et al., 2008; Galletti et al., 2011). Variation in elicitation events and pathogenic mechanisms leads to two major types of plant defence response. Classification of these responses is, somewhat crudely based into those mediated by salicylic acid (SA) or those dually influenced by jasmonate (JA)/ethylene (Et) (Pieterse and Van Loon, 2004). SA-mediated defences are prominent against (hemi)biotrophic pathogens that undergo a subtle interaction with the host, variously involving aspects such as the production of non-necrotizing toxins or distinct infection structures such as the fungal haustorium (O’Connell and Panstruga, 2006). SA has many roles in plant stress responses including the hypersensitive response (HR), a form of host programmed cell death – which can be triggered following plant recognition via a resistance gene product of avirulence (Avr) gene product encoded by the pathogen. In contrast, JA/Et acts in necrotrophic pathogens where rapid tissue maceration or pathogen-regulated host cell death are prominent infection mechanisms, although these do not preclude the exhibition of subtle responses during the interaction (Oliver and Solomon, 2004).
Over the last decade, nitric oxide has emerged as a major signal in plant defence against pathogens. It has established roles in influencing the HR form of cell death against bacterial pathogens (Delledonne et al., 1998, 2001; Mur et al., 2005, 2006), necrosis induced by oomycete pathogens (Foissner et al., 2000), and responses to the necrotrophic pathogens such as Botrytis cinerea (Yoshioka et al., 2009) as well as symbiotic relationships involving legumes and Sinorhizobium (Puppo et al., 2002; Uchiumi et al., 2005).
In considering how NO production and distribution could be regulated during plant defence, most effort has concentrated on understanding its means of generation. In this context, cytosolic nitrate reductase (NR; Modolo et al., 2005) has emerged as a major source of NO during plant–pathogen interactions. For efficient NO production, NR requires low concentrations of nitrate and high concentrations of nitrite because NR has a 2-fold higher affinity for nitrite in comparison to nitrate (Rockel et al., 2002). As an alternative, oxidative pathways are based on a nitric oxide synthase (NOS)-like enzyme as well as both hydroxylamine- or polyamine-mediated NO generation (Moreau et al., 2010; Gupta et al., 2011a). In all oxidative NO production pathways, l-arginine acts as substrate or intermediate, thus inhibitors of mammalian NOS that are based on analogues of l-arginine are effective at suppressing NO production in plants (for example, Mur et al., 2005). However, the genetic bases of these biochemically-defined pathways remain to be elucidated and these remain major targets for the plant NO scientist.
At the same time, mechanisms through which NO can be removed from plant cells need to be considered. NO can be eliminated by oxidation to NO3 following the formation of an oxidized form of Hb (methaemoglobin), from which the reduced form may be regenerated by monodehydroascorbate reductase (Igamberdiev et al., 2011). As such, Hb represents the prime candidate through which plants can modulate patterns of NO production during pathogen interactions (Hebelstrup et al., 2007). Plants contain three classes of Hb. In classes 1 and 2 (Hunt et al., 2001), Hb has protein structures similar to classical vertebrate globins with a 3-on-3 α-helical sandwich embracing a haem-group (Harutyunyan et al., 1995; Hargrove et al., 2000). Class 3 has truncated globins with a 2-on-2 α-helical sandwich (Wittenberg et al., 2002). Arabidopsis encodes three Hb genes, GLB1, GLB2, and GLB3 respectively in each class (Trevaskis et al., 1997; Watts et al., 2001). Previous studies have shown that GLB1 and GLB2 expression is particularly strong in specific cells: leaf hydathodes, meristems, and at lateral root branch points (Hebelstrup et al., 2006). However, expression is also detected at a lower level by quantitative real-time PCR (qRT-PCR) in other cells such as leaf mesophylls. Arabidopsis plants with silencing of Hb class 1 (GLB1) gene expression develop stunted organs (Hebelstrup et al., 2006) and flowering is delayed (Hebelstrup and Jensen, 2008). These phenotypes are associated with accumulation of NO, suggesting that Hb class 1 plays an essential role in adjustment of NO concentration in plants. These phenotypes are not present in plants with full loss-of-function mutations in class 2 (glb2) or 3 (glb3) Hb genes, demonstrating a specific role for GLB1 in NO removal (Hebelstrup et al., 2006; Wang et al., 2011).
Roles for Hb have already been suggested in plant defence. Class 1 Hb expression in cotton (Gossypium hirsutum) has been shown to be induced by exogenously applied defence hormones SA, JA, Et, H2O2, and NO (Seregelyes et al., 2003). Tobacco plants overproducing alfalfa class 1 Hb exhibited reduced leaf necrosis when treated with NO donor and reduced cell death in response to challenge with Pseudomonas syringae pv. phaseolicola or tobacco necrosis virus (Seregelyes et al., 2003). Some groups have used transgenic Arabidopsis lines overexpressing bacterial Hmp flavohaemoglobin genes from either Escherichia coli or Erwinia chrysanthemi (Zeier et al., 2004; Bocarra et al., 2005). At that time, due to the absence of well-characterized NO generation mutants, Hmp transgenics wereused to unequivocally demonstrate that NO influenced the HR (Zeier et al., 2004; Bocarra et al., 2005). Given these data, it was perhaps surprising that in transgenic plants overexpressing Arabidopsis Hb1, the major NO scavenging form, a HR elicited by the bacterial pathogen P. syringae pv. tomato DC3000 (Pst) AvrRpm1 was not affected (Perazzolli et al., 2004). However, in this study, it should be noted that cell death was only assessed using trypan blue staining and that production was only qualitatively assessed at one early time point (2 h) using the dye 4-amino-5-methylamino-2',7'-difluorescein.
This study presents a more extensive characterization of the effects of different Hb genes during pathogenic challenge in Arabidopsis, incorporating on-line, quantitative measurements of NO production. GLB1 expression is suppressed rapidly in response to P. syringae and more slowly following inoculation with B. cinerea and in both cases this is likely to potentiate NO effects on both SA- and JA/Et-mediated defences.
Materials and methods
Plant material
Plants were cultivated in Levington Universal compost in trays with 24 compartment inserts. Plants were maintained in Conviron growth rooms (Controlled Environments, UK) at 24 °C with a light intensity of 110 μmol/m/s and an 8/16 light/dark cycle for 4 weeks. For ease of treatment, plants were transferred to Polysec growth rooms (Polysec Cold Rooms, UK) and maintained under the same conditions. Aerial plant parts were treated at the fully expanded rosette stage at 5 weeks (stage 3.7 as defined by Boyes et al. (2001). All of the genotypes were of identical size expected for glb1 which were slightly smaller (Supplementary Fig. S1, available at JXB online) but exhibited no evidence of necrotic flecking which was visible to the naked eye. Plants used for NO and Et measurements were transported to Radboud University, The Netherlands via road and car ferry by the authors. Plants were then kept at Radboud University for 2 days under identical growth conditions as at Aberystwyth to allow plant physiology to normalize prior to gas measurements being attempted.
Pseudomonas syringae culture and inoculation
Arabidopsis plants of various genotypes were inoculated with avirulent P. syringae pv. tomato (Pst) strain DC3000 AvrRpm1 or the disease-forming Pst as described previously (Mur et al., 2000) using inocula of 2 × 106 bacterial cells/ml 10 mM MgCl2. Mock-inoculated controls consisted of injecting leaves with 10 mM MgCl2. Bacterial inoculations for NO or Et measurements involved vacuum infiltration of six 5-week-old short-day (8 h light period) Arabidopsis rosettes (excised just above the soil line) in plants treated with 2 × 106 bacterial cells/ml and 10 mM MgCl2 or 10 mM MgCl2 alone (mock inoculation). Rosettes were immersed in a bell vacuum infiltrator and a vacuum applied for 5 min. The vacuum was carefully released during which the intracellular spaces of the leaves were observed to become filled with bacterial suspension or 10 mM MgCl2 alone as appropriate. The rosettes were then quickly dried with a paper towel and then enclosed within a cuvette so that measurement could commence. The mass of plant material used was in the range of 4–6 g per cuvette.
B. cinerea culture and inoculation
This study used the grape B. cinerea isolate IMI169558 (Thomma et al., 1997). B. cinerea was cultured and harvested as stated in Johnson et al. (2007) and diluted to a concentration of 1 × 105 spores/ml in potato dextrose broth (PDB, Formedium, UK). For assessments of infection phenotypes, single leaves (leaf stage 7 or 8 as defined by Boyes et al., 2001) were inoculated with 5 μl of spore suspension, pipetted onto the adaxial surface of the leaf. Controls were inoculated with PDB. Plants remained under Stewart Micropropagators to sustain a relative humidity of 50–80% and lightly watered every 24 h. For the NO and Et measurements, 1 × 105 spores/ml suspension or PDB controls were sprayed onto a whole plant to run off.
Estimations of electrolyte leakage and in planta bacterial populations
Cell death was estimated by electrolyte leakage in 1-cm-diameter cores as described in Mur et al. (2000).
Scoring B. cinerea lesion phenotypes
A weighted scoring method was used to categorize B. cinerea lesion phenotypes (Lloyd et al., 2011). Susceptible symptoms (water-soaking, chlorosis, and spreading necrosis) were conferred a range of negative scores and the resistant symptoms (necrosis limited to inoculation site) were given positive scores. A weighted score could be produced arithmetically from the lesion scores of replicates.
Salicylic acid and jasmonic acid measurements
SA and JA concentrations in samples were determined by liquid chromatography–mass spectrometer (LC-MS) using the Micromass LCT–Time of Flight, as described in Clarke et al. (2004) and Allwood et al. (2006), respectively. Absolute SA and JA concentrations were derived by comparison with deuterated standards (d6-SA, C/D/N Isotopes, Quebec, Canada; d6-JA standard were kindly provided by Claus Wasternack Leibniz Institute of Plant Biochemistry, Halle, Germany),whichwere added to the samples at first extraction.
Ethylene measurements using photoacoustic laser spectroscopy
Ethylene production was monitored in real time using a gas flow-through in-line system fitted with a photoacoustic laser-based ethylene detector (ETD-300, Sensor Sense), which is able to detect on-line 300 parts per trillion volume of ethylene within 5 s (Cristescu et al., 2008). Gas was regulated by an automated valve control box (VC-6, Sensor Sense). Ethylene emanation from a single inoculated rosette within a glass cuvette (254 ml volume) was alternately monitored for 15 min (5 s per acquisition point), at a controlled continuous flow rate of 1.5 l h–1 by flushing with air and preventing accumulation-induced effects. KOH and CaCl2 scrubbers were incorporated into the system to remove CO2 and H2O respectively. As assays of Et production from Arabidopsis plants inoculated with B. cinerea involved measuring both the plant and the 18-cm3 module with Levington Universal compost, a reference cuvette was set up containing only a module of compost. The negligible level of ethylene produced from this compost was subtracted from experimental cuvettes. Each experiment was repeated to give similar results and the outcomes of one representative experiment are shown.
Nitric oxide measurements using a quantum cascade laser-based sensor
The configuration of the quantum cascade laser (QCL)-based sensor for NO detection is shown in Supplementary Fig. S2. A detailed description is given elsewhere (Cristescu et al., 2008). Briefly, the QCL emitting around 1900 cm−1 passes through an absorption multi-pass cell in which enters the airline transporting the NO released by a single inoculated rosette within a glass cuvette (~500 ml). The NO production is directly detected by measuring the attenuation of the laser intensity due to the NO absorption in the cell.
Infected materials were placed in glass cuvette and flushed with air at a controlled continuous flow rate of 1 l h–1. Multiple cuvettes could be monitored in sequence, each being measured for ~13 min. Each experiment was repeated to give similar results and the outcomes of one representative experiment are shown.
Gene expression analysis and Western blotting
The expression of GLB1 and GLB2 was assessed in rosette leaves from Arabidopsis (Col-0) using qRT-PCR. The method has previously been described in (Hebelstrup et al., 2010). Total RNA was purified using a FastRNA Pro Green kit (MP Biomedicals, France). Total RNA (3 μg) was used to generate first-strand cDNA by using Superscript Reverse Transcriptase II (Invitrogen) and random hexamer oligonucleotide primers in a total volume of 20 μl, as described in the manufacturer’s guidelines. The finished product was diluted 10 times to be ready for qRT-PCR. qRT-PCR was performed in an ABI Prism 7900HT Sequence Detection System. Power SYBR Green PCR Master Mix (Applied Biosystems) was used as the basis for the reaction in a total volume of 10 μl (5 μl PCR Master Mix, 1 μl primer mix containing 5 μm each primer, 3 μl H2O, 1 μl cDNA). Expression levels were normalized using the housekeeping gene ACT2 by the method described by Pfaffl (2001). The following primer pairs were used to amplify the genes of interest: GLB1: 5'-CTCTTCATCAAGATCTTTGAGATTGC-3' and 5'-GACAAAAACAGACATTGCGTGAGG-3'; GLB2: 5'-TCACTTCTTCTCACAGATACTGGA-3' and 5'-CTTGAAGACTTTAACAGCATGAGC-3'; ACT2: 5'-AGCGCTGAGGCTGATGATATTCAAC-3' and 5'-TCTAGAAACATTTTCTGTGAACGATTC-3'.
For Western blotting, plants were grown on 1 × Murashige-Skoog medium with 0.8% agar under 18/6 light/dark cycle with fluorescent lighting at intensities between 50 and 100 μmol/m2/s. Polyclonal rabbit antiserum against purified recombinant GLB1 and GLB2 proteins (Trevaskis et al., 1997) were used for protein detection. The proteins were extracted by grinding plant material in 0.01 M NaPO4 buffer (pH 7.0) and 1mM EDTA, centrifuging (10 000 g for 5 min), and collecting the supernatants. Total protein (50 μg, as measured using the BioRad protein detection reagent) was used per lane for SDS PAGE separation (15% acrylamide) and Western blots were prepared and probed as described previously (Trevaskis et al., 1997).
Statistical analysis
Data were subjected to analysis of variance using MiniTab version 14, after which residual plots were inspected to confirm data conformed to normality.
Results
Resistance to P. syringae is influenced by GLB1
These investigations into the role of Hb during pathogen challenge in Arabidopsis were based on the RNAi-suppressed line glb1, the glb2 Spm mutant (Hebelstrup et al., 2006), a glb3 T-DNA tagged line (Wang et al., 2011), and also CaMV 35S-GLB1 and 35S-GLB2 overexpression lines (Hebelstrup and Jensen, 2008; Supplementary Fig. S3). Where described collectively, these will be referred to as Glb lines. Each Glb line and Col-0 controls were challenged with the virulent P. syringae pv. tomato DC3000 (Pst) and the HR-eliciting strain P. syringae pv. tomato DC3000 AvrRpm1 (Pst AvrRpm1). With inoculation of Pst, typical chlorotic symptoms were observed at 48 h post inoculation (hpi) in Col-0 but were noticeably reduced in glb1 and accentuated in 35S-GLB1 where greater cell death was observed (Fig. 1A). With inoculation of Pst AvrRpm1 at 48 hpi, cellular collapse was observed with no observable differences between each Arabidopsis line (Fig. 1A) which were in agreement with the observations of Perazzolli et al. (2004). Following inoculation with Pst AvrRpm1, no observable change in HR forming in glb1 and 35S-GLB1 compared to that in the wild-type line. Following careful demarcation of the area of inoculation, this study found no evidence of altered lesion spread in the Glb lines (data not shown). Phenotypes in glb2, glb3, and 35S-GLB2, following inoculation with either Pst strain, were identical to those in Col-0 (data not shown).
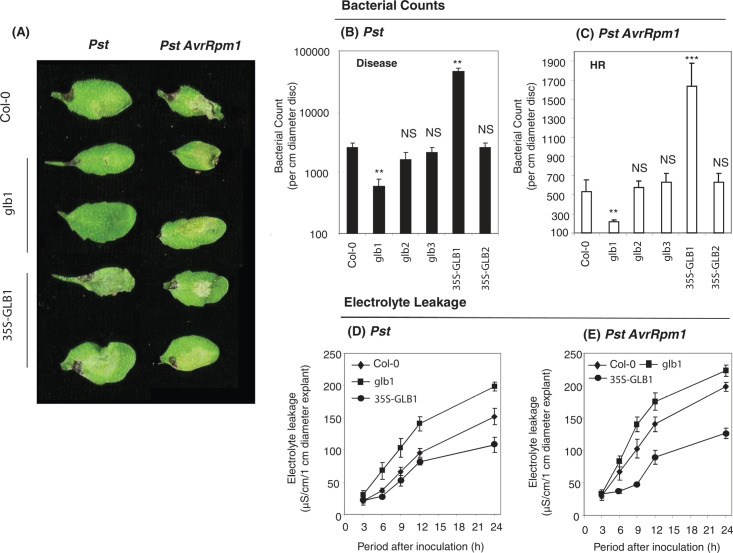
Fig. 1.
Haemoglobin effects on Pseudomonas syringae pv. tomato interactions with Arabidopsis. (A) Lesionphenotypes at 48 h post inoculation (hpi) with the virulent disease-forming strain P. syringae pv. tomato DC3000 (Pst) and the avirulent, hypersensitive response (HR)-eliciting strain Pst AvrRpm1 in Col-0, the haemoglobin (Hb) GLB1 RNAi-suppressed Arabidopsis line glb1, and the GLB1 overexpression line 35S-GLB1. Single representative examples are given for Pst AvrRpm1 and Pst-elicited lesions forming in Col-0, but two examples are given for lesions forming in glb1 and 35S-GLB1 to represent the range of phenotypes that were obtained. (B and C) Bacterial populations at 48 hpi of Pst (B, filled bars) and Pst AvrRpm1 (C, open bars) with from 1-cm-diameter cores sampled from inoculated tissue of Col-0, the glb1 RNAi-suppressed line, the Hb glb2 and glb3 mutants, and the 35S-GLB1 and -GLB2 overexpression lines. Results are mean ± SE (n = 6). Levels of significant difference to results obtained for Col-0 are indicated: NS, no significant difference; * P < 0.05; ** P <0.01; *** P < 0.001. (D and E) Electrolyte leakage from 1-cm-diameter discs sampled from leaves of Col-0 (filled diamonds), glb1 (filled squares), or 35S-GLB1 (filled triangles) immediately following inoculation with Pst (D) or Pst AvrRpm1 (E). Results are mean ± SE (n = 6).
Bacterial population sizes with the inoculated leaves were assessed at 48 hpi (Fig. 1B and C). The numbers of Pst AvrRpm1 and Pst in glb1 were significantly reduced and increased in 35S-GLB1 significantly. With all other genotypes, bacterial numbers were not significantly different to those in Col-0. The kinetics of cell death were quantified using electrolyte leakage (Fig. 1D and E). When inoculating with either Pst AvrRpm1 or Pst, cell death was increased in glb1 and decreased in 35S-GLB1. Electrolyte leakage did not significantly differ from Col-0 in Pst and Pst AvrRpm1 inoculations of glb2, glb3, and 35S-GLB2 (data not shown). These data were consistent with GLB1 expression influencing both HR-associated resistance and basal defences deployed during disease development.
Modification of GLB expression can influence plant-derived NO production
Since Hb has a well-established NO dioxygenase activity (Gupta et al., 2011b), and to focus on HR-linked defence, this study sought to related observed responses to Pst AvrRpm1 in glb1 and 35S-GLB1 to NO production. NO production was determined using a QCL, the underlying principles of which and its use to measure plant-derived NO have been recently described (Yordanova et al., 2010; Mur et al., 2011). As noted previously (Mur et al., 2006) in Col-0, NO generation was rapidly initiated and achieved maximal rates of production at 1–2 hpi (Fig. 2A). NO production from mock-inoculated uninfected glb1, but not glb2 (or glb3 plants, data not shown) was significantly increased compared with Col-0 and, in the case of glb1, was produced at similar rates as during the HR in Col-0. Following challenge of glb1 with Pst AvrRpm1, NO production was approximately double that seen with Col-0, but in glb2, the patterns of NO production did not differ from Col-0. Measuring NO in Pst AvrRpm1-inoculated 35S-GLB1 and 35S-GLB2 lines (Fig. 2A) indicated that in both instances, production was reduced compared to Col-0. NO production was also assessed from Pst-challenged Arabidopsis (Supplementary Fig. S4A). Increased NO production was not observed until after 6 hpi with Col-0 and was markedly reduced in 35S-GLB1 but increased in glb1. This correlated with the patterns of symptom development (Fig. 1A).
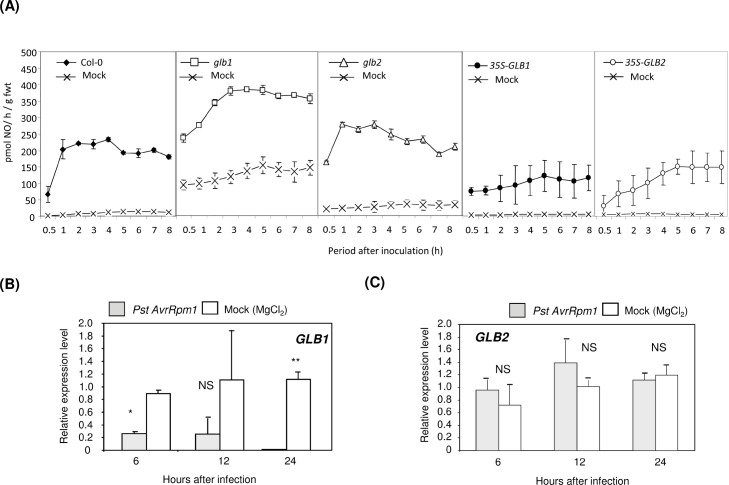
Fig. 2.
Nitric oxide production from Arabidopsis lines with modified haemoglobin expression on inoculation with strains of Pseudomonas syringae pv. tomato. (A) NOproductionwasdetermined from Arabidopsis Col-0and the haemoglobin (Hb) GLB1 RNAi-suppressed Arabidopsis line glb1, the Hb mutant glb2, and the 35S-GLB1 and -GLB2 overexpression lines, following inoculation with the hypersensitive response (HR)-eliciting P. syringae pv. tomato DC3000 (Pst) AvrRpm1 strain or mock inoculation with 10 mM MgCl2. NO was determined using a quantum cascade laser system. ANOVA of NO production from Pst avrRpm1-challenged glb1 and 35S-GLB1 suggested significant differences (in both cases P < 0.01) to that from Col-0 plants. (B and C) Quantitative real-time PCR was used to assess gene expression 24 h post inoculation in three biological replicates with either the HR-eliciting Pst AvrRpm1 (grey blocks) or mock inoculation (MgCl2; white blocks) of GLB1 (B) and GLB2 (C). Results are mean ± SE of three replicates of three different plants. Levels of significant difference to mock-inoculated controls at the same time point are indicated: NS, no significant difference; * P < 0.05; ** P <0.01; *** P < 0.001.
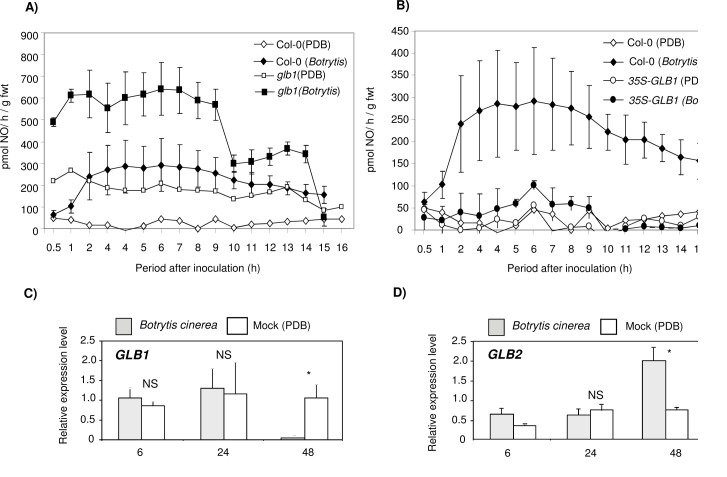
The expression of GLB1 (Fig. 2B) and GLB2 (Fig. 2C) was assessed in Col-0 at 24 h after inoculation with Pst AvrRpm1 by qRT-PCR. Inoculation with Pst AvrRpm1 significantly reduced GLB1 compared to controls within 6 hpi and was barely detectable by 24 hpi (Fig. 2B). GLB2 expression did not significantly differ from controls throughout the first 24 hpi with Pst AvrRpm1 (Fig. 2C).
NO contributes to salicylic acid-mediated defence potentiation in HR
Increased Hb expression has been shown to suppress pathogen-initiated SA accumulation (Seregelyes et al., 2003). Thus, this study sought to relate GLB1-associated effects to SA accumulation in response to Pst AvrRpm1 at 48 hpi (Fig. 1B). SA concentrations in Pst AvrRpm1-challenged glb1 were significantly elevated and reduced in 35S-GLB1. SA accumulation in mock-inoculated glb1 was also increased compared to Col-0 (Fig 3A).
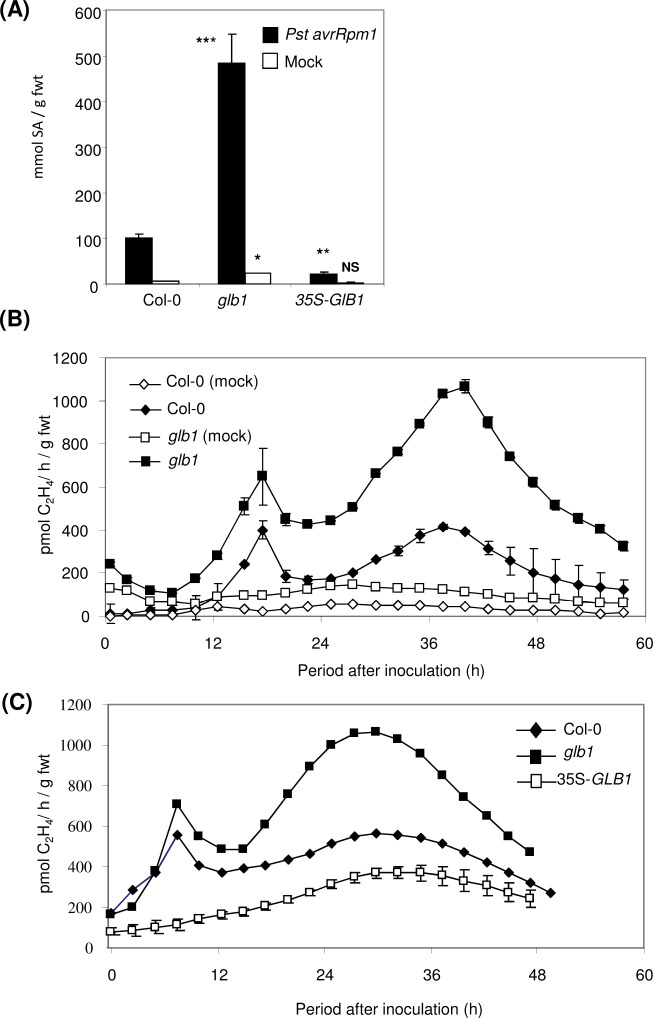
Fig. 3.
Haemoglobin effects onsalicylic acid (SA) accumulation and ethylene production elicited by Pseudomonas syringae pv. tomato. (A) SA accumulation at 48 h post inoculation of Arabidopsis Col-0, the haemoglobin (Hb) GLB1 RNAi-suppressed Arabidopsis line glb1, and the GLB1 overexpression line 35S-GLB1, inoculated with P. syringae pv. tomato DC3000 avrRpm1 (Pst avrRpm1) or mock inoculated with 10 mM MgCl2. Results are mean ± SE (n = 6). Statistical comparisons were made between inoculated Col-0 and glb1 or 35S-GLB1 plants and also between mock-inoculated and glb1 or 35S-GLB1 plants. Levels of significant difference are indicated: NS = no significant difference; * P < 0.05, ** P <0.01 and *** P < 0.001. (B and C) Ethylene productionwasdetermined using laser photoacoustic detection with Arabidopsis Col-0and either glb1 (B) or 35S-GLB1 (C) following inoculation with Pst avrRpm1 or mock inoculation with 10 mM MgCl2.
Both NO and SA are required to enhance Et biosynthesis during a P. s. pv. phaseolicola-elicited HR in tobacco (Mur et al., 2008) so Et production was measured in Pst AvrRpm1-challenged Arabidopsis Col-0, glb1, and 35S-GLB1 using laser photoacoustic detection. Challenge of Col-0 led to the biphasic rise in Et production (Mur et al., 2009) and both peaks were increased in glb1 (Fig. 3B) but decreased in 35S-GLB1 (Fig. 3C). Hence, one effect of increased NO accumulation in glb1 may be increase SA-influenced Et production. To substantiate this observation, this study examined SA and Et levels in Pst-challenged plants (Supplementary Fig. S4B and C). In glb1, Pst-elicited SA accumulation (Supplementary Fig. S4B) and ethylene production (Supplementary Fig. S4C) were increased, which correlated with the increased resistance to this virulent strain observed on this plant genotype (Fig. 1).
Resistance to the necrotrophic fungus B. cinerea is influenced by GLB1
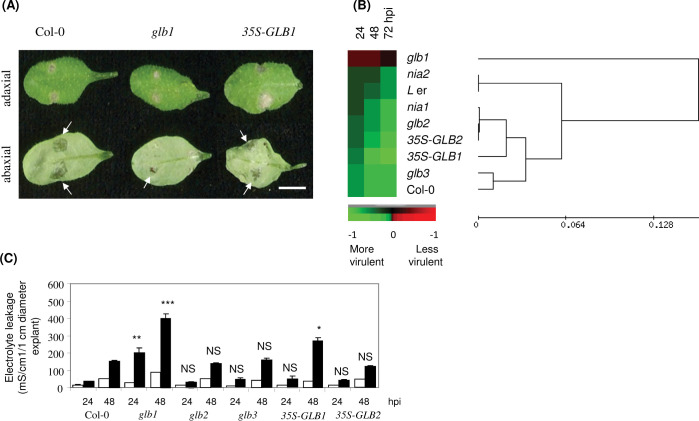
Et plays a minor role in resistance to Pst AvrRpm1 (Mur et al., 2009), unlike the situation following challenge with B. cinerea where it is a major defence determinant (Thomma et al., 1997). Inoculations with B. cinerea are carried out by drop inoculation on to adaxial surfaces so that the degree of host resistance is indicated by the size of lesion and the speed with which the pathogen penetrates through to the abaxial surface (Fig. 4A). At 72 hpi, lesion development in glb1 was markedly delayed compared to controls, with little penetration through to the abaxial surface. In 35S-GLB1 plants, abaxial penetration was rapid and whole leaf collapse was evident (Fig. 4A). A semi-quantitative scoring scheme has recently been developed to describe B. cinerea lesion development (Lloyd et al., 2011), which facilitates comparisons between responses to infection in different genotypes (Fig. 4B). This comparison included the NR Col-0 nia1 mutant, which is most compromised in NO generation (Neill et al., 2008; Miao et al., 2010; Lu et al., 2011). Also included was the L er nia2 mutant which is mutated in the gene encoding the most active form of NR but apparently is not a major source of NO (Neill et al., 2008). Lesion scores at 24, 48, and 72 hpi were identical in Col-0 and glb3, further suggesting that GLB3 does not play an observable role in plant defence. In contrast, the glb1 line was the most resistant phenotype and 35S-GLB1 the most susceptible line, whilst responses in nia1 and glb2 were very similar. In line with the negligible effect of NIA2 in NO-mediated events, lesions in nia2 and L er were identical.
Fig. 4.
Haemoglobin effects on Botrytis cinerea interactions with Arabidopsis. (A) Lesiondevelopmentin Arabidopsis Col-0, the haemoglobin (Hb) GLB1 RNAi-suppressed line glb1, and the GLB1 overexpression line 35S-GLB1 at 72 h post-inoculation (hpi) with B. cinerea strain IMI169558. Attached leaves were inoculated on the adaxial side with 5 μl of 1 × 105 spores ml–1 in potato dextrose broth. Leaves were detached only for photography and representative lesion phenotypes on the adaxial and abaxial sides are shown. (B) B. cinerea lesion phenotypes occurring in Col-0, the glb1 RNAi-suppressed line, the Hb glb2 and glb3 mutants ,and the 35S-GLB1 and -GLB2 overexpression lines and also the nitrate reductase mutants nia1 and nia2 (Landsberg erecta [L er] is included because the nia2 mutant is derived from this ecotype), scored at 24, 48, and 72 hpi according to Lloyd et al. (2011): see Materials and Methods. A heat map and dendogram was derived to show the relationships between patterns of lesion development. (C) Electrolyte leakage from 1-cm-diameter discs sampled from leaves of Col-0, glb1, glb2, glb3, 35S-GLB1, or 35S-GLB2 following inoculation with B. cinerea (filled bars) or mock inoculated (open bars) with potato dextrose broth. Results are mean ± SE (n = 6). Levels of significant differences to results equivalent treatments of Col-0 are indicated: NS, no significant difference; * P < 0.05; ** P <0.01; *** P < 0.001.
To further quantify the differences in B. cinerea lesion development amongst the Glb lines, cell death was estimated by electrolyte leakage in explants (Fig. 4C). Compared to Col-0 controls, glb1, but not glb2, glb3 or 35S-GLB2, electrolyte leakage was significantly increased at both 24 and 48 hpi. Electrolyte leakage was also significantly increased in 35S-GLB1 but this may reflect the rapid fungal penetration in this line (Fig. 4A). The equivocal nature of such results limited the value of the electrolyte leakage assay with B. cinerea inoculations of Glb lines.
NO potentiates ethylene- and jasmonic acid-mediated plant defence against B. cinerea
As above (Fig. 2), the observed changes in lesion development were related to patterns of NO generation. Using QCL, NO production was determined from Col-0, glb1 (Fig. 5A), and 35S-GLB1 (Fig. 5B) challenged with B. cinerea compared to uninfected controls. Spraying plants with B. cinerea led to rapid production of NO in Col-0, achieving similar levels to those seen with control glb1 plants. NO production in B. cinerea-infected glb1 was significantly higher and in 35S-GLB1 significantly lower than in infected wild-type controls. GLB1 (Fig. C) and GLB2 (Fig. 5D) expression in response to B. cinerea was investigated using qRT-PCR. At 48 h, GLB1 expression was reduced in B. cinerea-inoculated samples but GLB2 was increased compared to mock-inoculated controls.
Fig. 5.
Nitric oxide production from Arabidopsis lines with modified haemoglobin expression on inoculation with Botrytis cinerea. (A and B) NOproductionwasdetermined from Arabidopsis Col-0and the haemoglobin (Hb) GLB1 RNAi-suppressed Arabidopsis line glb1 or the GLB1 overexpression lines 35S-GLB1 following inoculation with B. cinerea strain IMI169558 or mock inoculated with potato dextrose broth (PDB). NO was determined using a quantum cascade laser system. Results in Fig. 5A are derived from two experiments conducted within a 48-h period and in Fig. 5B represent the pooling of two similarly contemporaneous experiments undertaken 1 week after those represented in Fig. 5A, albeit with equivalently aged plants. Results are mean ± SE (n = 3) except for controls, where only the result from a single replicate is depicted. ANOVA of NO production from B. cinerea-infected glb1 and 35S-GLB1 suggested significant differences (P < 0.05 and P < 0.01, respectively) to measurements from wild-type plants. (C and D) Quantitative real-time PCR was used to assess gene expression 48 h post inoculation in three biological replicates with either B. cinerea (filled bars) or mock inoculation (PDB; open bars) of GLB1 (C) and GLB2 (D). Results are mean ± SE of three PCR replicates. Levels of significant difference to mock-inoculated controls at the same time point are indicated: NS, no significant difference; * P < 0.05; ** P <0.01; *** P < 0.001.
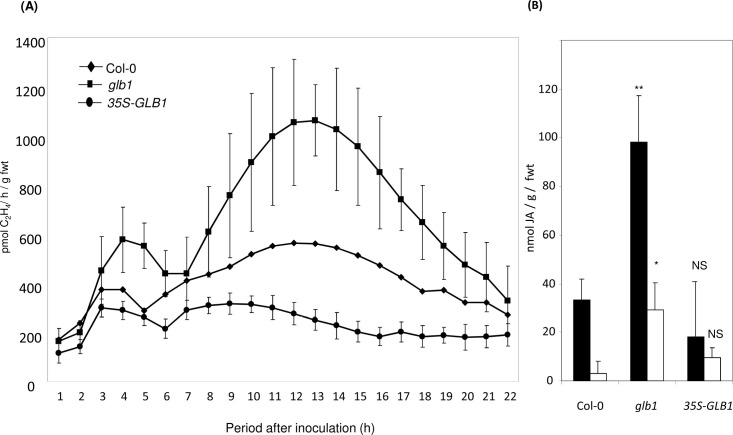
Et synthesis was determined in Col-0, glb1, and 35S-GLB1 following inoculation with B. cinerea using laser photoacoustic detection (Fig. 6A). In all genotypes, a minor, transient rise in Et biosynthesis was observed 3–6 hpi only to increase for a second time at ~5–7 hpi. In Col-0 and glb1, this rise in Et production persisted for at least 12h before subsiding. In 35S-GLB1, the second increase in Et generation greatly reduced. As Et acts with JA in conferring resistance to B. cinerea (Thomma et al., 1997), the concentration of JA was measured in Col-0, glb1, and 35S-GLB1 at 48 hpi (Fig. 6B). JA accumulation in mock-inoculated and inoculated 35S-GLB1 did not appear to significantly differ from equivalent treatments in Col-0. However, in B. cinerea-inoculated glb1 JA accumulation was significantly increased over infected Col-0.
Fig. 6.
Haemoglobin effects onethylene production and jasmonic acid (JA) accumulation elicited by Botrytis cinerea . (A) Ethylene productionwasdetermined using laser photoacoustic detection (LPAD) from Arabidopsis Col-0, the haemoglobin (Hb) GLB1 RNAi-suppressed Arabidopsis line glb1, and the GLB1 overexpression line 35S-GLB1 following inoculation with B. cinerea strain IMI169558 or mock inoculated with potato dextrose broth. Results are mean ± SE (n = 3). (B) JA accumulation at 48 h post inoculation of Arabidopsis with B. cinerea (filled bars) or mock inoculated with potato dextrose broth (open bars). Results are mean ± SE (n = 6). Statistical comparisons were made between B. cinerea-infected Col-0 and glb1 or 35S-GLB1 plants and also between controls in Col-0 and glb1 or 35S-GLB1 plants. Levels of significant difference are indicated: NS, no significant difference; * P < 0.05; ** P <0.01; *** P < 0.001. Results in Part A are derived from directly contemporaneous experiments as two LPAD were used in parallel.
Discussion
GLB1 negatively affects plant responses to pathogens
Over the last decade, NO has emerged as a major signal that mediates plant defence against pathogens. Previously, plant lines overexpressing bacterial flavohaemoglobin genes have been used as a means of demonstrating the importance of NO to the system under investigation, whether defence (Zeier et al., 2004; Bocarra et al., 2005), senescence (Mishina et al., 2007), or UV-B protection (Tossi et al., 2011). In contrast, when Perazzolli et al. (2004) overexpressed endogenous Hb in Arabidopsis, no effect on a HR elicited by Pst AvrRpm1 was observed. However, in Perazzolli’s study, both cell death and NO production were qualitatively estimated at a single time point. Thus, the present study embarked on a fuller, quantitative assessment of the potential roles of Hb in plant–pathogen interactions, focusing on defences employed against a hemibiotrophic bacterial pathogen Pst AvrRpm1 and the necrotrophic fungus B. cinerea.
This study used a variety of different Arabidopsis lines with silencing, overexpression, or mutation of the three Hb genes GLB1, GLB2, and GLB3. No GLB1 loss-of-function mutant has been identified in any mutation-tagging studies, suggesting that such a mutation is lethal. Instead a previously described silencing line, where gene expression level is limited to 2–3% of that of the wild-type line, was used (Hebelstrup et al., 2006). A number of developmental phenotypes, which may interfere indirectly with pathogen susceptibility, have been described for this line (Hebelstrup et al., 2006; Hebelstrup and Jensen, 2008). However, most of these phenotypes disappear when the plants are grown under short-day conditions, as done in this study, where the only difference is a small decrease in size of the glb1 line (Supplementary Fig. S3). Interestingly, the mock-inoculated glb1-suppressed line emitted a constant level of NO which was elevated compared to controls and which also featured to a lesser extent with the glb2 line. This latter observation is in line with the previous observation that GLB2 is less important for NO removal compared to GLB1 and that basal production of NO requires removal for efficient plant growth (Hebelstrup et al., 2006; Hebelstrup and Jensen, 2008).
To ascertain the relative roles of each individual Hb gene on NO removal, the corresponding suppressed lines were challenged with virulent Pst stains and HR-eliciting Pst AvrRpm1 (Fig. 1). As suggested by Perazzolli et al. (2004), the HR phenotype elicited by Pst AvrRpm1 did not appear to differ between controls and Glb modulated lines. However, quantitatve estimates of cell death using electrolyte leakage as well as of bacterial numbers suggested that, in GLB1-suppressed lines, resistance and cell death in response to both strains were significantly enhanced. Phenotypes obtained following inoculation of the Glb lines with Pst AvrRpm1 correlated with the observed rates of NO production. Thus, for example, overproduction of GLB1 and to a lesser extent GLB2 could suppress NO production (Fig. 2A) and this was associated with reduced host cell death and increased bacterial numbers, reaffirming the role of NO in resistance against Pst strains.
Whilst the regulation of NO is undoubtedly important to defence, the qRT-PCR data suggested that, with Pst AvrRpm1-inoculated Arabidopsis at least, modulation of Hb expression can also influence NO levels (Fig. 3A and 3D). Within 6 hpi with Pst AvrRpm1, GLB1 expression was significantly reduced in Col-0 and nearly undetectable by 24 hpi, suggesting that in a natural avirulent Pst–Arabidopsis Col-0 interaction GLB1 expression is suppressed in a manner analogous to the glb1 mutant with, presumably, comparable effects on NO accumulation. As GLB2 expression was not significantly affected throughout the time course, it was unlikely that the observed reduction of GLB1 expression was due either to plant cell collapse during the HR or to global changes in gene expression.
The data for GLB1 expression following inoculation with B. cinerea indicated that suppression was not observed until 48 hpi, when necrosis at the infection site is first evident (Lloyd et al., 2011). This suppression clearly did not match the observed patterns of NO production (Fig. 5A) and it may be that it reflected different kinetics when spotting Botrytis on Arabidopsis as opposed to spraying. Unfortunately, it did not prove possible to detect NO production from spot Botrytis-inoculated Arabidopsis plants (data not shown). As infections of 35S-GLB1 with Botrytis were markedly affected (Fig. 4A), this later GLB1 suppression may act to increase NO production to aid in confining the pathogen to the site of inoculation. Indeed, with the 35S-GLB1 line, Botrytis was less well confined to the point of inoculation (Fig. 4A).
Some indirect evidence could suggest that NO plays no role in resistance to B. cinerea. Thus, Benito et al. (2010) demonstrated that a Botrytis Hb mutant was not affected in its ability to infect its host. Further, B. cinerea also transiently produces NO early in its infection process (Benito et al., 2010). Indeed, it may be that the fungus responds to external, in planta-generated NO to promote pathogenesis of host tissue (Turrion-Gomez and Benito, 2011). This offers an apparently contradictory scenario where NO is being used by the host for defence and by the pathogen to promote virulence. Rationalization of these disparate data may require careful spatiotemporal measurement of NO concentrations, as the relative concentration of NO could play a vital role in governing its action (Lamattina and Beligni, 2001; Turrion-Gomez and Benito, 2011). It may be that altered host GLB1 expression at specific points in the infection process could play a role in favour of defence over promotion of pathogen invasiveness.
NO is a potentiator of both SA- and Et/JA-associated defences
A central event in the potentiation of defence and cell death against bacterial pathogens is the initiation of SA biosynthesis (Mur et al., 2000). The initiation of SA biosynthesis by NO is a well-established event (Klessig et al., 1998; Zottini et al., 2007), so unsurprisingly, SA levels were increased in glb1 and decreased in 35S-GLB1 following inoculation with Pst AvrRpm1 (Fig. 3A). Such SA production has previously been linked with a potentiated oxidative burst (Mur et al., 2000), but establishing the in planta kinetics of the oxidative burst in Arabidopsis is technically demanding. The use of the H2O2-detecting stain 3,3-diaminobenzidine did not suggest any changes in Pst AvrRpm1-challenged glb1 or 35S-GLB1 compared to Col-0 (data not shown). However, the kinetics of Et production shares many features of the oxidative burst (Mur et al., 2008, 2009). The first transient phase of Et is PAMPs-elicited whilst the second persistent rise was seen only with HR-eliciting bacteria Further, Et production was potentiated by SA (Mur et al., 2008, 2009). The current study observed increased Et production in glb1 but this was greatly compromised in 35S-GLB1 (Fig. 3C). The mammalian NOS inhibitor L-NAME was previously used to demonstrate a link between bacterially elicited NO and Et production (Mur et al., 2008). However, those experiments failed to suppress the first transient rise in Et production leading to the hypothesis that this could be influenced by other signals, particularly reactive oxygen species (ROS). However, in glb1, both the transient and persistent rise in Et production was augmented and, crucially, both were compromised in 35S-GLB1, which demonstrates the limitations of using L-NAME in plant studies. Somewhat surprisingly, Qu et al. (2006) noted that overexpression of a cotton Hb gene in Arabidopsis led to enhanced disease resistance to pathogenic challenge and the activation of defence genes PR1 and PDF1.2. This contrasts with the well-established role of NO in the initiation of SA biosynthesis for which PR1 is a well-established gene marker (Klessig et al., 1998), because Hb overexpression leads to scavenging of NO, as demonstrated in this work and several previous studies (Hebelstrup et al., 2007) This could indicate that cotton Hb can have distinctive effects than scavenging of NO when expressed in Arabidopsis. Equally, it should be noted that no NO measurements were done by Qu et al. (2006).
Et is much more prominent in conferring resistance to B. cinerea than to Pst AvrRpm1 (Thomma et al., 1997). The current measurements established that the degree of resistance correlated with the NO and Et rates of production (Fig 4A, Fig. 5A and 5B). Interestingly, Et production was more rapid in these experiments than in Lloyd et al., 2011). As suggested above for NO production, this may indicate that applying fungal spore suspensions resulted in more rapid responses than simply spotting the pathogen on to the leaf surface as in Lloyd et al. (2011). JA accumulation in glb1 and 35S-GLB1 suggested that, following challenge with B. cinerea, JA accumulation correlated with the rate of NO production (Fig. 6). Thus, NO seems likely to contribute to both Et and JA defences against necrotrophs. This situation appears different to that in Nicotinia attenuata grazed by tobacco hornworm (Meduca sexta). Silencing of NOA1, a gene linked with NO generation (Guo and Crawford, 2005) in N. attenuata increased JA biosynthesis during M. sexta feeding (Wunsche et al., 2011a,b) Similarly, silencing S-nitrosoglutathione reductase (GSNOR), which removes NO-derived S-nitrosylaton adducts, so that the effects of NO would be amplified, less JA biosynthesis was observed in N. attenuata on feeding with M. sexta (Wunsche et al., 2011a). Although these observations appear at odds with the current results obtained using B. cinerea and Arabidopsis (Fig. 6B), it may be that specific pest/pathogen and host species interactions influence the outcome of NO–JA interactions.
The role of ROS during NO–Et/JA interactions following attack with Botrytis also requires consideration. Asai and Yoskioka (2009) demonstrated how NO acting with SA is important in suppressing B. cinerea disease development in Nicotiana benthamiana. In contrast, ROS production actively aided disease progression. Similarly, Govrin and Levine (2000) showed that host ROS generation is an important determinant of B. cinerea virulence. Thus, NO could reduce B. cinerea aggressiveness by reducing H2O2 production. Indeed, Malolepsza and Rozalska (2005) observed that NO reduced H2O2 concentration in tomato infected with B. cinerea. When the current study attempted to assess ROS levels in Col-0, glb1, and 35S-GLB1 lines with B. cinerea using 3,3-diaminobenzidine staining, no differences could be detected (data not shown) but this could reflect the insensitivity of this assay for ROS.
Taking all of these observations together suggests that NO acts as a broadly acting defence initiator which acts to potentiate multiple signalling pathways. Further, NO production is regulated at least in part through modulated host Hb expression.
Supplementary Material
Acknowledgements
The authors would like to thank Prof. John Mansfield (Imperial College, UK) for the gift of the Pst strains and Bart Thomma (Wageningen, The Netherlands) for the B. cinerea strain IMI169558. As ever, they appreciate the support provided by the Penglais gardeners – Ray Smith and Tom Thomas (Aberystwyth, UK) – in providing well-maintained plant material. Thanks also to Prof. Michael Hall and Dr. Paul Kenton (both Aberystwyth, UK) for critically reading this manuscript. The work described in this manuscript was made possible through support provided by the BBRSC, the Centre for Integrated Research in the Rural Environment (CIRRE, http://www.cirre.ac.uk/), The Danish Council for Independent Research Technology and Production Sciences, and the EU-FP6-Infrastructures-5 programme (project FP6-026183 ‘Life Science Trace Gas Facility’).
Footnotes
Supplementary material
Supplementary material is available at JXB online.
Supplementary Fig. S1. Arabidopsis rosettes used to assess trace gas emissions following pathogen attack.
Supplementary Fig. S2. The quantum cascade laser-based sensor adapted for nitric oxide detection.
Supplementary Fig. S3. GLB1 and GLB2 expression in the overexpression lines.
Supplementary Fig. S4. Nitric oxide, salicylic acid, and ethylene production and from Arabidopsis lines with modified haemoglobin expression on inoculation with Pseudomonas syringae pv. tomato.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
© 2012 The Authors.
References
- Allwood JW, Ellis DI, Heald JK, Goodacre R, Mur LA. Metabolomic approaches reveal that phosphatidic and phosphatidyl glycerol phospholipids are major discriminatory non-polar metabolites in responses by Brachypodium distachyon to challenge by Magnaporthe grisea . The Plant Journal. 2006;46:351–368. doi: 10.1111/j.1365-313X.2006.02692.x. [DOI] [PubMed] [Google Scholar]
- Asai S, Yoshioka H. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botrytis cinerea in Nicotiana benthamiana . Molecular Plant–Microbe Interactions. 2009;22,:619–629. doi: 10.1094/MPMI-22-6-0619. [DOI] [PubMed] [Google Scholar]
- Benito EP, Turrion-Gomez JL, Eslava AP. The flavohemoglobin BCFHG1 is the main NO detoxification system and confers protection against nitrosative conditions but is not a virulence factor in the fungal necrotroph Botrytis cinerea . Fungal Genetics and Biology. 2010;47:484–496. doi: 10.1016/j.fgb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Boccara M, Mills CE, Zeier J, Anzi C, Lamb C, Poole RK, Delledonne M. Flavohaemoglobin HmpX from Erwinia chrysanthemi confers nitrosative stress tolerance and affects the plant hypersensitive reaction by intercepting nitric oxide produced by the host. The Plant Journal. 2005;43:226–237. doi: 10.1111/j.1365-313X.2005.02443.x. [DOI] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. The Plant Cell. 2001;13,:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis . The Plant Cell. 2000;12,:2175–1290. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SM, Mur LA, Wood JE, Scott IM. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana . The Plant Journal. 2004;38:432–447. doi: 10.1111/j.1365-313X.2004.02054.x. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. Journal of Experimental Botany. 2006;57:581–588. doi: 10.1093/jxb/erj045. [DOI] [PubMed] [Google Scholar]
- Cristescu SM, Persijn ST, te Lintel Hekkert S, Harren FJM. Laser-based systems for trace gas detection in life sciences. Applied Physics B. 2008;92:343–349. [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394,:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences, USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Molecular Plant. 2008;1:423–445. doi: 10.1093/mp/ssn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. The Plant Journal. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Galletti R, Ferrari S, De Lorenzo G. Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide- or flagellin-induced resistance against Botrytis cinerea . Plant Physiology. 2011;157:804–814. doi: 10.1104/pp.111.174003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea . Current Biology. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- Guo FQ, Crawford NM. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. The Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT. On the origins of nitric oxide. Trends in Plant Science. 2011a;16:160–168. doi: 10.1016/j.tplants.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Hebelstrup KH, Mur LAJ, Igamberdiev AU. Plant hemoglobins: important players at the crossroads between oxygen and nitric oxide. FEBS Letters. 2011b;585:3843–3849. doi: 10.1016/j.febslet.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Hargrove MS, Brucker EA, Stec B, Sarath G, Arredondo-Peter R, Klucas RV, Olson JS, Phillips GN., , Jr Crystal structure of a nonsymbiotic plant hemoglobin. Structure. 2000;8:1005–1014. doi: 10.1016/s0969-2126(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Harutyunyan EH, Safonova TN, Kuranova IP, et al. The structure of deoxy- and oxy-leghaemoglobin from lupin. Journal of Molecular Biology. 1995;251:104–115. doi: 10.1006/jmbi.1995.0419. [DOI] [PubMed] [Google Scholar]
- Hebelstrup KH, Christiansen MW, Carciofi M, Tauris B, Brinch-Pedersen H, Holm PB. UCE: a uracil excision (USER (TM))-based toolbox for transformation of cereals. Plant Methods. 2010;6:15. doi: 10.1186/1746-4811-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebelstrup KH, Hunt P, Dennis E, Jensen SB, Jensen EO. Hemoglobin is essential for normal growth of Arabidopsis organs. Physiologia Plantarum. 2006;127,:157–166. [Google Scholar]
- Hebelstrup KH, Igamberdiev AU, Hill RD. Metabolic effects of hemoglobin gene expression in plants. Gene. 2007;398:86–93. doi: 10.1016/j.gene.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Hebelstrup KH, Jensen EO. Expression of NO scavenging hemoglobin is involved in the timing of bolting in Arabidopsis thaliana . Planta. 2008;227,:917–927. doi: 10.1007/s00425-007-0667-z. [DOI] [PubMed] [Google Scholar]
- Hunt PW, Watts RA, Trevaskis B, Llewelyn DJ, Burnell J, Dennis ES, Peacock WJ. Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Molecular Biology. 2001;47:677–692. doi: 10.1023/a:1012440926982. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Bykova NV, Hill RD. Structural and functional properties of class 1 plant hemoglobins. IUBMB Life. 2011;63:146–152. doi: 10.1002/iub.439. [DOI] [PubMed] [Google Scholar]
- Johnson HE, Lloyd AJ, Mur LAJ, Smith AR, Causton DR. The application of MANOVA to analyse Arabidopsis thaliana metabolomic data from factorially designed experiments. Metabolomics. 2007;3:517–530. [Google Scholar]
- Klessig DF, Durner J, Wendehenne D. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamattina L, Beligni MV. Nitric oxide in plants: the history is just beginning. Plant, Cell and Environment. 2001;24,:267–278. [Google Scholar]
- Lloyd AJ, William Allwood J, Winder CL, et al. Metabolomic approaches reveal that cell wall modifications play a major role in ethylene-mediated resistance against Botrytis cinerea . The Plant Journal. 2011;67:852–868. doi: 10.1111/j.1365-313X.2011.04639.x. [DOI] [PubMed] [Google Scholar]
- Lu D, Dong J, Jin H, Sun L, Xu X, Zhou T, Zhu Y, Xu M. Nitrate reductase-mediated nitric oxide generation is essential for fungal elicitor-induced camptothecin accumulation of Camptotheca acuminata suspension cell cultures. Applied Microbiology and Biotechnology. 2011;90:1073–1081. doi: 10.1007/s00253-011-3146-1. [DOI] [PubMed] [Google Scholar]
- Malolepsza U, Rozalska S. Nitric oxide and hydrogen peroxide in tomato resistance. Nitric oxide modulates hydrogen peroxide level in o-hydroxyethylorutin-induced resistance to Botrytis cinerea in tomato. Plant Physiology and Biochemistry. 2005;43:623–635. doi: 10.1016/j.plaphy.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Miao C, Hao FS, Zhao SL, Dong H, Zhang H, Sun LR. Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis . Journal of Integrative Plant Biology. 2010;52,:298–307. doi: 10.1111/j.1744-7909.2010.00920.x. [DOI] [PubMed] [Google Scholar]
- Mishina TE, Lamb C, Zeier J. Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis . Plant, Cell and Environment. 2007;30:39–52. doi: 10.1111/j.1365-3040.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- Modolo LV, Augusto O, Almeida IM, Magalhaes JR, Salgado I. Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae . FEBS Letters. 2005;579,:3814–3820. doi: 10.1016/j.febslet.2005.05.078. [DOI] [PubMed] [Google Scholar]
- Moreau M, Lindermayr C, Durner J, Klessig DF. NO synthesis and signaling in plants – where do we stand? Physiologia Plantarum. 2010;138:372–383. doi: 10.1111/j.1399-3054.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- Mur LA, Brown IR, Darby RM, Bestwick CS, Bi YM, Mansfield JW, Draper J. A loss of resistance to avirulent bacterial pathogens in tobacco is associated with the attenuation of a salicylic acid-potentiated oxidative burst. The Plant Journal. 2000;23:609–621. doi: 10.1046/j.1365-313x.2000.00825.x. [DOI] [PubMed] [Google Scholar]
- Mur LA, Carver TL, Prats E. NO way to live; the various roles of nitric oxide in plant–pathogen interactions. Journal of Experimental Botany. 2006;57:489–505. doi: 10.1093/jxb/erj052. [DOI] [PubMed] [Google Scholar]
- Mur LA, Laarhoven LJ, Harren FJ, Hall MA, Smith AR. Nitric oxide interacts with salicylate to regulate biphasic ethylene production during the hypersensitive response. Plant Physiology. 2008;148:1537–1546. doi: 10.1104/pp.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Lloyd AJ, Cristescu SM, Harren FJ, Hall MA, Smith AR. Biphasic ethylene production during the hypersensitive response in Arabidopsis: a window into defense priming mechanisms? Plant Signaling and Behavior. 2009;4:610–613. doi: 10.4161/psb.4.7.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LAJ, Mandon J, Cristescu SM, Harren FJM, Prats E. Methods of nitric oxide detection in plants: a commentary. Plant Science. 2011;181:509–519. doi: 10.1016/j.plantsci.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Mur LA, Santosa IE, Laarhoven LJ, Holton NJ, Harren FJ, Smith AR. Laser photoacoustic detection allows in planta detection of nitric oxide in tobacco following challenge with avirulent and virulent Pseudomonas syringae pathovars. Plant Physiology. 2005;138:1247–1258. doi: 10.1104/pp.104.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. Journal of Experimental Botany. 2008;59,:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Panstruga R. Tête à tête inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. The New Phytologist. 2006;171:699–718. doi: 10.1111/j.1469-8137.2006.01829.x. [DOI] [PubMed] [Google Scholar]
- Oliver RP, Solomon PS. Does the oxidative stress used by plants for defence provide a source of nutrients for pathogenic fungi? Trends in Plant Science. 2004;9:472–473. doi: 10.1016/j.tplants.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. The Plant Cell. 2004;16,:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT- PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, Van Loon LC. NPR1: the spider in the web of induced resistance signaling pathways. Current Opinion in Plant Biology. 2004;7:456–464. doi: 10.1016/j.pbi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Puppo A, Herouart D, Baudouin E, Frendo P, Harrison J, Santos R, Jamet A, Van de Sype G, Touati D. Reactive oxygen species, nitric oxide and glutathione: a key role in the establishment of the legume–Rhizobium symbiosis? Plant Physiology and Biochemistry. 2002;40:619–624. [Google Scholar]
- Qu ZL, Zhong NQ, Wang HY, Chen AP, Jian GL, Xia GX. Ectopic expression of the cotton non-symbiotic hemoglobin gene GhHbd1 triggers defense responses and increases disease tolerance in Arabidopsis . Plant and Cell Physiology. 2006;47:1058–1068. doi: 10.1093/pcp/pcj076. [DOI] [PubMed] [Google Scholar]
- Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro . Journal of Experimental Botany. 2002;53:103–110. [PubMed] [Google Scholar]
- Seregelyes C, Barna B, Hennig J, Konopka D, Pasternak TP, Lukacs N, Feher A, Horvath GV, Dudits D. Phytoglobins can interfere with nitric oxide functions during plant growth and pathogenic responses: a transgenic approach. Plant Science. 2003;165,:541–550. [Google Scholar]
- Thomma BP, Nurnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. The Plant Cell. 2011;23,:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Tadesse YSH, Cammue BPA, Broekaert WF. Susceptibility of an ethylene response mutant of Arabidopsis thaliana to Botrytis cinerea and Alternaria brassicicola . Plant Physiology. 1997;114:1177–1177. [Google Scholar]
- Tossi V, Amenta M, Lamattina L, Cassia R. Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant, Cell and Environment. 2011;34:909–921. doi: 10.1111/j.1365-3040.2011.02289.x. [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Watts RA, Andersson CR, Llewellyn DJ, Hargrove MS, Olson JS, Dennis ES, Peacock WJ. Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proceedings of the National Academy of Sciences, USA. 1997;94:12230–12234. doi: 10.1073/pnas.94.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrion-Gomez JL, Benito EP. Flux of nitric oxide between the necrotrophic pathogen Botrytis cinerea and the host plant. Molecular Plant Pathology. 2011;12:606–616. doi: 10.1111/j.1364-3703.2010.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiumi T, Shimoda Y, Nagata M, Suzuki A, Abe M, Sato S, Kato T, Tabata S, Higashi S. Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus . Plant and Cell Physiology. 2005;46:99–107. doi: 10.1093/pci/pci001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Elhiti M, Hebelstrup KH, Hill RD, Stasolla C. Manipulation of hemoglobin expression affects Arabidopsis shoot organogenesis. Plant Physiology and Biochemistry. 2011;49,:1108–1116. doi: 10.1016/j.plaphy.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Watts RA, Hunt PW, Hvitved AN, Hargrove MS, Peacock WJ, Dennis ES. A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proceedings of the National Academy of Sciences, USA. 2001;98,:10119–10124. doi: 10.1073/pnas.191349198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg JB, Bolognesi M, Wittenberg BA, Guertin M. Truncated hemoglobins: a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes, and plants. Journal of Biological Chemistry. 2002;277:871–874. doi: 10.1074/jbc.R100058200. [DOI] [PubMed] [Google Scholar]
- Wunsche H, Baldwin IT, Wu J. S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata . Journal of Experimental Botany. 2011a;62:4605–4616. doi: 10.1093/jxb/err171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunsche H, Baldwin IT, Wu J. Silencing NOA1 elevates herbivory-induced jasmonic acid accumulation and compromises most of the carbon-based defense metabolites in Nicotiana attenuata (F) Journal of Integrative Plant Biology. 2011b;53:619–631. doi: 10.1111/j.1744-7909.2011.01040.x. [DOI] [PubMed] [Google Scholar]
- Yordanova ZP, Iakimova ET, Cristescu SM, Harren FJ, Kapchina-Toteva VM, Woltering EJ. Involvement of ethylene and nitric oxide in cell death in mastoparan-treated unicellular alga Chlamydomonas reinhardtii . Cell Biology International. 2010;34:301–308. doi: 10.1042/CBI20090138. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Asai S, Yoshioka M, Kobayashi M. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Molecules and Cells. 2009;28:321–329. doi: 10.1007/s10059-009-0156-2. [DOI] [PubMed] [Google Scholar]
- Zeier J, Delledonne M, Mishina T, Severi E, Sonoda M, Lamb C. Genetic elucidation of nitric oxide signaling in incompatible plant–pathogen interactions. Plant Physiology. 2004;136:2875–2886. doi: 10.1104/pp.104.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottini M, Costa A, De Michele R, Ruzzene M, Carimi F, Lo Schiavo F. Salicylic acid activates nitric oxide synthesis in Arabidopsis . Journal of Experimental Botany. 2007;58,:1397–1405. doi: 10.1093/jxb/erm001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.