Abstract
It is well established that the plant hormone ethylene plays a key role in cucumber sex determination. Since the unisexual control gene M was cloned and shown to encode an ethylene synthase, instead of an ethylene receptor, the ‘one-hormone hypothesis’, which was used to explain the cucumber sex phenotype, has been challenged. Here, the physiological function of CsACS2 (the gene encoded by the M locus) was studied using the transgenic tobacco system. The results indicated that overexpression of CsACS2 increased ethylene production in the tobacco plant, and the native cucumber promoter had no activity in transgenic tobacco (PM). However, when PM plants were treated with exogenous ethylene, CsACS2 expression could be detected. In cucumber, ethylene treatment could also induce transcription of CsACS2, while inhibition of ethylene action reduced the expression level. These findings suggest a positive feedback regulation mechanism for CsACS2, and a modified ‘one-hormone hypothesis’ for sex determination in cucumber is proposed.
Keywords: CsACS2, Cucumis sativus L., ethylene induction, one-hormone hypothesis, positive feedback regulation, sex determination
Introduction
In angiosperms, the mechanisms of sex determination, which have been extensively studied in recent years, involve a number of different genetic and epigenetic factors (Tanurdzic and Banks, 2004). As a result of its diversity in sex types, cucumber (Cucumis sativus L.) has been researched as a model for more than half a century (Atsmon, 1968; Tsao, 1988; Perl-Treves, 1999). Cucumber plants generally produce male and female flowers separately on the same individual (monoecious); however, certain lines (hermaphrodite and andromonoecious) also produce bisexual flowers (Galun, 1961; Shifriss, 1961; Kubicki, 1969). Morphologically, all floral buds are initially hermaphroditic with staminate and pistillate primordia (Kubicki, 1969). A systematic morphogenetic analysis of male and female flower development revealed that the origin of floral reproductive organ differentiation and sex determination occurs after the carpel primordia initiation stage (Bai et al., 2004). The selective arrest of either the staminate or pistillate organs results in female or male flowers, respectively. Furthermore, if the arrest does not happen, hermaphroditic flowers are formed (Atsmon and Galun, 1960; Goffinet, 1990; Perl-Treves, 1999).
Early genetic studies indicated that three major genes are responsible for sex expression and segregation in cucumber: F/f, M/m, and A/a. The F gene promotes femaleness, while the recessive m gene regulates the appearance of bisexual flowers on the plant. Furthermore, in combination with the homozygous recessive f gene, the recessive a gene can intensify the androecious nature in cucumber plants (Galun, 1961; Kubicki, 1969; Robinson et al., 1976). Additionally, sex expression can also be regulated by many environmental factors such as photoperiod, temperature, and plant hormones such as ethylene, indole-3-acetic acid, and gibberellic acid (Atsmon, 1968; Takahashi et al. 1983; Perl-Treves, 1999; Yamasaki et al., 2005).
Preliminary physiological investigations revealed that a strong correlation exists between the plant hormone ethylene and the sex phenotype in cucumber. Elevated endogenous levels of ethylene can promote femaleness, whereas the inhibitors of ethylene biosynthesis or the ethylene response suppress the development of female reproductive organs (Atsmon and Tabbak, 1979; Takahashi and Jaffe, 1984). The arresting action of ethylene on stamen development can also be found in some other plant species. Overexpression of a cucumber ethylene synthesis gene, CsACO2 (Cucumis sativus ACC oxidase gene 2), can restrain Arabidopsis stamen development, mimicking female cucumber flowers (Duan et al., 2008).
The ‘one-hormone hypothesis’ posits that the F locus, which controls the gynoecious phenotype, regulates ethylene levels, promoting the development of the pistillate primordial state; and the dominant M locus, which is important in determining the monoecious condition, mediates the inhibition of stamen development by ethylene. The ethylene signal is not transmitted if the genotype is homozygous for m; as a result, stamen development is not inhibited, and the plant produces hermaphroditic flowers (Yin and Quinn, 1995; Yamasaki et al., 2001). According to this hypothesis, the F locus encodes a gene for ethylene production, and the M locus encodes an ethylene-response factor, which might be a receptor of ethylene.
Recently, the F locus has been cloned and shown to encode 1-aminocyclopropane-1-carboxylic acid synthase (ACS) (Trebitsh et al., 1997; Mibus et al., 2004), which has been verified as being involved in ethylene biosynthesis (Li et al., 2009). Furthermore, an ethylene receptor gene, CsETR1 (Cucumis sativus ethylene receptor 1), has been observed to play a key role in stamen arrest (Wang et al., 2010). A cucumber nuclease-encoding gene, CsCaN (Cucumis sativus calcium-dependent nuclease), has been shown to respond to the ethylene signal and is involved in the anther-specific DNA damage in the primordia of developing female flowers (Gu et al., 2011). However, the cloning of the M locus, which was previously thought to encode an ethylene receptor, reveals that it is not part of the ethylene signalling pathway, but rather encodes an enzyme involved in ethylene biosynthesis. This gene, CsACS2, also encodes an ACS (Boualem et al., 2009; Li et al., 2009). So, the response relationship between the CsACS2 gene and ethylene needs to be clarified.
Previous work indicated that the CsACS2 gene product is enzymatically active in Escherichia coli and can synthesize ACC (1-aminocyclopropane-1-carboxylic acid), which is a precursor of the ethylene molecule (Li et al., 2009). In this study, the function of this gene was studied using the transgenic tobacco system. The results indicate that the overexpression of CsACS2 increases ethylene production in tobacco, and these transgenic plants (P35) display short internodes and delayed flowering. Moreover, the original cucumber promoter of CsACS2 was not active in transgenic tobacco (PM), and did not promote transcription of the gene. However, when the PM plants were treated with exogenous ethylene, expression of CsACS2 could be detected. In cucumber plants, a certain amount of ethylene treatment could induce the transcription of CsACS2, while inhibition of ethylene action could reduce expression levels to varying degrees. These findings suggest a positive feedback regulation mechanism for CsACS2, which could be involved in flower development in cucumber.
Materials and methods
Plant materials
Sterile-grown seedlings of tobacco Nicotiana tabacum pv. Bairihong, which were provided by Professor Lingxia Zhao (Shanghai Jiaotong University, Shanghai), were used for Agrobacterium-mediated transformation. A gynoecious cucumber inbred line G06 (FFMM) was used for isolation of the wild-type CsACS2 gene and its promoter. A monoecious cucumber line M06 (ffMM), which was the near-isogenic line (NIL) with G06 for the F locus, was used to study the exogenous ethylene-inducing expression of CsACS2 in cucumber. A second gynoecious line, G34 (FFMM), together with the gynoecious line G06, was used to analyse the level of transcription of the CsACS2 gene during inhibition of ethylene action. A hermaphroditic cucumber line H34 (FFmm), together with its NIL G34 for the M locus, was used to study the promoters sequence and compare the relative expression of CsACS2 in near genotypic backgrounds, except for M/m. A traditional andromonoecious line ‘Lemon’ (ffmm) was used to analyse the gene expression and response to the ethylene-related chemical treatments. All cucumber lines were maintained by inbreeding at the Plant Department of Shanghai Jiaotong University and the College of Horticulture at Northwest A&F University. Conditions for germination and growth were according to Li et al. (2009).
Cloning of CsACS2 and its promoter
Cucumber apical shoots without any immature leaves were excised from the shoot apices with an Olympus SZ61 stereomicroscope (Olympus, Japan). As described previously (Li et al., 2009), apical shoot total RNA was isolated from G06 cucumber plants at the four-leaf stage (∼20 d after sowing). Reagents for RNA extraction were purchased from TIANGEN (China). DNase I (TaKaRa, China) was used to remove contaminating DNA, and first-strand cDNA was synthesized with the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas). The CDS (coding sequence) of the CsACS2 gene was amplified using a Long-Distance PCR Kit (Takara, China) with the appropriate primers (Supplementary Table S1 available at JXB online).
To isolate the CsACS2 promoter, genomic DNA was extracted as described previously (Li et al., 2008). The promoter fragment of the CsACS2 gene from G06 was amplified using a Long-Distance PCR Kit (Takara, China). The promoter regions from the NILs G34 and H34 were also amplified to compare the homologous cis-acting elements. Putative cis-acting elements in the CsACS2 promoter were identified by searching the PLACE database (http://www.dna.affrc.go.jp/PLACE/) (Higo et al., 1999).
All the DNA fragments were subcloned into the T/A-cloning vector pUCm-T (Fermentas) and sequenced as previously described (Li et al., 2008).
Generation of transgenic tobacco plants
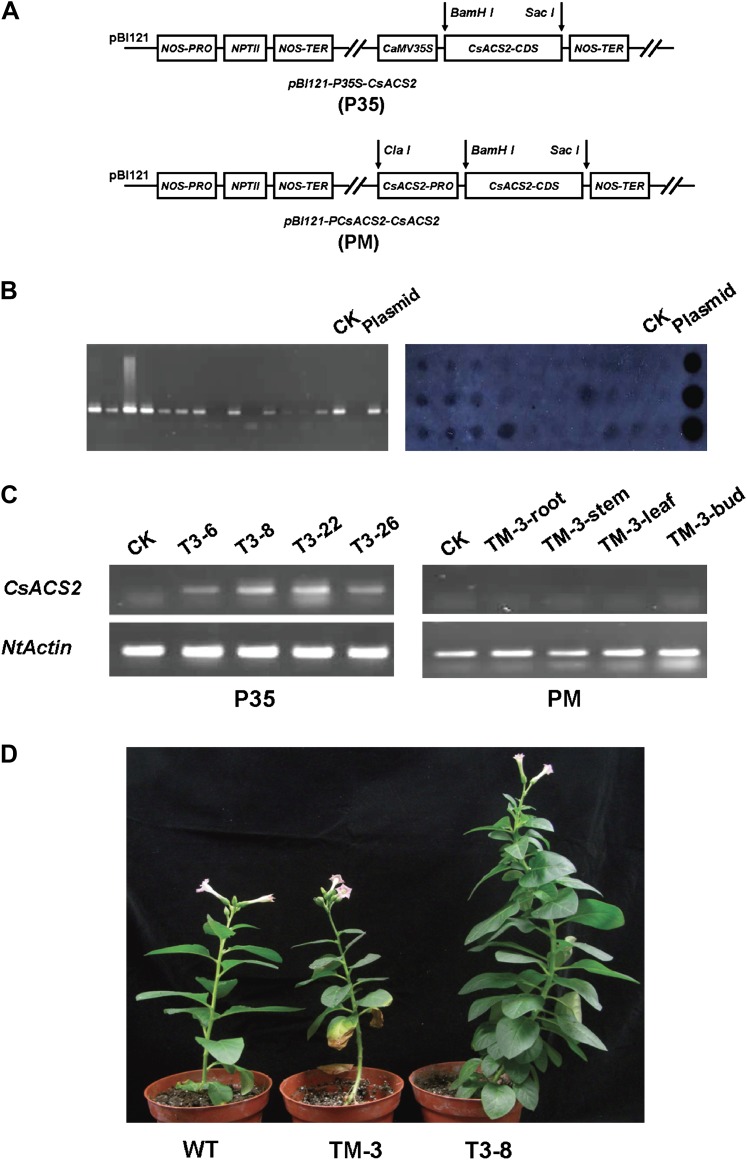
The CDS of CsACS2 was inserted into the pBI121 plasmid in the sense orientation under the control of the Cauliflower mosaic virus (CaMV) 35S promoter to give the pBI121-P35S-CsACS2 construct (Fig. 1A), which resulted in the transgenic tobacco plants designed P35. Using the restriction sites (ClaI and BamHI) flanking the CaMV 35S promoter, the cucumber promoter fragment was inserted to produce the pBI121-PCsACS2-CsACS2 plasmid (Fig. 1A), which resulted in the transgenic tobacco plants designed PM. The two expression cassettes were introduced into tobacco N. tabacum pv. Bairihong by Agrobacterium-mediated transformation (Hoekema et al., 1983).
Fig. 1.
Transgenic CsACS2 tobacco plants. (A) The two plasmid vectors used for tobacco transformation. The CsACS2 coding sequence was digested with BamHI and SacI, and ligated into pBI121 to produce the pBI121-P35S-CsACS2 construct. The CsACS2 promoter fragment was then digested with ClaI and BamHI to give the pBI121-PCsACS2-CsACS2 vector. (B) PCR and dot blotting analysis of transgenic tobacco plants. A total of 36 regenerated plants (27 P35 plants and nine PM plants) were assayed to find the positive transgenic events. Wild-type tobacco is indicated as CK, and this figure revealed part of the positive results. (C) Analysis of CsACS2 expression in transgenic plants. The left panel shows the expression pattern in four P35 plants (T3-6, T3-8, T3-22, and T3-26). No expression was detected in any positive PM transgenic tobacco plants, and the right panel shows the analysis of root, stem, leaf, and bud tissues from the TM-3 plant. (D) Phenotypes of the transgenic tobacco plants. TM-3 was a plant derived from the PM transformation (CsACS2 under control of the cucumber native promoter), and T3-8 was from the transformation of P35 (CsACS2 under control of the 35S promoter). (This figure is available in colour at JXB online.)
DNA was isolated from leaves of kanamycin-resistant regenerated plants to assay for positive transformation events by PCR using gene-specific primers (Supplementary Table S1 at JXB oline). PCR-positive transgenic plants were subjected to dot blotting analysis. Five P35 and three PM transgenic tobacco plants were chosen and self-pollinated to obtain homozygous T2 offspring.
Dot blotting and quantitative RT-PCR
Genomic DNA and total RNA of transgenic, wild-type tobacco and cucumber plants were extracted using the method described previously (Li et al., 2008, 2009).
Dot blotting
Tobacco genomic DNA samples (40 μg) were blotted onto Hybond-N+ nylon membranes with the Minifold I™ Dot-Blot System (Amersham, China). Hybridizations were performed using the protocol provided by the manufacturer (Gene Images random priming labeling module and Gene Images CDP-Star detection module from Amersham, China).
Semi-quantitative RT-PCR
First-strand cDNA was synthesized according to a method described previously(Li et al., 2009). Using the appropriate gene-specific primers (Supplementary Table S1 at JXB online), PCR was performed for 30 cycles (94 °C, 30 s; 55 °C, 30 s; 72 °C, 30 s). The products were separated on 1% agarose gels stained with ethidium bromide. The results were analysed using Gene Tools software from Syngene (CA, USA). The reverse transcription-PCRs (RT-PCRs) for two housekeeping genes (tobacco Actin gene AY189972 and cucumber Actin3 gene DQ115883) were performed under the same conditions as described above to estimate whether equal amounts of RNA had been used in the RT-PCRs.
Quantitative RT-PCR
Cucumber RNA extraction and first-strand cDNA synthesis were described previously. PCR was performed in a 96-well plate using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, USA), with SYBR Green Realtime PCR Master Mix (TaKaRa, China). The amplification was initiated by heating to 94 °C for 10 min, followed by 40 cycles at 94 °C for 5 s and 62 °C for 34 s. The amplification specificity was tested by a dissociation curve (65–90 °C). To compare results from different reactions and samples, CsACS2 amplification was normalized to CsActin3 using the CT values.
Ethylene production in transgenic tobacco and cucumber cultivars
The time course of ethylene evolution was examined in transgenic tobacco and cucumber cultivars according to Yamasaki et al. (2001). To examine ethylene production from the two transgenic tobacco lines, 10 g samples of leaf tissue from each of three P35 plants (T3-8, T3-22, and T3-26) and one PM plant (TM-3) were cut into 1 cm2 pieces and enclosed individually in 5 ml vessels sealed with rubber stoppers. After incubation at 25 °C for 1 h, 1 ml of head gas was withdrawn from each vessel using a gas-tight syringe. The gas samples were collected once every hour during a 3 h incubation period. To examine ethylene production from the five cucumber cultivars G06, M06, G34, H34, and ‘Lemon’, shoot apices were excised at the four-leaf stage. Head gas was sampled after incubation at 25 °C for 16 h. All samples were injected into a gas chromatograph (GC-2010, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and an activated alumina column. The instrument was calibrated with standard ethylene gas. Each result was derived from three independent biological replicates.
Chemical treatments
To investigate the effect of ethylene on CsACS2 expression, ethephon (an ethylene-releasing agent) was applied to transgenic tobacco and cucumber plants (M06) at various concentrations. AgNO3 (ethylene action inhibitor) and aminoethoxyvinyl glycine (AVG, ethylene biosynthesis inhibitor) were also applied to two cucumber lines, G06 and G34. Ethephon and AgNO3 were purchased from Sigma (China). AVG was provided by Professor Qiguang Wen (IPPE, CAS, Shanghai).
Ethephon treatment of transgenic plants
Briefly, 50 μl aliquots of 0.5 mM and 1.0 mM ethephon in water containing 0.1% (v/v) Tween-20 were applied to the apex of flower buds of the PM transgenic plant line TM-3 using a micropipette once a day for 3 d. The same treatment with water containing 0.1% (v/v) Tween-20 was the control (and was also used in the experiment below). The apex buds, leaves adjacent to the buds, and untreated lower leaves were then excised for RNA extraction.
Ethephon treatment of cucumber plants
As the expression of CsACS2 was not detected in apical shoot, the monoecious cucumber line M06 was selected to analyse the effect of ethylene on CsACS2 transcription. The apical shoots of four-leaf-stage M06 seedling were soaked in water containing 0.1, 0.5, and 1.0 mM ethephon with 0.1% (v/v) Tween-20 for 2, 4, and 6 h, respectively. The apices were then excised for RNA extraction.
AgNO3 and AVG treatment of cucumber plant
The cucumber lines G06 and G34, which both express CsACS2 at high levels, were selected to analyse the inhibitory effect of ethylene on CsACS2 expression. Aliquots of 50 μl of 1.0 mM AgNO3 and 1.0 mM AVG in H2O containing 0.1% (v/v) Tween-20 were applied to the apices of the two cucumber lines at the four-leaf stage using a micropipette once a day for 3 d. The apices were then excised for RNA extraction.
To study the effects of chemical application on the sexual expression of cucumber plants, several of the treated plants were allowed to grow to maturity. These included G06 and G34 treated with AgNO3 (1.0 mM), M06 treated with ethephon (0.5 mM and 1.0 mM), H34 treated with AgNO3 (1.0 mM) and ethephon (0.5 mM and 1.0 mM), and ‘Lemon’ treated with AgNO3 (1.0 mM) and ethephon (0.5 mM and 1.0 mM). The application method was as given above for AgNO3 and AVG treatment of cucumber plants. Every treatment employed five plants, and the number of male, female, and bisexual flowers was recorded up to the 30th node on the main stem.
Results
Overexpression of CsACS2 in transgenic tobacco plants
Transgenic tobacco plants were generated to analyse the biological function of CsACS2 under the control of two different promoters. After identification by PCR and dot blotting (Fig. 1B), five P35 and three PM transgenic tobacco plants were chosen for self-pollination to produce the homozygous T2 offspring.
RT-PCR revealed a high level of expression of CsACS2 in the P35 transgenic lines, T3-6, T3-8, T3-22, and T3-26 (Fig. 1C), and the plants showed delayed flowering compared with wild-type tobacco (Fig. 1D). The overexpression lines also had a shorter internode distance and increased leaf number before flowering (Fig. 1D). These phenotypes might have a relationship with an endogenous hormone balance disorder.
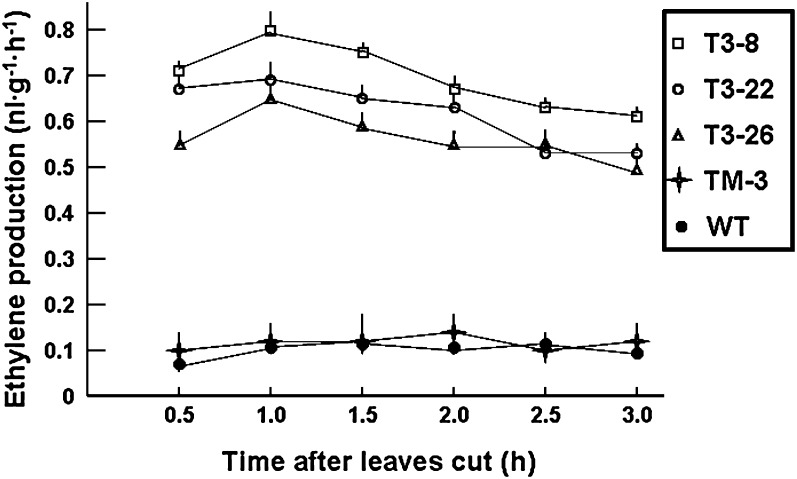
The P35 plants also showed higher ethylene production. Using gas chromatography analysis, it was found that leaves of three overexpression lines, T3-8, T3-22, and T3-26, produced more ethylene than did the wild-type (Fig. 2). This result indicated that the CsACS2 gene is involved in ethylene biosynthesis in planta, and it could lead to more ethylene production in the transgenic tobacco system.
Fig. 2.
Ethylene production in transgenic tobacco plants. Cut leaf sections from three P35 lines (T3-8, T3-22, and T3-26) and one PM line (TM-3) were used to analyse ethylene production by gas chromatography. Vertical bars indicated the standard deviation of the means for triplicate samples.
Induction of CsACS2 expression in transgenic tobacco
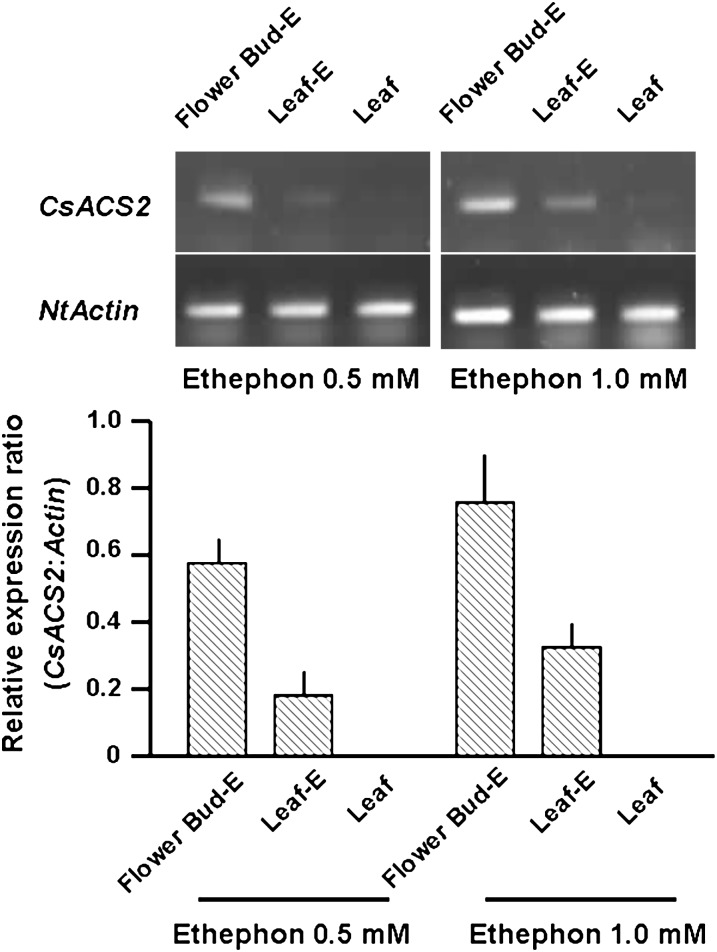
Using RT-PCR, it was not possible to detect any expression of CsACS2 in the PM lines; and the right-hand part of Fig. 1B shows this result in root, stem, leaf, and flower bud from the TM3 line. This is consistent with the fact that there was no visible change in phenotype of transgenic PM lines (Fig. 1D), and indicated that the native CsACS2 promoter from cucumber had no activity in tobacco. Moreover, ethylene production in TM3 was not significantly difference from that in wild-type tobacco (Fig. 2). However, after ethephon treatment, some differences in the expression level could be detected in the flower bud and its adjacent leaf (Fig. 3). It was supposed that treatment with 1.0 mM ethephon could induce more accumulation of CsACS2-specific mRNA due to transcription than 0.5 mM ethephon treatment, and that induction in flower bud was more sensitive than in leaf.
Fig. 3.
RT-PCR gel images (upper) and relative expression ratio (bar graph) of CsACS2 normalized to the Actin gene internal control in an ethephon-treated PM transgenic plant (TM-3). Leaf, untreated lower leaf; Flower Bud-E, ethephon-treated flower bud; Leaf-E, leaf adjacent to the ethephon-treated flower bud. Significance at P ≤ 0.05 is indicated by mean standard error bars (n=3).
Induced expression of CsACS2 in cucumber
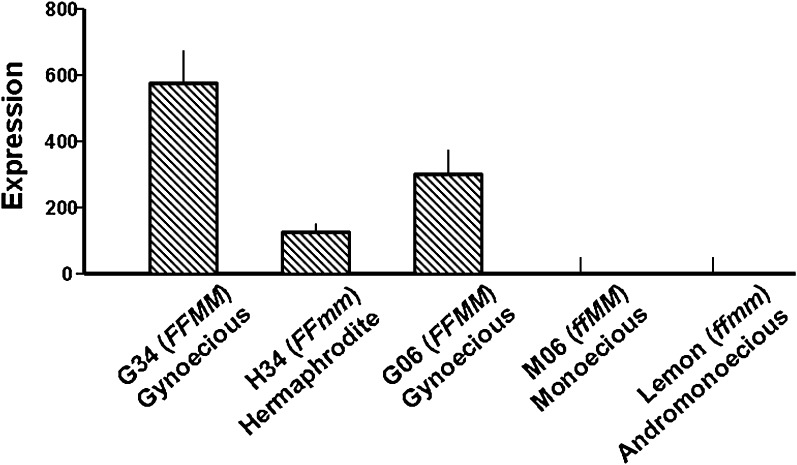
To analyse the effects of ethylene on CsACS2 expression in cucumber, the basal level of transcription needed to be determined initially. As a result of the different expression patterns of CsACS2 (Fig. 4), the cucumber line M06 was selected for the ethylene induction experiments, while lines G34 and G06 were used to study the inhibitory effect of ethylene on CsACS2 expression.
Fig. 4.
Quantitative RT-PCR of CsACS2 expression in five cucumber lines: G06 (FFMM), M06 (ffMM), G34 (FFMM), H34 (FFmm), and ‘Lemon’ (ffmm). Total RNA was extracted from shoot apices of cucumber plants at the four-leaf stage. Each result was the average of three independent biological replicates, and the significance at P ≤ 0.05 is indicated by mean standard error bars (n=3).
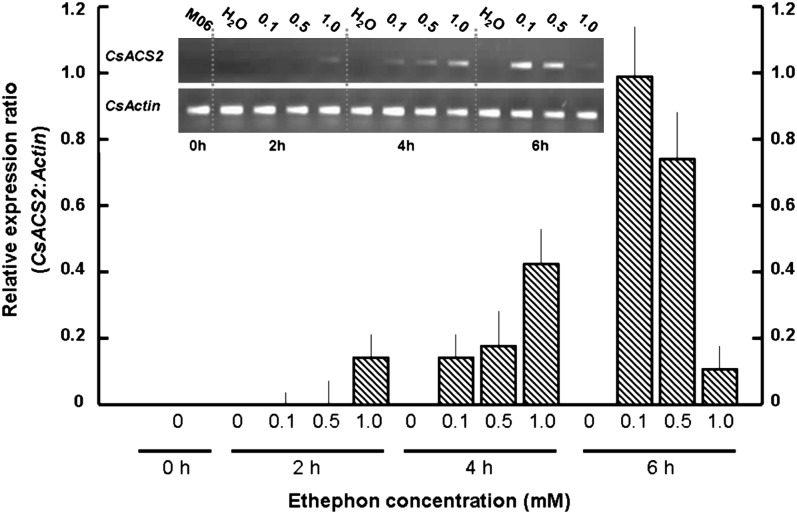
In M06, after 2 h treatment with 1.0 mM ethephon, the expression of CsACS2 could be detected; the transcription level peaked at 4 h after treatment, and then declined rapidly at 6 h. With 0.1 mM and 0.5 mM ethephon treatment, transcription was first detected at 4 h, and reached the highest level at 6 h (Fig. 5). This result indicated that certain concentrations of exogenous ethylene can induce the expression of CsACS2 in cucumber, and that higher concentrations result in more rapid induction and accumulation of CsACS2-specific mRNA. Possible causes for the observed decline in CsACS2 expression after treatment with 1.0 mM ethephon for 6 h will be discussed below.
Fig. 5.
RT-PCR gel images (upper) and relative expression ratio (bar graph) of CsACS2 normalized to the Actin gene control in shoot apices of cucumber line M06 (ffMM). Apical shoots of four-leaf stage seedlings were soaked in water containing 0, 0.1, 0.5, and 1.0 mM ethephon for 2, 4, and 6 h, respectively. The apices were then excised for RNA extraction. Data analysis was as given in Fig. 3.
Reduced expression of CsACS2 in cucumber in response to chemical treatment
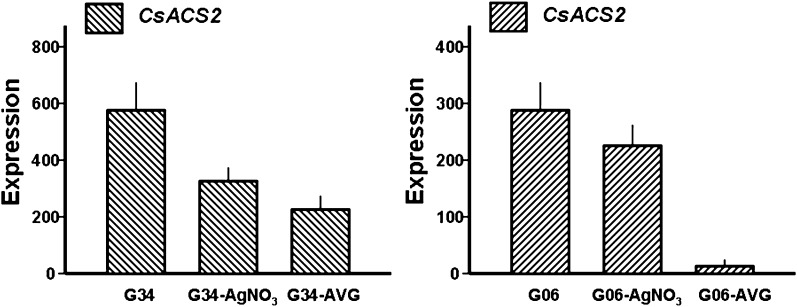
After 3 d of treatment with AgNO3 and AVG, a decline in CsACS2 expression was observed in cucumber shoot apices. For the AgNO3 treatment, expression decreased by 42% and 18% in G34 and G06, respectively. However, treatment with AVG had a more pronounced inhibitory effect than was observed for AgNO3; CsACS2 expression decreased by 60% and 95% in G34 and G06, respectively (Fig. 6). Therefore, the inhibitory effects of the endogenous ethylene response and biosynthesis negatively controlled CsACS2 expression.
Fig. 6.
Quantitative RT-PCR analysis of CsACS2 expression in two cucumber lines homozygous for the F locus after AgNO3 or AVG treatment. Left panel: expression of CsACS2 in cucumber line G34 (FFMM) resulting from different chemical treatments. Right panel: CsACS2 gene expression in G06 (FFMM). AgNO3 treatments are marked with ‘-AgNO3’, and AVG treatments with ‘-AVG’. Plant materials, RNA extraction, and expression analysis were as described in Fig. 4 and in the Materials and methods.
Effects of chemical treatment on sex determination in cucumber
Besides the effects on the expression of CsACS2, ethylene has a significant correlation with sex determination in cucumber plants, and it can induce femaleness. Table 1 shows the effect of ethylene-related treatments on the gynoecious (G06 and G34), monoecious (M06), hermaphrodite (H34), and andromonoecious (‘Lemon’) cucumber lines. AgNO3 is known to interfere with ethylene action, and its application promoted the conversion of female/hermaphrodite flowers into male flowers, which increased male flower number in all four cucumber cultivars tested (G06, from 0.5 to 13.5; G34, from 0 to 10.4; H34, from 0 to 13.5; and ‘Lemon’, from 16.6 to 28.4). Moreover, ethephon could induce development of female flowers in M06, with female flowers produced on 5.6 and 7.4 nodes after treatment with 0.5 mM and 1.0 mM ethephon, respectively. In bisexual lines, 0.5 mM ethephon treatment resulted in more bisexual flowers (16.5) in ‘Lemon’. Unexpectedly, when the concentration was increased to 1.0 mM, some female flowers (8.9) were produced, along with a further reduction of both male and bisexual flowers. A similar phenomenon was found with line H34, in which female flowers were first produced at 0.5 mM ethephon (3.2 nodes), and increased to 11.3 nodes at 1.0 mM. The number of bisexual flowers on H34 plants decreased to 24.3 and 12.6 nodes, respectively, at 0.5 mM and 1.0 mM ethephon. These results confirm the action of ethylene in arresting stamen development, which was expressed as the conversion of male/hermaphrodite into female flowers in cucumber plants with ethephon treatment, and suggest that there is a significant correlation between ethylene content and sex expression in cucumber.
Table 1.
Effects of chemical treatment on sex expression in cucumber plants
| Cultivar and genotype | Treatment | No. of flowers per plantz | |||
| Male flowers | Bisexual flowers | Female flowers | Abortion | ||
| G06 (FFMM) | Control | 0.5±0.3 a | 0.0 a | 28.8±1.0 b | 0.8±0.8 a |
| AgNO3 (1.0 mM) | 13.5±2.1 b | 0.0 a | 15.5±2.2 a | 1.0±0.9 a | |
| G34 (FFMM) | Control | 0.0 a | 0.0a | 28.5±0.8 b | 1.1±0.4 a |
| AgNO3 (1.0 mM) | 10.4±1.7 b | 0.0 a | 18.2±1.8 a | 0.9±0.8 a | |
| M06 (ffMM) | Control | 28.8±0.7 b | 0.0 a | 0.6±0.5 a | 0.6±0.5 a |
| Ethephon (0.5 mM) | 18.2±2.8 a | 0.0 a | 5.6±1.2 b | 3.6±1.8 a,b | |
| Ethephon (1.0 mM) | 15.7±5.6 a | 0.0 a | 7.4±3.2 b | 6.9±3.2 b | |
| H34 (FFmm) | Control | 0.0 a | 29.6±0.5 d | 0.0 a | 0.5±0.4 a |
| AgNO3 (1.0 mM) | 13.5±1.0 b | 16.3±1.0 b | 0.0 a | 0.4±0.2 a | |
| Ethephon (0.5 mM) | 0.0 a | 24.3±1.2 c | 3.2±0.5 b | 2.4±1.3 a | |
| Ethephon (1.0 mM) | 0.0 a | 12.6±2.0 a | 11.3±0.8 c | 6.1±2.1 b | |
| ‘Lemon’ (ffmm) | Control | 16.6±1.7 b | 13.1±2.0 c | 0.0 a | 0.5±0.3 a |
| AgNO3 (1.0 mM) | 28.4±1.7 c | 1.3±1.5 a | 0.0 a | 0.5±0.3 a | |
| Ethephon (0.5 mM) | 12.3±3.0 a | 16.5±2.5 c | 0.0 a | 1.8±0.5 a | |
| Ethephon (1.0 mM) | 10.3±2.0 a | 8.4±1.3 b | 8.9±2.0 b | 2.4±3.0 a | |
AgNO3 and ethephon were applied to the shoot apices of cucumber plants at the four-leaf stage. The number of male, female, and bisexual flowers was recorded up to the 30th node on the main stem. Nodes with no flowers were defined as ‘abortion’.
Mean values of five independent trials. Means followed by different letters are significantly different at the 5% level by LSD test.
CsACS2 promoter sequence analysis
The 1958 bp promoter fragments derived from G34 and H34 genomic DNA were sequenced, and no DNA polymorphisms were identified between them (GenBank accession no. FJ529216). Additionally, analysis of the conserved CsACS2 promoter sequence indicated that there were two EREs (ethylene-responsive elements; AWTTCAAA) at positions –154 to –147 and –1334 to –1327, respectively (Table 2). The EREs were identified as an ethylene-responsive enhancer element in Solanum lycopersicum (Montgomery et al., 1993), Dianthus caryophillus (Itzhaki et al., 1994), and Lycopersicon chilense (Tapia et al., 2005). These two cis-acting elements might take part in the ethylene-regulated expression of CsACS2. Moreover, one cytokinin (CTK) response element and one LEAFY element were also found in the CsACS2 promoter. These findings could be in agreement with the spatial expression pattern of CsACS2 in the cucumber shoot apical meristem (Saito et al., 2007).
Table 2.
Specific putative cis-acting elements in the promoter of CsACS2 (negative values indicate the first nucleotide position of the cis-elements with respect to the transcription initiation site located at position +1)
| Response | cis-element | Sequence (5' to 3') | Position |
| CTK response | CPBCSPOR [TATTAG] | TATTAG | –1407a |
| Ethylene response | ERE [A(A/T)TTCAAA] | AATTCAAA | –154,–1334 |
| LEAFY response | LEAFYATAG [CCAATGT] | CCAATGT | –1715a |
Sequence of the complementary strand.
Discussion
There is a long history of research into the mechanisms of sex determination in cucumber, and extensive physiological studies indicate that the plant hormone ethylene is the key point in the regulatory pathways. Based on the studies of Yin and Quinn (1995) and Yamasaki et al. (2001), the ‘one-hormone hypothesis’ was proposed, in which the core view is that ethylene regulates expression of the three cucumber flower phenotypes (female, male, and hermaphrodite). According to this hypothesis, the F locus is involved in ethylene synthesis, and the M locus is part of the ethylene signalling pathway. It was previously shown that the products of both CsACS1G (the F locus gene) and CsACS2 (the M locus gene) possess ACS enzyme activity, and that they might both be involved in ethylene biosynthesis (Li et al., 2009). In the present study, overexpression of CsACS2 in transgenic tobacco resulted in production of increased levels of ethylene gas, thus confirming that CsACS2 encodes an ethylene synthesis-related enzyme. Therefore, the regulation pattern and the response relationship between CsACS2 and ethylene needs to be clarified.
The present results demonstrated that the native CsACS2 promoter from cucumber could be induced by ethephon. This effect was detected in both transgenic tobacco and cucumber. These results suggest that the ethylene-responsive elements (possibly the EREs) in the cucumber CsACS2 promoter had a conserved function in the two plants. Unexpectedly, in cucumber plant, it was found that when the ethephon concentration was increased to 1.0 mM, and the treatment extended to 6 h, the expression of CsACS2 declined significantly. This is in agreement with the results of the study of Wang et al. (2010), in which treatment with ethephon at 1.0 mM for 6 h caused DNA damage in cucumber protoplasts.
The results of experiments in which chemical treatments interfered with endogenous ethylene action also confirmed this conclusion. To summarize, it was demonstrated that CsACS2 could cause increased ethylene production, and that expression of the CsACS2 gene can be induced by ethylene. Thus, a positive feedback mechanism could exist in the regulation of the CsACS2 gene. In a previous study, CsACS2 expression analysis indicated significant differences (>9-fold) between NILs for the M locus (Li et al., 2009). The experiments were repeated with more accurate shoot apex materials, and it was confirmed that the transcription level was much higher in the gynoecious line, G34 (FFMM), than in its hermaphrodite NIL partner, H34 (FFmm) (Fig. 4). Considering the very close genetic relationship, the difference in expression probably derives from the CsACS2 (M locus gene) itself. In this study, the allelic gene promoter fragments isolated from G34 and H34 did not show any sequence polymorphism. In previous work, it was also demonstrated that the recessive m allele, which is mutated at a single nucleotide, encoded an inactive product (Li et al., 2009). Thus, the putative positive feedback mechanism suggested here could explain this difference between the NILs. It is proposed that the ethylene derived from the wild-type M locus (CsACS2) induced self-expression, which was absent in the mm genotype because of the non-functional mutant.
The involvement of ethylene in the development of the gynoecium has been reported in hermaphrodite plants such as Arabidopsis and tobacco (De Martinis and Mariani, 1999; Tsuchisaka and Theologis, 2004). Moreover, ethylene production can directly disrupt stamen development in Arabidopsis (Duan et al., 2008). However, in the present study, relatively high concentrations of ethylene did not have an obvious arresting effect on tobacco stamen development (Supplementary Fig. S1 at JXB online), and ethephon treatments also did not cause any significant changes in filament length in PM transgenic plants (unpublished data). This leads to the proposal that stamen development in tobacco is less sensitive to ethylene than in cucumber and Arabidopsis.
The action of ethylene in arresting stamen development in cucumber was confirmed in the present study. The classical chemical application tests were repeated, and it was found that AgNO3 could induce stamen development, which led to an increase in the number of male flowers in all four cucumber genotypes. It was also shown that ethylene could induce pistil development and stamen arrest in three genetic backgrounds. As observed by Yamasaki et al. (2001), treatment with a low concentration of ethephon (0.5 mM in the present study) could induce the production of female flowers in the monoecious line (M06), but was not able to convert hermaphrodite into female flowers in the andromonoecious line (‘Lemon’). Unexpectedly, it was found that relatively high concentrations of ethephon could induce the change from bisexual flowers to female flowers in the hermaphrodite (H34 at 0.5 mM and 1.0 mM) and andromonoecious (‘Lemon’ at 1.0 mM) lines. As the M locus encodes an ethylene synthase and caused ethylene production in planta, it is proposed that there could be a local ethylene threshold to control the action of ethylene in arresting stamen development in cucumber. Under natural conditions, the ethylene derived from the continuous expression of CsACS2, which is induced by CsACS1G and maintained by positive feedback regulation, stays above the threshold and arrests the stamen development from stage 5 to stage 12 shown by Bai et al. (2004) and Satio et al. (2007), leading to female flowers in cucumber. In the mm genotype (hermaphrodite and andromonoecious lines), the ethylene threshold is not maintained, and stamen development does not arrest. In this case, the appropriate concentration of exogenous ethylene could compensate for this threshold and arrest stamen development, which was revealed when the lines H34 and ‘Lemon’ were treated with 1.0 mM ethephon. Moreover, in the H34 line, 0.5 mM ethephon induced female flowers, but this concentration was ineffective in ‘Lemon’. This finding was consistent with higher endogenous levels of ethylene in the H34 line than in ‘Lemon’ (Supplementary Fig. S2 at JXB online).
Saito et al. (2007) performed a comprehensive study on the expression pattern of CsACS2. Their results showed that the CsACS2 mRNA initially accumulated just beneath the pistil primordia of flower buds at the bisexual stage, and this coincided with the sex determination stage (stage 6) in cucumbers (Bai et al., 2004). It was noticed in the study that the continuous accumulation of CsACS2 mRNA was correlated with the final establishment of female flowers. These results imply the relationship between the expression of CsACS2 and the continuous arrest of stamen development. As has been proposed from this study, the positive feedback mechanism which leads to a constant level of transcription of CsACS2 could supply continuous ethylene, and could therefore constantly arrest stamen development.
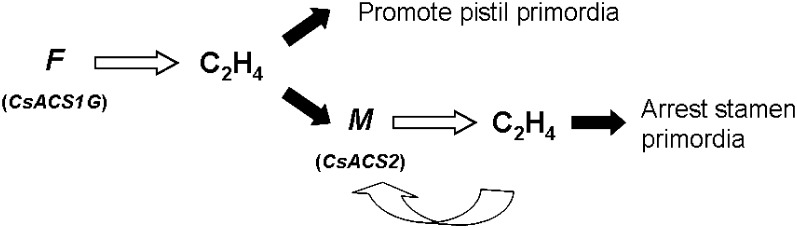
Combining all the previous data for F and M, the following modified model for sex determination in cucumber is proposed (Fig. 7).
Fig. 7.
Model of F and M functions during the development of female flowers in cucumber. Both F and M encode ACC synthases. F is the first specific gene activated and its eventual product, ethylene, promotes pistil primordia development. Concomitantly, ethylene activates M, and CsACS2 then begins its positive feedback activating expression. The continuous accumulation of ethylene constantly arrests the development of stamen primordia, resulting in the production of female flowers.
(i) Both F (CsACS1G) and M (CsACS2) encode ACC synthases, and their cooperation produces female, male, or bisexual flowers.
(ii) In the primordial buds that will develop into female flowers, F may be the first specific gene activated, and the eventual product, ethylene, promotes pistil primordia development. At the same time, ethylene activates M, which then begins its positive feedback expression. The continuous accumulation of ethylene beneath the region of the pistil primordia by translation of the M gene mRNA constantly arrests stamen primordia development, resulting in female flowers.
(iii) In the pre-determined stamen primordial buds, F will not be activated, and there will not be enough ethylene to start pistil primordia development and promote the expression of M. As a result, the pistil primordia do not develop and the arrest of stamen development does not happen. Thus, male flowers develop.
(iv) In the F-mm genotype, F will be activated normally, and pistil primordia develop without resistance. However, there will not be enough ethylene accumulation to eliminate the development of stamen primordia because of the inactive ACC synthase mutant in the mm genotype. Thus, bisexual flowers develop.
The detailed mechanism of how ethylene arrests stamen development is still unknown in cucumber, and the ethylene threshold proposed here is speculative. The hypothesis stated above is probably not the final one, and it can only explain the current research results in cucumber. Identifying more mutations in the sex expression pathway and cloning the a gene in cucumber may help to reveal more complete information in this area.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Flower phenotypes in transgenic tobacco plants.
Figure S2. Ethylene production from shoot apices of the five cucumber cultivars.
Table S1. Primers used in this study
Acknowledgments
The authors wish to thank Professor Lingxia Zhao (Shanghai Jiaotong University, Shanghai) for the tobacco Nicotiana tabacum pv. Bairihong; Professor Qiguang Wen (Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, Shanghai) for the chemical reagent AVG; and Dr Shoujun Wu (Northwest A&F University, Yangling) for his critical reading of this work. This work was supported by the National Natural Science Foundation of China (no. 31101540), Research Fund for the Doctoral Program of Higher Education of China (no. 20110204120004), and Starting Research Fund from the Northwest A&F University (no. 2010BSJJ060).
References
- Atsmon D. The interaction of genetic, environmental, and hormonal factors in stem elongation and floral development of cucumber plants. Annals of Botany. 1968;32:877–882. [Google Scholar]
- Atsmon D, Galun E. A morphogenetic study of staminate, pistillate and hermaphrodite flowers in Cucumis sativus L. Phytomorphology. 1960;10:110–115. [Google Scholar]
- Atsmon D, Tabbak C. Comparative effects of gibberellin, silver nitrate and aminoethoxyvinyl glycine on sexual tendency and ethylene evolution in cucumber plant (Cucumis sativus L.) Plant and Cell Physiology. 1979;20:1547–1555. [Google Scholar]
- Bai SL, Peng YB, Cui JX, Gu HT, Xu LY, Li YQ, Xu ZH, Bai SN. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.) Planta. 2004;220:230–240. doi: 10.1007/s00425-004-1342-2. [DOI] [PubMed] [Google Scholar]
- Boualem A, Troadec C, Kovalski I, Sari MA, Perl-Treves R, Bendahmane A. A conserved ethylene biosynthesis enzyme leads to andromonoecy in two cucumis species. PLoS ONE. 2009 doi: 10.1371/journal.pone.0006144. 4, e6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis D, Mariani C. Silencing gene expression of the ethylene-forming enzyme results in a reversible inhibition of ovule development in transgenic tobacco plants. The Plant Cell. 1999;11:1061–1071. doi: 10.1105/tpc.11.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan QH, Wang DH, Xu ZH, Bai SN. Stamen development in Arabidopsis is arrested by organ-specific overexpression of a cucumber ethylene synthesis gene. CsACO2. Planta. 2008;228:537–543. doi: 10.1007/s00425-008-0756-7. [DOI] [PubMed] [Google Scholar]
- Galun E. Study of the inheritance of sex expression in the cucumber: the interaction of major genes with modifying genetic and non-genetic factors. Genetica. 1961;32:134–163. [Google Scholar]
- Goffinet MC. Comparative ontogeny of male and female flowers of Cucumis sativus . In: Bates EM, Robinson RW, Jeffrey C, editors. Biology and utilization of the Cucurbitaceae. New York: Cormell University Press; 1990. pp. 288–304. [Google Scholar]
- Gu HT, Wang DH, Li X, He CX, Xu ZH, Bai SN. Characterization of an ethylene-inducible, calcium-dependent nuclease that is differentially expressed in cucumber flower development. New Phytologist. 2011;192:590–600. doi: 10.1111/j.1469-8137.2011.03825.x. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Research. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema P, Hirsch P, Hooykaas R, Schilperoort A. A binary plant vector strategy based on separation of vir and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature. 1983;303:179–180. [Google Scholar]
- Itzhaki H, Maxson JM, Woodson WR. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene. Proceedings of the National Academy of Sciences, USA. 1994;91:8925–8929. doi: 10.1073/pnas.91.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki B. Investigation of sex determination in cucumber (Cucumis sativus L.) Genetica Polonica. 1969;10:5–143. [Google Scholar]
- Li Z, Huang SW, Liu SQ, et al. Molecular isolation of the M gene suggests that a conserved-residue conversion induces the formation of bisexual flowers in cucumber plants. Genetics. 2009;182:1381–1385. doi: 10.1534/genetics.109.104737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Pan JS, Guan Y, Tao QY, He HL, Si LT, Cai R. Development and fine mapping of three co-dominant SCAR markers linked to the M/m gene in the cucumber plant (Cucumis sativus L.) Theoretical and Applied Genetics. 2008;117:1253–1260. doi: 10.1007/s00122-008-0859-3. [DOI] [PubMed] [Google Scholar]
- Mibus H, Tatlioglu T. Molecular characterization and isolation of the F/f gene of femaleness in cucumber (Cucumis sativus L.) Theoretical and Applied Genetics. 2004;109:1669–1676. doi: 10.1007/s00122-004-1793-7. [DOI] [PubMed] [Google Scholar]
- Montgomery J, Goldman S, Deikman J, Margossian L, Fischer RL. Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proceedings of the National Academy of Sciences, USA. 1993;90:5939–5943. doi: 10.1073/pnas.90.13.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl-Treves R. Male to female conversion along the cucumber shoot: approaches to studying sex genes and floral development in Cucumis sativus . In: Ainsworth CC, editor. Sex determination in plants. Oxford: BIOS Scientific Publishers; 1999. pp. 189–286. [Google Scholar]
- Robinson RW, Munger HM, Whitaker TW, Bohn GM. Genes of Cucubitaceae. HortScience. 1976;11:554–568. [Google Scholar]
- Saito S, Fujii N, Miyazawa Y, Yamasaki S, Matsuura S, Mizusawa H, Fujita Y, Takahashi H. Correlation between development of female flower buds and expression of the CS-ACS2 gene in cucumber plants. Journal of Experimental Botany. 2007;58:2897–2907. doi: 10.1093/jxb/erm141. [DOI] [PubMed] [Google Scholar]
- Shifriss O. Sex control in cucumber. Journal of Heredity. 1961;52:5–12. [Google Scholar]
- Takahashi H, Jaffe MJ. Further studies of auxin and ACC induced feminization in the cucumber plant using ethylene inhibitors. Phyton. 1984;44:81–86. [PubMed] [Google Scholar]
- Takahashi H, Saito T, Suge H. Separation of effects of photoperiod and hormones on sex expression in cucumber. Plant and Cell Physiology. 1983;24:142–154. [Google Scholar]
- Tanurdzic M, Banks JA. Sex-determining mechanisms in land plants. The Plant Cell. 2004;16:S61–S71. doi: 10.1105/tpc.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia G, Verdugo I, Yanez M, Ahumada I, Theoduloz C, Cordero C, Poblete F, Gonzalez E, Ruiz-Lara S. Involvement of ethylene in stress-induced expression of the TLC1.1 retrotransposon from Lycopersicon chilense Dun. Plant Physiology. 2005;138:2075–2086. doi: 10.1104/pp.105.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebitsh T, Staub JE, O‘Neill SD. Identification of a 1-aminocyclopropane-1-carboxylic acid synthase gene linked to the Female (F) locus that enhances female sex expression in cucumber. Plant Physiology. 1997;113:987–995. doi: 10.1104/pp.113.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao TH. Sex expression in flowering. Acta Phytophysiologica Sinica. 1988;14:203–207. [Google Scholar]
- Tsuchisaka A, Theologis A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiology. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DH, Li F, Duan QH, Han T, Xu ZH, Bai SN. Ethylene perception is involved in female cucumber flower development. The Plant Journal. 2010;61:862–872. doi: 10.1111/j.1365-313X.2009.04114.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Fujii N, Takahashi H. Hormonal regulation of sex expression in plants. Vitamins and Hormones. 2005;72:79–110. doi: 10.1016/S0083-6729(05)72003-3. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Fujii N, Matsuura S, Mizusawa H, Takahashi H. The M locus and ethylene-controlled sex determination in andromonoecious cucumber plants. Plant and Cell Physiology. 2001;42:608–619. doi: 10.1093/pcp/pce076. [DOI] [PubMed] [Google Scholar]
- Yin T, Quinn JA. Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae) American Journal of Botany. 1995;82:1537–1546. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.