Abstract
Various transcriptional networks and plant hormones have been implicated in controlling different aspects of potato tuber formation. Due to its broad impact on many plant developmental processes, a role for auxin in tuber initiation has been suggested but never fully resolved. Here, auxin concentrations were measured throughout the plant prior to and during the process of tuber formation. Auxin levels increase dramatically in the stolon prior to tuberization and remain relatively high during subsequent tuber growth, suggesting a promoting role for auxin in tuber formation. Furthermore, in vitro tuberization experiments showed higher levels of tuber formation from axillary buds of explants where the auxin source (stolon tip) had been removed. This phenotype could be rescued by application of auxin on the ablated stolon tips. In addition, a synthetic strigolactone analogue applied on the basal part of the stolon resulted in fewer tubers. The experiments indicate that a system for the production and directional transport of auxin exists in stolons and acts synergistically with strigolactones to control the outgrowth of the axillary stolon buds, similar to the control of above-ground shoot branching.
Key words: Auxin, auxin content, gibberellins, potato, strigolactones, tuberization physiology, YUC.
Introduction
Potato tuber formation is the result of interplay between environmental cues and endogenous signals. In many potato species, short photoperiods promote the initiation of potato tuber organogenesis whereas high night temperatures and high nitrogen levels have an inhibiting effect (Gregory, 1965; Demagante and Vander Zaag, 1988; Ewing and Struik, 1992). Under favourable environmental conditions, a graft-transmissible signal is produced in the leaves and transported to the stolon where it induces tuber formation (Gregory, 1956). This graft-transmissible signal has recently been identified to be an FT-like protein, encoded by an FT orthologue in potato called StSP6A (Navarro et al., 2011). Several plant hormones have been implicated in tuber initiation; in particular gibberellic acid (GA) was shown to have a strong inhibiting effect, and degradation of active GAs in the stolon tip at tuber formation is important for tuberization to proceed normally (Ewing and Struik, 1992; Carrera et al., 2000; Kloosterman et al., 2007). Abscisic acid (ABA) has been shown to have a promoting effect on tuberization when applied exogenously and may act antagonistically to GA (Xu et al., 1998a). Application of a cytokinin (zeatin riboside) to an in vitro tuberization experiment resulted in an increase in tuber formation (Mauk and Langille, 1978). Moreover, potato plants expressing the cytokinin biosynthesis gene ipt yielded more tubers, but with reduced tuber weight and nitrogen content (Tao et al., 2010). Xu et al. (1998a) reported that when auxin was applied to single nodal in vitro potato explants, an earlier tuberization phenotype was observed with sessile and slightly smaller tubers. These findings suggest a role for auxin in tuber formation. However, the precise mode of action for auxin in conjunction with other plant hormones in stolons has not been established, partly due to lack of knowledge on auxin concentrations in the stolon. A microarray-based expression study provided additional data to support an important role for auxin in tuber development. Many auxin-related genes are differentially expressed during early tuber developmental stages. Genes involved in auxin transport (PIN gene family), auxin response factors (ARF), and Aux/IAA genes exhibited differential expression profiles (Kloosterman et al., 2008). The ARF6 gene was found to have high expression prior to visible swelling, followed by down-regulation during subsequent tuber growth (Faivre-Rampant et al., 2004).
Auxin has been shown to play a key role in many different aspects in plant architecture, such as lateral root formation (Marchant et al., 2002), embryogenesis (Luo et al., 2011), and flower development (Krizek, 2011). Tuber organogenesis is divided into three stages, tuber induction, tuber initiation, and tuber growth (Ewing and Struik, 1992). During the stage of tuber initiation, changes in the plane of cell division occur in the region of the stolon that will give rise to the young tuber through swelling (Xu et al., 1998b). Auxin is produced in plants by at least two different metabolic routes, a tryptophan-dependent and a tryptophan-independent pathway (reviewed in Lehmann et al., 2010). Although these pathways are not yet fully understood, several biosynthetic genes have been identified, such as the YUCCA gene family (Steven, 2001; Zhao et al., 2001; Cheng et al., 2006). Recently, YUC proteins were identified as catalysing a rate-limiting step in the main indole-3-acetic acid (IAA) biosynthesis pathway in Arabidopsis (Mashiguchi et al., 2011). Shoot apical meristems (SAMs) are the main sites of auxin biosynthesis, along with cotyledons, expanding leaves, and root tissues (Ljung et al., 2001). Specific subcellular localization of auxin influx and efflux carriers modulates the transport directionality (Chen et al., 1998; Gälweiler et al., 1998). Auxin transport inhibitors, such as 2,3,5-triiodobenzoic acid (TIBA), which interrupt the constitutive cycling of the PIN proteins between the plasma membrane and the endosomes, interfere with directional auxin transport, and have been essential tools in formulating the concept of asymmetrical auxin distribution (Dhonukshe et al., 2008). In addition to polar auxin transport inhibitors, auxin antagonists such as α-(phenylethyl-2-one)-IAA (PEO-IAA), competing for the same binding site as auxin, has been used to unravel the role of auxin in various developmental procedures, such as root node development (Benková E, Bielach A, 2010) and the gravitropic response (Nishimura et al., 2009).
Studies on plant stem architecture have led to a model that describes the principles of shoot branching (reviewed in Domagalska and Leyser, 2011). Two key players in the model are auxin and strigolactones (SLs) (Cook et al., 1972; Bouwmeester et al., 2003; Gomez-Roldan et al., 2008; Rameau, 2010). Auxin was the first plant hormone shown to have an inhibitory effect on shoot branching (Snow, 1937), through the establishment of polar auxin transport (Gälweiler et al., 1998). SLs have recently been identified as the secondary signal that, in concert with auxin, appears to regulate shoot branching (Gomez-Roldan et al., 2008). Moreover, SLs have been detected in root exudates and extracts of several plant species including Arabidopsis and tomato (López-Ráez et al., 2008; Kohlen et al. 2011). It has been proposed that SLs act either directly on axillary bud outgrowth or indirectly via dampening of auxin transport and canalization of auxin from the buds (Brewer et al., 2009; Prusinkiewicz et al., 2009).
In this study, auxin concentrations were determined during early tuberization events in potato plants in vivo, in parts of the stolon and in parts of the stem. Furthermore, the impact of auxin, synthetic strigolactone (GR24) and inhibitors of auxin transport and perception on tuber formation was examined using a modified in vitro tuberization approach. Based on these results, a similar system of apical dominance in underground stolons to that described for shoots is proposed.
Materials and methods
Plant materials and in vitro tuberization
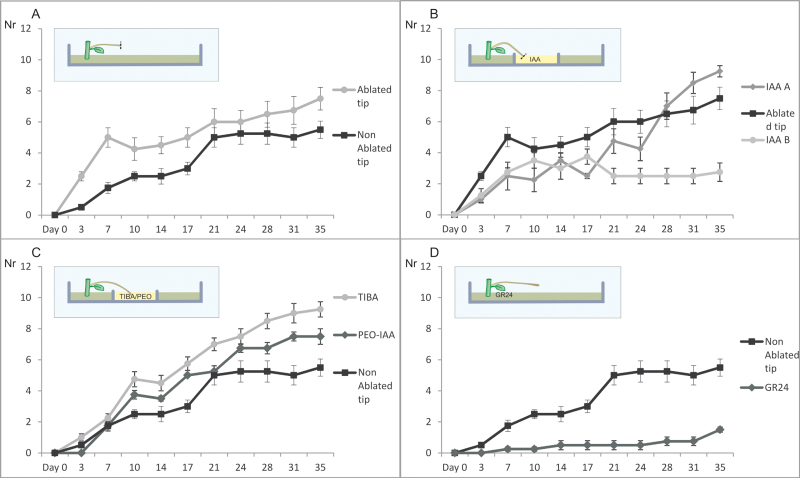
Single-node cuttings from short-day (SD)-grown potato plants (Solanum tuberosum L. var Bintje) were propagated in vitro, on standard Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) with 2% (w/v) sucrose. Potato plantlets were grown for 4 weeks prior to harvesting single-node explants. After 10–12 d of growth in the dark on 2% sucrose MS medium with 1 mg l-1 benzylaminopurine (BAP), the explants formed etiolated shoots/stolons which were transferred to Petri dishes containing different tuberization media and kept in the dark at 18 °C. Explants with stolons were placed into Petri dishes containing dual media based on KI medium (Hendriks et al., 1991). Media supplemented with the compounds under investigation were applied to the base of the explants or to the stolon tip (Fig. 4, inset). The final concentrations of the studied compounds were: 80 µM TIBA, 1 µM PEO-IAA, 1 µM IAA, and 5µM GR24. BAP, IAA, and TIBA were purchased from Sigma. PEO-IAA (Hayashi et al., 2008) was a kind gift from Dr Kenichiro Hayashi. The explants for each treatment were divided into four groups of two Petri dishes each, with six explants in each Petri dish (total 48 explants per treatment). The number of explants producing tubers was monitored for a period of 39 d.
Fig. 4.
Comparison of in vitro tuberization frequency between the ablated and not ablated stolon tips exposed to various treatments (A–D). In all panels, the y-axis (labelled ‘Nr’) represents the number of explants, and the x-axis (labelled ‘Days’) represents the days under tuber-inducing conditions. (A) The effect of stolon tip ablation on the production of in vitro tubers. (B) Effect of the application of 1 µM auxin on ablated stolon tips where explants were either transferred to fresh medium after 19 d (IAA B) or remained on the same medium throughout the experimental period (IAA A). The controls (black graph) were explants with ablated tips without hormone treatment. (C) Numbers of explants that produced tubers after applying TIBA and the IAA antagonist PEO-IAA. The controls are the same as in B. (D) The effect of GR24 on in vitro tuberization efficiency. In all cases, error bars represent the standard error of the mean of four replicated measurements. The insets are diagrammatic representations of the location of treatment application, and the ablation of the stolon (dotted line). (This figure is available in colour at JXB online.)
Detection of strigolactones in potato roots
Solanum tuberosum L. var. Karnico, Bintje and S. tuberosum group andigena were grown in vitro for 20–30 d and then transferred to an aeroponic system (Nutricolture Co. UK) on Hoagland’s solution, as previously described (López-Ráez et al., 2008). After 24 d of growth, the plants were exposed to phosphate starvation as described for tomato (López-Ráez et al., 2008). Seven days after the phosphate starvation, roots were harvested for extraction of SLs. The extraction of SLs and the liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis were performed as described previously (López-Ráez et al., 2008).
Assessment of auxin concentrations
Potato plants (S. tuberosum group andigena) were propagated in vitro and grown for 2.5 weeks in MS20 before being transferred to soil-filled pots in the greenhouse. After 9 weeks, the shoot apex, middle, and basal internode stem segments, the stolon region 1 cm below the apex [subswelling region (SSR)], and the stolon apical meristem (STAM) were harvested and immediately frozen in liquid nitrogen (day 0). The remaining plants were then transferred to SD conditions (8 h light). The same set of plant tissues was harvested 5, 16, and 26 d after the switch to SD conditions. Fully matured tubers were harvested 8 weeks after switch to SD conditions, and samples of the tuber apex, heel, pith, and the perimedullary region were collected. For all samples, two biological repeats were collected and tissues from five different plants were pooled for each repeat, except for the stolon tip on day 5 where only one biological repeat was collected due to low sample weight. All samples were ground to a fine powder and stored at –80 °C. The auxin extraction was performed starting with 200–250 mg of ground material. A 1 ml aliquot of MeOH with labelled auxin (IAA-IS; 0.1 nmol ml-1 in a sample a 0.5 nmol ml-1) was added and samples were briefly vortexed. The samples were then sonicated for 10 min. After sonification, the samples were placed in a shaker (~150 rpm) in a cold room (4° C) for 1 h. The samples were then centrifuged at 2000 rpm for 10 min and the supernatant was transferred to a 4 ml glass vial. Extraction was repeated with 1 ml of MeOH without IAA-IS, shaken for 1 h in a cold room, centrifuged, and the supernatants of the same sample were pooled. The columns (Solid Phase Extraction cartridges, Grace Davison Discovery Sciences) were placed on a Solid Phase Extraction Apparatus and pre-equilibrated by applying in order: 5 ml of hexane, 5 ml of acetonitrile, 5 ml of deionized water, and 5 ml of imidazole buffer. The sample was applied on the column and washed by applying the following in this order: 5 ml of hexane, 5 ml of ethyl acetate, 5 ml of acetonitrile, and 5 ml of methanol. The samples were eluted with 4 ml of 98% methanol and 2% acetic acid. Solvents were evaporated in a speedvac. The samples were finally eluted in 200 µl of acetonitrile:H2O:formic acid, 25:75:0.1 and filtered in vials using a RC4 Minisart 0.2 µm filter. The LC-MS/MS analysis was performed as described in Ruyter-Spira et al. (2010).
DR5::GUS transgenic plants and GUS staining
Transgenic plants harbouring the DR5::GUS (Ulmasov et al., 1997) and the promoter from a potato PINgene fused to the β-glucuronidase (GUS) reporter (pStPINV::GUS) were obtained by Agrobacterium-mediated transformation of S. andigena. StPINV is apotato PIN gene located on chromosome 5 (genome sequence coordinates PGSC0003DMB000000051: 1580317–1584014 V.3.0), previously found to be expressed during early stages of tuber initiation (Kloosterman et al., 2008). A region of 1310 bp upstream of the translational start site of StPINV was used to drive the GUS gene expression in the pStPINV::GUS construct. The GUS staining assays were done as described previously (Stomp, 1992). The incubation of the tissues in the GUS substrate X-Gluc was performed overnight at 37 °C. The tissues were washed with 70% ethanol prior to imaging.
Quantitative RT-PCR
Solanum tuberosum L. group andigena plants were grown in the greenhouse, and transferred to SD conditions to induce tuberization. Stolon tips were harvested at day 0 (switch to SD), 2, 4, 6, and 8. RNA was extracted using the Qiagen RNaesy Plant mini kit and DNase I treated. cDNA synthesis was performed using a Bio-Rad iScript cDNA synthesis kit, and qRT-PCR was performed using the Bio-Rad cycler. As a reference gene, eIF3e was used (forward primer seq GGAGCACAGGAGGAAGATGAAGGAG, reverse primer seq CGTTGGTGAATGCGGCAGGAAGGAG). A StYUC-like1 (GenBank accession number JN935396) gene expression study was performed with the following primers: forward primer seq TGTTTTGGACATTGGTGCAT, reverse primer seq AACGGTGCCACATGAAAACT.
Auxin transport assays
Polar auxin transport was measured as previously described (Okada et al., 1991), with some modifications. Stolon tips were cut into 25 mm pieces, put into 1 ml brown glass vials, and incubated at either the proximal or distal end with 200 µl of quarter strength Hoagland medium containing 14C-labelled IAA (American Radiolabeled Chemicals Inc., St Louis, MO, USA), with or without 2.5 µM naphthylphthalamic acid (NPA), for 18 h at room temperature. The final concentration of IAA was adjusted to 1 µM (0.2 nCi ml-1). For comparable access, the tip at the distal end (0.5 mm) was removed before introduction to the radioactive medium. The 5 mm end not in direct contact with the radioactive medium was incubated at 50 °C for 2 h using 0.5 ml of Lumasolve (Lumac Systems AG, Basel, Switzerland) and the radioactivity was counted in 4 ml of Ultima Gold™ (PerkinElmer Life Sciences, Inc., Boston, MA, USA) using a tri-carb 2100TR liquid scintillation counter (PerkinElmer Life Sciences, Inc.).
Results
Auxin measurement in potato plants under inductive and non-inductive conditions
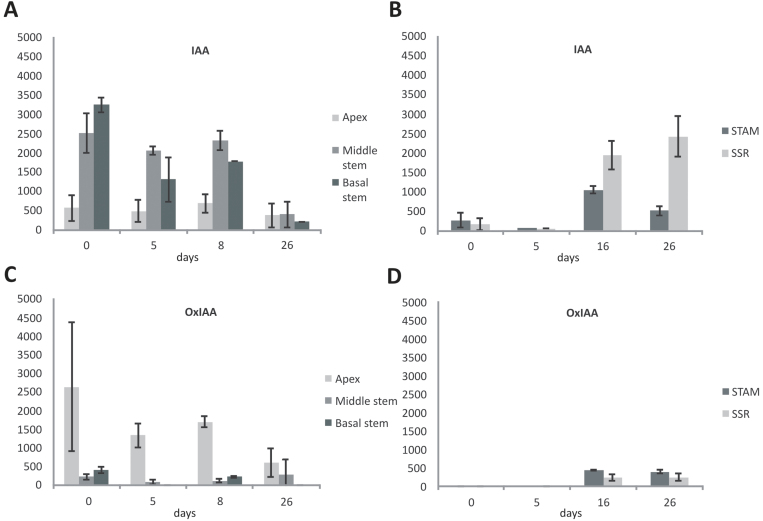
The concentration of the free auxin (IAA) was measured in in vivo plants at different time points after a switch from long-day (LD, non-inductive) to SD conditions (inductive). Under non-inductive LD conditions, the IAA concentration increased from the apex to the lower parts of the plant (Fig. 1A). Average IAA concentrations of 560, 2510, and 3250 pmol of IAA g-1 fresh weight (FW) were measured for the shoot apex, middle, and basal part of the stem, respectively. After the switch to SD conditions, the IAA concentration gradient decreased and, at day 26, the difference in IAA concentrations between shoot apex and basal stem was lost (Fig. 1A). The concentration of oxidized auxin (oxindole-3-acetic acid; referred as OxIAA hereafter) was complementary to that of IAA (Fig. 1C). At day 0, the concentration of OxIAA in the shoot apex, middle stem, and basal stem was 2630, 220, and 400 pmol, respectively. As with free IAA, the concentration of OxIAA reduced over time, such that by day 26 the concentrations were 590, 280, and 0 pmol in the shoot apex, middle stem, and basal stem, respectively.
Fig. 1.
Concentration of free IAA and OxIAA (A and C) in three different parts of the stem (apex, middle, and base) and two parts of the stolon [subswelling region (SSR) and the stolon apical meristem (STAM)] (B and D). Plants were grown for 9 weeks under non-inductive conditions, before a switch to inductive SD conditions. IAA and OxIAA concentrations are in pmol g-1 FW. Samples were harvested under LD conditions (day 0) just before switching to inductive SDs and after 5, 8, and 26 d in SD conditions (SD day 5, 8, and 26, respectively). Error bars represent the standard error of the mean of two replicated measurements.
IAA concentrations were also measured in in vivo grown stolons at the same developmental stages as the above-ground tissues. IAA was measured in the STAM and the SSR (Fig. 1B). Under LD conditions, the free auxin concentration in the STAM was 270 pmol g-1 FW. After a small initial decrease after the switch to SD conditions (day 5; 70 pmol g-1 FW), IAA levels increased dramatically to a maximum of 1050 pmol g-1 FW on day 16, at which time the first tubers were observed. On day 26 when tubers are ~1 cm in diameter, IAA concentrations were reduced to 510 pmol g-1 FW (day 26, Fig. 1B). Interestingly, the increase in auxin concentration is not restricted to the STAM but also extends to the section behind the stolon tip (SSR) that follows a similar profile and reaches a peak in auxin concentration at day 26 (2430 pmol g-1 FW; Fig. 1B).
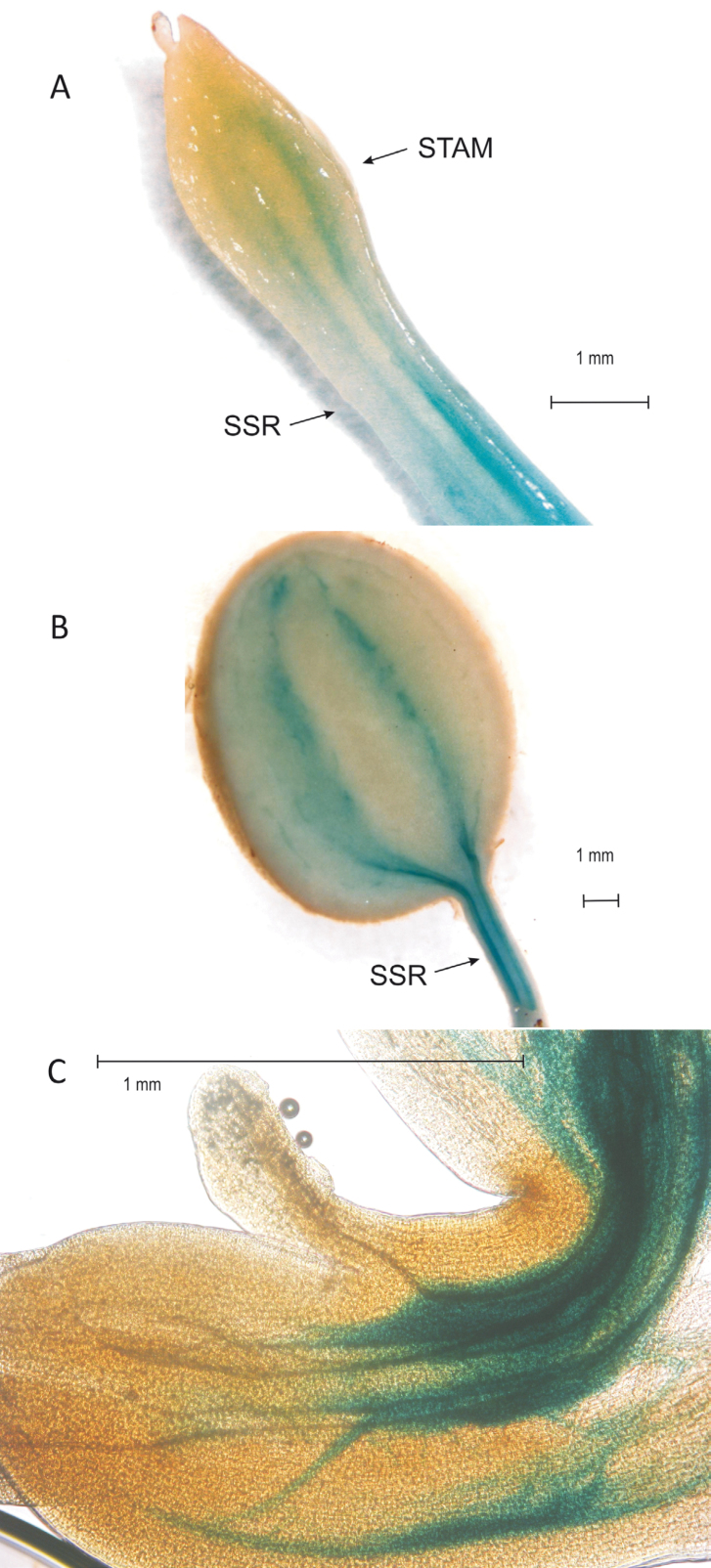
Potato plants were transformed with the DR5::GUS construct to visualize the local auxin concentration. In the stolon a strong GUS staining was observed in the SSR and around the vascular tissues, clearly visible in the swelling tip (Fig. 2A). In young tubers, blue staining is clearly visible around the vascular bundles and a strong staining is observed in the stolon region below the growing tuber (Fig. 2B), consistent with the auxin measurements.
Fig. 2.
Staining of DR5::GUS in transgenic stolon after 9 d in SDs (A) and in a young tuber after 20 d in SDs (B). The arrows indicate the subswelling region (SSR) and the stolon apical meristem (STAM). In (C), staining of promPINV::GUS transgenic plants in the stolon apical hook under LD conditions is shown. The GUS staining is located around the vascular tissue in the STAM, an indication of auxin transport from the site of biosynthesis in the STAM to the basal parts of the stolon. (This figure is available in colour at JXB online.)
Potato plants were also transformed with a construct comprising 1.3 kbp of the regulatory sequences of a PIN gene from chromosome 5 of potato (pStPINV) driving the GUS reporter gene (pPINV::GUS construct) in order to visualize the locations of expression of the StPINV gene that is involved in auxin transport. In the stolon apical hook, GUS staining was observed around the vascular bundles, indicating that StPINV expression is strongly correlated with the DR5::GUS staining and showing auxin transport from the stolon apical meristem (Fig. 2C).
IAA levels were measured in different parts of mature tubers to evaluate auxin distribution within the tuber. The tuber apex had the lowest concentrations of free IAA (110 pmol g-1 FW) but in similar concentration ranges to those in the perimedullary region (120 pmol g-1 FW) and the pith (170 pmol g-1 FW). The highest concentration of IAA observed in the tuber heel (240 pmol) is consistent with the GUS staining of younger tubers (Fig 2B). IAA levels of whole tuber samples were ~160 pmol g-1 FW, significantly less than at tuber swelling (1050 pmol g-1 FW).
Interestingly, there were no detectable levels of OxIAA in the STAM or in the SSR at days 0 and 5. Only at day 16 was OxIAA detected (430 pmol g-1 FW and 220 pmol g-1 FW for STAM and SSR, respectively), but concentrations were lower than those found for free IAA and remained relatively stable during further tuber growth (day 26; 390 pmol g-1 FW and 240 pmol g-1 FW for STAM and SSR, respectively).
Expression of an auxin biosynthesis gene (StYUC-like1) in the potato stolon
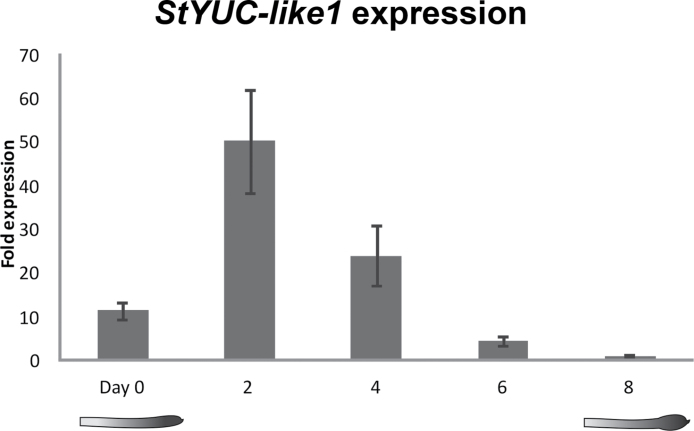
In order to identify genes responsible for the increase in IAA content in the stolon prior to tuber onset, the potato orthologues of an auxin biosynthesis gene family (YUCCA) were identified using the potato genome sequence. Based on expression analysis of five YUCCA-like gene orthologues during the tuber development stages described by Kloosterman et al. (2005), one YUC-like gene (here referred to as StYUC-like1) gene showed a 200-fold increase at stage 2, 5 d after induction of tuberization compared with stage 8, 25 d after tuber induction (Supplementary Fig. S1 available at JXB online). The expression profile of this StYUC-like1 gene in stolon tips during tuber development was verified in S. tuberosum L. var. andigena plants used for auxin measurements, by qRT-PCR (Fig. 3). A peak in the expression of StYUC-like1 was observed already 2 d after the switch to SD conditions. After day 2, the expression levels decreased rapidly (Fig. 3). At the tuber swelling stage (day 8) expression levels were almost 50-fold lower in comparison with the peak at day 2 and 10-fold less compared with non-inductive LD conditions on day 0. StYUC-like1 expression peaks prior to visible stolon swelling, while the strong increase in IAA levels was not observed before swelling. However, it is important to note that auxin measurements were performed on plants grown in the greenhouse and required a longer period in SD conditions before stolon swelling could be observed in comparison with plants grown in the climate cell used for the tuber developmental series (day 0–8). Therefore, StYUC-like1 remains a strong candidate for involvement in the increase in local auxin concentrations in the stolon tip after the switch to SD conditions. Expression data for alternative auxin biosynthesis pathways using the publicly available RNA-seq data (Potato Genome Sequencing Consortium, 2011) revealed that either these orthologues are poorly expressed in the case of potato TAA and TAR orthologues, or their expression in stolons and tubers does not change over time as in the case of potato AMI1 gene orthologues (data not shown). Therefore, we concluded that alternative pathways are unlikely to play a significant role in the initiation of tuber formation.
Fig. 3.
Relative fold expression of the StYUC-like 1 gene, under LD conditions (day 0) and 2, 4, 6, and 8 d after a switch to SDs in a climate chamber. Error bars show the standard error of the mean of three technical repeats.
The effects of IAA, SL, and their inhibitors on stolon axillary bud outgrowth and tuberization in vitro
In standard in vitro tuberization, apical tubers and subsequently lateral tubers are formed on etiolated shoots/stolons in the dark due to the promoting effect of high sucrose levels. Based on the StYUC-like1 gene expression profile and the IAA measurements in the stolon, the STAM is a likely site of auxin biosynthesis. To study the role of the STAM and auxin biosynthesis in the process of lateral tuber initiation under tuber inductive conditions, the stolon tip was removed from the in vitro stolon explants. As a result, the axillary buds of the stolons grew out and formed more tubers in comparison with the control (Fig. 4A). When IAA was applied on the ablated stolon tips, axillary bud outgrowth and thus tuberization was suppressed (Fig. 4B). Interestingly, when explants were not transferred to fresh IAA-containing media after 19 d, final tuber numbers were substantially increased (Fig. 4B, IAA A). These results indicate that stolon tips are a site of auxin biosynthesis and that auxin regulates the process of axillary tuberization in a similar way to axillary shoot growth in the aerial stem.
TIBA is a widely used auxin transport inhibitor that interrupts polar transport of auxin. When TIBA was applied on the basal part of the explants, a higher number of explants formed tubers in comparison with the control (Fig. 4C). Furthermore, the tubers were sessile and formed on the distal axillary buds of the stolons. Similarly, when the auxin antagonist PEO-IAA was applied on the basal part of the explants, a promoting effect on the tuberization was observed, but not as strong as with TIBA-treated explants (Figure 4C). TIBA and PEO-IAA are likely to decrease the inhibitory effect of auxin, as auxin transport (TIBA) or perception (PEO-IAA) is reduced. As a result, the inhibition on stolon bud outgrowth is reduced and axillary buds are able to grow out and form tubers under inductive conditions.
To investigate a possible role for SLs in the stolon axillary bud outgrowth and initiation of lateral tuber formation, GR24, a synthetic SL analogue, was applied in the in vitro tuber induction system. Application of GR24 on the basal part of the stolon explants resulted in significantly fewer tubers (Fig. 4D). At day 39, <2 explants per group of 12 treated with GR24 formed tubers, in contrast to >5 in the control plants. This result reveals a strong inhibitory effect of GR24 on stolon axillary bud outgrowth.
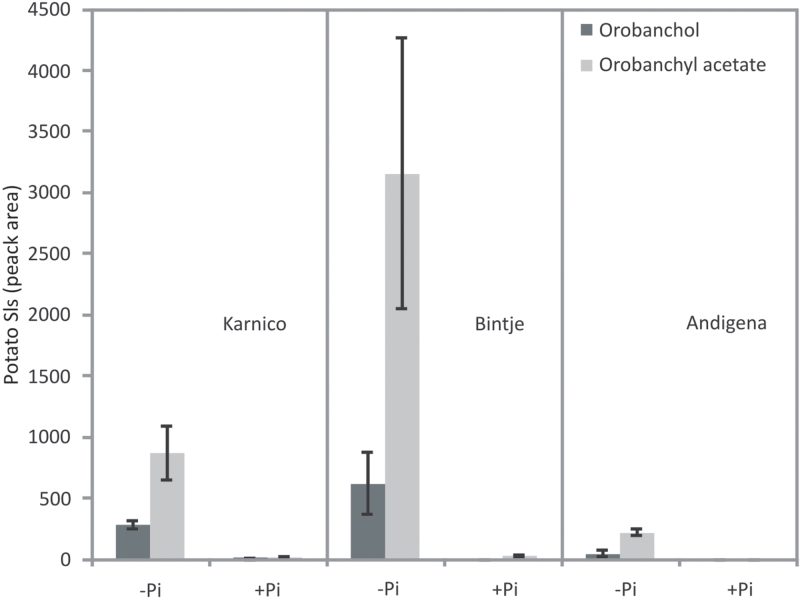
Root extracts of potato plants were investigated for the presence of SLs known to be present in tomato roots (López-Ráez et al., 2008). In all three potato cultivars analysed, two different SLs, namely orobanchol and orobanchyl acetate, were detected (Fig. 5). Phosphate starvation using an aeroponics system increased the level of both SLs. Interestingly, the potato cultivars exhibited different levels of the two SLs in both normal and phosphate-depleted growing conditions.
Fig. 5.
Identification of the strigolactones orobanchol and orobanchyl acetate in potato root extracts of three genotypes (Karnico, Bintje, and Andigena) under normal growth conditions (+P) and under phosphate starvation (–P). The relative concentration is calculated by the peak areas measured by LC-MS/MS analysis. Error bars represent the standard error of the mean of two replicated measurements.
IAA transport assays
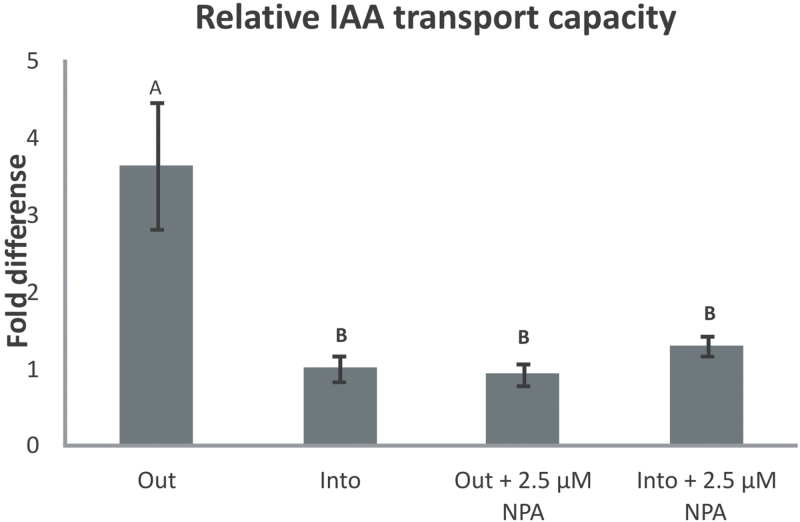
In order to investigate the direction of movement of auxin in the stolons, the transport of 14C-labelled IAA in the presence or absence of the polar auxin transport inhibitor NPA (2.5 µM) was scored in stolon tips placed vertically in Hoagland medium, with their basal or apical part placed in the medium containing the 14C-labelled IAA. Scoring the transport of 14C-labelled IAA revealed that the efflux of IAA from the stolon tip is 3.5 times more compared with the influx into the stolon tip (Fig. 6). This indicated that the main direction of IAA movement in the stolon tissue is from the stolon tip to the basal part of the stolon. NPA is a polar auxin transport inhibitor that acts by disturbing the localization of the PIN proteins. Application of NPA did not alter the transport of IAA into the stolon tip, but decreased the outflux of IAA to the same levels as the influx, indicating that the polar movement of IAA out of the stolon tip is mediated by the PINs, and influx in probably due to diffusion. The results of the 14C-labelled IAA assays verified that IAA is moving in a polar direction out of the stolon tip, a movement that is mediated by the PIN proteins.
Fig. 6.
Fold difference in the transport of auxin out of and into the stolon tip in the absence and presence of the auxin transport inhibitor NPA. Influx of IAA into the stolon tip is set as 1. Error bars are the standard error of the mean for 10 replicates per treatment. Statistically significant differences between treatments are denoted by different letters, A or B (t-test, α=0.05).
Discussion
The free IAA and oxidized IAA (OxIAA) content was studied in three different parts of the stem of potato. The concentrations of free IAA in these three stem parts were 10- to 50-fold higher than the concentrations found in Arabidopsis (Mashiguchi et al., 2011). Furthermore, a negative correlation was shown between the concentrations of free IAA and OxIAA in the same tissue. It is particularly noteworthy that in potato a gradient of IAA levels was found in the stem, with the highest concentration at the base (Fig. 1A). Since the basipetal transport of auxin is well established, in potato the high IAA concentration in the basal stem at the time of harvest may indicate an auxin accumulation due to a bottleneck of transport in the lower stem. This gradient is diminished after the switch to SD conditions. Whether this is due to a reduced biosynthesis of auxin, degradation, or increased transport into other tissues such as the roots remains to be resolved.
Indeed, assessment of the concentration of free IAA in stolon tips before and in three different stages after the switch to SD conditions shows a peak of IAA concentration in the STAMs and the SSR 16 d after the induction of tuberization. Auxin is known to participate in many developmental processes, such as flower development and lateral root formation involving cell division and changes in the orientation of plane of cell division (Marchant et al., 2002; Krizek, 2011; Luo et al., 2011). During stolon swelling, the plane of cell division also changes from transversal to longitudinal (Xu et al., 1998b). The change in cell division plane is likely to be at least partly controlled by auxin and the increase in auxin content that was noticed in these cells.
The question of whether in planta auxin is transported from the shoot apex into the stolons or whether auxin is synthesized autonomously by the stolon tip was answered by the finding that the auxin biosynthesis gene StYUC-like1 increases transiently after the switch to SD conditions and by the IAA transport assays. The StYUC-like1 gene expression appears to be highly specific for stolons (Potato Genome Sequencing Consortium, 2011) and thus may be a causative factor in the increase in IAA concentration in the stolon tip that was noticed a few days later. The phase shift between StYUC-like1 gene expression and auxin accumulation is consistent with the delay between expression and enzyme activity. The difference in StYUC-like1 gene expression at day 0 and day 26 may in part be due to the dilution of cells with active transcription of this gene in tubers in comparison with the stolon tissue. However, a gradual reduction of StYUC-like1 gene expression in the young tubers is in line with a stabilization of IAA concentration and a slow decrease during further tuber growth. The finding that the expression of genes from alternative IAA biosynthesis pathways is not tissue specific or differentially expressed during tuber development makes it unlikely that these pathways play a key role in tuberization. In addition, 14C-labelled IAA assays verified that the main direction of IAA movement is from the stolon tip to the basal part of the stolon. NPA application that is known to disrupt PIN protein polarity also disrupts IAA efflux from the stolon tip (Fig. 6). As a result, the direction of IAA movement and the mechanism that mediates polar auxin transport in stolons and in stems appear to be similar.
The high auxin levels in the swelling stolon are confirmed by the induction of DR5::GUS (Fig. 2). It is interesting to note that during further tuber development, high levels of GUS staining are maintained in the tuber heel and proximal stolon section. The pPINV-driven GUS staining in the apical hook indicates that auxin is transported from the STAM, being the probable site of auxin biosynthesis, towards the basal part of the stolon (Fig. 2C). The high auxin levels in the stolon sections not destined to form a part of the tuber, both prior to and after tuberization, imply the need for additional factors for development and differentiation of this organ. Clearly, other hormones, sucrose, and a recently identified FT-orthologue all play essential roles in tuber induction and formation of the potato tuber (Navarro et al., 2011).
In vitro tuberization has been used extensively for studies concerning tuberization (Yasunori Koda, 1983; Hendriks et al., 1991; Ewing and Struik, 1992; Xu et al., 1998a, b) and has been shown to provide a good representation of processes occurring in vivo. It has the advantages of producing many tubers with temporal synchronicity. Explants in the present experimental design were grown in the dark to produce stolons that would produce tubers upon induction by high sucrose concentration. It is possible that sucrose merely provides the energy source needed to produce the tuber and the dark condition is the stimulus that induces tuber induction, or alternatively that sucrose might be the stimulus itself (Ewing and Struik, 1992). Here the use of both mechanical and chemical ablation of the stolon tip in in vitro potato stolons to investigate the role of auxin in the architecture of the stolon under tuber-inducing conditions is described. In addition, the 14C-labelled IAA assays verified that IAA is transported from the stolon tip to the basal parts of the stolon (Fig. 6). When auxin transport is inhibited, through either ablation or chemical inhibition, there is a release of dormancy of axillary stolon buds and an increase in the number of tubers (Fig. 4). The application of IAA on the mechanically decapitated stolon tips reduces the numbers of explants producing tubers compared with explants with decapitated stolons. This suggests that the re-establishment of polar auxin transport restores the apical dominance on the distal axillary buds.
Another major player in the control of shoot branching are the SLs (Hayward et al., 2009). SLs, working together with auxin, have been shown to have an inhibitory role on shoot branching (Gomez-Roldan et al., 2008). In the present in vitro tuberization experiments, a synthetic SL analogue (GR24) was applied, leading to a marked inhibition of axillary stolon bud outgrowth and subsequent potato tuber formation. Whether SLs are present in stolons of in vivo growing plants and the role these may play in tuberization remains unclear. However, SLs were measured for the first time in potato roots and it was possible to demonstrate their presence in root extracts. Taken together, these results point to a significant role for auxin in tuber formation. Furthermore, in vitro tuberization experiments indicate an additional role for auxin together with SL in the regulation of stolon axillary bud growth.
Supplementary Material
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Expression analysis of five StYUC-like genes during early stages in tuber development
Acknowledgements
We thank Tatsiana Charnikhova, Francel Verstappen, and Wendy Jo-Chen Liao for their technical support. The synthetic strigolactone analogue GR24 was kindly provided by Binne Zwanenburg (Department of Organic Chemistry, Radboud University, Nijmegen, The Netherlands). The strigolactone standards used in this study were kindly provided by Koichi Yoneyama (Weed Science Center, Utsunomiya University, Japan). PEO-IAA was kindly provided by Dr Kenichiro Hayashi, Department of Biological Sciences, Tokyo Metropolitan University, Tokyo, Japan. We thank Dr Hendrik Rietman for providing the plant material for the 14C-labelled IAA assays. We thank Professor Salome Pratt for providing the Solanumm tuberosum L. var. andigena RNA of the developmental range, days 0–8. Part of this work was done in the framework of the EU-SOL project (PL 016214-2 EU-SOL) and co-financed by The Netherlands Organization for Scientific Research (NWO; VICI grant, 865.06.002 and Equipment grant, 834.08.001 to H.B.). HB was co-financed by the Centre for BioSystems Genomics (CBSG), which is part of The Netherlands Genomics Initiative/Netherlands Organization for Scientific Research. ER gratefully acknowledges financial support from the Bakalas Foundation, and the Stichting Veenhuizen-Tulp fonds.
References
- Benková E, Bielach A. Lateral root organogenesis - from Cell to organ. Current Opinion in Plant Biology. 2010;13:677–683. doi: 10.1016/j.pbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. Secondary metabolite signalling in host–parasitic plant interactions. Current Opinion in Plant Biology. 2003;6,:358–364. doi: 10.1016/s1369-5266(03)00065-7. [DOI] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiology. 2009;150,:482–493. doi: 10.1104/pp.108.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Bou J, García-Martínez JL, Prat S. Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. The Plant Journal. 2000;22:247–256. doi: 10.1046/j.1365-313x.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proceedings of the National Academy of Sciences, USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YF, Dai XH, Zhao YD. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes and Development. 2006;20:1790–1799. doi: 10.1101/gad.1415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Wall M, Egley GH, Coggon P, Luhan PA, McPhail AT. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea) Journal of the American Chemical Society. 1972;94:6198–6199. [Google Scholar]
- Demagante AL, Vander Zaag P. The response of potato (Solanum spp.) to photoperiod and light intensity under high temperatures. Potato Research. 1988;31:73–83. [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proceedings of the National Academy of Sciences, USA. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nature Reviews Molecular Cell Biology. 2011;12:211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- Ewing EE, Struik PC. Tuber formation in potato: induction, initiation, and growth. Horticulture Reviews. 1992;14:89–133. [Google Scholar]
- Faivre-Rampant O, Cardle L, Marshal D, Viola R, Taylor MA. Changes in gene expression during meristem activation processes in Solanum tuberosum with a focus on the regulation of an auxin response factor gene. Journal of Experimental Botany. 2004;55:613–622. doi: 10.1093/jxb/erh075. [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–94. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Gregory LE. Some factors for tuberization in the potato plant. American Journal of Botany. 1956;43:281–288. [Google Scholar]
- Gregory LE. Physiology of tuberization in plants. Handbuch Pflanzenphysiologie. 1965:1328–1354. [Google Scholar]
- Hayashi K-i, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H. Small-molecule agonists and antagonists of F-box protein—substrate interactions in auxin perception and signaling. Proceedings of the National Academy of Sciences, USA. 2008;105:5632–5637. doi: 10.1073/pnas.0711146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A, Stirnberg P, Beveridge C, Leyser O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiology. 2009;151:400–412. doi: 10.1104/pp.109.137646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks T, Vreugdenhil D, Stiekema WJ. Patatin and four serine proteinase inhibitor genes are differentially expressed during potato tuber development. Plant Molecular Biology. 1991a;17:385–394. doi: 10.1007/BF00040633. [DOI] [PubMed] [Google Scholar]
- Kloosterman B, De Koeyer D, Griffiths R, et al. Genes driving potato tuber initiation and growth: identification based on transcriptional changes using the POCI array. Functional and Integrative Genomics. 2008;8,:329–340. doi: 10.1007/s10142-008-0083-x. [DOI] [PubMed] [Google Scholar]
- Kloosterman B, Navarro C, Bijsterbosch G, Lange T, Prat S, Visser RGF, Bachem CWB. StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. The Plant Journal. 2007;52:362–373. doi: 10.1111/j.1365-313X.2007.03245.x. [DOI] [PubMed] [Google Scholar]
- Kloosterman B, Vorst O, Hall RD, Visser RG, Bachem CWB. Tuber on a chip: differential gene expression during potato tuber development. Plant Biotechnology Journal. 2005;3:505–519. doi: 10.1111/j.1467-7652.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, Verstappen F, Leyser HMO, Bouwmeester HJ, Ruyter-Spira C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in on-AM host Arabidopsis thaliana. Plant Physiology. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA. Auxin regulation of Arabidopsis flower development involves members of the AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) family. Journal of Experimental Botany. 2011;62:3311–3319. doi: 10.1093/jxb/err127. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Hoffmann M, Hentrich M, Pollmann S. Indole-3-acetamide-dependent auxin biosynthesis: a widely distributed way of indole-3-acetic acid production? European Journal of Cell Biology. 2010;89:895–905. doi: 10.1016/j.ejcb.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. The Plant Journal. 2001;28:465–474. doi: 10.1046/j.1365-313x.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Gómez-Roldán V, et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytologist. 2008;178:863–874. doi: 10.1111/j.1469-8137.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- Luo Y, Qin G, Zhang J, et al. d-myo-inositol-3-phosphate affects phosphatidylinositol-mediated endomembrane function in arabidopsis and is essential for auxin-regulated embryogenesis. The Plant Cell. 2011;23:1353–1372. doi: 10.1105/tpc.111.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell. 2002;14,:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, et al. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2011;108,:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk CS, Langille AR. Physiology of tuberization in Solanum tuberosum L: cis-zeatin riboside in the potato plant: its identification and changes in endogenous levels as influenced by temperature and photoperiod. Plant Physiology. 1978;62,:438–442. doi: 10.1104/pp.62.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Navarro C, Abelenda JA, Cruz-Oro E, Cuellar CA, Tamaki S, Silva J, Shimamoto K, Prat S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature. 2011;478,:119–122. doi: 10.1038/nature10431. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nakano H, Hayashi K, Niwa C, Koshiba T. Differential downward stream of auxin synthesized at the tip has a key role in gravitropic curvature via TIR1/AFBs-mediated auxin signaling pathways. Plant and Cell Physiology. 2009;50:1874–1885. doi: 10.1093/pcp/pcp129. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. The Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potato Genome Sequencing Consortium. Genome sequence and analysis of the tuber crop potato. Nature. 2011;475,:189–195. doi: 10.1038/nature10158. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O. Control of bud activation by an auxin transport switch. Proceedings of the National Academy of Sciences, USA. 2009;106:17431–17436. doi: 10.1073/pnas.0906696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau C. Strigolactones, a novel class of plant hormone controlling shoot branching. Comptes Rendus Biologie. 2010;333:344–349. doi: 10.1016/j.crvi.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another below-ground role for strigolactones? Plant Physiology. 2010;155:721–734. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. On the nature of correlative inhibition. New Phytologist. 1937;36:283–300. [Google Scholar]
- Steven GK. YUCCA: a flavin monooxygenase in auxin biosynthesis. Trends in Biochemical Sciences. 2001;26:218. doi: 10.1016/s0968-0004(01)01814-x. [DOI] [PubMed] [Google Scholar]
- Stomp AM. Histochemical localization of β-glucuronidase activity. In: Gallagher SR, editor. GUS protocols: using the GUS gene as a reporter of gene expression. San Diego: Academic Press; 1992. pp. 103–113. [Google Scholar]
- Tao G, Letham DS, Yong JWH, Zhang K, John PCL, Schwartz O, Wong SC, Farquhar GD. Promotion of shoot development and tuberisation in potato by expression of a chimaeric cytokinin synthesis gene at normal and elevated CO2 levels. Functional Plant Biology. 2010;37:43–54. [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9,:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, van Lammeren AA, Vermeer E, Vreugdenhil D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro . Plant Physiology. 1998a;117,:575–84. doi: 10.1104/pp.117.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Vreugdenhil D, van Lammeren AAM. Cell division and cell enlargement during potato tuber formation. Journal of Experimental Botany. 1998b;49:573–582. [Google Scholar]
- Yasunori Koda YO. Influences of invironmental, hormonal and nutritional factors on potato tuberization in vitro . Japanese Journal of Crop Science. 1983;52,:582–91. [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291,:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.