Abstract
Cell wall isolated from pea roots was used to separate and characterize two fractions possessing class III peroxidase activity: (i) ionically bound proteins and (ii) covalently bound proteins. Modified SDS–PAGE separated peroxidase isoforms by their apparent molecular weights: four bands of 56, 46, 44, and 41kDa were found in the ionically bound fraction (iPOD) and one band (70kDa) was resolved after treatment of the cell wall with cellulase and pectinase (cPOD). Isoelectric focusing (IEF) patterns for iPODs and cPODs were significantly different: five iPODs with highly cationic pI (9.5–9.2) were detected, whereas the nine cPODs were anionic with pI values between pH 3.7 and 5. iPODs and cPODs showed rather specific substrate affinity and different sensitivity to inhibitors, heat, and deglycosylation treatments. Peroxidase and oxidase activities and their IEF patterns for both fractions were determined in different zones along the root and in roots of different ages. New iPODs with pI 9.34 and 9.5 were induced with root growth, while the activity of cPODs was more related to the formation of the cell wall in non-elongating tissue. Treatment with auxin that inhibits root growth led to suppression of iPOD and induction of cPOD. A similar effect was obtained with the widely used elicitor, chitosan, which also induced cPODs with pI 5.3 and 5.7, which may be specifically related to pathogen defence. The differences reported here between biochemical properties of cPOD and iPOD and their differential induction during development and under specific treatments implicate that they are involved in specific and different physiological processes.
Abbreviations:
- cPOD
covalently bound peroxidase
- DAB
3,3'-diaminobenzidine
- DEPMPO
spin-trap (5-diethoxy-phosphoryl-5-methyl-1-pyrroline-n-oxide)
- EPR
electron paramagnetic resonance
- HRP
horseradish peroxidase
- IAA
indole-3-acetic acid
- HRP
horseradish peroxidase
- IEF
isoelectric focusing
- iPOD
ionically bound peroxidase
- NAA
naphthalene acetic acid
- PNGase F
peptide N-glycosidase F
- PR
pathogen-related
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- SHAM
salicylhydroxamic acid
- TMB
tetramethyl benzidine
- WGA
wheat germ agglutinin
Key words: Auxin, cell wall peroxidase, elicitor, IEF–PAGE, pea root, Pisum sativum, Auxin, Auxin
Introduction
The primary cell wall is a complex extracellular structure mostly made of cellulose, hemicellulose, and pectin, with proteins embedded in the polysaccharide matrix. The most abundant enzymes among extracellular proteins are class III peroxidases (EC 1.11.1.7). They are normally involved in the dissipation of hydrogen peroxide (H2O2); however, they also possess a capacity to produce H2O2 via one-electron reduction of oxygen while oxidizing reductants [NAD(P)H, ascorbate, or auxin]. H2O2 participates in oxidative reactions in the apoplast both as a substrate in polymerization processes of the cell wall and also as an intermediary molecule in the process of cell wall loosening. In addition, H2O2 participates in defence reactions and signalling processes in the apoplast and at the plasma membrane (Mika et al., 2010). Various peroxidase reactions are linked to the cell wall, such as those involved in lignin and suberin synthesis (Espelie et al., 1986; Christiansen et al., 1998; Brownleader et al., 2000; Quiroga et al., 2000), indole-3-acetic acid (IAA) catabolism (Hinnman and Lang, 1965; Nakajima and Yamazaki, 1979; Brooks, 1986, Alba et al., 1998), and/or oxidative cross-linking of cell wall components (Cooper and Varner, 1984; Lopez-Serrano et al., 2004). Although certain peroxidase functions could be related to the specific isoforms, such as those in wounding (Bernards et al., 1999; Minibayeva et al., 2009) or during pathogen attack (Lamb and Dixon, 1997; Bolwell et al., 1999), it is generally difficult to assign a specific function to the individual peroxidase isoforms in vivo. Numerous studies have examined the functions of peroxidases and tried to attribute some function in vivo to the anionic, cationic, neutral, ionically or covalently bound, or soluble peroxidase isoforms (Brooks, 1986; Narita et al., 1995; Sanchez et al., 1995; Groppa et al., 1999; Quiroga et al., 2000). Alternatively, there are several reasons in favour of factors other than enzyme structure being important in determination of peroxidase activity: microenvironment, a high redundancy found in peroxidase genes, very similar immunological properties of different isoenzymes, and failure to relate down-regulated specific peroxidases to phenotype (Cosio and Dunand, 2009). The aim of this work was to elucidate if localization within the cell wall and the type of binding to polysaccharide or isoform structure determine peroxidase function during plant growth.
Based on the type of their interaction with cell wall constituents, several groups of proteins may be distinguished. Soluble proteins extracted with an intercellular washing fluid are probably loosely or not bound to cell wall constituents and move freely in apoplastic space. Some cell wall peroxidases are weakly bound to a polysaccharide matrix by Van der Waals interaction, hydrogen bonds, or hydrophobic interactions (Jamet et al., 2006). Finally, proteins can be strongly bound to the cell wall using either ionic bonds or cross-linking by covalent bonds (Jamet et al., 2006). Peroxidase activity is found in all cell wall protein fractions. One of the most common classifications of extracellular peroxidases is based on their isoelectrophoretic mobilities, being classified as anionic, neutral, and cationic. Also one of the classifications that is often used is soluble peroxidase found in the apoplast, and forms which are ionic or covalently bound to the cell wall (Ros Barcelo et al., 1998). As a working hypothesis, a specific role for covalently bound cell wall peroxidase in the process of cleavage of cell wall polymers during growth has been proposed. Recent work demonstrated that peroxidases covalently bound to the cell wall may spontaneously generate hydroxyl radicals (Kukavica et al., 2009) that have a specific role in the process of cleavage of cell wall polymers.
In the present study, peroxidases that are either ionically (iPOD) or covalently bound (cPOD) to the cell wall of pea roots were extracted and their structural and biochemical characteristics studied. Changes in isoperoxidase induction were analysed after auxin or chitosan treatment and also during plant growth and along root zones.
Materials and methods
Plant materials and growth condition
Pea seeds (Pisum sativum L. Mali Provansalac) were washed under tap water and germinated at 18 °C in the dark for 3 d. Seedlings were then placed in Hoagland solution, which was changed after a week, and grown hydroponically for 3, 6, 10, 17, and 23 d in a growth chamber with a photoperiod of 16h/8h (light/darkness) at 24 °C and 18 °C. Irradiance of 80 µmol m–1 s–1 was provided by white fluorescent tubes. For auxin treatments, plants were grown in the presence of 10 µM 1-naphthaleneacetic acid (NAA) that was added to the Hoagland solution for 11 d. Elicitation was performed with chitosan using plants of different ages (3, 6, 10, 17, and 23 d). Plants were treated with 1g l–1 chitosan (Sigma, Deisenhofen, Germany) before harvesting and cell wall isolation from the root for 16h.
Cell wall isolation
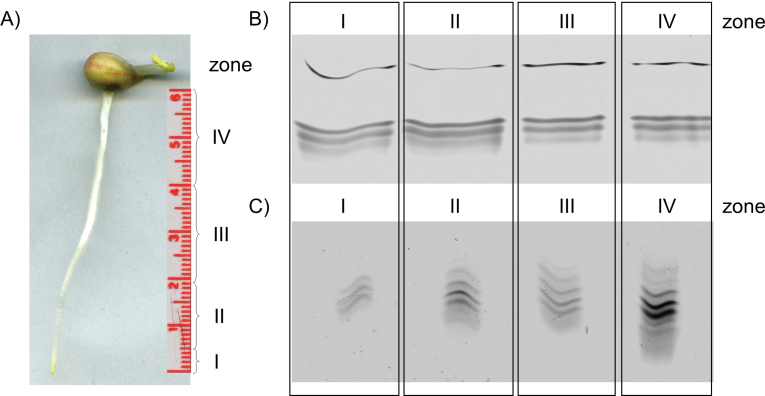
The cell wall fraction was isolated from roots by a method modified from Kukavica et al. (2009). Roots were cut and kept in TRIS buffer [50mM TRIS pH 7.2, 50mM NaCl, 0.05% Tween-20, 1mM phenylmethylsulphonyl fluoride (PMSF)] at 4 ºC and immediatelly homogenized in a Waring blender (VWR, Darmstadt, Germany) for 2min. The homogenate was filtered through two layers of cloth and centrifuged at 1000 g for 20min. The pellet with cell wall fragments was washed four times in 50mM TRIS (pH 7.2). To extract the ionically bound protein fraction, the pellet was suspended in 1M NaCl, incubated for 30min at 4 ºC, and then centrifuged at 1000 g for 15min. The supernatant was used for analysis of iPOD. After salt treatment, the pellet was washed four times with TRIS buffer. The covalently bound protein fraction was released after incubation of cell wall isolate with 0.5% cellulase (Sigma, Taufkirchen, Germany) and 2.5% pectinase (Fluka, Taufkirchen, Germany) in a cold room for 24h. After centrifugation of the suspension at 1000 g for 15min, the supernatant was used to analyse cPOD. Both iPODs and cPODs were extracted from roots of 3-day-old pea seedlings divided into four zones: I, 5mm from the root tip; II, 1.5cm; III, 2cm; and IV, 2cm according to Cordoba-Pedregosa et al. (2003) (Fig. 6A). For other experiments, whole roots were used to isolate the cell wall and the ionically and covalently bound fractions. For enzyme analysis, 3–4 cell wall isolates were used.
Fig. 6.
Zonal distribution of cell wall-bound isoperoxidases. Allocation of root zones (A) used for peroxidase analysis. Distribution of iPOD (B) and cPOD (C) isoforms along the root zones separated on an IEF gel and stained with α-chloro-naphthol. Pea seedlings were used for cell wall isolation.
Extraction of apoplastic fluid
Freshly cut roots were vacuum infiltrated for 10min in distilled water, blotted, placed into tips mounted on top of Eppendorf tubes, and centrifuged for 20min at 500 g. No significant contamination of the isolated apoplastic fluid and cell wall fragments was detected using glucose-6-phosphate dehydrogenase activity as a cytoplasmic marker. The reaction mixture consisted of 5mM MgSO4, 5mM glucose-6-phosphate, and 0.1mM NADH in 0.1M K-phosphate buffer pH 8, and an absorbance decrease at 340nm indicated NADH oxidation (ε = 6.22mM–1 cm–1).
Extraction of soluble proteins
For extraction of soluble proteins from roots, frozen roots (0.5g) were powdered in a mortar containing liquid N2 and suspended in 100mM potassium phosphate buffer (pH 6.5). The homogenate was centrifuged at 10 000 g for 15min at 4 ºC. The supernatant was used for electron paramagnetic resonance (EPR) measurments.
Modified SDS–PAGE
Modified SDS–PAGE was used to separate peroxidase isoforms by molecular weight using the prosthetic haem group according to Mika and Lüthje (2003). The final concentration of SDS was 0.1% (w/v) in all solutions and gels. Samples were diluted in loading buffer to final concentrations of 62.5mM TRIS-HCl, 0.1% (w/v) SDS, 10% (w/v) glycerol, and 0.002% (w/v) bromophenol blue without reducing compounds and loaded onto the gels without heating. It was shown that all isoforms stayed active after separation on modified SDS–PAGE by staining with 0.01% α-chloro-naphthol used as a substrate for peroxidase reaction and 0.03% H2O2 in 0.1M Na-phosphate buffer pH 6.5. This enabled the determination of the apparent molecular weights of peroxidase isoforms using molecular mass standards (Broad Range, Bio-Rad, Munich, Germany) according to Laemmli (1970). The other half of the gel was used for haem staining with 6.3mM tetramethyl benzidine (TMB) and 30mM H2O2 (Thomas et al., 1976).
Isoelectric focusing of peroxidase isoforms
Isoelectric focusing (IEF) was performed with mini gels [7.5% (w/v) acrylamide with 3M urea and 2% ampholyte; Serva, Heidelberg, Germany] at 4 °C for 3h. IEF was performed at a gradually increasing voltage: 90min at 100V; 60min at 250V; and 30min at 500V. The anode buffer was 10mM phosphoric acid and the cathode buffer was 20mM NaOH. For more precise resolution of iPOD isoforms, a pH gradient from 9 to 11 and for cPODs a pH gradient from 3 to 7 were used. Before loading on the gel, samples were mixed with loading buffer containing 40% glycerol and 20% ampholyte.
The thermostability of iPOD and cPOD was determined on the IEF gel by staining for peroxidase activity after incubation of aliquots at 25, 50, 60, and 80 °C for 15min.
Determination of IAA oxidase activity
IAA oxidase peroxidase activity on the gel was visualized according to the protocol of Hoyle (1977). Staining solutions were: (A) Fast Blue Salt BB (4mg ml–1) dissolved in ethanol; (B) 2 µM p-coumaric acid, 2 µM IAA, and 2 µM H2O2 were diluted in 50ml; (C) 2M sodium acetate buffer (pH 4.2). Final staining solution consisted of 25ml of solution A, 50ml of solution B, and 25ml of solution C.
Peroxidase activity
The peroxidase activity of iPOD and cPOD was measured spectrophotometrically in a reaction mixture consisting of 100mM Na-phosphate buffer pH 6.5, 0.01M pyrogallol, and aliquots of fractions bound ionically and covalently to the cell wall. The reaction was started by addition of 3.3mM H2O2, and an increase in absorbance at 430nm was followed. Peroxidase activity was calculated using the extinction coefficient for purpurogallin (ε = 12mM–1cm–1).
Oxidation of the hydroxycinnamic acids (chlorogenic, caffeic, or ferulic) by iPOD or cPOD was measured in a reaction mixture (3ml) containing 50 µl of a suspension of cell wall isolates or ionic fraction, 3.3mM H2O2, and 4mM acids in 100mM potassium phosphate buffer (pH 6.5). An increase in absorbance was followed at 410, 450, and 356nm, respectively (Bestwick et al., 1998).
To investigate the effect of lectins on peroxidase activity, aliquots of ionically and covalently bound cell wall fractions were incubated for 3min with different concentrations (1 µg ml–1 and 5 µg ml–1) of concanavalin A (Con A) and wheat germ agglutinin (WGA).
For deglycosylation treatment, endoglycosidases F1, F2, and F3 (Endo F1, F2, and F3) and peptide N-glycosidase F (PNGase F) were used. Aliquots of samples were incubated with different proteolytic enzymes for 2h and peroxidase activity was measured.
Protein concentrations were determined according to Bradford (1976).
NADH-POD oxidase activity
Measurement of NADH-POD oxidase activity in ionic and covalent cell wall fractions was done according to Fecht-Christoffers et al. (2006) with some modifications. The reaction mixture contained 100mM sodium acetate buffer, pH 5, aliquot of samples, 0.15mM NADH, 1.6mM p-coumaric acid, and 16mM MnCl2. Enzyme activity was calculated using the extinction coefficient for NADH (ε = 6.22mM–1cm–1).
During NADH-POD oxidase activity, the formation of H2O2 occurs (Fecht-Christoffers et al., 2006). After 1min of NADH oxidation by peroxidase, 10mM pyrogallol was added and an increase in absorbance at 430nm was followed for 2min. The amount of H2O2 generated by oxidase activity was estimated according to the enzyme activity determined under the same conditions in the presence of a known H2O2 concentration.
EPR spectroscopy
EPR measurements were performed using a custom-made Teflon flat cell with one cell side made of oxygen-permeable thin Teflon foil. The spectra were recorded at room temperature using a Varian E104-A EPR spectrometer operating at X-band (9.51 GHz) and using the following settings: modulation amplitude, 2 G; modulation frequency, 100kHz; microwave power, 10 mW; centre of magnetic field, 3410 G; scan range, 200 G, the same in all EPR spin-trap measurements. Spectra were recorded and analysed using EW software (Scientific Software). DEPMPO spin-trap (5-diethoxy-phosphoryl-5-methyl-1-pyrroline-n-oxide) (Alexis Biochemicals, Switzerland) was added to a final concentration of 42.5mM. Distilled and deionized 18 MΩ water was used in all experiments.
Statistic analysis
All data were subjected to analysis of variance (ANOVA), and means were compared by the Holm–Sidak test (SigmaPlot 11.0, Systat Software, Inc., USA). The level of significance was set at P < 0.05. Two-way ANOVA was carried out to assess the difference in means from various concentrations of inhibitors and from various inhibitors at the same concentration, followed by multiple comparisons using the Holm–Sidak test (P < 0.05) test.
Results
Modified SDS–PAGE and IEF separation of iPOD and cPOD
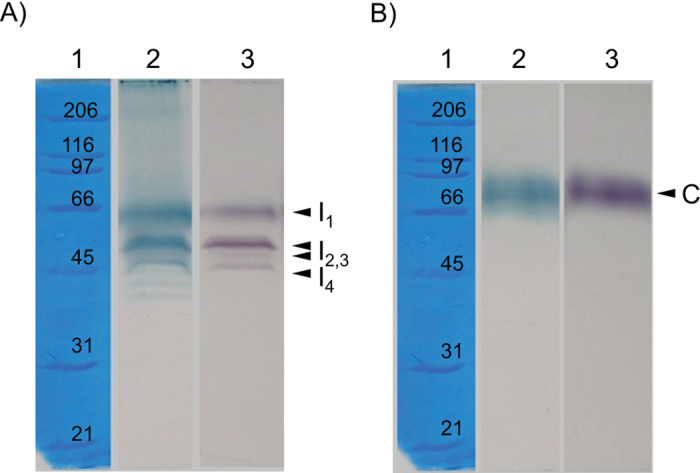
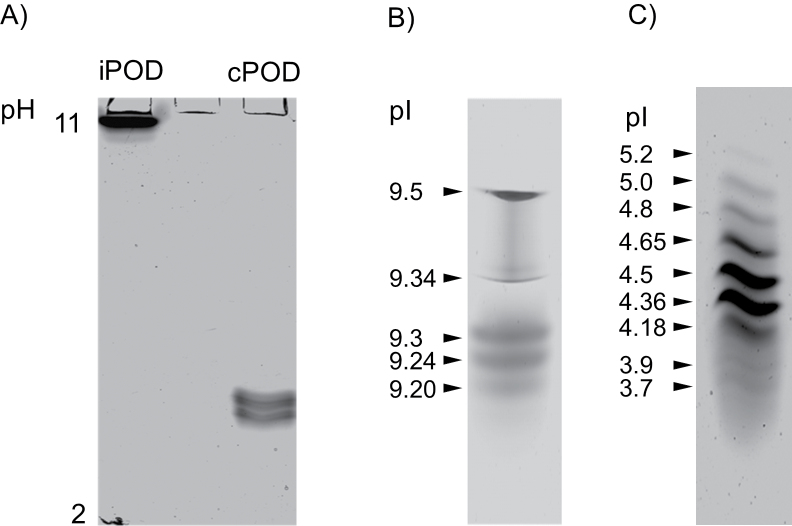
After the cell wall had been isolated from roots of 2-week-old pea plants, it was used to extract two protein fractions: ionically and covalently bound to the cell wall. Ionically bound cell wall proteins were salt extracted (1M NaCl) and covalently bound proteins were released with 0.5% cellulase and 2.5% pectinase. Peroxidase isoforms were separated by modified SDS–PAGE and stained with α-chloro-naphthol for detection of peroxidase activity. According to the work of Mika and Lüthje (2003) modified SDS–PAGE with a low SDS concentration (0.1%) can be used for estimation of enzyme molecular weight. Four peroxidase isoforms with apparent molecular masses of 56, 46, 44, and 41kDa were identified in the ionic fraction (Fig. 1A). In the covalent fraction, one peroxidase isoform was detected with an apparent molecular mass of 70kDa (Fig. 1B). When the same gels were stained with TMB (an indicator of the haem prosthetic group) no other haem enzyme was obtained in the covalent fraction. However, in the ionic fraction, two bands in addition to those with peroxidase activity were separated (Fig. 1A). Modified SDS–PAGE with a low SDS concentration (0.1%) can also be used for detection of peroxidase isoforms on the gel and can distinguish peroxidase isoforms from other oxidases stained with TMB (Fig. 1). IEF of peroxidase isoforms extracted from either ionic or covalent cell wall protein fractions showed that iPODs had pI values distinct from those of cPOD (Fig. 2A). Isoenzyme profiles obtained in the pH gradient 2–11 showed that iPODs and cPODs had significantly different pI values: iPODs were highly cationic, while cPODs were all anionic (Fig. 2A). Using ampholite with a narrow range of pH gradient, acidic for cPODs or alkaline for iPODs, five iPOD isoforms (Fig. 2B) and nine cPOD isoforms (Fig. 2C) could be separated.
Fig. 1.
Separation of peroxidase isoforms from ionic (A) and covalent (B) cell wall fractions by modified SDS–PAGE. Lane 1, protein standards with their corresponding molecular weight. Gels were stained for haem with TMB (lane 2) and with α-chloro-naphthol for peroxidase activity (lane 3). Arrows indicate different peroxidase isoforms. Four ionic peroxidases isoforms (I1–I4) and one covalent isoform (C) were identified.
Fig. 2.
IEF–PAGE of peroxidase isoforms of ionic and covalent cell wall fractions. Separation of ionically and covalently bound peroxidases obtained using a wide pH range (A), an alkaline pH range (9–11) for iPOD (B), and an acidic pH range (3–7) for cPOD (C). Peroxidase isoforms on the IEF gel were visualized with α-chloro-naphthol.
Substrate and inhibitor studies
Peroxidases are enzymes with a wide spectrum of substrates, capable of oxidizing hydroxycinnamic derivatives and other phenolic compounds with different specificities. Besides pyrogallol as an artificial electron donor, common endogenous hydroxycinamic acids present in the pea root (Kukavica et al., 2009) were used to analyse the total activity of soluble and cell wall-bound peroxidases (Table 1). The oxidation rate of chlorogenic, caffeic, and ferulic acids was expressed as an absorbance increase at 410, 450, and 356nm, respectively. An absorbance increase, rather than a absorbance decrease, was measured (Bestwick et al., 1998; Hadži-Taškovic Šukalovic et al., 2003; Mika and Lüthje, 2003) for two reasons: (i) the substrate concentration was above the measured K m values; and (ii) there is no possibility of overlapping in the absorbance spectra of substrate and products, as found to be the case for chlorogenic acid (data not shown). The analyses of enzyme affinity for different substrates by determining the K m (Table 1) showed that all peroxidases had a similar K m for pyrogallol that differs from that of horseradish peroxidase (HRP). It was determined that only cPOD had a lower affinity for H2O2 (K m=2.4mM) when compared with all other fractions and HRP (K m 1.1–1.4mM; Table 1). The lowest activity for all peroxidases was measured with ferulic acid as reducing substrate; cPOD had the highest affinity for ferulic acid compared with all others peroxidases (Table 1). Compared with HRP, all pea root peroxidases had twice as high K m values for caffeic acid.
Table 1. Km values of apoplastic (aPOD), ionic (iPOD), and covalent (cPOD) cell wall-bound peroxidases from pea roots and HRP for different reducing substrates (pyrogallol, chlorogenic, caffeic, and ferulic acids) in the presence of 3.3mM H 2 O 2 K m values for H2O2 were obtained with pyrogallol as a substrate.
| CGA, chlorogenic acid; CA, caffeic acid; FA, ferulic acid. | |||||
| K m (mM) | |||||
| Fraction | H2O2 | Pyrogallol | CGA | CA | FA |
| aPOD | 1.4±0.2 | 2.6±0.4 | 0.6±0.2 | 2.7±0.5 | 4.0±0.7 |
| iPOD | 1.3±0.1 | 2.5±0.3 | 1.0±0.2 | 2.4±0.3 | 1.2±0.3 |
| cPOD | 2.4±0.4 | 2.7±0.3 | 0.7±0.12 | 2.1±0.2 | 0.6±0.2 |
| HRP | 1.4±0.3 | 1.1±0.1 | 1.6±0.3 | 0.9±0.2 | 1.1±0.4 |
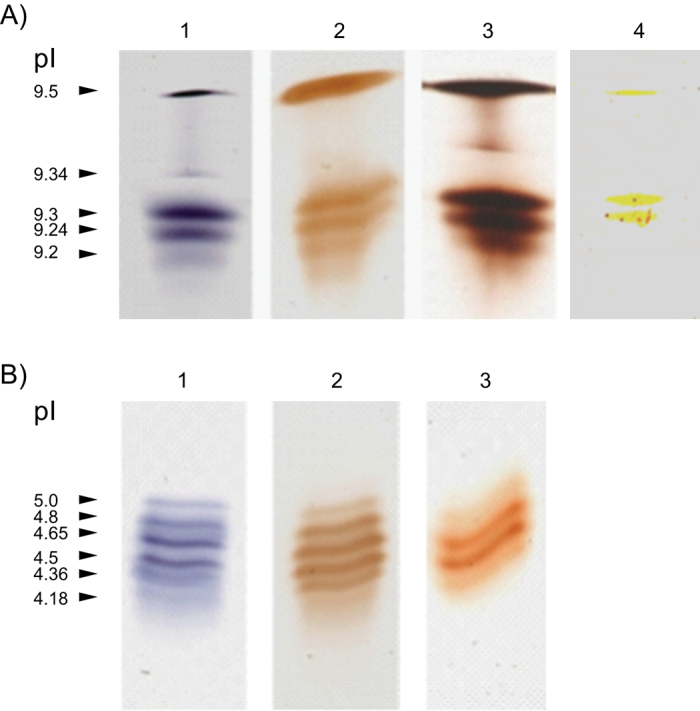
IEF of peroxidase isoforms extracted from either ionic or covalent cell wall protein fractions showed that both fractions consisted of several isoforms (Fig. 2), which might differ in their substrate specificity. To test this possibility, IEF gels with separated iPODs and cPODs were stained for peroxidase activity using different substrates: α-chloro-naphthol, 3,3′-diaminobenzidine (DAB), guaiacol, or IAA (Fig. 3). Four iPOD isoforms (pI 9.5, 9.3, 9.24. and 9.2) had similar activity with each of the substrates, while the isoform with pI 9.34 had no activity with DAB (Fig. 3A). Two cPOD isoforms (pI 4.5 and 4.65) had the highest activity with guaiacol and α-chloro-naphthol, while four isoforms (pI 4.8, 4.65, 4.5. and 4.36) had similar activity with DAB (Fig. 3B). The results suggested that only the protein with a pI 9.34 differed from all other iPODs regarding its substrate specifity, while among six cPODs, only two had similar affinity for all three substrates. For staining of IAA oxidase activity on the IEF gel, the procedure was carried out as described by Hoyle (1977). Comparison of staining patterns of IAA oxidase and peroxidase activities showed that three of five iPOD isoforms (pI 9.5 and 9.3, and 9.24) had also oxidizing activity, while none of the cPOD isoforms had IAA oxidase activity (Fig. 3). It was also shown that iPODs and cPODs differed in their K m for H2O2 (Table 1), with significantly higher affinity for H2O2 of cPODs.
Fig. 3.
Substrate affinity of iPOD (A) and cPOD (B) isoforms on an IEF gel. After separation of the isoforms, gels were stained with 18mM α-chloro-naphthol (lane 1), 1mM 3,3-diaminobenzidine (DAB; lane 2), 20mM guaiacol (lane 3), and 1 µM IAA (lane 4).
Catalase-like activity was measured in all cell wall fractions, with the highest rate obtained in the cell wall fraction with cPODs, compared with HRP, soluble peroxidases, and iPODs (Table 2). The catalase-like activity of peroxidase was proposed to act as a major protective mechanism for HRP-C against inactivation by H2O2, being optimal at pH > 6.5 (Hernandez-Ruiz et al., 2001). The present study showed that the highest rate of O2 evolution coincided with the highest resistance to H2O2-induced inhibition of peroxidase reaction (Table 2).
Table 2. Catalase-like activity of the cell wall fraction with cPODs, iPODs, soluble extract from pea roots (sol. ex.), and commercial peroxidase from horseradish (HRP). Differences in peroxidase sensitivity on inactivation with H2O2 were determined by measurements of pyrogallol-dependent peroxidase activity as described in the Materials and methods after aliquots of all fractions were incubated for 30min in 10mM H2O2 in phosphate buffer, pH 6.5 at 30 °C. Catalase-like activity was determined by measuring oxygen production with a Clark-type electrode in the presence of 3mM H2O2 at 30 °C.
| cPOD | iPOD | sol. ex. | HRP | |
| O2 evolution (nmol min–1 g–1 protein) | 230±38 | 43±6 | 40±3 | 17±0.6 |
| Inactiation of POD activity (%) | 9±3 | 64±11 | 76±9 | 81±4 |
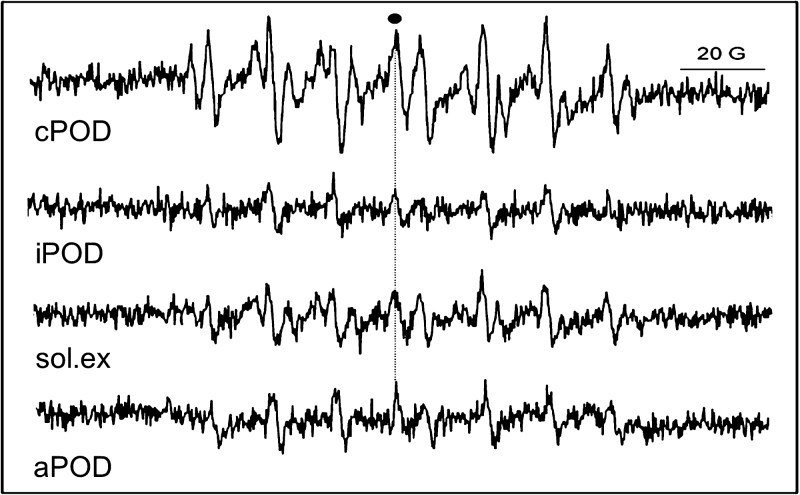
It has been shown that isolated cell wall fragments containing cPOD spontaneously generated ·OH radicals as demonstrated by the spectrum of the DEPMPO/OH adduct signal (Kukavica et al., 2009). In addition to cPOD and iPOD, the apoplastic and soluble fractions have a capacity to generate ·OH (Fig. 4), although to a much lesser extent (10% of the signal being induced by cPOD). In all of the cases studied, the generation of the DEPMPO/OH adduct signal was oxygen dependent, with measurements in a nitrogen atmosphere abolishing the DEPMPO/OH adduct signal (as in Veljović-Jovanović et al., 2005). It was also previously demonstrated that purified commercial HRP did not generate either of the two adducts, DEPMPO/OOH or DEPMPO/OH, while addition of H2O2 induced the generation of both adducts (Kukavica et al., 2009). Also the addition of H2O2 to cell wall fragments led to increased production of ·OH signal, while the signal O2· – can only be seen after the addition of very high concentrations of H2O2 (10mM) (data not shown).
Fig. 4.
EPR spectra of the DEPMPO/OH adduct obtained from cPOD bound to the cell wall, iPOD, soluble protein extract from roots (sol. ex.), and apoplastic fluid isolated from intact pea roots (aPOD). Aliquots with the same peroxidase units in different isolates were used for EPR measurements.
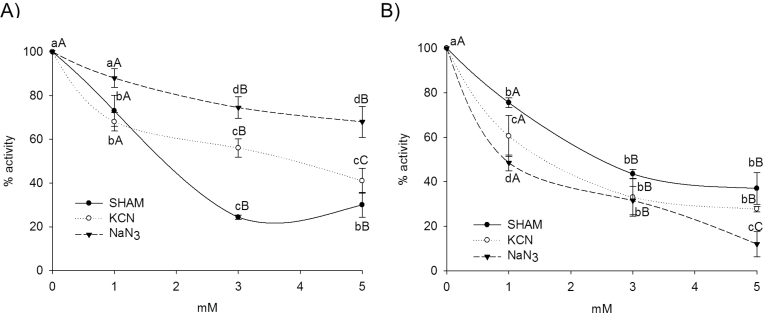
The effects of three common peroxidase inhibitors KCN, salicylhydroxamic acid (SHAM), and NaN3, on the activity of iPOD and cPOD isoforms were tested on IEF gels. After separation of peroxidase isoforms, IEF gels were incubated in different inhibitor concentrations and, after thorough washing, gels were stained for peroxidase activity as described in the Materials and methods. The effects of inhibitors on the activity of peroxidase isoforms were similar among isoforms of the same type of binding to the cell wall. The inhibition of total peroxidase activity in the ionic fraction increased with increasing concentration of inhibitor, which is especially pronounced in the case of SHAM (Fig. 5A). All iPODs were more sensitive to the inhibition by SHAM compared with cPODs (Fig. 5B). The iPOD with pI 9.3 and iPOD with pI 9.24 were the least sensitive to all three inhibitors, whereas the isoforms at pI 9.34 and pI 9.2 were the most sensitive (data not shown). The two isoforms among cPODs (pI 4.5 and 4.65) were the most resistant to inhibition with azide.
Fig. 5.
Inhibition of iPOD (A) and cPOD (B) activity after 15min incubation in solution with different concentrations of inhibitors (SHAM, KCN, and NaN3). The peroxidase activity after treatment with inhibitor is expressed as a percentage of the control. Peroxidase activity represents the sum of the relative activity of each peroxidase isoform calculated by using the program TotaLab. Two-way ANOVA was carried out to assess the difference of means from various concentration of inhibitors and from various inhibitors at the same concentration, followed by multiple comparisons using the Holm–Sidak test (P < 0.05). Different lower case letters denote the results for the same concentration of different inhibitors (and controls), which are significantly different (P < 0.05, Holm–Sidak), and different upper case letters denote the results for the different concentration of the same inhibitors (and controls), which are significantly different (P < 0.05, Holm–Sidak).
When aliquots of ionic and covalent cell wall protein fractions were pre-incubated at various temperatures (4–80 °C) and peroxidase isoforms separated on an IEF gel, a distinctive sensitivity of iPODs and cPODs to high temperatures was obtained (Supplementary Fig. S1 available at JXB online). At extremely high temperatures (80 °C), only the two iPOD isoforms (pI 9.5 and 9.34) were completely inhibited, while all cPODs were inhibited at 80 °C and significantly inhibited also at 60 °C.
Glycosylation and binding properties of iPOD and cPOD isoforms
Class III peroxidases are haem-containing glycosylated proteins. Carbohydrates may modulate the physicochemical and biochemical properties of the enzyme and may change its activity, resistance to protease attack, or thermal stability. The effects of deglycosylation on iPOD and cPOD activity were compared to determine if they also differed in a binding site for the carbohydrate moiety. For deglycosylation treatments, Endo F1, F2, and F3 and PNGase F were used. After the deglycosylation treatment lasting for 24h, the activity of cell wall peroxidases was measured (Table 3). The results showed that the treatment with endoglycosidases caused inhibition of cPOD activity (from 26% to 38%), while they did not affect the activity of iPOD. In contrast to this, PNGase F inhibited iPODs ~63% and even slightly increased cPODs activity (Table 3). Additionally, to test a possible protein glycosylation, the changes in peroxidase activity caused by binding of lectins to sugar moieties were determined. The result showed different effects of the lectins Con A and WGA on the activity of iPOD and cPOD (Table 4). While the activity of cPOD was inhibited, that of iPOD was even slightly increased after treatment with both molecules, Con A and WGA. Increasing concentrations of Con A and WGA further inhibited cPOD activity (Table 4).
Table 3. The effect of native protein deglycosylation on the peroxidase activity in ionic and covalently cell wall-bound fractions.
| Means followed by an asterisk are significantly different from control at P < 0.05, according to Holm–Sidak test. | ||||
| iPOD | cPOD | |||
| Activity (ΔA min–1) | % of control | Activity (ΔA min–1) | % of control | |
| Control | 0.2697±0.0197 | 100 | 0.01113±0.001 | 100 |
| +F1 | 0.3068±0.056 | 114 | 0.0083±0.0014* | 74 |
| +F2 | 0.3132±0.03 | 116 | 0.0071±0.0003* | 64 |
| +F3 | 0.318±0.036 | 118 | 0.0069±0.0002* | 62 |
| +PNGase | 0.1±0.014* | 37 | 0.0118±0.0023 | 106 |
Table 4. Effects of the lectins, concanavalin A (Con A), and wheat germ agglutinin (WGA) on iPOD and cPOD activity. Peroxidase activity was determined by absorbance change measurement at 430nm in the presence of 20mM pyrogallol.
| Means followed by an asterisk are significantly different from control at P < 0.05, according to Holm–Sidak test. | ||||
| iPOD | cPOD | |||
| Activity (µmol min–1 mg–1 protein) | % of control | Activity (µmol min–1 mg–1 protein) | % of control | |
| Control | 102±2 | 100 | 4.1±0.12 | 100 |
| +1 µg ml–1 ConA | 107±3.4 | 105 | 3.6±0.3* | 88 |
| +5 µg ml–1 ConA | 104±1 | 101 | 3.1±0.5* | 76 |
| +1 µg ml–1 WGA | 106.6±1.6 | 104 | 2.9±0.15* | 71 |
| +5 µg ml –1WGA | 103±1.6 | 101 | 7±1* | 173 |
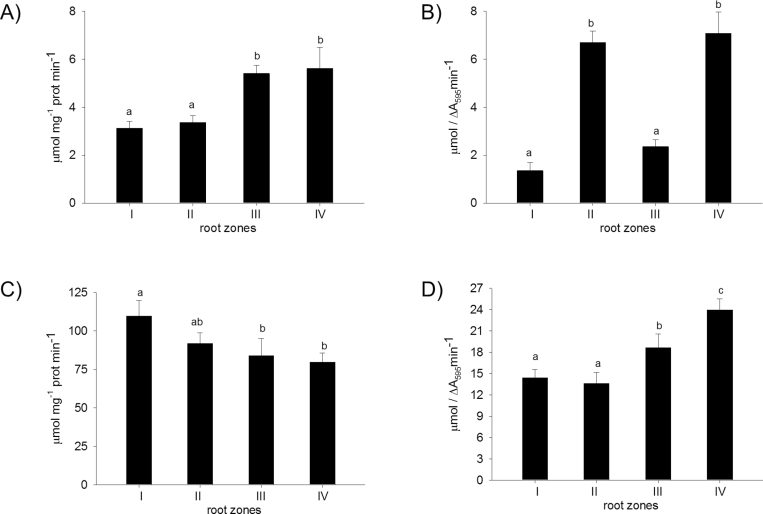
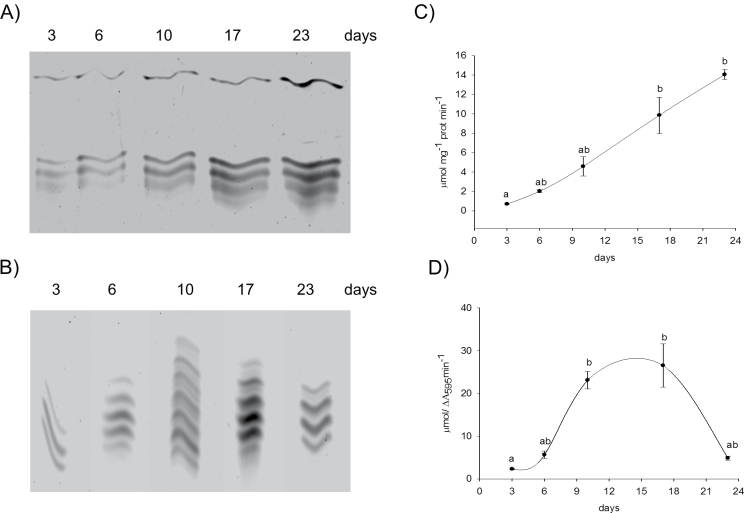
Zonal distribution of iPOD and cPOD along the root
Root growth is divided into phases with several zones along the root moving upwards towards the stem: the root tip, the zone of active division, the zone of cell elongation, and the zone of maturation or differentiation. Cell wall peroxidases are involved in elongation and also in differentiation processes: cell wall loosening is necessary for elongation and cell wall stiffening for differentiation. In the present study, the cell wall was extracted from four zones along the root of 3-day-old seedlings and the distribution of iPODs and cPODs in the samples was analysed by separation of the isoforms by IEF–PAGE (Fig. 6). Isoperoxidase profiles of iPOD did not change significantly along the root (Fig. 6B), whereas a significant increase in abundance of cPODs was obtained in zone II and in the last zone (IV) with differentiated cells (Fig. 6C). In root tips, only three cPODs (pI 4.5, 4.36, and 4.18) were detected with the lowest activity (Figs 6B, 7B). Induction of new cPOD isoforms with pI 4.6, 4.8, and those with pI 3.9–3.7 occurred in the root base consisting of differentiated vascular tissue (Supplementary Fig. S3 at JXB online). The total activity of iPODs and cPODs was measured with pyrogallol as substrate (Fig. 7A, B) and corresponded to the intensity of bands from the gel. In addition to peroxidase activity, iPODs and cPODs were analysed for their ability to oxidize NADH and generate H2O2 in the oxidative cycle of peroxidase (Elstner and Heupel, 1976; Halliwell, 1978). Although there is no proof for the occurrence of NADH in the apoplast under standard conditions, release of NADH into the apoplast could be demonstrated after wounding (Zhang and Mou, 2009); NADH-POD oxidase activity of iPOD decreased, while that of cPOD increased along the root from the tip to the base (Fig. 7C, D).
Fig. 7.
Total peroxidase (A, B) and NADH-POD oxidase activity (C, D) of ionic (A, C) and covalent (B, D) cell wall fractions. Cell wall fractions were isolated from different root zones of 3-day-old pea seedlings. Peroxidase activity was measured using pyrogallol as substrate as described in the Material and methods. Oxidizing activity of peroxidase was measured with NADH in the presence of catalytic concentrations of Mn and p-coumaric acid. Values with different letters are significantly different at P < 0.05, according to Holm–Sidak test.
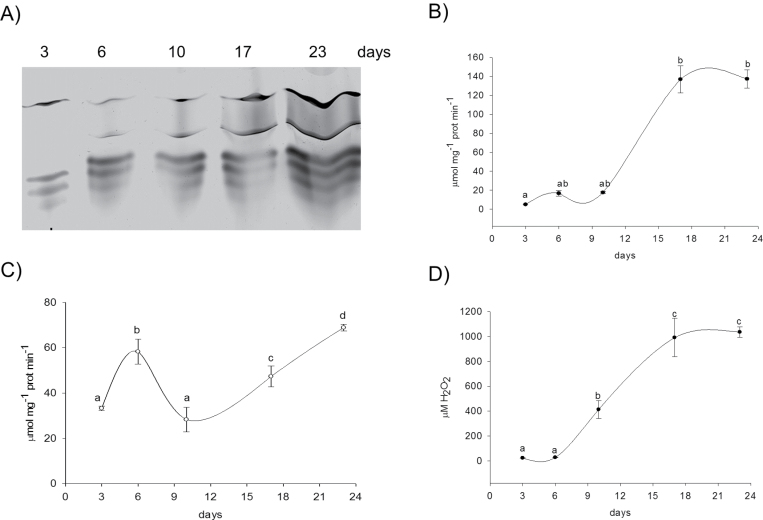
Induction of iPODs and cPODs in root during plant growth
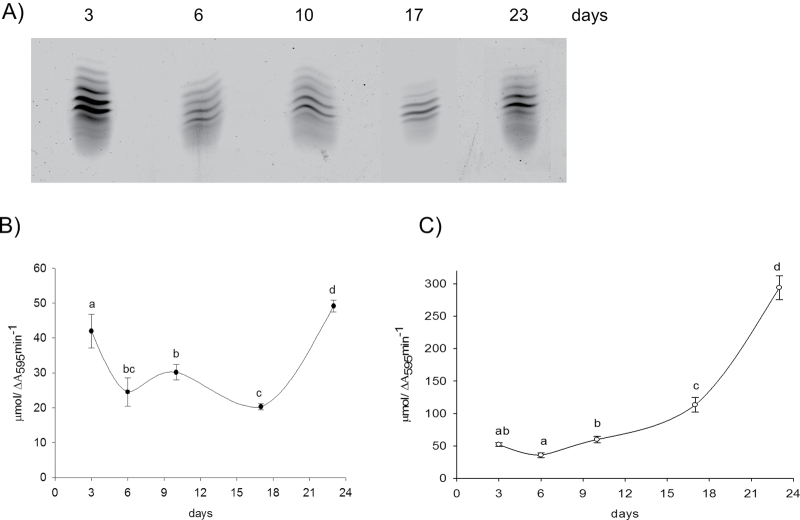
Root growth begins as soon as seedling germinate by division of meristemic cells in the root tip and by elongation of cells in zone II of the primary root. After 6 d, secondary roots started to branch from the original primary root and continued with growth during a 3 week period (Supplementary Fig. S2 at JXB online). Induction of specific cell wall peroxidases during root growth was determined using IEF separation of iPODs and cPODs extracted from the root of pea seedlings and the whole root system of plants 6, 10, 17, and 23 days old (Figs 8, 9). Total iPOD activity increased 3-fold with the appearance and elongation of secondary roots (after 6 d and 10 d). The induction of a new ionic isoform with pI 9.34 and an increase in abundance of one with pI 9.5 accompanied elongation of primary and secondary roots (Fig. 8A).
Fig. 8.
IEF pattern of iPOD (A), total peroxidase (B), and NADH-POD oxidase activity (C), and capacity of iPOD to produce H2O2 (D) in root cell wall isolated from plants of different ages as indicated. Whole roots were used for cell wall isolation. Peroxidase activity was measured spectrophotometrically with pyrogallol as a substrate. Measurement of NADH-POD oxidase activity and capacity for formation of H2O2 was done according to Fecht-Christoffers et al. (2006). Values with different letters are significantly different at P < 0.05, according to Holm–Sidak test.
In the covalent fraction, high peroxidase activity was measured in the seedlings, and then it decreased during elongation of primary and secondary roots (Fig. 9B). After 17 d, lignification of endodermal cells started, as well as formation of the Casparian strip (data not shown). Only on 23rd day, the central cylinder was almost fully formed and lignified (Supplementary Fig. S3 at JXB online) which was accompanied by the highest activity of cPODs (Fig. 9A, B). The peroxidase activity was also visualized by staining with α-chloro-naphthol of the root cross-section in the area of the central cylinder (Supplementary Fig. S3). However, the Casparian strip was not completely formed, implying that the root was still growing.
Fig. 9.
IEF pattern of cPOD (A), total peroxidase (B), and NADH-POD oxidase activity (C) in root cell wall isolated from plants of different ages as indicated. Whole roots were used for cell wall isolation. Peroxidase activity was measured spectrophotometrically with pyrogallol as a substrate. Measurement of NADH-POD oxidase activity was done according to Fecht-Christoffers et al. (2006). Values with different letters are significantly different at P < 0.05, according to Holm–Sidak test.
NADH oxidase activity in both protein fractions increased significantly with plant growth. The ratio of NADH oxidase and peroxidase activity for the ionic fraction was the highest for 6-day-old plants, when secondary roots started to develop (Supplementary Fig. S2 at JXB online). Although in the covalent fraction the ratio of oxidase/peroxidase activity increased with the age of the plants, the NADH oxidase reaction was not accompanied by H2O2 production. Only iPODs had the capacity to produce H2O2 during NADH oxidation (Fig. 8C).
Auxin and chitosan induced changes in the peroxidase profile
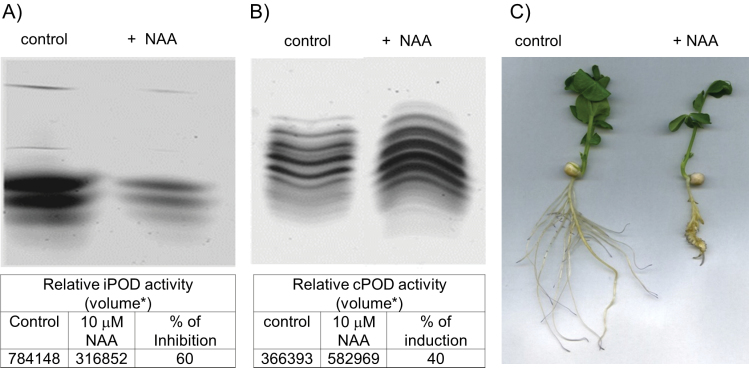
Treatment of pea plants with auxin (10 µM NAA) for 11 d led to a significant inhibition of root growth, which was similar to the observations of Kukavica et al. (2007). The inhibition and an intensified lignification were accompanied by a decrease in iPOD and an increase in cPOD activities (Fig. 10). Two iPOD isoforms with pI 9.5 and 9.3 almost disappeared, while NAA induced a new cPOD isoform with pI 5.2 along with a >2-fold increase obtained in the intensity of all cPODs (Fig. 10B).
Fig. 10.
Inhibiton of root growth of 10-day-old plants induced by NAA (10 µM) was accompanied by a decrease in iPOD (A) and an increase in cPOD (B) isolated from root cell wall. NAA was present in hydroponic solution from the beginning of the experiment. The tables show the total peroxidase activity that represents the sum of the relative activity of each peroxidase isoform. Data were calculated by TotaLab, and the percentage of inhibition of iPOD and induction of cPOD activity is given. *The raw volume of the uncalibrated quantity of material in the image feature after the background intensity has been removed (the units of the numbers are expressed as relative activity units). (C) Roots of control and treated pea plants. Roots were incubated for 15 min in 0.1 M Na-phosphate buffer pH 7.5 containing 0.005% NBT to indicate in vivo production of superoxide anion radical.
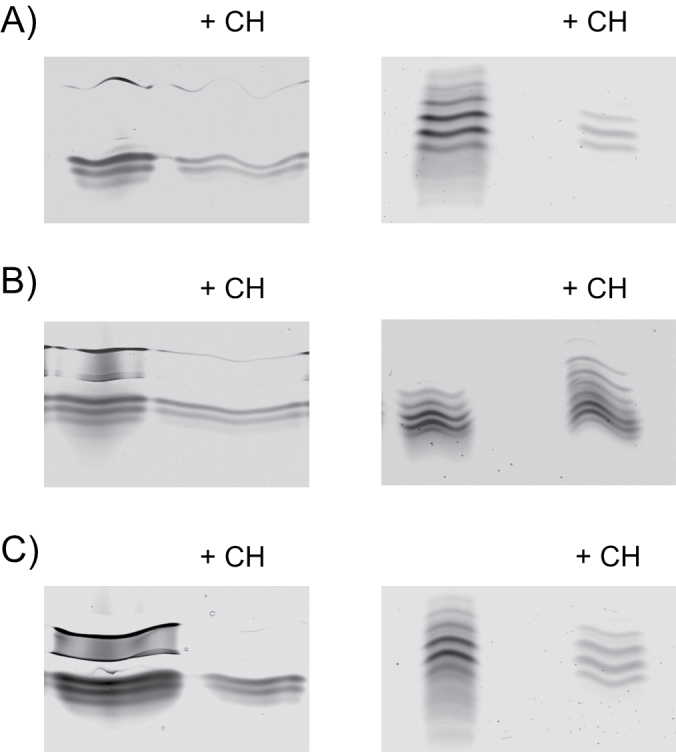
Chitosan is known to have eliciting activities leading to a variety of defence responses in host plants to microbial infections, including the accumulation of phytoalexins, pathogen- related (PR) proteins, and proteinase inhibitors, lignin synthesis, and callose formation. In maize and pea plants, chitosan led to slightly increased total peroxidase activity of the membrane fraction, while the total activity of soluble peroxidase did not change (Mika et al., 2010; Meisrimler et al., 2011). The present study shows a remarkable effect of chitosan on cell wall-bound peroxidases, both iPODs and cPODs, compared with the control (Fig. 11). In Fig. 12, the effects of 1g l–1 chitosan on peroxidase patterns in the ionic and covalent fraction of the cell wall isolated from plants of different ages are shown. Plants were grown in Hoagland solution till the treatment was applied on the third, sixth, 10th, 17th, and 23rd day, lasting for 16h.
Fig. 11.
Effect of chitosan (CH) on the IEF pattern of iPOD (left) and cPOD (right) isolated from root cell wall. Plants of different ages, 3 (A), 10 (B), and 23 (C) d, were treated with 1g l–1 of chitosan for 16h.
Fig. 12.
Chitosan induced distinct changes in iPOD (A) and cPOD (B) isoform activities in roots of plants of different ages as indicated. Data on iPOD (C) and cPOD (D) activity obtained spectrophotometrically with pyrogallol as a substrate. Values with different letters are significantly different at P < 0.05, according to Holm–Sidak test.
Chitosan induced significant inhibition of activity of all iPODs regardless of plant age (Fig. 11, left panels; Fig. 12A). However, the effect of chitosan on the activity of cPODs was dependent on the plant age: it decreased in the youngest and oldest plants. When chitosan was applied on the 10th day, cPOD isoforms (pI 5.2 and pI 5.0) were induced (Fig. 11, right panels; Fig. 12B). In other words, an opposite effect of chitosan was obtained that depends on plant age: a decrease in number and intensity of cPOD isoforms in root of 3- and 23-day-old plants and an increase in number and intensity of cPODs extracted from 10- and 17-day-old roots (Figs 11, 12).
Discussion
Biochemical and binding properties of cell wall peroxidases
The cell wall of pea root contains two groups of peroxidases according to their isoelectrophoretic mobility: cationic peroxidases that are retained within the polysaccharide matrix by ionic interactions and anionic peroxidases that are cross-linked by covalent bonds. In the present study, five cationic isoforms were detected with pI values between 9.2 and 9.5 (apparent mol. wts from 41kDa to 56kDa) and nine anionic isoforms with pI values ranging from 3.7 to 5.2 with apparent mol. wts of ~70kDa (Figs 1, 2). A wide range of peroxidase isoforms regarding pI (from pI 2 to 11.6) were found in many species (Welinder and Mazza, 1975; Heidrich et al., 1983; Floris et al., 1984; Quiroga et al., 2001; Dicko et al., 2006). HRP extract from commercial sources contained 42 isoforms with a different range of pI values, from 2 to 10 (Hoyle, 1977). The present study showed that isoform patterns differ depending on the type of binding to the cell wall constituents. Among cell wall polysaccharides, with most of them being neutral, only pectins provide negative charges for interactions with basic proteins. As such interactions may be modified by pH, Ca2+ concentration (Penel and Greppin, 1996), or pectin esterification (Ros Barcelo et al., 1988; Penel and Greppin, 1994), pectin may be involved in the regulation of peroxidase distribution within the apoplast. A distinct charge of these two groups of isoforms, which are bound to the cell wall of pea root, might imply different domains within the cell wall and, accordingly, a specific physiological function. Indeed, the results from the literature are in favour of the hypothesis that anionic and cationic isoforms have specific and distinct physiological functions in situ. There are numerous articles regarding the specific roles of either cationic (Church and Galston, 1988; Abeles and Biles, 1991; Sato et al., 1995; Ros Barcelo et al., 1998) or anionic isoperoxidase (Ferrer et al., 1991; McDougall, 1992; Mohan et al., 1993; Ros Barcelo, 1997) in lignification and suberization (Espelie and Kolattukudy, 1985; Bernards et al., 1999). However, Quiroga et al. (2001) demonstrated that both the basic isoform with pI 9.6 and the acidic isoform with pI 3.6 from tomato root showed a similar catalytic efficiency with coniferyl alcohol as substrate. It was also shown that both iPODs and cPODs have a similar K m for pyrogallol, clorogenic acid, and cumaric acid (Table 1). The only difference in substrate affinity between iPODs and cPODs was found for H2O2, with the K m of cPODs being 2-fold higher than that of iPODs.
The differences in biochemical properties between isoperoxidases ionically and covalently bound to the cell wall are summarized in Table 5. Besides their different electrophoretic mobility and apparent molecular weights, comparative analysis of their oxidizing and catalase-like activities, sensitivity to deglycosylation, and the effects of inhibitors or high temperature indicate that iPODs and cPODs possess characteristic and specific structure and also different binding domains within the cell wall. Taken together, the results implicate their specific role in root physiology during development and defence response.
Table 5. Biochemical properties of pea cell wall peroxidases.
| +++, highest inhibition; +, lowest inhibition | ||
| iPOD | cPOD | |
| pI | Cationic (9.2–9.5) | Anionic (5.2–3.7) |
| Thermostability | Very high (80 °C) | High (60 °C) |
| Deglycosylation (F1, F2, F3) | No effect | +++ |
| Deglycosylation (PNGase) | +++ | No effect |
| Binding lectins (Con A, WGA) | No effect | +++ |
| OH production | No | Yes |
| Catalase-like activity | No | Yes |
| IAA oxidase activity | Yes | No |
| Capacity for H2O2 production | Yes | No |
| K m for H2O2 | 1.3±0.1 mM | 2.4±0.4 mM |
| SHAM | +++ | + |
| KCN | ++ | ++ |
| NaN3 | + | + |
Peroxidases are bifunctional enzymes, possessing the capacity to catalyse either oxidative or peroxidative reactions, which in vitro depends on the presence of activators (diphenols and Mn2+ and NADH as reductant). A novel function of peroxidase in the hydroxylic cycle has been postulated (Chen and Schopfer, 1999; Liszkay et al., 2003). It was found that both cell wall fractions had oxidizing activity when NADH was used as reductant (Fig. 7). However, no H2O2 production during NADH oxidation was detected with anionic isoforms. Also when IAA was used as a reductant to stain the oxidizing reaction on the gel, the activity was obtained only with cationic isoforms. On the other hand, it was shown that anionic or cPODs bound to the cell wall were the most efficient in ·OH production compared with iPOD, apoplastic peroxidase, or HRP (Fig. 4). A failure of iPODs or commercial HRP (VI and II type) to promote ·OH production in the absence of NAD(P)H even when added to the deproteinated cell wall may implicate that a specific property of cPODs is inevitable for this reaction (Kukavica et al., 2009). In addition to that, it was also shown that cPODs had the highest catalase-like activity (Table 2), being the most stable against H2O2 inactivation. Besides that, the cPOD isoforms possess a different affinity for H2O2 compared with the peroxidases in all other fractions (Table 1). Since the K m can be used to approximate the value of the intercellular level of the substrate (Segal, 1976); a higher K m of H2O2 for cPODs (K m = 2.36mM) than for all other fractions may indicate their specific role in physiological processes dependent on H2O2 concentration (Schopfer, 1994). It is hypothesized that iPODs and cPODs differ in their capacity to gain an oxidative or hydroxylic cycle, with iPODs that favour the oxidative cycle coupled to the peroxidative, while cPODs are preferentialy involved in the hydroxylic cycle.
Developmental- and elicitor-induced changes in anionic and cationic cell wall peroxidases
A significant increase in the activity of anionic isoforms was obtained only in the fourth zone of the root with differentiated cells, while cationic isoforms did not significantly change along the 3-day-old root (Fig. 6). On the other hand, growth of pea plants during 3 weeks was accompanied by an increase in the abundance and activities of cationic isoforms, both peroxidative and oxidative. Additionally, a new isoform with pI 9.34 appeared on the sixth day and increased with root elongation (Fig. 8). The capacity of cationic peroxidases to oxidize NADH in the presence of diphenol and Mn2+ and to produce H2O2 also increased with time. However, the anionic isoforms were most abundant in the roots of seedlings and in the roots of the oldest plants used in the experiment. The results suggest that cationic isoforms with pI 9.34 and 9.5 are specifically involved in root elongation, and anionic isoforms are involved in the formation of the cell wall and lignification. It has been shown that peroxidases covalently bound to the cell wall are preferentialy involved in generation of ·OH, when endogenous H2O2 is produced by MnSOD (superoxide dismutase), which is also bound to the cell wall (Kukavica et al., 2009). In addition to the roles in the process of lignification, extracellular anionic and cationic peroxidases are associated with other physiological processes such as defence reaction in response to pathogen attack (Young et al., 1995; Morimoto et al., 1999). The changes in the peroxidase profile after two treatments, with the phytohormone auxin on root growth, and chitosan, a potent elicitor of plant resistance against fungal pathogens, are in favour of the specific roles of anionic and cationic peroxidases in root physiology. Exogenous auxin or auxin-like substances usually inhibit root growth (Pilet and Elliot, 1981; Lüthen and Böttger, 1993). There are two hypotheses for the mechanism of auxin-induced growth nhibition and accompanying cell wall stiffening: one involves alkalization of the apoplast (Evans et al., 1980) and the other is an acid-independent mechanism affected by auxin (Lüthen and Böttger, 1993). The present study showed that a cessation of pea root growth by auxin was accompanied by a significant decrease in cationic peroxidase and induction of the activity of anionic peroxidase (Fig. 10). This is in agreement with the hypothesis that cationic peroxidases are responsible for elongation of root cells and anionic peroxidases for stiffening of the cell wall. Similar to the auxin effect, applying chitosan to plants for 16h induced strong inhibition of cationic isoperoxidase, with the most pronounced effect on isoforms with pI 9.34 and 9.5, isoforms that increased during development (Fig. 11). It has been reported that chitosan, which enhances plant defence, also induced lignification (Barber et al., 1989). Taken together, the results of the present study strongly indicate that the ratio of cationic and anionic peroxidases in the cell wall may be an important parameter for determination of root growth. Developmental and environmental factors induced differences in the level of peroxidase activity of those enzymes bound either ionically or covalently to the cell wall. The different stability of the particular isoforms presented in this work implies a different regulation mechanism of their physiological roles. The results further confirm the heterogenity of the peroxidase system including specific in muro localization (Gibson and Liu, 1978). The results on differentially induced or inhibited peroxidase isoforms during root development and under the effects of auxin or elicitor indicate that cationic isoperoxidases are responsible for cell wall loosening during root elongation, while anionic isoperoxidases are involved in cell wall stiffening and lignification or suberization.
Supplementary Material
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Thermostability of cell wall peroxidases.
Figure S2. Appearance of pea roots dependent on plant development.
Figure S3. Cross-sections of zone IV of 3- and 23-day-old pea roots.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG Lu 668/4-4, Lu 668/8-1, Lu 668/9-1) and by the Ministry of Education and Science of the Republic of Serbia (to SVJ, Project no. III43010). BK and SVJ greatfully acknowledge Miloš Mojović for EPR measurments, Jovana Glušac (University of Banja Luka) for statistical analysis and Dino Hasanagić (University of Banja Luka) for histological analysis. The authors are grateful to Hartwig Lüthen (University of Hamburg, Germany) for stimulating discussions.
References
- Abeles FB, Biles CL. Characterization of peroxidase in lignifying peach fruit endocarp. Plant Physiology. 1991;95:269–273. doi: 10.1104/pp.95.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba CM, De Forchetti SM, Quesada MA, Valpuesta V, Tigier HA. Localization and general properties of developing peach seed coat and endosperm peroxidase isoenzymes. Journal of Plant Growth Regulation. 1998;17:7–11. [Google Scholar]
- Barber MS, Bertram RE, Ride JP. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiology and Molecular Plant Pathology. 1989;34:3–12. [Google Scholar]
- Bernards M, Fleming W, Lewellyn D, Prifer R, Yang X, Sabatino A, Plourude G. Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiology. 1999;121:135–145. doi: 10.1104/pp.121.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick C, Brown IR, John W. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiology. 1998;118:1067–1078. doi: 10.1104/pp.118.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F, Rowntree EG, Wojtaszek P. Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Radical Research. 1999;31:137–145. doi: 10.1080/10715769900301431. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram of protein utilizing the principle of protein–dye binding . Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brooks JL. Oxidase reactions of tomato anionic peroxidase. Plant Physiology. 1986;80:130–133. doi: 10.1104/pp.80.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownleader MD, Hopkins J, Mobasheri A, Dey PM, Jackson P, Trevan M. Role of extension peroxidase in tomato (Lycopersicon esculentum Mill.) seedling growth. Planta. 2000;210:668–676. doi: 10.1007/s004250050058. [DOI] [PubMed] [Google Scholar]
- Chen S, Schopfer P. Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. European Journal of Biochemistry. 1999;260:726–773. doi: 10.1046/j.1432-1327.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Bauw G, Welinder KG, Van Montagu M, Boerjan W. Purification and characterization of peroxidases correlated with lignification in poplar xylem. Plant Physiology. 1998;118:125–135. doi: 10.1104/pp.118.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DL, Galston AW. 4-Coumarate:coenzyme A ligase and isoperoxidase expression in Zinnia mesophyll cells induced to differentiate into tracheary elements. Plant Physiology. 1988;88:679–684. doi: 10.1104/pp.88.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JB, Varner JE. Cross-linking of soluble extensin in isolated cell walls. Plant Physiology. 1984;76:414–417. doi: 10.1104/pp.76.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Pedregosa MC, Cordoba F, Villalba JM, Gonzalez- Reyes JA. Zonal changes in ascorbate and hydrogen peroxide contents, peroxidase, and ascorbate-related enzyme activities in onion roots. Plant Physiology. 2003;131:697–706. doi: 10.1104/pp.012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. Journal of Experimental Botany. 2009;60:391–408. doi: 10.1093/jxb/ern318. [DOI] [PubMed] [Google Scholar]
- Dicko MH, Gruppen H, Hilhorst R, Voragen, AGJ, van Berkel WJH. Biochemical characterization of the major cationic sorghum peroxidase. FEBS Journal. 2006;273:2293–2307. doi: 10.1111/j.1742-4658.2006.05243.x. [DOI] [PubMed] [Google Scholar]
- Elstner E, Heupel A. Formation of hydrogen peroxide by isolated cell wall from horseradish (Armoracia lapathifolia Gilib.) Planta. 1976;130:175–180. doi: 10.1007/BF00384416. [DOI] [PubMed] [Google Scholar]
- Espelie KE, Franceschi VR, Kolattukudy PE. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiology. 1986;81:487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelie KE, Kolattukudy PE. Purification and characterization of an abscisic acid-inducible anionic peroxidase associated with suberization in potato (Solanum tuberosum) Archives of Biochemistry and Biophysics. 1985;240:539–545. doi: 10.1016/0003-9861(85)90060-8. [DOI] [PubMed] [Google Scholar]
- Evans ML, Mulkey TJ, Vesper MJ. Auxin action on proton influx in corn roots and its correlation with growth. Planta. 1980;148:510–512. doi: 10.1007/BF00552667. [DOI] [PubMed] [Google Scholar]
- Fecht-Christoffers MM, Führs H, Braun HP, Horst WJ. The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiology. 2006;140:1451–1463. doi: 10.1104/pp.105.070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer MA, Calderón AA, Muñoz R, Ros Barceló A. 4-Methoxy-α-naphthol as a specific substrate for kinetic, zymographic and cytochemical studies on plant peroxidase activities. Phytochemical Analysis. 1990;1:63–69. [Google Scholar]
- Floris G, Medda R, Rinaldi A. Peroxidase from Ipomoea batatas seedlings: purification and properties. Phytochemistry. 1984;23:1523–1529. [Google Scholar]
- Gibson DM, Liu EV. Substrate specification of peroxidase isozymes in the developing pea seedlings. Annals of Botany. 1978;42:1075–1083. [Google Scholar]
- Groppa MD, Tomaro ML, Fernández ME. Activity and expression of peroxidases from sunflower: effect of development. Revista Brasileira de Fisiologia Vegetal. 1999;11:55–59. [Google Scholar]
- Hadži-Taškovicć Šukalovicć V, Vuleticć M, Vučinicć Ž. Plasma membrane-bound phenolic peroxidase of maize roots: in vitro regulation of activity with NADH and ascorbate. Plant Science. 2003;165:1429–1435. [Google Scholar]
- Halliwell B. Lignin synthesis: the generation of hydrogen peroxidase and superoxide by horseradish peroxidase and its stimulation by manganese (II) and phenols. Planta. 1978;140:81–88. doi: 10.1007/BF00389384. [DOI] [PubMed] [Google Scholar]
- Heidrich E, Lorenz G, Schreier P. Ultrathin-layer isoelectric focusing of partially purified peroxidase from tomato fruit. Food Chemistry. 1983;10:285–296. [Google Scholar]
- Hernandez-Ruiz J, Arno MB, Hiner ANP, Garcia-Canovas F, Acosta M. Catalase-like activity of horseradish peroxidase: relationship to enzyme inactivation by H2O2. Biochemical Journal. 2001;354:107–114. doi: 10.1042/0264-6021:3540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnman RL, Lang J. Peroxidases catalyzed oxidation of indole-3-acetic acid. Biochemistry. 1965;4:144–158. doi: 10.1021/bi00877a023. [DOI] [PubMed] [Google Scholar]
- Hoyle MC. High resolution of peroxidase-indoleacetic acid oxidase isoenzymes from horseradish by isolectric focusing. Plant Physiology. 1977;60:787–793. doi: 10.1104/pp.60.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet E, Canut H, Boudart G, Pont-Lezica RF. Cell wall proteins: a new insight through proteomics. Trends in Plant Science. 2006;11:33–39. doi: 10.1016/j.tplants.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kukavica B, Mojovicć M, Vučinicć Ž. , Maksimovicć V, Takahama U, Veljovicć-Jovanovicć S. Generation of hydroxyl radical in isolated pea root cell wall, and the role of cell wall-bound peroxidase, Mn-SOD and phenolics in their production. Plant and Cell Physiology. 2009;50:304–317. doi: 10.1093/pcp/pcn199. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Liszkay A, Kenk B, Schopfer P. Evidence for involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta. 2003;217:658–667. doi: 10.1007/s00425-003-1028-1. [DOI] [PubMed] [Google Scholar]
- Lopez-Serrano M, Fernandez M, Pomar F, Pedrano M, Ros Barcelo A. Zinnia elegans uses the same peroxidase isoenzyme complement for cell wall lignification in both single-cell tracheary elements and xylem vessels. Journal of Experimental Botany. 2004;55:423–431. doi: 10.1093/jxb/erh036. [DOI] [PubMed] [Google Scholar]
- Lüthen H, Böttger M. The role of protons in the auxin-induced root growth inhibition—a critical reexamination. Botanica Acta. 1993;106:58–63. [Google Scholar]
- McDougall GJ. Changes in cell wall-associated peroxidases during the lignification of flax fibres. Phytochemistry. 1992;31:3385–3389. [Google Scholar]
- Meisrimler CN, Planchon S, Renaut J, Sergeant K, Lüthje S. Alteration of plasma membrane-bound redox systems of iron deficient pea roots by chitosan. Journal of Proteomics. 2011;74:1437–1449. doi: 10.1016/j.jprot.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Mika A, Boenisch MJ, Hopff D, Lüthje S. Membrane-bound guaiacol peroxidases are regulated by methyl jasmonate, salicylic acid, and pathogen elicitors. Journal of Experimental Botany. 2010;61:831–841. doi: 10.1093/jxb/erp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Lüthje S. Properties of guiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiology. 2003;132:1489–1498. doi: 10.1104/pp.103.020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minibayeva F, Lüthje S, Kolesnikov O, Chasov A, Beckett RP, Vylegzhanina N, Buck F, Böttger M. Wound-induced apoplastic peroxidase activities: their roles in the production and detoxification of reactive oxygen species. Plant, Cell and Environment. 2009;32:497–508. doi: 10.1111/j.1365-3040.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- Mohan R, Bajar AM, Kolattukudy PE. Induction of a tomato anionic peroxidase gene (tap 1) by wounding in transgenic tobacco and activation of tap1:GUS and tap2:GUS chimeric gene fusions in transgenic tobacco by wounding and pathogen attack. Plant Molecular Biology. 1993;21:341–354. doi: 10.1007/BF00019949. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Tateishi N, InuyamaM, Taura F, Tanaka H, Shoyama Y. Identification and molecular characterization of novel peroxidase with structural protein-like properties. Journal of Biological Chemistry. 1999;37:26192–26198. doi: 10.1074/jbc.274.37.26192. [DOI] [PubMed] [Google Scholar]
- Nakajima R, Yamazaki I. The mechanism of indole-3-acetic acid oxidation by horseradish peroxidase. Journal of Biological Chemistry. 1979;254:872–878. [PubMed] [Google Scholar]
- Narita H, Asaka Y, Ikura K, Matsumoto S, Sasaki R. Isolation, characterization and expression of cationic peroxidase isozymes released into the medium of cultured tobacco cells. European Journal of Biochemistry. 1995;228:855–862. [PubMed] [Google Scholar]
- Penel C, Greppin H. Binding of plant isoperoxidases to pectin in the presence of calcium. FEBS Letters. 1994;343:51–55. doi: 10.1016/0014-5793(94)80605-5. [DOI] [PubMed] [Google Scholar]
- Penel C, Greppin H. Pectin binding proteins: characterization of the binding and comparison with heparin. Plant Physiology and Biochemistry. 1996;34:479–488. [Google Scholar]
- Pilet PE, Elliot MC. Some aspect of the control of root growth and georeaction: the involvement of indoleacetic acid and abscisic acid. Plant Physiology. 1981;67:1047–1050. doi: 10.1104/pp.67.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga M, de Forchetti SM, Taleisnik E, Tigier HA. Tomato root peroxidase isoforms: kinetic studies of the coniferylalcohol peroxidase activity, immunological properties and role in salt stress. Journal of Plant Physiology. 2001;158:1007–1013. [Google Scholar]
- Ros Barceló A. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta. 1998;207:207–216. [Google Scholar]
- Ros Barceló A, Pedreño MA, Muñoz R, Sabater F. Lupin peroxidases. II. Binding of acidic isoperoxidases to cell walls. Physiologia Plantarum. 1988;73:238–244. [Google Scholar]
- Sánchez M, Revilla G, Zarra I. Changes in peroxidase activity associated with cell walls during pine hypocotyl growth. Annals of Botany. 1995;75:415–419. [Google Scholar]
- Sato Y, Sugiyama M, Komamine A, Fukuda H. Separation and characterization of the isoenzymes of wall-bound peroxidase from cultured Zinnia cells during tracheary element differentiation. Planta. 1995;196:141–147. [Google Scholar]
- Schopfer P. Histochemical demonstration and localization of H2O2 in organs of higher plants by tissue printing on nitrocellulose paper. Plant Physiology. 1994;104:1269–1275. doi: 10.1104/pp.104.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahy G, Wagner A. Evidence that hydrohyl radicals mediate auxin-induced extension growth. Planta. 2002;214:821–828. doi: 10.1007/s00425-001-0699-8. [DOI] [PubMed] [Google Scholar]
- Segel IH. 1976. Biochemical calculations, 2nd edn. New York: : John Wiley & Sons; [Google Scholar]
- Thomas PE, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Analytical Biochemstry. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Veljovicć-Jovanovicć S, Kukavica B, Cveticć T, Mojovicć M, Vučinicć Ž. Ascorbic acid and the oxidative processes in pea root cell wall isolates. Characterization by fluorescence and EPR spectroscopy. Annals of the New York Academy of Sciences. 2005;1048:501–505. doi: 10.1196/annals.1342.076. [DOI] [PubMed] [Google Scholar]
- Wellinder K, Mazza G. Similarities and differences of five peroxidases from turnip and horseradish peptide mapping studies on glycoproteins. European Journal of Biochemistry. 1975;77:415–421. doi: 10.1111/j.1432-1033.1975.tb02316.x. [DOI] [PubMed] [Google Scholar]
- Young SA, Cuo A, Cuikema JA, White FF, Leach JE. Rice cationic peroxidase accumulates in xylem vessels during incompatible interactions with Xanthomonas oryzae pv oryzae . Plant Physiology. 1995;107:1333–1341. doi: 10.1104/pp.107.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mou Z. Extracellular pyridine nucleotides induce PR gene expression and disease resistance in Arabidopsis. The Plant Journal. 2009;57:302–312. doi: 10.1111/j.1365-313X.2008.03687.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.