Abstract
There is a need to develop rice plants with improved photosynthetic capacity and efficiency in order to enhance potential grain yield. Alterations in internal leaf morphology may be needed to underpin some of these improvements. One target is the production of a ‘Kranz-like’ anatomy, commonly considered to be required to achieve the desired levels of photosynthesis seen in C4 crops. Kranz anatomy typically has two or three mesophyll cells interspersing adjacent veins. As a first step to determining the potential for such anatomical modifications in rice leaves, a population of rice deletion mutants was analysed for alterations in vein patterning and mesophyll cells in the interveinal regions. Significant variation is demonstrated in vein arrangement and the sequential distribution of major and minor veins across the leaf width, although there is a significant correlation between the total number of veins present and the width of the leaf. Thus the potential is demonstrated for modifying rice leaf structure. Six distinct rice mutant lines, termed altered leaf morphology (alm) mutants, were analysed for the architecture of their interveinal mesophyll cell arrangement. It is shown that in these mutant lines, the distance between adjacent minor veins and adjacent minor and major veins is essentially determined by the size of the interveinal mesophyll cells rather than changes in mesophyll cell number across this region, and hence interveinal distance changes as a result of cell expansion rather than cell division. This observation will be important when developing screens for traits relevant for the introduction of Kranz anatomy into rice.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details)
Key words: Leaf morphology, mesophyll cell, Oryza sativa L., rice, vein spacing, Leaf morphology
Introduction
Rice (Oryza sativa L.) feeds more people directly than any other agricultural crop and yet in the last two decades rice yields have stagnated (Mitchell and Sheehy, 2006). In a background of increasing human population in rice-growing areas of the world, especially south-eastern Asia and Africa (Dawe, 2007), the requirement to increase rice yields is critical to maintain food security. To achieve this, an increase in total biomass of the rice crop is considered essential and yet it may be difficult to achieve step changes by traditional breeding approaches. In recent years more direct process-based approaches to increasing rice yields have been suggested. In particular an increase in leaf photosynthetic capacity and efficiency is needed to underpin any rise in crop biomass. It is possible to identify a number of targets from leaf and canopy structure to cellular and plastidic components (Murchie et al., 2009). However, in C3 leaves, one key factor is the availability of CO2 within the leaf: at low pCO2 and in the presence of O2, Rubisco catalyses the oxygenation reaction producing phosphoglycollate and initiating photorespiration which substantially reduces photosynthetic productivity. One radical approach that has been taken to overcome this is the introduction of the C4 photosynthetic pathway into rice (Surridge, 2002; Mitchell and Sheehy, 2006; Hibberd et al., 2008; Furbank et al., 2009).
The rice plant carries out C3 photosynthetic carbon fixation even though it is a tropical to warm temperate grass (Sage, 2001). Consequently rice productivity suffers because of the enhanced levels of photorespiration, which reduces photosynthetic capacity by around 30–35% when the ambient temperature is 30–35 °C (Sage, 2001). Introducing C4 characteristics into rice to overcome the limitations imposed by photorespiration is a highly challenging project and requires multifaceted modifications to rice leaf and chloroplast development, not least the alteration of internal leaf architecture and cellular morphology to establish Kranz anatomy, which is found in the vast majority of C4 plants (Brown, 1975; Nelson and Langdale, 1992; Muhaidat et al., 2007). Such an anatomy requires the development of two distinct chloroplast-containing cell types within the leaf; the mesophyll cells, which make up the bulk of the cells within the leaf and lie predominantly between the parallel vascular strands, and the bundle sheath cells, which form a ring around the vascular tissue forming a column of cells encasing each vascular strand throughout the leaf’s length. In C4 plants there is a distinctly different pattern of gene expression in these two cell types (Hibberd and Covshoff, 2010), a prominent feature of which is the expression of PEP carboxylase only in the mesophyll cell and Rubisco only in the bundle sheath cell chloroplasts. This anatomical feature of C4 plants facilitates two distinct places for CO2 fixation, with a C4 carbon shunt from the mesophyll cell into the bundle sheath chloroplasts, increasing the concentration of CO2 at the active site of Rubisco in the bundle sheath cell chloroplasts, thereby largely obliterating the oxygenase activity of Rubisco and hence the photorespiratory pathway (Edwards and Walker, 1983; Nelson and Langdale, 1992). Single-cell C4 species exist (Voznesenskaya et al., 2001). However, C4 species that show high rates of productivity all possess Kranz-type leaf anatomy and it is highly likely that this will be required to enable a yield-enhancing C4 photosynthetic pathway. The basic structure of rice leaves follows the general pattern displayed by monocotyledonous species (Sakaguchi and Fukuda, 2008; Sage and Sage, 2009) in that the arrays of parallel veins are surrounded by bundle sheath cells, which is a feature of all leaves including dicotyledonous leaves such as Arabidopsis thaliana (Kinsman and Pyke, 1998) although these cells and the chloroplasts within them are less well developed in rice. Another consistent aspect of Kranz-type anatomy which appears to be important is the distance between the veins in relation to the numbers of mesophyll cells present (Langdale et al., 1988; Sakaguchi and Fukuda, 2008). The typical arrangement of Kranz anatomy in C4 grasses generally results in an enlarged cylinder of bundle sheath cells encircling the vascular strand and with rarely more than two mesophyll cells between adjacent veins (Nelson and Langdale, 1992). This cell positioning, together with the fact that chloroplasts in bundle sheath cells are often located on the cell surface closest to the mesophyll cells in crop species, presumably optimizes transport of molecules involved in the C4 cycle between the mesophyll cells and the bundle sheath cells.
The introduction of the C4 mechanism would be likely to have large benefits. However, there are other aspects of rice leaf morphology that deserve investigation. For example the presence of mesophyll cell lobing has been suggested to be involved with CO2 recycling (Sage and Sage, 2009) and there is recent evidence that mesophyll conductance is a variable factor in gas exchange in leaves (Flexas et al., 2008). All of these lines of work suggest that alteration in leaf morphology will be required to enable a rise in photosynthetic rate, either by C3 or C4 photosynthesis.
Currently it is not known whether rice or the rice genome possesses sufficient ‘plasticity’ with respect to these morphological features. As an initial step, this study set out to identify mutants of rice with alterations in their vascular patterning and leaf morphology, in order to assess the extent to which the cellular architecture within the rice leaf can be altered and test the plasticity of the rice genome in being able to generate such variability. Such mutants with features that are reminiscent of Kranz anatomy within their leaf structure will be crucial to efforts to enhance manipulation of rice leaf architecture to phenocopy a Kranz-type anatomy.
Materials and methods
Plant material
Rice lines characterized in this study were originally identified from a large population of mutants in the widely grown rice cultivar IR64, generated using a variety of different mutagens including gamma radiation and diepoxybutane (DEB) (Wu et al., 2005). Pre-scoring of 38,000 M4 lines from this mutant population produced a subpopulation of 500 mutant lines in the M4 generation that had pale, wider or narrower leaves compared to wild type, a rationale which was considered more likely to reveal novel patterns of vein development. Forty-six of these M4 mutant rice lines were used in this study to assess the nature of any altered leaf development and differences in vascular patterning and gross leaf morphology. Seeds were kindly supplied by Dr Hei Leung at IRRI.
Plant growth conditions
Plants were grown in a walk-in growth room at 27 °C with a 12/12 light/dark photoperiod and illuminated by a bank of 400 W metal halide lamps, with a light intensity at plant level of 400 µm m–2 s–1. Rice seeds were washed thoroughly in distilled water and germinated on moist filter paper in 5-cm Petri dishes placed in the growth room. Young seedlings were then transferred to a tank hydroponics system (Murchie et al., 2005). The nutrient composition was the same as Murchie et al. (2005) with the exception that no silica was added. The sixth fully expanded true leaf emerging from the primary tiller of each rice plant was harvested around 30 days after transfer into the hydroponic system and used for detailed analysis.
Leaf sectioning
An initial, rapid screen was carried out using hand-cut sections viewed immediately in water under a light microscope. This was used to measure vein spacing and ‘arrangement’ and leaf thickness. It was not possible to measure detailed characteristics such as mesophyll cell size.
A second more detailed analysis of select lines was carried out following cell clearance as follows. Sections of the mature rice leaf 6 were hand cut from the widest part of the leaf using a razor blade, mounted on a microscope slide, and cleared using a solution of 85% (w/v) lactic acid saturated with chloral hydrate. Slides were placed in covered Petri dishes and heated on a water bath at 70°Cfor 1 hour. Sections were then washed several times with distilled water and stained with solution of 1% toluidine blue in 1% (w/v) disodium tetraborate for 15 s and then washed thoroughly with distilled water. Sections were viewed using a Optiphot Microscope and images captured using a DXM 1200 digital camera (Nikon). Composite images of leaf sections were created using Adobe Photoshop software. Measurements of leaf thickness, vein spacing, and vein size were measured from composite images of leaf sections imported into Infinity Analyze software.
Analysis of separated leaf cells
Preparations of separated cells from samples of rice leaves were made adapting the method of Pyke and Leech (1987). Pieces of rice leaf 6 (1-mm wide) were fixed in 3.5% (w/v) glutaraldehyde for 1 hour in the dark, and were then transferred to a solution of 0.1M NaEDTA (pH 9) and heated for 6 hours at 60 °C. Samples were then left to cool in a fridge at 4°C overnight before small pieces of leaf were gently macerated on a microscope slide in distilled water and individual cells imaged and their plan area measured using the microscope systems and software as described above.
Statistical analyses were performed using analysis of variance (ANOVA) using Genstat and T-tests were conducted using Microsoft Excel. Multiple plants were sectioned for each line, and this value is described by n in each case.
Results
Leaf anatomy
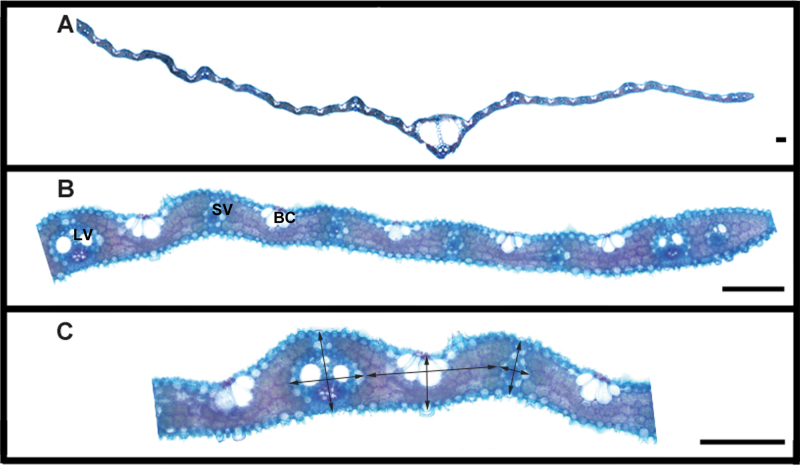
Transverse sectioning of the rice leaf reveals four major tissue types; the vascular tissue, the chlorenchyma composed of mesophyll cells, bulliform cells, and the epidermis (Fig. 1), of which the vascular tissue and chlorenchyma are of particular interest to this study. The vascular bundles can be categorized into three types according to their size; the midrib, the major veins, and the minor veins (Scarpella et al., 2003). In wild-type rice leaves, the midrib is central and minor and major veins run parallel to it between the midrib and the leaf margin (Fig. 1). The sequence and repeating pattern of minor and major veins is normally symmetrical about the midrib and well conserved between leaves with variation generally only observed close to the leaf margins. Typically, two minor veins are present adjacent to the midrib, before the first major vein, after which there is repeating pattern of five minor veins between each major vein until the final major vein is reached before the leaf margin. The ratio of major veins to minor veins in wild-type leaves is thus 1:3.3.
Fig. 1.
(A) Hand-cut and cleared transverse section through a wild-type rice leaf, showing the midrib (MR) centrally placed. (B) Enlarged transverse section of rice leaf showing detail of the internal leaf anatomy and different tissues: BC, Bulliform cells; LV, large vein; M, mesophyll cells forming the chlorenchyma; SV, small vein. (C) Arrows showing how leaf thickness, width of small and large veins, and interveinal distance were measured. Bars, 100 µm.
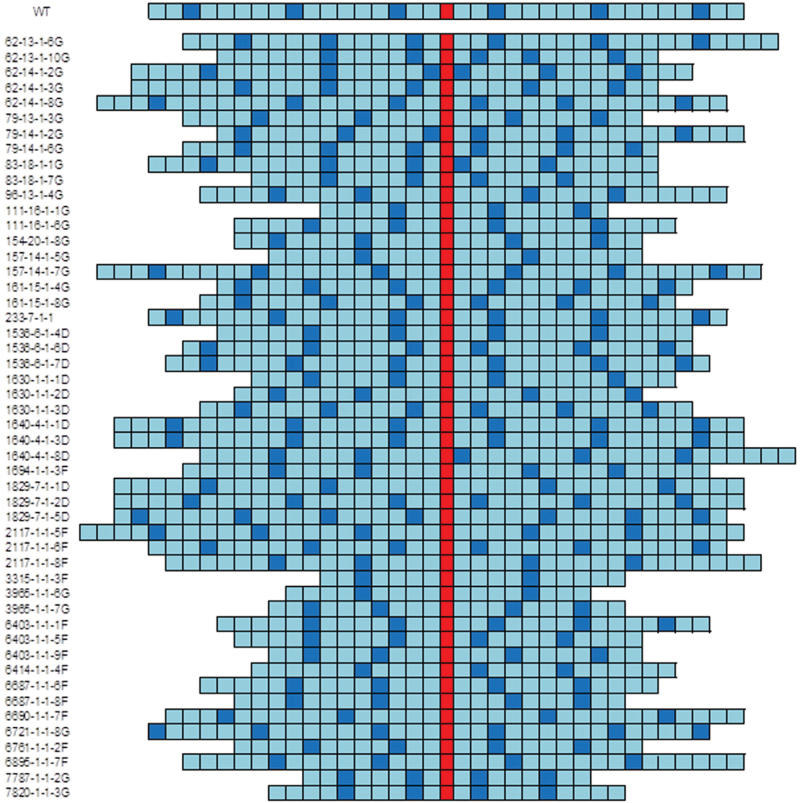
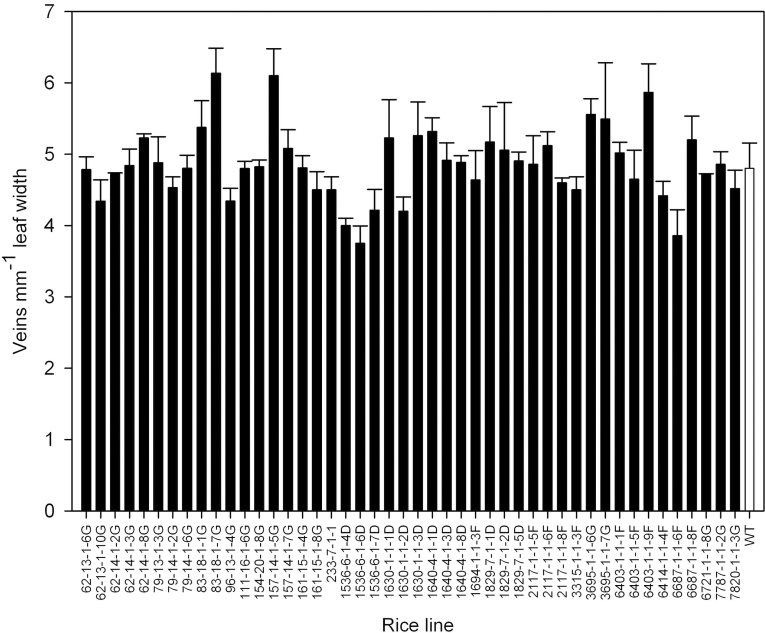
Transverse sections of leaf 6 were taken at the widest part and the vascular patterning within each leaf was determined in order to establish the extent of variation in vascular patterning within the mutant population. The variation in patterning among the mutants is shown schematically in Fig. 2 and was extensive with differences in the number of major and minor veins present as well as their relative positioning. In some mutant lines, major veins were produced immediately adjacent to the midrib rather than being separated by one or more minor veins, as seen in wild-type leaves. Interestingly, major veins were never produced immediately adjacent to each other in any of these mutant lines (Fig. 2). Some lines showed large differences in the ratio of minor to major veins, for example mutant 3315-1-1-3F had a major/minor vein ratio of 1:7.5 (Fig. 2). Many mutant lines displayed an increased asymmetry of vascular patterning about the midrib and in some mutant lines the midrib was not present in the centre of the leaf. The variation in both leaf width and the total number of veins present across the leaf was used to calculate average vein density (Fig. 3). In the rice mutant population, total vein density varied from 3.7–6.1 veins (mm leaf width)–1, compared to a density of 4.8 veins (mm leaf width)–1 in wild-type rice leaves.
Fig. 2.
Variation in leaf vascular patterning in all wild-type and mutant rice lines analysed in this study. Only sequence and number of veins are shown: not to scale. The midrib is red, major veins are dark blue, and the minor veins are light blue. A large degree of variation was visible between mutant lines in the patterning and number of veins across the leaf width and the degree of asymmetry of major and minor veins in relation to the midrib.
Fig. 3.
Variation in vein density in leaf 6 in a population of rice mutants compared to wild-type rice leaves (WT). Error bars are standard errors (n = 3).
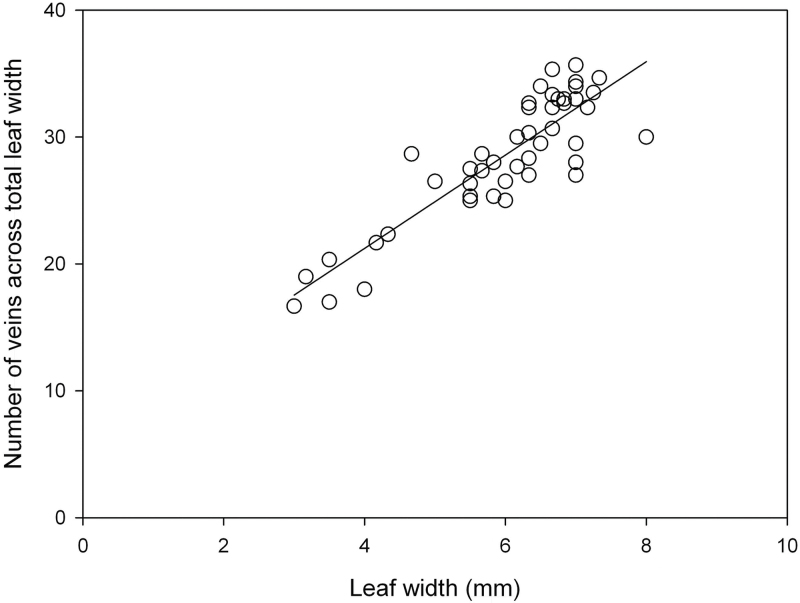
This study examined possible allometric relationships that could explain this variation: for example could the relationship between cell size and leaf width be a factor? A small change in mesophyll cell size alone could result in narrow leaves with high vein density. Fig. 4 shows a significant correlation between vein number and leaf width, showing that the vein density itself may be fairly conserved. The distance between each adjacent vein was then measured, this time distinguishing between the major and minor veins (Supplementary Fig. S1, available at JXB online). The average distance between two adjacent minor veins in leaves of the mutant population ranged from 91.2 to 196.6 µm, and in the wild type a mean ± SE of 152.4 ± 7.7 µm. The distance between adjacent major veins and minor veins ranged from 105 to 205 µm, and in the wild type a mean ± SE of 146 ± 13.8 µm. Three lines had significantly altered vein spacing (P < 0.05) (Table 1).
Fig. 4.
The relationship between the total number of veins present across a rice leaf and the leaf width for leaves from a population of rice mutants and wild type. r 2 = 0.76; n = 47; P < 0.01.
Table 1.
Anatomical analysis of WT type rice leaves and six alm rice mutants, with altered leaf morphologies
| Line | Number | Mutagen | Mean interveinal minor vein distance (▪m) | Mean minor vein width (▪m) | Mean major vein width (▪m) | Leaf thickness at major vein (▪m) |
| Wild type | IR64 | – | 152.4 ± 7.7 | 41.9 ± 1.8 | 104.6 ± 8.7 | 133.5 ± 8.8 |
| alm1 | 83-18-1-7G | Gamma | 116.8 ± 6.0* | 35.5 ± 0.4* | 74.3 ± 3.4 | 89.6 ± 7.9 |
| alm2 | 111-16-1-1G | Gamma | 135.0 ± 28.4 | 72.1 ± 30.1 | 91.8 ± 4.9 | 138.2 ± 1.1 |
| alm3 | 233-7-1-1D | DEB | 171.3 ± 7.7 | 45.2 ± 1.5 | 104.0 ± 4.0 | 173.7 ± 3.8* |
| alm4 | 1536-6-1-6D | DEB | 152.2 ± 5.7 | 51.1 ± 1.6* | 108.3 ± 0.8 | 191.5 ± 2.4* |
| alm5 | 3965-1-1-6G | Gamma | 113.8 ± 8.0* | 49.4 ± 2.0* | 99.1 ± 10.2 | 138.9 ± 6.0 |
| alm6 | 3965-1-1-7G | Gamma | 91.2 ± 6.9* | 57.3 ± 14.9 | 86.1 ± 6.4 | 142.9 ± 7.1 |
Values are mean ± SD, (n = 5).
*Anatomical features significantly different to wild type: P < 0.05. DEB, Diepoxybutane.
Leaf thickness was also measured at the major vein position, the minor veins, and the bulliform cells (Supplementary Fig. S2). No significant variation in minor vein or bulliform cell thickness was identified amongst the mutant population or wild-type leaves. However, two lines produced leaves which were significantly thicker at the major vein (P < 0.01): 233-7-1-1D and 1536-6-1-6D (Table 1).
Altered leaf morphology
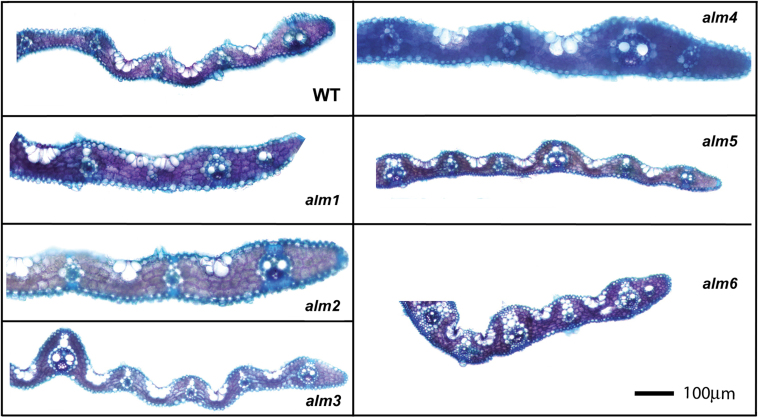
Six mutant rice lines that showed greatest significant differences compared to wild type in a number of morphological parameters were selected for further examination and designated as altered leaf morphology (alm) mutants (Fig. 5, Table 1). These phenotypes were shown to be heritable through three generations in this study, whilst maintaining the same gross morphology through four generations during pre-screening at IRRI. Three of these alm mutants had significantly altered minor vein spacing alm1 (P < 0.05), alm5 (P < 0.05), and alm6 (P < 0.01) compared to wild type. Leaves of alm 1 had minor veins which were significantly narrower than WT (P < 0.05). alm5 produced minor veins which were significantly wider than the wild type (P < 0.05), and both alm1 and alm5 displayed an altered ‘gross’ leaf morphology; leaves were narrower with a greatly reduced midrib compared to wild type. alm2 was also identified as having an altered gross leaf morphology: although the sequence of major–minor veins was similar to wild type, the midrib itself was positioned off centre such that the numbers of veins on either side of the midrib was different. Leaves of alm3 and alm4 were significantly thicker at the major vein than wild-type leaves (P < 0.01).
Fig. 5.
Cleared, leaf transverse sections for wild type (WT) and alm1–6 mutants of rice and wild type (WT). These are representative examples only; full statistical analysis is shown in table 1. Bar, 100 µm.
The individual mesophyll cells which comprise the chlorenchyma in rice leaves are relatively small when compared to other grass species: e.g. they are approximately half the size of those of wheat mesophyll cells (data not shown). Rice mesophyll cells are extensively lobed along their longest axis, in a similar way to mesophyll cells from other cereal leaves. Rice mesophyll cells have a length to width ratio of approximately 2:1 and are orientated such that the long axis of the cell is parallel to the leaf surface. When observed in transverse leaf sections, they appear in rows between the vascular bundles and are densely packed, with relatively few airspaces visible between them (Fig. 1). The bulliform cells are present on the adaxial side of the leaf, interrupting the rows of mesophyll cells between successive veins (Fig. 1B).
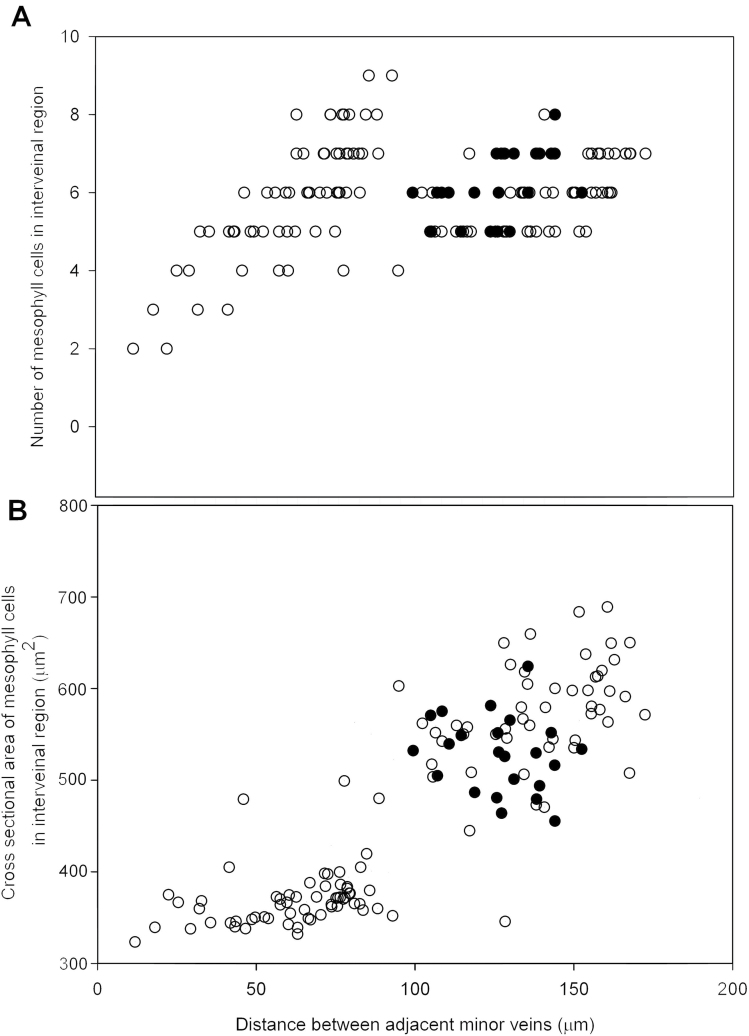
Since vein spacing and a reduced number of interveinal mesophyll cells is considered an important consistent aspect of Kranz anatomy in C4 leaves (Sakaguchi and Fukuda, 2008), the relationship between variation in vein spacing in these alm mutants and the nature of the intervening mesophyll cells was determined. Counts of mesophyll cell number in interveinal regions of minor veins from transverse leaf sections showed a poor correlation with veinal spacing in the leaves of alm mutants and wild type (Fig. 6A), with most interveinal regions containing between four and eight mesophyll cells.
Fig. 6.
(A) The relationship between interveinal distance between adjacent minor veins in leaves of wild type (closed circles) and alm mutants (open circles) and the number of intervening mesophyll cells. r 2 = 0.07; n = 158; P > 0.05. (B) The relationship between interveinal distance in leaves of wild-type rice (closed circles) and alm mutants (open circles) and average plan area of mesophyll cells measured in isolated cell preparations. r 2 = 0.7; n = 158; P < 0.001.
To investigate how mesophyll cell size relates to interveinal distance of minor veins, isolated cell preparations were made from alm mutant leaves and wild-type leaves and the plan area of individual mesophyll cells in preparations were measured. Interveinal distance and mesophyll cell size (Fig. 6B) shows a significant correlation(r 2 = 0.70, P < 0.001) suggesting that most of the variation in interveinal distance shown by these rice mutants was a result of differences in the extent of mesophyll cell expansion rather than changes in the extent of cell division and cell number.
Discussion
Improvements of yield per hectare in the world’s major crop species will need to be underpinned by increased leaf photosynthetic rate (Murchie et al., 2009; Zhu et al., 2010). Whilst the basic processes in model species can be identified, these processes need to be applied to crop species where knowledge is limited and may be species-specific. Leaf morphology underlies many photosynthetic traits and can limit nitrogen content, surface area, and resistance for gas exchange. In the case of C4 plants, morphology is central to the CO2 concentration mechanism that permits superior crop biomass production in warm environments (Hibberd et al., 2008). This study seeks variation in an existing rice mutant population for leaf morphology features relevant to photosynthetic capacity, in particular those typical of C4 anatomy. It is shown that ,while there is substantial variation in vein density, it was driven by a change in mesophyll cell size.
Is there plasticity in the rice mutant population for key traits?
The lines alm5 and alm6 provide some encouragement for the prospects of introduction of a Kranz-type anatomy by showing that vascular density can be increased. However, the reduction in interveinal distance was shown to be a function of cell size rather than a reduction in cell number. Therefore, screening on the basis of vein density alone is insufficient and it must be considered a pre-screen to more in-depth morphological analysis, although this study represents a small number of mutants that were pre-selected on the basis of leaf shape (see Materials and methods). The full deletion mutant collection includes around 60,000 lines (Wu et al., 2005). It would be expected that, in order to reveal mutants that possessed a reduced number of mesophyll cells, a larger screen is needed and indeed this is being carried out at IRRI.
Screening of wild relatives of rice at IRRI has revealed species with an interveinal mesophyll cell number as low as five. Whilst an interveinal mesophyll cell number of five is still greater than the two cells typically displayed in Kranz anatomy plants such as maize or sorghum, these do provide a potentially important step. Rice lines displaying a reduction in interveinal mesophyll cell number also draw comparison with species of the Flaveria genus, which is known to contain seven C4 species, four C3 species, and a number of C3-C4 intermediates (McKown et al., 2005). F. robusta, the closest relative to the C4 Flaveria species, possesses a closer vein spacing than is typically observed in C3 plants. The same was also true of the C3 plant Cleome sparsifolia, a close relative of the C4 plant Cleome gynandra (Sanchez-Acebo, 2005). It was therefore suggested by Sage and Sage (2008) that an initial increase in vein density plays an essential role in C4 preconditioning, even if not a typical Kranz anatomy.
However, the narrow range of interveinal mesophyll cell numbers observed in screened populations to date suggests that reorganization of the rice vascular and mesophyll cell arrangement in order to resemble that of C4 plants with Kranz anatomy still represents a significant challenge. None of the genes controlling C4 leaf anatomy have been identified (Kajala et al., 2011), even though detailed studies of vascular and cell differentiation have been done in the C4 plant maize, in which signals emanating from the vasculature are hypothesized to control the subsequent differentiation of the surround parenchyma cells (Langdale et al., 1988; Langdale and Nelson, 1991; Nelson, 2011).
As the genes directly responsible for the control of Kranz anatomy have not been identified, it is therefore impossible here to infer the likely impact on vein spacing within each mutant line. The mutational agents employed here produce predictable genetic lesions with DEB producing multiple small deletions and point mutations, and gamma radiation resulting in larger deletions in the range of 1kb and point mutations (Wu et al., 2005). Multiple genes may therefore be responsible for the phenotypes shown.
Leaf development
In the development of the rice leaf (Itoh et al., 2005), vascular arrangement is determined at an early stage: differentiation of vascular tissue may first be observed in the P2 leaf primordium, which therefore suggests that the altered morphologies displayed by plants of the alm1, alm5, and alm6 lines are the result of changes during this stage of development. The expression of OsPNH1 precedes the morphological development of vascular tissue by approximately one plastochron (Nishimura et al., 2002) and OsPNH1 is strongly expressed in the pro-vascular regions. In plants expressing an antisense transcript of OsPNH1, vascular development is incomplete and a disordered arrangement of vascular bundles within the leaf primordium results, suggesting that OsPNH1 plays a significant role in leaf vascular development. OsPNH1 antisense plants displayed vascular defects only in the leaves and not in the stem or root tissue, suggesting that OsPNH1 functions only in leaves. The molecular function of OsPNH1 is not known.
Interveinal spacing in the alm lines was shown to be correlated with mesophyll cell size, suggesting that the reductions in interveinal spacing were not due to changes in OsPNH1 expression as the relative position of vascular bundles was unchanged. Since vein spacing appears to be primarily regulated by individual mesophyll cell size rather than the number of mesophyll cells within this region, it is possible that the production of a Kranz-type anatomy displaying a low interveinal mesophyll cell number will require alteration in the localization of OsPNH1 expression during production of the P1 leaf primordium.
Conserved anatomical features of the rice leaf
Mesophyll cells of the rice leaf were consistently small when compared to those of other crop species (Chonan, 1970; Burundukova et al., 2003). They are also highly lobed and the combination of these factors appears to result in 97% if the mesophyll cell surface area being covered by chloroplasts (Sage and Sage, 2009), which is much higher than the 70% cover seen in wheat (Ellis and Leech, 1985). It is thought that this high level of chloroplast cover has evolved in rice as a response to the high photorespiratory potential to which it is typically subject as a result of high irradiance, high temperatures, and high photosynthetic capacity. This is thought to aid photorespiratory CO2 scavenging and/or maximization of CO2diffusion into the chloroplast stroma (Evans and von Caemmerer, 1996; Flexas et al., 2008).
None of the rice lines observed during the mutant screen displayed greatly enlarged mesophyll cells, and the fact that small mesophyll cell size and deep lobing of the cells were demonstrated in every plant of the alm lines and the wild-type rice plants suggests that these characteristics are highly conserved in rice. This strong adaptation to minimization of and recovery from photorespiration could explain why the C4 photosynthetic pathway has never evolved in the Oryza genus of grasses.
This high degree of specialization in minimization of photorespiratory impact would need to be overcome in any C4 engineering project. The small mesophyll cell size and high degree of lobing displayed by rice are not consistent with C4 grasses (Chonan, 1970), and mesophyll cells within the typical Kranz anatomy are much larger than those of rice. Therefore an understanding of the genetic and physiological factors that determine mesophyll cell size will be critical.
This study carried out an analysis of morphological variation in a subset of the IR64 deletion mutant population and showed significant variation in vein density, leaf thickness, and cell size. The close relationship shown here between mesophyll cell size and vein density should be viewed as an important factor in the development of large-scale screens for mutants with altered mesophyll cell number. The narrow leaved lines demonstrated a higher likelihood of increased vein density. The small size of rice mesophyll cells, in comparison to other species, was highly conserved and understanding the regulatory factors of this feature in rice should be a priority.
Acknowledgments
Supplementary material
Supplementary materials are available at JXB online.
Supplementary Fig. S1. The mean interveinal distance between adjacent minor veins and adjacent minor and major veins for a population of rice leaf mutants and wild type
Supplementary Fig. S2. The thickness of rice leaves from mutant and wild-type plants measured at the major vein and bulliform cells.
Acknowledgements
This work was funded by a BBSRC studentship awarded to IS. The authors thank Hei Leung and his team for supplying the mutant lines, Hei Leung, Paul Quick, and all the C4 rice team both at IRRI and internationally for advice and useful discussions, GemaVizcay-Barrena (Nottingham) for technical help.
References
- Brown WV. Variations in anatomy, associations, and origins of Kranz tissue. American Journal of Botany. 1975;62:395–402. [Google Scholar]
- Burundukova OL, Zhuravlev YN, Solopov NV, P’yankov VI. A method for calculating the volume and surface area in rice mesophyll cells. Russian Journal of Plant Physiology. 2003;50:133–139. [Google Scholar]
- Chonan N. Studies on the photosynthetic tissues in the leaves of cereal crops. V. Comparison of the mesophyll structure among seedling leaves of cereal crops. Proceedings of the Crop Science Society of Japan. 1970;39:418–425. [Google Scholar]
- Dawe D. Agricultural research, poverty alleviation and key trends in Asia’s rice economy. In: Sheehy JE, Mitchell PL, Hardy B, editors. Charting new pathways to C4 rice. Los Banos, Philippines: International Rice Research Institute; 2007. pp. 37–53. [Google Scholar]
- Edwards GE, Walker DA. C3, C4: Mechanism and cellular and environmental regulation of photosynthesis. Oxford: Blackwell Scientific Publications; 1983. [Google Scholar]
- Ellis JR, Leech RM. Cell size and chloroplast size in relation to chloroplast replication in light-grown wheat leaves. Planta. 1985;165:120–125. doi: 10.1007/BF00392220. [DOI] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S. Carbon dioxide diffusion inside leaves. Plant Physiology. 1996;110:339–346. doi: 10.1104/pp.110.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmés J, Medrano H. Mesophyll conductance to CO2 current knowledge and future prospects. Plant, Cell and Environment. 2008;31:602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Furbank RT, von Caemmerer S, Sheehy J, Edwards GE. C-4 rice: a challenge for plant phenomics. Functional Plant Biology. 2009;36:845–856. doi: 10.1071/FP09185. [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Covshoff S. The regulation of gene expression required for C4 photosynthesis. Annual Review of Plant Biology. 2010;61:181–207. doi: 10.1146/annurev-arplant-042809-112238. [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA. Using C4 photosynthesis to increase the yield of rice–rationale and feasibility. Current Opinion in Plant Biology. 2008;11:228–231. doi: 10.1016/j.pbi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y. Rice plant development: from zygote to spikelet. Plant Cell Physiology. 2005;46:23–47. doi: 10.1093/pcp/pci501. [DOI] [PubMed] [Google Scholar]
- Kajala K, Covshoff S, Karki S, et al. Strategies for engineering a two-celled C4 photosynthetic pathway into rice. Journal of Experimental Botany. 2011;62:3001–3010. doi: 10.1093/jxb/err022. [DOI] [PubMed] [Google Scholar]
- Kinsman EA, Pyke KA. Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development. 1998;125:1815–1822. doi: 10.1242/dev.125.10.1815. [DOI] [PubMed] [Google Scholar]
- Langdale JA, Nelson T. Spatial regulation of photosynthetic development in C4 plants. Trends in Genetics. 1991;7:191–196. doi: 10.1016/0168-9525(91)90435-s. [DOI] [PubMed] [Google Scholar]
- Langdale JA, Zelitch I, Miller E, Nelson T. Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO Journal. 1988;7:3643–3651. doi: 10.1002/j.1460-2075.1988.tb03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. American Journal of Botany. 2005;92:1911–1928. doi: 10.3732/ajb.92.11.1911. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Sheehy J. Supercharging rice photosynthesis to increase yield. New Phytologist. 2006;171:688–693. doi: 10.1111/j.1469-8137.2006.01855.x. [DOI] [PubMed] [Google Scholar]
- Muhaidat R, Sage RF, Dengler NG. Diversity of Kranz anatomy and biochemistry in C-4 eudicots. American Journal of Botany. 2007;94:362–381. doi: 10.3732/ajb.94.3.362. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Hubbart S, Peng S, Horton P. Acclimation of photosynthesis to high irradiance in rice: gene expression and interactions with leaf development. Journal of Experimental Botany. 2005;56:449–460. doi: 10.1093/jxb/eri100. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Pinto M, Horton P. Agriculture and the new challenges for photosynthesis research. New Phytologist. 2009;181:532–552. doi: 10.1111/j.1469-8137.2008.02705.x. [DOI] [PubMed] [Google Scholar]
- Nelson T. The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C4 leaves. Journal of Experimental Botany. 2011;62:3039–3048. doi: 10.1093/jxb/err072. [DOI] [PubMed] [Google Scholar]
- Nelson T, Langdale JA. Developmental genetics of C4 photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1992;43:25–47. [Google Scholar]
- Nishimura A, Ito M, Kamiya N, Sato Y, Matsuoka M. OsPNH1 regulates leaf development and maintenance of the shoot apical meristem in rice. The Plant Journal. 2002;30:189–201. doi: 10.1046/j.1365-313x.2002.01279.x. [DOI] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. The control of chloroplast number in wheat mesophyll cells. Planta. 1987;170:416–420. doi: 10.1007/BF00395035. [DOI] [PubMed] [Google Scholar]
- Sage RF. Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biology. 2001;3:202–213. [Google Scholar]
- Sage R, Sage TL. Learning from nature to develop strategies for the directed evolution of C4 rice. In: Sheehy JE, Mitchell PL, Hardy B, editors. Charting pathways to C4 rice. Los Banos, Philippines: International Rice Research Institute; 2008. pp. 195–216. [Google Scholar]
- Sage TL, Sage RF. The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant and Cell Physiology. 2009;50:756–772. doi: 10.1093/pcp/pcp033. [DOI] [PubMed] [Google Scholar]
- Sakaguchi J, Fukuda H. Cell differentiation in the longitudinal veins and formation of commissural veins in rice (Oryza sativa) and maize (Zea mays) Journal of Plant Research. 2008;121:593–602. doi: 10.1007/s10265-008-0189-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Acebo L. A Phylogenetic study of the new world Cleome (Brassicaceae Cleomoideae) Annals of the Missouri Botanical Garden. 2005;92:179–201. [Google Scholar]
- Scarpella E, Rueb S, Meijer A. The RADICLELESS1 gene is required for vascular pattern formation in rice. Development. 2003;130:645–658. doi: 10.1242/dev.00243. [DOI] [PubMed] [Google Scholar]
- Surridge C. Agricultural biotech: the rice squad. Nature. 2002;416:576–578. doi: 10.1038/416576a. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature. 2001;414:543–546. doi: 10.1038/35107073. [DOI] [PubMed] [Google Scholar]
- Wu J-L, Wu C, Lei C, et al. Chemical- and irradiation-induced mutants of Indica rice IR64 for forward and reverse genetics. Plant Molecular Biology. 2005;59:85–97. doi: 10.1007/s11103-004-5112-0. [DOI] [PubMed] [Google Scholar]
- Zhu X-G, Long S, Ort DR. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology. 2010;61:235–261. doi: 10.1146/annurev-arplant-042809-112206. [DOI] [PubMed] [Google Scholar]