Abstract
Skeletal muscle and kidney-enriched inositol polyphosphate phosphatase (SKIP) has previously been implicated in the regulation of insulin signaling in skeletal muscle. Here, we present the first report of the mechanisms by which SKIP specifically suppresses insulin signaling and the subsequent glucose uptake. Upon insulin stimulation, SKIP is translocated to the membrane ruffles, where it binds to the active form of Pak1, which mediates multiple protein complex formation with phosphatidylinositol 3,4,5-triphosphate (PIP3) effectors such as Akt2, PDK1, and Rac1; this leads to inactivation of these proteins. SKIP also promotes the inhibition of Rac1-dependent kinase activity and the scaffolding function of Pak1, which results in the dissociation of Akt2 and PDK1 from Pak1. Thus, specific suppression of insulin signaling is achieved via the spatiotemporal regulation of SKIP through the scaffolding function of Pak1. These interactions are the foundation of the specific and prominent role of SKIP in the regulation of insulin signaling.
INTRODUCTION
Type II diabetes mellitus is caused by impairment of the activity of insulin, which stimulates glucose transport in skeletal muscle and adipose tissues (24). Skeletal muscle is responsible for approximately 75% of all insulin-induced glucose uptake in the body. Thus, regulation of insulin signaling is critical for systemic glucose homeostasis.
Activation of insulin receptors leads to the phosphorylation of insulin receptor substrate family proteins on tyrosine residues, which are responsible for activation of the phosphatidylinositol 3-kinase (PI 3-kinase) pathway. PI 3-kinase phosphorylates phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and increases intracellular phosphatidylinositol 3,4,5-trisphosphate (PIP3) at the plasma membrane. PIP3 leads to the activation of Akt and 3-phosphoinositide-dependent protein kinase (PDK1), both of which are required for the metabolic activity of insulin (5, 19). Akt translocates from the cytosol to the plasma membrane through its binding with PIP3, where it is phosphorylated at Thr-308 and Ser-473, which is required for the full activation of Akt (25, 26). Among the 3 Akt family species (Akt1 to -3), only Akt2 is implicated in the regulation of insulin-dependent glucose homeostasis. Smaller body size has been observed among Akt1 knockout mice; on the other hand, Akt2 knockout mice exhibited metabolic defects such as insulin resistance and a diabetic phenotype (3, 7). In addition, cultured adipocytes from Akt2 knockout mice displayed significant defects in glucose uptake (36). A loss-of-function mutation in Akt2 produces insulin resistance in humans (8). Recently, identification of a de novo activating E17K mutation of Akt2, which induced human hypoglycemia, has been reported (11). Akt2 E17K mutation exhibited plasma membrane localization even under serum-deprived conditions, which led to the increase in its kinase activity (11). This mutation in the GRP1 PH domain is reported to increase its phosphoinositide-binding affinity (23). Therefore, phosphoinositides are the key regulators of Akt2 activity and blood glucose levels.
In addition to glucose uptake, insulin stimulates actin cytoskeletal rearrangements. In myoblasts, insulin activates the small GTPase Rac1 and stimulates membrane ruffle formation via PI 3-kinase signaling; both of these steps are necessary for insulin-mediated GLUT4 translocation to the plasma membrane and subsequent glucose uptake. Binding of PIP3 to the N-terminal PH domain of the Rac nucleotide exchange factor Tiam1 results in the subsequent activation of Rac1. Active Rac can then interact with the members of the p21-activated kinase 1 (Pak1) family, comprising serine/threonine protein kinases that regulate the actin cytoskeleton and cell motility (1). Under resting conditions, the N-terminal autoinhibitory region of Pak1 inhibits the kinase in trans, thus forming heterodimers (20). In the presence of extracellular stimuli, these are dissociated upon activation by the GTP-bound form of Rac1, resulting in phosphorylation of the kinase domain at Thr-422 and subsequent full activation that triggers membrane ruffle formation (21). Recently, it has been reported that Pak acts as a scaffold by binding to PDK1, Akt1, and Akt2; further, Pak regulates the localization and specificity of the PI 3-kinase-PDK1-Akt signaling pathway (10). In that model, growth factors cause activation of Rac1, which binds to the N-terminal CRIB domain of Pak and releases the C-terminal kinase domain, resulting in its association with Akt and PDK1, which appears to be independent of Pak1 kinase activity (10). The Pak1 kinase domain serves as a scaffold to aid the recruitment of Akt to the plasma membrane and its phosphorylation by PDK1. This function of Pak is under the control of upstream signals, which take place in a spatially and temporally restricted manner.
PIP3 phosphatases hydrolyze PIP3 and negatively regulate the PI 3-kinase-Akt signaling pathway. Skeletal muscle and kidney-enriched inositol polyphosphate 5-phosphatase (SKIP) is one of the PIP3 5-phosphatases that negatively regulates insulin-dependent PIP3 generation, glucose incorporation, and membrane ruffle formation in skeletal muscle (12, 14). We have recently determined that among PIP3 phosphatases, SKIP predominantly regulated insulin signaling and insulin-mediated glucose uptake (13). SKIP is localized to the endoplasmic reticulum (ER) under basal conditions and translocates to the membrane ruffles in response to insulin stimulation (9). These data indicate that membrane localization of SKIP controls the strength of insulin action through the hydrolysis of PIP3 (9). SKIP heterozygous knockout mice have higher levels of systemic glucose uptake and insulin sensitivity and are resistant to high-fat diet (HFD)-induced hyperglycemia (15). Isolated skeletal muscle from SKIP heterozygous knockout mice shows high levels of insulin-dependent glucose uptake.
In this study, we investigated the mechanisms by which SKIP negatively regulates insulin signaling and the subsequent glucose uptake. We found that Pak1 provides the site for spatiotemporal regulation of SKIP, thus mediating the specific regulation of insulin signaling.
MATERIALS AND METHODS
Materials.
SKIP antibodies raised against the C terminus were developed in our laboratory for immunoblotting. Antibodies specific for Akt, Akt1, Akt2, phospho-Akt (Ser-473), phospho-Akt (Thr-308), Pak1, Nck, AS160, phospho-AS160 (Thr-642), Bad, phospho-Bad (Ser-112), glycogen synthase kinase 3β (GSK3β), phospho-GSK3β (Ser-9), and PTEN were purchased from Cell Signaling Technology (Beverly, MA). Anti-insulin receptor β, Rac1, and PDK1 antibodies were purchased from BD Biosciences (Franklin Lakes, NJ).
Constructs.
The SKIP expression vector was generated by introducing cDNA encoding human SKIP into the p3×FLAG-CMV8 vector (Sigma-Aldrich, St. Louis, MO). The D310G, D361A, and position 318 to 448 mutants were generated by PCR and were subcloned into expression vectors. Murine Pak1 and Rac1 cDNAs were obtained by reverse transcription-PCR (RT-PCR) and subcloned into the p3×FLAG-CMV8 or pEGFP-C1 vector (BD Clontech, Franklin Lakes, NJ).
Transfection and RNA interference (RNAi).
Small interfering RNA (siRNA) duplexes were purchased from Life Technologies (Carlsbad, CA). The following siRNA oligonucleotides were used in this study: control, 5′-GAGCAACTGCGTGTCGAATCTCTTA-3′; mouse SKIP, 5′-GAGTCAACGTCTGCCTGAAGCTTTA-3′; rat SKIP, 5′-CCATGGAGCAGTTTCTTCATGGATA-3′; and mouse Pak1, 5′-CAAGGTGCTTCAGGCACAGTGTATA-3′.
Twenty nanomoles of control, SKIP, and Pak1 siRNA species was transfected into mouse C2C12 or rat L6 myoblasts. Cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) for 24 h and then were serum starved for another 24 h. Cells were stimulated with insulin (100 nM) at various times.
Immunoprecipitation.
Cells were washed twice with phosphate-buffered saline (PBS) and lysed with cell lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 5 mM NaF, 1 mM Na3VO4, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1% Triton X-100. Lysates were centrifuged at 10,000 × g for 10 min, and the supernatants were used for Western blot analysis. The supernatants were subjected to immunoprecipitation with various antibodies and 25 μl of protein A- or protein G-agarose (Thermo Fisher Scientific, Waltham, MA).
Measurement of Akt isoform phosphorylation.
C2C12 cells were cultured in 60-mm plates in DMEM supplemented with 10% fetal bovine serum at 37°C in 5% CO2; they were then treated with insulin (0 to 100 nM) at 37°C for 10 min. The cells were washed twice with Tris-buffered saline and then lysed for 10 min in a lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1% Triton X-100, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Lysates were centrifuged after brief sonication. The supernatants were used for Western blotting and immunoprecipitation. Lysates were immunoprecipitated with Akt1 or Akt2 antibody for 1 h at 4°C. The precipitates were washed with wash buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, and 1 mM PMSF) 5 times and then used for Western blot analysis. Densitometry was used to quantify protein levels.
Glucose incorporation analysis.
[3H]2-deoxyglucose and [14C]mannitol (GE Healthcare, Fairfield, CT) were used to measure glucose incorporation into the L6 myoblasts as described previously (11). Cells were stimulated with insulin (10 nM) for 60 min.
PIP3 phosphatase assay.
Recombinant SKIP protein (25 μM) in the presence or absence of recombinant Pak1 (25 μM) was incubated with PIP3-C8 (Echelon Co. Ltd.) for 10 min at 37°C. The malachite green assay was used to quantify released phosphate.
In vitro binding assay.
Recombinant SKIP and Pak1 proteins were purified from Escherichia coli. Approximately 10 μg of immobilized glutathione S-transferase (GST)-conjugated proteins was incubated with 20 μg of recombinant proteins for 2 h at 4°C in binding buffer (50 mM Tris-HCl [pH 7.0], 150 mM NaCl, and 2 mM EDTA).
Immunofluorescence.
Twenty-four hours after infection with plasmids, C2C12 cells were serum starved with serum-free medium for 24 h and then stimulated with insulin (100 nM). Cells were fixed with 3.7% formaldehyde and then visualized by immunofluorescence. The cells were permeabilized with PBS containing 0.2% Triton X-100 for 10 min and incubated with 1% FBS in PBS for 30 min to block nonspecific antibody binding. Finally, the cells were immunostained with the first antibody for 1 h at room temperature and then incubated with fluorescein-labeled secondary antibodies. F-actin was visualized with Alexa Fluor 647-labeled phalloidin (Life Technologies). After a brief wash with PBS, coverslips were mounted onto slides by using PermaFluor mountant medium (Thermo Fisher Scientific), and the cells were observed under a FluoView 1000-D confocal microscope (IX81; Olympus) equipped with 473-, 568-, and 633-nm diode lasers (Olympus) through an objective lens (60-Å oil immersion objective; numerical aperture [NA], 1.35; Olympus) and with Fluoview software (Olympus). Acquired images were processed with Photoshop (Adobe).
Preparation of the active GTP and inactive GDP forms of Rac1 on the affinity column.
Conversion of purified GST-Rac1 protein to the active GTP conformation was performed by the nucleotide exchange method as described previously (4). Immobilized GST-Rac1 combined with GDP immobilized to glutathione-Sepharose 4B beads was washed with nucleotide exchange buffer containing 20 mM HEPES (pH 7.5), 100 mM NaCl, 10 mM EDTA, 5 mM MgCl2, 1 mM DTT, and 10 mM GTPγS [guanosine 5′-O-(3-thiotriphosphate)] or GDP and further incubated for 30 min at room temperature in nucleotide exchange buffer containing 1 mM GTPγS or GDP. Then, to stabilize GTPγS-Rac1 and remove EDTA, beads were washed with nucleotide stabilization buffer containing 20 mM HEPES (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 1 mM GTPγS or GDP for 20 min.
Pak1 kinase activity assay.
The in vitro kinase activity of Pak1 was measured using GST-Bad (amino acids [aa] 105 to 141, S136A) mutant protein as a substrate protein. The substrate protein was incubated with Pak1 or the kinase domain of Pak1 (Pak1 aa 247 to 545) in kinase buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 30 mM 2-mercaptoethanol, and 0.2 mM ATP) at 37°C for 30 min. Phosphorylation of the substrate protein was detected by immunoblotting with anti-phospho-Bad (Ser-112) antibody.
Statistical analysis.
Prior to analysis, we visualized all data to ensure that they were normally distributed. Differences between treatments were investigated using Student's t tests. All values are listed as the means ± standard errors of the means (SEMs) or means ± standard deviations (SDs).
RESULTS
SKIP regulates phosphorylation of Akt2 but not of Akt1.
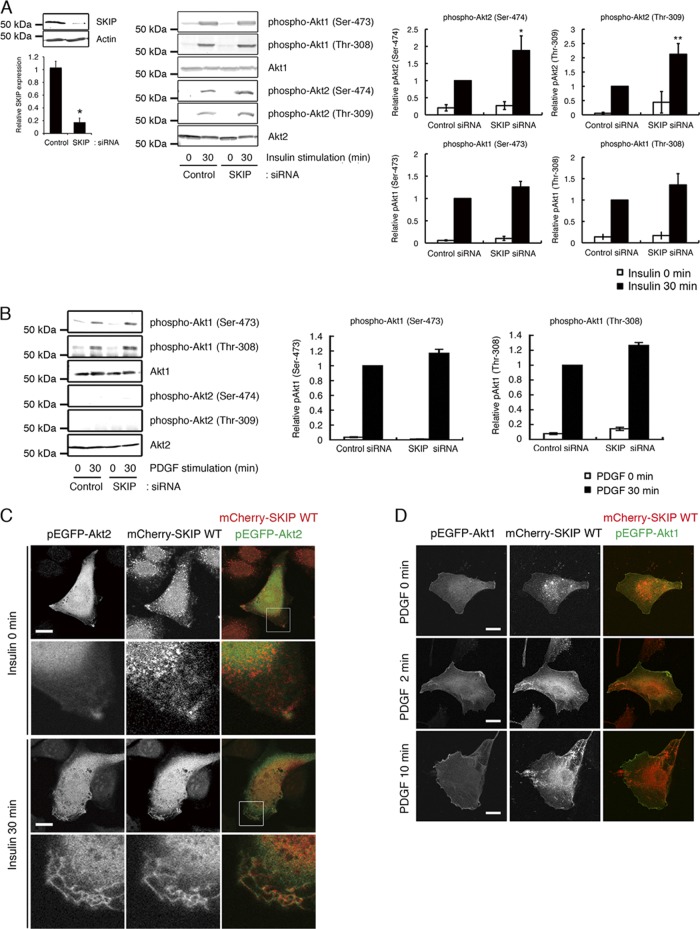
To explore the mechanisms by which SKIP regulates insulin signaling, we first examined the effect of SKIP on insulin-induced phosphorylation of Akt1 and Akt2 in C2C12 cells. Insulin stimulation caused phosphorylation of Akt1 at Ser-473 and Thr-308 and of Akt2 at Ser-474 and Thr-309. Among the Akt species, activation of Akt2 is necessary and sufficient for insulin-dependent glucose uptake. Knockdown of SKIP significantly increased phosphorylation of Akt2, but not of Akt1, in response to insulin, implying that SKIP predominantly regulates insulin-induced glucose uptake (Fig. 1A). Platelet-derived growth factor (PDGF) treatment activated Akt1 but did not induce phosphorylation of Akt2 to any significant extent; further, these patterns were not altered by the silencing of SKIP (Fig. 1B). Thus, it appears that SKIP regulates phosphorylation of Akt2 but not of Akt1. SKIP localization differed markedly in insulin- and PDGF-treated cells. Localization of mCherry-SKIP could be observed throughout the endoplasmic reticulum (ER) under resting conditions. Upon insulin treatment, SKIP was translocated to the plasma membrane, in particular to the membrane ruffles, where colocalization of SKIP and Akt2 could be observed (Fig. 1C). In contrast, PDGF stimulation did not trigger the relocation of SKIP to the plasma membrane, where GFP-Akt1 was concentrated (Fig. 1D).
Fig 1.
Negative regulation of insulin-dependent Akt2 phosphorylation by SKIP. (A) Effect of SKIP silencing on the phosphorylation of Akt1 at Ser-473 and Thr-308 and of Akt2 at Ser-474 and Thr-309 after insulin stimulation for 30 min. Values shown are normalized to total Akt1 or Akt2. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05; **, P < 0.01. (B) Effect of SKIP knockdown on PDGF-mediated phosphorylation of Akt1 in C2C12 cells. C2C12 cells transfected with control or SKIP siRNA were stimulated with PDGF for the indicated times. PDGF-induced phosphorylation of Akt2 was not detected. Results are presented as the means ± SDs of 3 independent experiments. (C) Insulin-dependent colocalization of SKIP with Akt2 at the membrane ruffles in C2C12 cells is shown in the upper panel. The scale bar indicates 10 μm. Enlarged images of boxed areas in the upper panel are shown in the lower panel. Yellow indicates regions of colocalization. (D) Lack of SKIP mobilization to the membrane ruffles in response to PDGF stimulation. C2C12 cells were transfected with EGFP-Akt1 and mCherry-SKIP WT for 48 h. Cells were stimulated with PDGF for the indicated times. The scale bar indicates 20 μm.
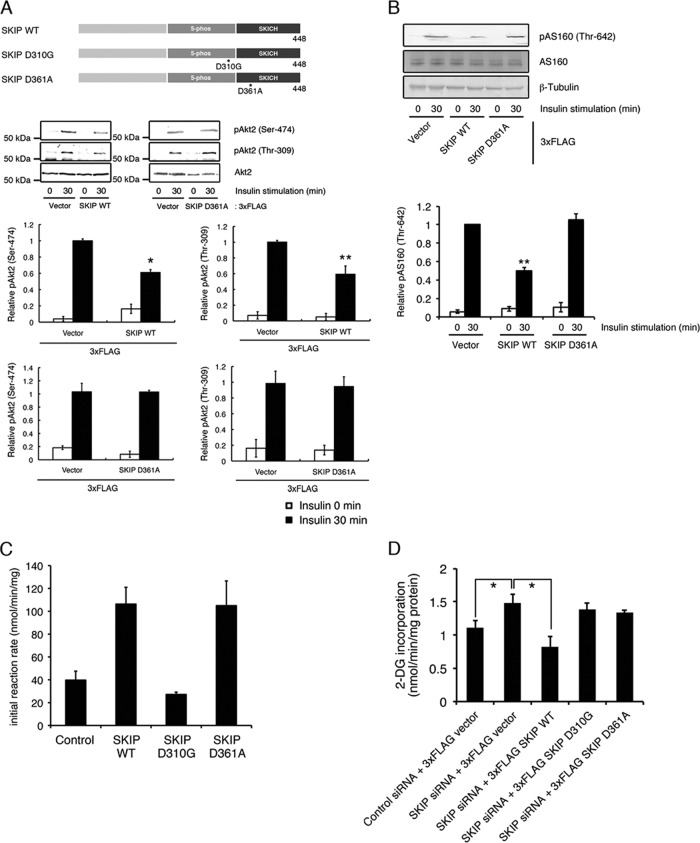
We next examined whether this membrane translocation is necessary for the regulation of insulin signaling. The SKIP C-terminal homology (SKICH) domain of SKIP has been reported to mediate its localization to the membrane ruffles (9). The SKIP D361A mutant, which has a mutation in a conserved aromatic residue within the SKICH domain, does not undergo insulin-mediated membrane localization (9). We found that expression of this mutant did not affect insulin-dependent Akt2 phosphorylation and the subsequent AS160 phosphorylation (Fig. 2A and B), although it possessed PIP3 phosphatase activity (Fig. 2C). Insulin-dependent glucose uptake into L6 cells was increased by the silencing of SKIP (13). Further expression of wild-type SKIP (SKIP WT), but not of the D361A mutant, showed a marked decrease in glucose incorporation (Fig. 2D). These results demonstrate that membrane localization of SKIP is necessary for the regulation of insulin signaling.
Fig 2.
SKICH domain mutant did not inhibit insulin signaling. (A) Role of the SKIP SKICH domain in the negative regulation of insulin signaling. Shown is a schematic representation of the SKIP constructs used in this study (upper panel). Also shown is insulin-induced Akt2 phosphorylation in C2C12 cells expressing 3× FLAG-tagged SKIP wild-type (SKIP WT) and the SKICH domain mutant (SKIP D361A) (lower panels). Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05; **, P < 0.01. (B) Effect of expression of SKIP D361A mutant on insulin-induced AS160 phosphorylation. C2C12 cells expressing SKIP WT or SKIP D361A mutant were stimulated with insulin for the indicated times. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05. (C) PIP3 phosphatase activity of the SKIP D310G and the D361A mutants. Results are presented as the means ± SDs of 3 independent experiments. **, P < 0.01. (D) Effect of the expression of the SKIP mutant on insulin-induced glucose uptake in L6 cells. Cells were transfected with SKIP siRNA in combination with the indicated human SKIP expression constructs. Cells were serum deprived for 24 h, and the insulin-induced glucose uptake was measured as described in Materials and Methods. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05.
Interaction of SKIP with Pak1 mediates specific regulation of Akt2.
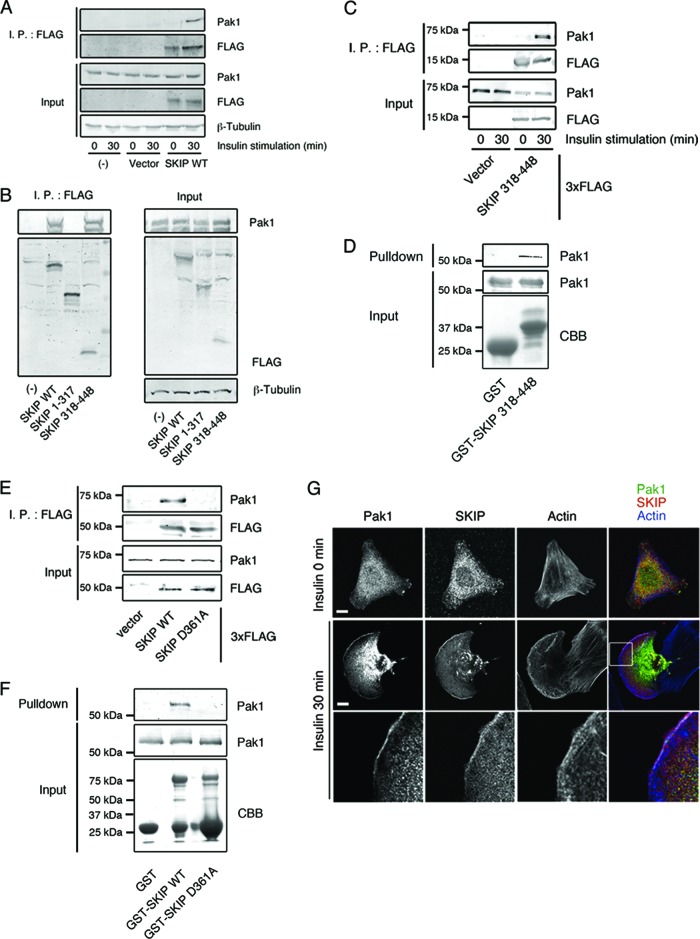
We have identified Pak1, a protein kinase that regulates membrane ruffle formation and cell motility, as a binding partner of SKIP. In C2C12 cells, an association between SKIP and Pak1 was observed only after 30 min of stimulation with insulin (Fig. 3A). Although Pak2 is also expressed in C2C12 cells, it was not found to coimmunoprecipitate with SKIP (data not shown). The SKICH domain (SKIP aa 318 to 448), not the catalytic domain (SKIP aa 1 to 317), is necessary for this insulin-dependent complex formation (Fig. 3B to D). In contrast, Pak1 did not form a complex with the SKIP D361A mutant (Fig. 3E and F), suggesting that SKIP interaction with Pak1 is necessary for its membrane ruffle localization. In insulin-stimulated C2C12 cells, colocalization of SKIP with Pak1 at the membrane ruffles could be observed (Fig. 3G).
Fig 3.
Insulin-dependent interaction between SKIP and Pak1. (A) Coimmunoprecipitation of Pak1 and SKIP in insulin-stimulated cells. C2C12 cells transfected with 3× FLAG SKIP WT for 48 h were stimulated with insulin for 30 min. Lysates were immunoprecipitated (I.P.) with anti-FLAG antibody. (B) Interaction between Pak1 and the SKICH domain of SKIP. Shown is a schematic representation of the SKIP constructs used in this study (upper panel). C2C12 cells transfected with 3× FLAG-tagged SKIP WT, the SKIP 1–317 mutant, and the SKIP 318–448 mutant were stimulated with insulin for 30 min. Lysates were immunoprecipitated with anti-FLAG antibody. (C) Insulin-mediated interaction between Pak1 and the SKICH domain of SKIP. C2C12 cells transfected with the 3× FLAG-tagged SKIP 318–448 mutant were stimulated with insulin for the indicated times. Lysates were immunoprecipitated with anti-FLAG antibody. (D) Binding of Pak1 to the SKICH domain of SKIP. The results of an in vitro binding assay of recombinant Pak1 and GST-SKIP 318–448 protein beads are shown. (E) Interaction between Pak1 and the SKICH domain mutant of SKIP. C2C12 cells transfected with 3× FLAG-tagged SKIP WT or SKIP D361A mutant for 48 h were stimulated with insulin for 30 min. Lysates were immunoprecipitated with anti-FLAG antibody. (F) The SKICH domain mutant does not bind to Pak1. The results of the in vitro binding assay between recombinant Pak1 and GST-tagged SKIP WT or D361A mutant are shown. (G) Immunofluorescence of endogenous SKIP and Pak1 in C2C12 cells. F-actin was visualized with Alexa Fluor 647-labeled phalloidin. Enlarged images of boxed areas are shown. White indicates regions of colocalization of SKIP, Pak1, and F-actin. Scale bar, 20 μm.
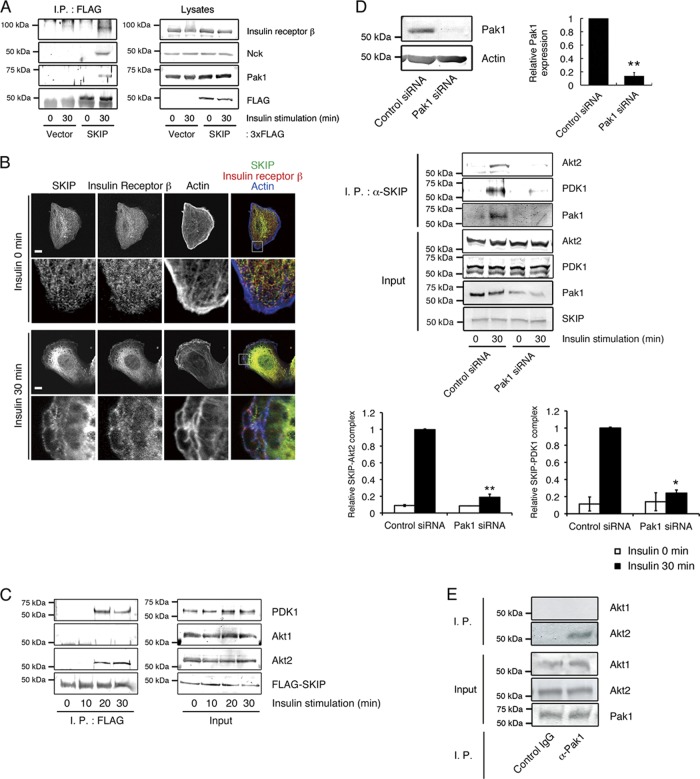
It has been reported that Pak1 mediates the formation of a protein complex including PDK1, Akt1, and Akt2 as well as those including insulin receptor substrate 1 (IRS-1) and the insulin receptor. Therefore, we have examined whether SKIP is recruited to these protein complexes. In cells treated with insulin, SKIP formed a protein complex with insulin receptor β and the adaptor protein Nck (Fig. 4A), the latter of which works to recruit Pak1 to the insulin receptor complex at the plasma membrane (30). Colocalization of SKIP and insulin receptor β was observed at the membrane ruffles of insulin-stimulated C2C12 cells (Fig. 4B). These results elucidate the process by which SKIP is recruited to the insulin receptor complex. In C2C12 cells, SKIP did not form a protein complex with Pak1 and Akt2 in the absence of insulin stimulation or after insulin stimulation for 10 min, at which time Akt2 is already phosphorylated (Fig. 4C). Coimmunoprecipitation between 3× FLAG-tagged SKIP and both Akt2 and PDK1 started to be observed after insulin stimulation for 20 min (Fig. 4C). Endogenous SKIP did not form a protein complex with Pak1 and Akt2 in the absence of insulin stimulation. However, after 30 min of insulin stimulation, we observed an interaction between these proteins (Fig. 4D). Pak1 has been reported to form a protein complex with Akt1 and, to a lesser extent, with Akt2 in serum-stimulated NIH 3T3 fibroblasts (10). However, in C2C12 cells, endogenous Pak1 formed a complex with Akt2, but not with Akt1, in response to insulin stimulation (Fig. 4E). Attenuation of Pak1 abolished the insulin-dependent association of SKIP with Akt2 and PDK1 (Fig. 4D), demonstrating that upon insulin stimulation, Pak1 mediates formation of a multiple-protein complex composed of SKIP, Akt2, PDK1, and insulin receptor at the membrane ruffles. It has been reported that upon insulin stimulation, the insulin receptor is recruited to the detergent-insoluble lipid raft fraction (17). Therefore, we have examined whether SKIP is recruited to this lipid raft fraction in C2C12 cells. In these cells, SKIP is hardly observed in the Triton-insoluble fraction either in the presence or in the absence of insulin stimulation (data not shown). Although the amount of insulin receptor in the lipid-raft fraction increased in response to insulin, it was predominantly found in the detergent-soluble nonraft fraction. Therefore, endogenous SKIP formed a protein complex with Pak1, Akt2, and the insulin receptor in the nonraft plasma membrane fraction.
Fig 4.
Insulin-dependent formation of a SKIP-Akt2 complex. (A) Induction of protein complex formation between SKIP, insulin receptor β, and Nck by insulin stimulation. C2C12 cells were transfected with the indicated constructs and stimulated with insulin for 0 or 30 min. Lysates from these cells were immunoprecipitated (I.P.) with anti-FLAG antibody. (B) Colocalization of endogenous SKIP and insulin receptor β in insulin-stimulated C2C12 cells. Cells were visualized by confocal microscopy. F-actin was visualized with Alexa Fluor 647-labeled phalloidin. Yellow indicates regions of colocalization of SKIP and insulin receptor β; white shows regions of colocalization of SKIP, insulin receptor β, and actin. Scale bar, 20 μm. (C) Insulin-dependent formation of a SKIP-Akt2 complex. C2C12 cells were transfected with 3× FLAG-tagged SKIP WT and then stimulated with insulin for the indicated times. Lysates were immunoprecipitated with anti-FLAG antibodies. (D) Pak1 mediates complex formation between endogenous SKIP and Akt2. C2C12 cells were transfected with control or Pak1 siRNA. Relative expression of Pak1 in these cells is shown (upper panels). These cells were stimulated with insulin for 0 or 30 min. Lysates were immunoprecipitated with anti-SKIP antibody, and the immunoprecipitates were detected with anti-Akt2, -PDK1, or -Pak1 antibodies. The amounts of Akt2 and PDK1 immunoprecipitated with SKIP are shown (lower panels). Results are presented as the means ± SEMs of 5 independent experiments. *, P < 0.05; **, P < 0.01. (E) Pak1 predominantly associated with Akt2 in C2C12 cells. C2C12 cells were serum deprived for 24 h and then stimulated with insulin for the indicated times. Lysates were immunoprecipitated with anti-rabbit IgG and anti-Pak1 antibody. Immunoprecipitates were subjected to Western blot analysis.
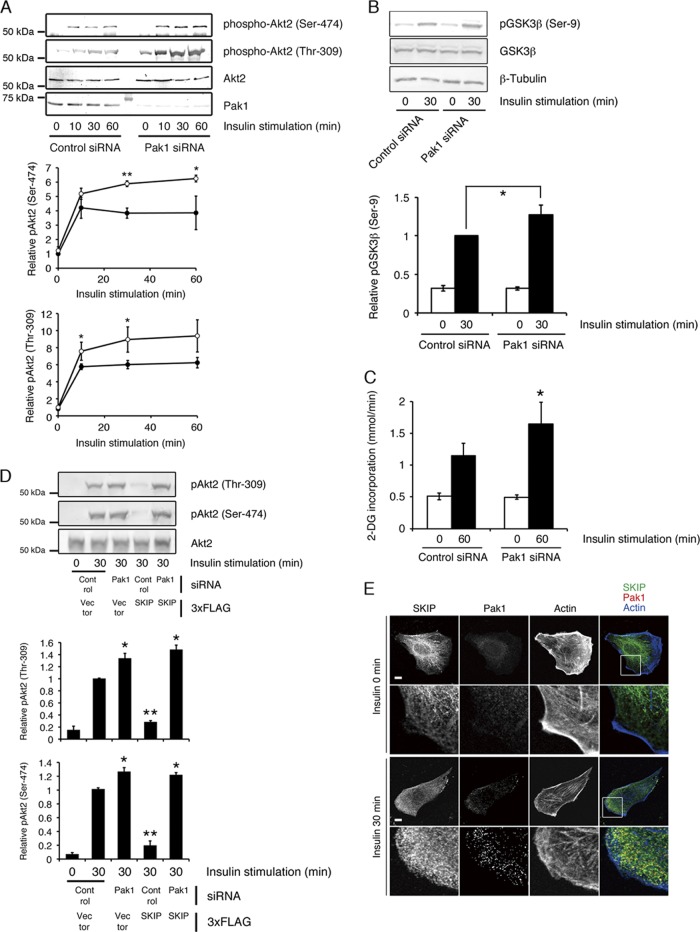
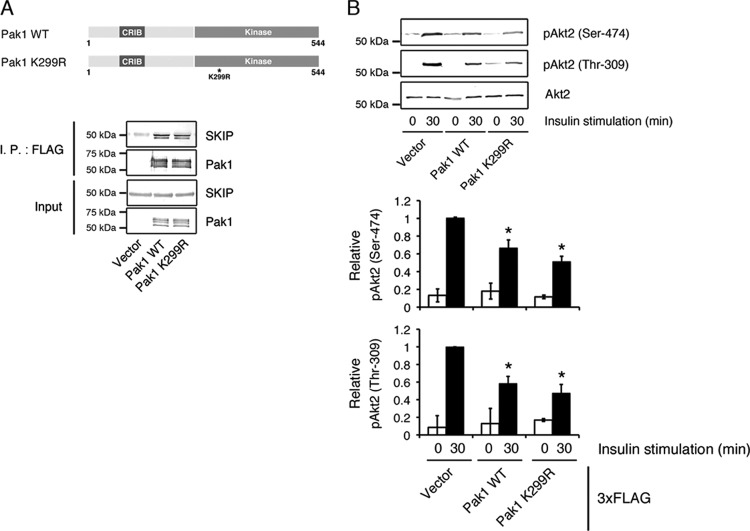
Next, we examined whether Pak1 binding is necessary for the inhibition of insulin-dependent Akt2 activity by SKIP. After 30 min of insulin stimulation, attenuation of Pak1 increased Akt2 phosphorylation at Ser-474 and Thr-309 to approximately twice the level seen in control cells (Fig. 5A). Silencing of Pak1 also increased insulin-dependent GSK3β phosphorylation and glucose uptake (Fig. 5B and C). Further transfection of 3× FLAG-tagged SKIP WT together with Pak1 siRNA failed to inhibit insulin-dependent Akt2 phosphorylation, implying that Pak1-mediated SKIP-Akt2 complex formation is necessary for the negative regulation of insulin signaling (Fig. 5D). In the absence of Pak1, almost no SKIP localized to the membrane ruffles (Fig. 5E); these data demonstrate that Pak1 mediates the negative regulation of Akt2 by SKIP. Pak1 has been reported to phosphorylate Akt2 (10); therefore, we have examined whether binding of SKIP affects kinase activity of Pak1 and subsequent phosphorylation of Akt2. Both Pak1 WT (3×FLAG-Pak1 WT) and its kinase-negative mutant (3×FLAG-Pak1 K299R) interacted with endogenous SKIP (Fig. 6A). Expression of these constructs decreased the insulin-induced phosphorylation of Akt2 (Fig. 6B) because they increased the formation of SKIP-Pak1-Akt2 complexes. Thus, negative regulation of Akt2 by SKIP appears to be mediated by a kinase-independent function of Pak1.
Fig 5.
SKIP negatively regulates Akt2 phosphorylation through Pak1. (A) Increased insulin-dependent Akt2 phosphorylation caused by Pak1 attenuation. C2C12 cells transfected with control (filled circles) or Pak1 (open circles) siRNA were stimulated with insulin for the indicated times. Results are presented as the means ± SDs of 5 independent experiments. *, P < 0.05; **, P < 0.01. (B) Silencing of Pak1 increased insulin-dependent GSK3β phosphorylation. C2C12 cells transfected with control or Pak1 siRNA were stimulated with insulin for 0 min or 30 min. Phosphorylation of GSK3β at Ser-9 was detected by Western blot analysis. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05. (C) Effect of Pak1 silencing on insulin-induced glucose uptake in L6 cells. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05. (D) Effect of SKIP expression on insulin-induced Akt phosphorylation in Pak1-silenced cells. C2C12 cells transfected with the p3×FLAG vector or SKIP WT in combination with control or Pak1 siRNA were stimulated with insulin for the indicated times. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05. (E) Inhibition of localization of SKIP at the membrane ruffle by the silencing of Pak1. C2C12 cells transfected with control or Pak1 siRNA were stimulated with insulin for 0 min or 30 min. Localization of endogenous SKIP is shown. F-actin was visualized with Alexa Fluor 647-labeled phalloidin. Scale bar, 20 μm.
Fig 6.
Effect of Pak1 kinase activity on the interaction with SKIP. (A) Association between SKIP and the WT and K299R mutant of Pak1. Shown is a schematic representation of Pak1 constructs used in this study (upper panel). Lysates from C2C12 cells expressing 3× FLAG-tagged Pak1 WT or 3× FLAG-tagged Pak1 K299R were subjected to immunoprecipitation (I.P.) with anti-FLAG antibody (lower panel). (B) Effects of 3× FLAG-tagged Pak1 WT and kinase-negative mutant (K299R) expression on insulin-induced Akt2 phosphorylation. C2C12 cells expressing 3× FLAG-tagged Pak1 WT or K299R were stimulated with insulin for the indicated times. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05.
Complex formation of SKIP with Pak1 and Rac1 mediates termination of insulin signaling.
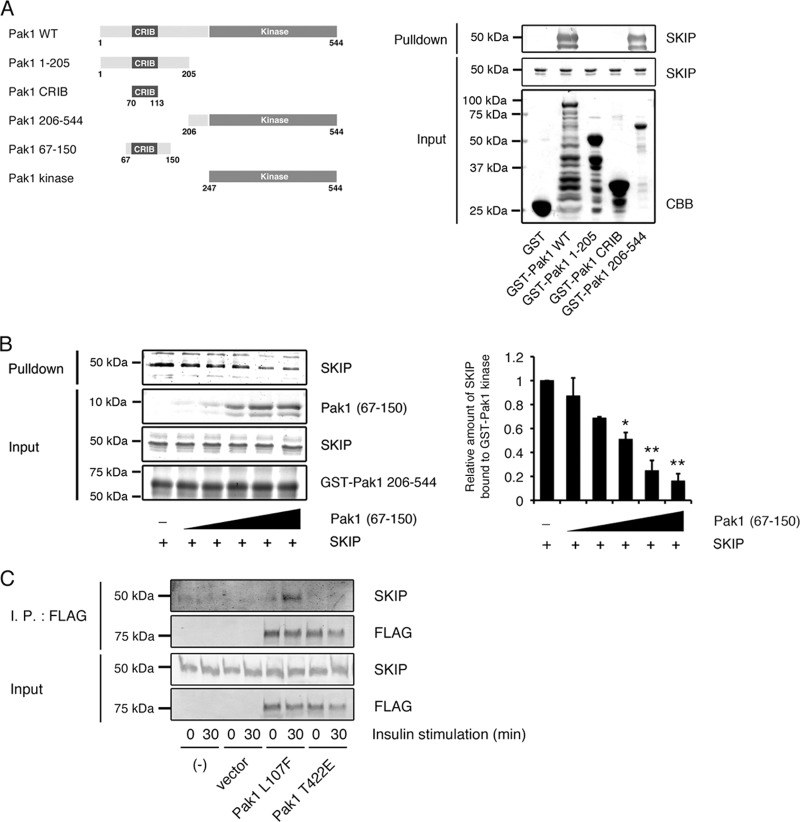
SKIP binds to the kinase domain of Pak1 (GST-Pak1 aa 206 to 544) but not to the N-terminal region, including the CRIB domain (GST-Pak1 aa 1 to 205) (Fig. 7A). However, addition of the SKIP protein to the GST-Pak1 kinase domain did not alter the phosphorylation of the substrate (GST-Bad aa 105 to 141, S136A) at Ser-112 (data not shown), showing that SKIP does not affect the kinase activity of Pak1. Under resting conditions, the kinase domain is masked by the autoinhibitory region of Pak1 (Pak1 aa 67 to 150) in trans, thus forming heterodimers (20). In the presence of extracellular stimuli, these are dissociated upon activation by Rac1, resulting in its open form (21). Akt2 and PDK1 have been reported to interact with the Rac1-induced activated form of Pak1 through a scaffolding function. To examine if SKIP binds to this open form of Pak1, we added the recombinant Pak1 autoinhibitory domain (Pak1 aa 67 to 150) to the SKIP-Pak1 (aa 206 to 544) protein complex. Addition of Pak1 aa 67 to 150 decreased the binding between SKIP and the GST-Pak1 kinase domain in a dose-dependent manner (Fig. 7B). In addition, an active form of Pak1 (p3×FLAG Pak1 L107F) formed a complex with SKIP in response to insulin stimulation, while the T422A destabilizing active-form mutant (mimicking nonphosphorylated threonine) did not, demonstrating that SKIP specifically binds to the active form of Pak1 (Fig. 7C). These results imply that insulin activation released the kinase domain of Pak1 from the N-terminal autoinhibitory domain, resulting in its association with SKIP.
Fig 7.
Insulin-dependent formation of an active Pak1-SKIP complex. (A) Pak1 constructs used in this study (left) and binding of SKIP to the Pak1 kinase domain (right). (B) Competitive binding for the Pak1 kinase domain between SKIP and the Pak1 inhibitory switch region (Pak1 aa 67 to 150). GST-Pak1 kinase domain (1 μg) immobilized to glutathione-Sepharose 4B beads was incubated with SKIP (1 μg) and various amounts of Pak1 aa 67 to 150. *, P < 0.05; **, P < 0.01 (t test). (C) Binding of SKIP to the constitutively active form of Pak1 (Pak1 L107F) in insulin-stimulated C2C12 cells. C2C12 cells transfected with 3× FLAG-tagged Pak1 L107F or Pak1 T422A mutant were stimulated with insulin for the indicated times. Lysates were immunoprecipitated (I.P.) with anti-FLAG antibody.
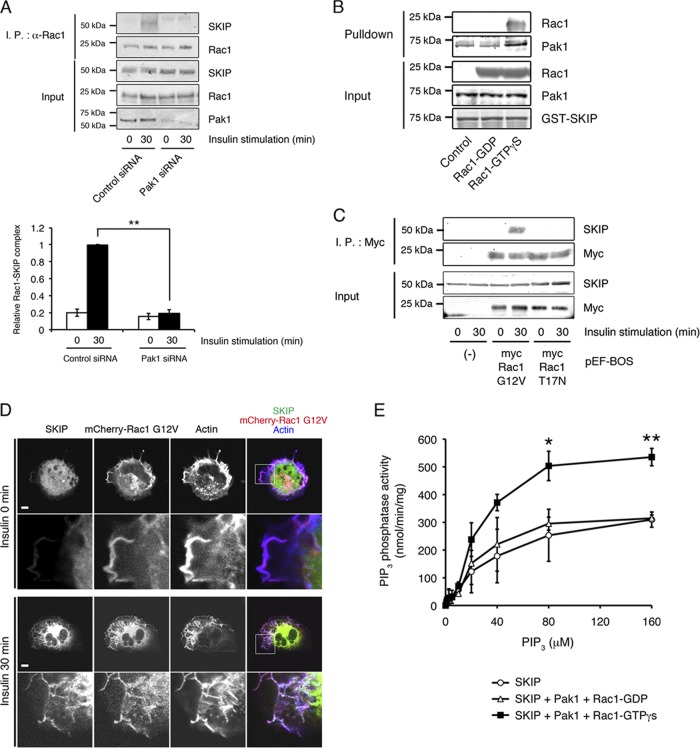
SKIP inactivates Pak1 and Rac1.
It has been reported that activation of the small GTPase Rac1 causes association of SKIP with Pak1 through the CRIB domain and triggers the formation of its active form. Endogenous SKIP was coimmunoprecipitated with Rac1 in insulin-stimulated cells, which was mediated by Pak1 (Fig. 8A). An in vitro binding assay showed that addition of GTPγS-loaded Rac1, which leads to the active conformation of Pak1, markedly increased binding between SKIP and Pak1 (Fig. 8B). Expression of the constitutively active mutant of Rac1 (Rac1 G12V), which leads to activation of Pak1, did not induce complex formation between SKIP and Rac1 under basal conditions. However, insulin stimulation triggered formation of a SKIP-Rac1 complex (Fig. 8C). Insulin stimulation relocalized SKIP to the membrane ruffles, where it colocalized with Rac1 (Fig. 8D). In contrast, the expression of dominant negative Rac1 (pEF-BOS-myc Rac1 T17N) did not form a protein complex with SKIP even after insulin stimulation (Fig. 8C). These results demonstrate that insulin induced an increase in GTP-Rac1-dependent activation of the scaffolding function of Pak1, leading to recruitment of SKIP to a protein complex that includes Pak1, Akt2, and PDK1. Furthermore, we found that SKIP is enzymatically activated by Pak1. Specifically, we observed increased PIP3 phosphatase activity of SKIP after the addition of an equal concentration of active Pak1 (SKIP-Pak1-Rac1-GTPγS, Km = 25.3 μM and Vmax = 714.2 nmol · min−1 · mg1 protein−1; SKIP-Pak1-Rac1-GDP, Km = 22.9 μM and Vmax = 384.6 nmol · min−1 · mg1 protein−1 [Fig. 8E]).
Fig 8.
Complex formation of SKIP and the active form of Rac1. (A) Insulin-dependent interaction between endogenous SKIP and Rac1, which is mediated by Pak1. C2C12 cells transfected with control or Pak1 siRNA were stimulated with insulin for the indicated times. Lysates were immunoprecipitated (I.P.) with anti-Rac1 antibody. Results are presented as the means ± SEMs of 3 independent experiments. **, P < 0.01. (B) SKIP binds to the activated form of Pak1. GST-SKIP domain (0.5 μg) immobilized to glutathione-Sepharose 4B beads was incubated with Pak1 (0.5 μg) in the presence of GDP-Rac1 or GTPγS-Rac1 (2 μg). (C) Complex formation of SKIP with the active form of Rac1 (pEF-BOS myc Rac1-G12V) but not with the dominant negative mutant (pEF-BOS myc Rac1-T17N). (D) Localization of SKIP in C2C12 cells expressing the constitutive active mutant of Rac1 (mCherry-Rac1 G12V). Pak1 activation by the expression of constitutive active Rac1 is not sufficient for the membrane ruffle localization of SKIP. F-actin was visualized with Alexa Fluor 647-labeled phalloidin. Scale bars, 20 μm. (E) Active Pak1 activated PIP3 phosphatase activity of SKIP. Recombinant SKIP (50 ng) in the presence or absence of Pak1 (70 ng) and GTPγS-Rac1 (100 ng) was used for the PIP3 phosphatase activity assay. Recombinant protein was incubated with various concentrations of PIP3 (0 to 160 μM). Results are presented as the means ± SEMs of 5 independent experiments. *, P < 0.05; **, P < 0.01.
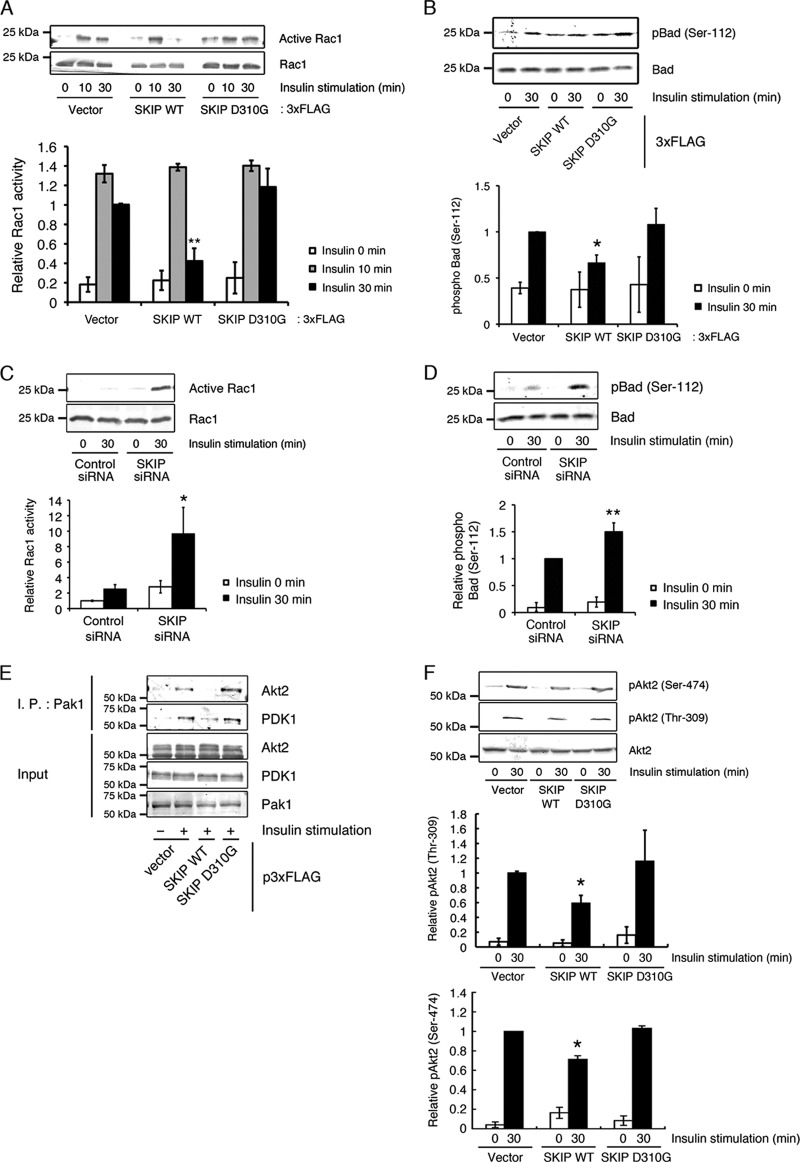
It has been reported that Pak1-Akt-PDK1 complex formation accelerates the efficiency and specificity of Akt signaling, and therefore, disassembly of this complex would lead to the termination of insulin signaling (10). We have previously reported that the expression of SKIP negatively regulates insulin-mediated membrane ruffle formation (14), implying that SKIP may negatively regulate Rac1 activity. Insulin-induced Rac1 activation is mediated by Tiam1 activation, which is dependent on PI 3-kinase activity. In this study, we found that the expression of SKIP WT, but not of the D310G mutant, significantly decreased Rac1 activity after 30 min of insulin treatment (Fig. 9A) but did not affect insulin-dependent Rac1 activation after 10 min. Likewise, expression of SKIP WT, but not of the D310G mutant, markedly decreased phosphorylation of Bad at Ser-112 by Pak1 after 30 min of insulin treatment (Fig. 9B). In contrast, Rac1 and Pak1 activities were markedly increased when SKIP was silenced (Fig. 9C and D). Expression of p3×FLAG-SKIP WT resulted in the dissociation of the Pak1-Akt2 complex (Fig. 9E). This, in turn, caused a significant decrease in the phosphorylation of Akt2 at Ser-474 and Thr-309 (Fig. 9F). In contrast, the expression of SKIP D310G did not affect the amount of Akt2 and PDK1 bound to Pak1 or insulin-induced phosphorylation of Akt2 (Fig. 9E and F). These results suggest that the dissociation of Akt2 and PDK1 from Pak1 is related to the PIP3 phosphatase activity of SKIP. These findings suggest that negative regulation of Rac1 activity by SKIP appears to enhance the formation of an inactive Pak1 dimer, resulting in the dissociation of Akt2 and PDK1 from Pak1.
Fig 9.
Dissociation of Akt2 from the Pak1 complex, as mediated by the PIP3 phosphatase activity of SKIP. (A) Decreased Rac1 activity in insulin-stimulated C2C12 cells caused by expression of SKIP WT but not the D310G mutant. Results are presented as the means ± SDs of 3 independent experiments. **, P < 0.01. (B) Suppression of Pak1 activity caused by expression of SKIP WT but not the D310G mutant. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05. (C) Increased Rac1 activity caused by the silencing of SKIP. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05. (D) Increased Pak1 activity, measured as insulin-induced phosphorylation of Bad at Ser-112, caused by SKIP attenuation in C2C12 cells. Results are presented as the means ± SDs of 3 independent experiments. **, P < 0.01. (E) Decreased binding of Akt2 and PDK1 to Pak1 caused by the PIP3 phosphatase activity of SKIP. (F) Suppression of Akt2 activity caused by expression of SKIP WT but not the D310G mutant. Results are presented as the means ± SDs of 3 independent experiments. *, P < 0.05.
DISCUSSION
SKIP is a PIP3 phosphatase that is abundantly expressed in skeletal muscle. We have recently showed that among PIP3 phosphatases, SKIP predominantly regulated insulin-dependent GLUT4 translocation and the subsequent glucose uptake in C2C12 and L6 myoblasts (13). In these cells, SKIP negatively regulated insulin-dependent phosphorylation of Akt and the downstream targets, including GSK3β, p70 S6 kinase, and AS160 (13, 14). SKIP heterozygous knockout mice showed resistance to diet-induced hyperglycemia and systemic insulin resistance (15). These mice exhibited increased insulin signaling and glucose uptake in skeletal muscle. Although it has been suggested that SKIP is involved in the specific regulation of insulin-mediated PI 3-kinase signaling in skeletal muscle, the underlying molecular mechanisms as to how and when it hydrolyzes PIP3 are still unknown. In this study, our results provide the first detailed look at the mechanisms by which SKIP affects both the timing and location of insulin signaling.
First, we demonstrated that SKIP specifically regulates insulin signaling by acting on Akt2 but not on Akt1. Akt2 is a crucial regulator of insulin signaling and glucose incorporation. Murine studies have suggested that the Akt2 isoform is closely related to insulin metabolic effects (3). A loss-of-function mutation of Akt2 produces severe insulin resistance (3), while a somatic activating E17K mutation of Akt2 induces hypoglycemia in humans (11). In contrast, the E17K mutation in Akt1 is commonly found in cancer (2), which is not likely to relate to hyperglycemia. Taken together, among Akt species, Akt2 activity is closely related to the regulation of blood glucose levels. Attenuation of SKIP increased insulin-dependent Akt2 phosphorylation without affecting the phosphorylation of Akt1. PDGF has also been shown to facilitate activation of PI 3-kinase-Akt1 signaling (16, 18, 32). PDGF induces GLUT4 translocation and glucose incorporation along with PI 3-kinase stimulation. However, its ability is not sufficient for the rapid decrease in the blood glucose levels in mice (32, 34). Unexpectedly, SKIP did not inhibit PDGF-dependent Akt1 phosphorylation and glucose incorporation. It remains to be determined whether PDGF utilizes the same signaling pathway as insulin. It has been reported that insulin activates Akt in both raft and nonraft regions, while PDGF activates Akt only in the raft region; these signaling differences may result from variations in spatiotemporal mechanisms (6). In C2C12 cells, endogenous SKIP and Akt2 predominantly existed in the nonraft fraction, where they form a protein complex with the insulin receptor. Predominant localization at the nonraft region might explain the specific regulation of insulin signaling, not PDGF signaling, by SKIP. Insulin stimulation triggers the relocation of SKIP from the ER to the membrane ruffles as well as the subsequent association of SKIP with Akt2, while PDGF stimulation did not mobilize SKIP to the membrane ruffles, which also explains why SKIP specifically regulates insulin signaling. In myoblasts, different mechanisms are responsible for Akt phosphorylation and actin remodeling by insulin versus PDGF, which might be explained by the function of SKIP (29). SKIP consists of the phosphatase domain and the C-terminal SKICH domain that mediates localization to membrane ruffles (9). PIP3 phosphatase activity of SKIP has been reported to be necessary for the negative regulation of insulin signaling (14). In addition, we found that the SKICH domain was also necessary for this regulation. A SKIP D361A mutant did not localize to the membrane ruffles in response to insulin, thus preventing it from regulating Akt2 phosphorylation and glucose incorporation. These data suggest that SKIP functions as a PIP3 phosphatase at membrane ruffles. Hence, spatiotemporal regulation of SKIP mediates the specific regulation of insulin signaling in skeletal muscle.
An important issue is how SKIP is spatiotemporally regulated in insulin signaling. The SKICH domain of SKIP has been identified as a membrane-localizing domain (9) whose function was unknown. We identified Pak1 as a binding protein of the SKIP SKICH domain, and this interaction was necessary for the localization of SKIP to the membrane ruffles. We have recently reported that among PIP3 phosphatases, only suppression of SKIP achieved an increase in insulin signaling (13), which could be explained by the existence of the SKICH domain. Under resting conditions, Pak1 formed an inactive homodimer in trans by the interaction between the autoinhibition domain and kinase domain; insulin stimulation triggered binding between Rac1 and the Pak1 CRIB domain to allow the kinase domain to adopt an active conformation (35). In this study, we observed an insulin stimulation-dependent SKIP-Pak1 interaction at the membrane ruffles. SKIP binds to the kinase domain of Pak1 without affecting its kinase activity in vitro. Further, we found that the active form (L107F) of Pak1 could interact with SKIP in response to insulin, while the T422A mutant that destabilizes the active conformation could not. Addition of the GTPγS-loaded activated form of Rac1 increased in vitro binding between SKIP and Pak1. SKIP only binds to the activated form of Pak1, the kinase domain of which is not masked by an autoinhibitory domain. Given that the expression of constitutively active Rac1 did not mobilize SKIP to the membrane ruffles in the absence of insulin, these results also indicate that Pak1 activation is necessary, but not sufficient, for the binding of SKIP to Pak1. Both Pak1 activation and translocation of SKIP are required for the formation of this protein complex. By these events, the time lag between the activation of insulin signaling and its negative regulation by SKIP occurs. It should be noted that the scaffolding function of Pak1, independent of its kinase activity, plays a central role in this mechanism.
Our results provide important data about the process by which SKIP effectively inhibits Akt2 activity. Insulin triggers the formation of SKIP-Akt2 and that of SKIP-PDK1 complexes at the membrane ruffles, both of which are mediated by Pak1. Both Akt2 and PDK1 possess PH domains that bind to PIP3, and their activation depends on PI 3-kinase signaling. SKIP is likely to hydrolyze PIP3, which efficiently activates Akt2 and PDK1. Proximal localization of SKIP to the substrate PIP3 and its effectors would facilitate the effective and rapid inactivation of Akt2. It should also be noted that the PIP3 phosphatase activity of SKIP is activated by Pak1, which might further lead to the inactivation of Akt2 and PDK1, resulting in the rapid termination of insulin signaling.
Another important role of SKIP is to regulate Rac1 activity. We have previously reported that insulin-mediated membrane ruffle formation was significantly inhibited by the expression of SKIP (14). In skeletal muscle cells, Rac1-mediated membrane ruffle formation is necessary for the insulin-mediated GLUT4 translocation and glucose uptake (31). We observed insulin-dependent complex formation between SKIP and the active form of Rac1, which was also mediated by Pak1. Insulin treatment for 10 min induced activation of Rac1 and Pak1, which was not affected by SKIP because SKIP is not recruited to the Pak1 protein complex at that time. However, expression of wild-type SKIP led to inhibition of Rac1 activity after insulin treatment for 30 min, demonstrating that formation of a complex between SKIP and Rac1 contributes to the rapid inactivation of Rac1. PIP3 phosphatase activity of SKIP is also necessary for Rac1 inactivation. Rac1 binds to Tiam1, which, because it has a PH domain that binds to PIP3, is necessary for membrane ruffle formation. Tiam1 may be one of the potential targets of PIP3 phosphatase activity of SKIP. Pak1 is also inactivated by the expression of SKIP through Rac1 inactivation, a process that might induce the inactive conformation of Pak1 and facilitate the dissociation of Akt2 from Pak1. Although the significance of this dissociation is unknown, the scaffolding function of Pak1 has been reported to be necessary for the binding with Akt and the activation of PI 3-kinase signaling (10). Therefore, inactivation of Pak1 by SKIP may mediate termination of the activation of PI 3-kinase signaling.
Alterations in insulin signaling may affect body weight, food intake, adiposity, and blood glucose levels. PIP3 phosphatases have been implicated in the regulation of insulin signaling. Among them, SHIP2 and PTEN negatively regulate insulin signaling. SHIP2 deficiency and muscle-specific knockdown of PTEN in mice resulted in protection against insulin resistance and obesity when the mice were fed an HFD, but these mice did not show increased insulin sensitivity or insulin-induced glucose uptake when fed a normal chow diet (27, 33). In contrast, SKIP heterozygous mice exhibited increased insulin-dependent glucose uptake in isolated skeletal muscle and were resistant to diet-induced hyperglycemia (15). These mice also showed increased insulin sensitivity and glucose uptake when they were fed a normal chow diet. Insulin resistance and hyperglycemia, two major features of type II diabetes, are associated with atherosclerosis and endothelial dysfunction (22, 28). Therefore, SKIP may be a potential pharmaceutical target for the intervention of hyperglycemia and associated diseases. Increased serine phosphorylation and decreased tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1) are the major features of HFD-induced limited insulin action and the PI 3-kinase-Akt signaling pathway, which lead to insulin resistance and hyperglycemia. Under such conditions, SKIP heterozygous mice exhibited increased systemic glucose uptake compared to that of wild-type mice, and isolated skeletal muscle showed increases in insulin signaling and glucose incorporation (15). These results imply that an activation of PI 3-kinase signaling is effective for the recovery from diet-induced insulin resistance and the increase in insulin-induced glucose incorporation. In this study, we identified the underlying mechanism for the efficient and specific regulation of insulin-induced activation of Akt2 and Rac1 by SKIP. Pak1 mediates the complex formation between SKIP, Akt2, and Rac1 and the negative regulation of Akt2 and Rac1 activity, both of which are necessary for the insulin-dependent glucose uptake into skeletal muscle cells.
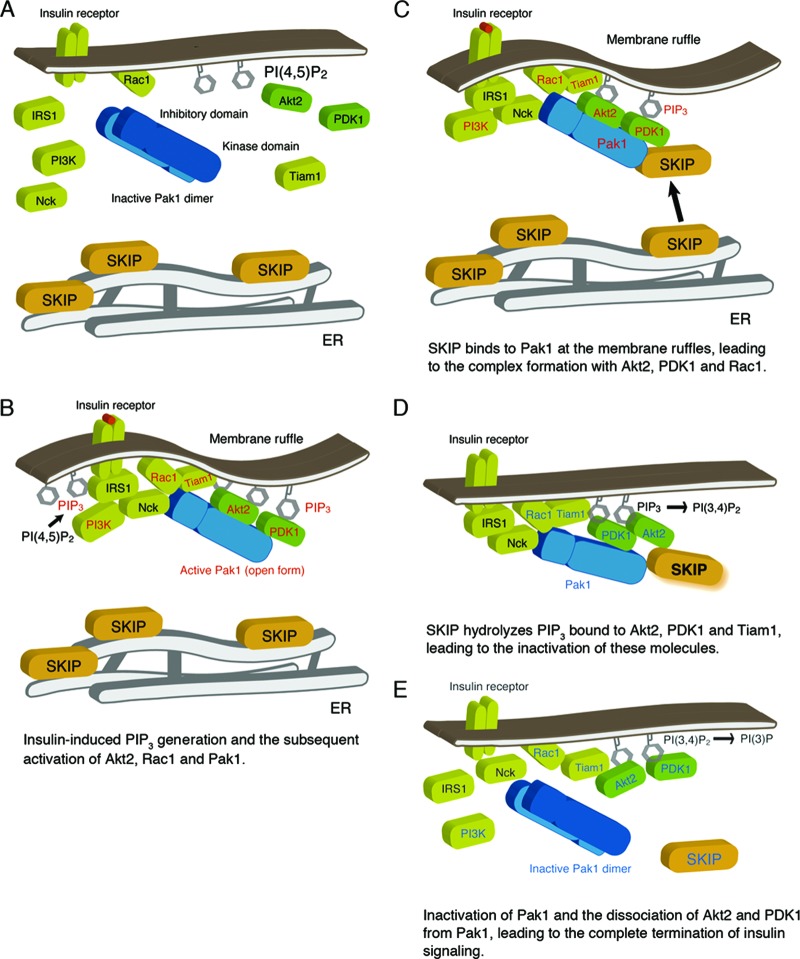
In summary, our results suggest a simple model whereby spatiotemporal regulation of SKIP by Pak1 leads to the specific regulation of insulin signaling in skeletal muscle cells. Under basal conditions, SKIP is predominantly localized to the ER (Fig. 10A). Insulin stimulation triggers PI 3-kinase activation, leading to PIP3 generation. Subsequently, PIP3-dependent Rac1 activation leads to the formation of membrane ruffles, to which Pak1 localizes in its active conformation (Fig. 10B). Insulin stimulation also triggers the translocation of SKIP from the ER to the membrane ruffles. There, SKIP interacts with an active form of Pak1 through the scaffolding function, leading to formation of protein complexes including Akt2 and PDK1 (Fig. 10C), two PIP3-binding proteins (10). SKIP is also recruited to the insulin receptor complex (Fig. 10C). The location of SKIP proximal to these PIP3 effectors determines the efficiency and specificity of the negative regulation of insulin signaling. Hydrolysis of PIP3 associated with these effectors to PI(3,4)P2 by SKIP is likely to facilitate the effective and rapid inactivation of Akt2 and insulin signaling (Fig. 10D). However, release of Akt2 from the plasma membrane may require further hydrolysis of PI(3,4)P2 by another phosphoinositide phosphatase because Akt2 can also bind to PI(3,4)P2. Formation of SKIP-Pak1-Rac1 complexes also contributes to the termination of insulin signaling (Fig. 10E). SKIP leads to inhibition of Rac1 activity, which results in the inactive conformation of Pak1 and the disassembly of the Akt2-PDK1-Pak1 complex (Fig. 10E). Because phosphorylation at Thr-309 by PDK1 is required for full activation of Akt2, dissociation of the PDK1-Pak1-Akt2 complex by SKIP at the plasma membrane may facilitate rapid termination of the activation of insulin signaling. Our results suggest that SKIP could be a promising target for efforts aimed at improving systemic insulin-dependent glucose uptake and treating hyperglycemia.
Fig 10.
Working model of SKIP regulation of insulin signaling. (A) Under resting conditions, SKIP is localized to the ER. (B) Insulin-dependent PIP3 generation and Rac1-dependent Pak1 activation. Pak1 activation triggers its recruitment to the insulin receptor complex and subsequent complex formation with Akt2 and PDK1. Activated signaling molecules are highlighted in red. (C) Insulin induces translocation of SKIP to the plasma membrane, where SKIP binds to active Pak1 (the open form). Formation of a complex including SKIP, Pak1, Akt2, and Rac1 is induced at the membrane ruffles. (D) SKIP hydrolyzes PIP3 bound to Akt2. Localization of SKIP in proximity to Akt2 facilitates its inactivation. Signaling molecules inactivated by SKIP are indicated in blue. (E) SKIP negatively regulates Rac1 and Pak1 activity, leading to the inactive conformation of Pak1. Dissociation of Akt2 and PDK1 from inactive Pak1 terminates insulin signaling.
ACKNOWLEDGMENTS
We thank Natsuko Goda and Takeshi Tenno (Nagoya University Graduate School of Science) for their assistance and Kazuya Tsujita, Daisuke Yamazaki, and Toshiki Itoh (Kobe University Graduate School of Medicine) for their helpful discussion and assistance.
This work is supported in part by grants to T.I. from the Japan Diabetes Foundation and the Hyogo Science and Technology Association.
Footnotes
Published ahead of print 2 July 2012
REFERENCES
- 1. Bishop AL, Hall A. 2000. Rho GTPases and their effector proteins. Biochem. J. 348(Part 2):241–255 [PMC free article] [PubMed] [Google Scholar]
- 2. Carpten JD, et al. 2007. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448: 439– 444 [DOI] [PubMed] [Google Scholar]
- 3. Cho H, et al. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728– 1731 [DOI] [PubMed] [Google Scholar]
- 4. Christoforidis S, Zerial M. 2000. Purification and identification of novel Rab effectors using affinity chromatography. Methods 20: 403– 410 [DOI] [PubMed] [Google Scholar]
- 5. Dugani CB, Klip A. 2005. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 6: 1137– 1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao X, Zhang J. 2008. Spatiotemporal analysis of differential Akt regulation in plasma membrane microdomains. Mol. Biol. Cell 19: 4366– 4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garofalo RS, et al. 2003. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Invest. 112: 197– 208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. George S, et al. 2004. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304: 1325– 1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gurung R, et al. 2003. Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization. The inositol 5-phosphatase skip localizes to the endoplasmic reticulum and translocates to membrane ruffles following epidermal growth factor stimulation. J. Biol. Chem. 278: 11376– 11385 [DOI] [PubMed] [Google Scholar]
- 10. Higuchi M, Onishi K, Kikuchi C, Gotoh Y. 2008. Scaffolding function of PAK in the PDK1-Akt pathway. Nat. Cell Biol. 10: 1356– 1364 [DOI] [PubMed] [Google Scholar]
- 11. Hussain K, et al. 2011. An activating mutation of AKT2 and human hypoglycemia. Science 334: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ijuin T, et al. 2000. Identification and characterization of a novel inositol polyphosphate 5-phosphatase. J. Biol. Chem. 275: 10870– 10875 [DOI] [PubMed] [Google Scholar]
- 13. Ijuin T, Takenawa T. 2012. Regulation of insulin signaling and glucose transporter 4 (GLUT4) exocytosis by phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, skeletal muscle, and kidney enriched inositol polyphosphate phosphatase (SKIP). J. Biol. Chem. 287: 6991– 6999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ijuin T, Takenawa T. 2003. SKIP negatively regulates insulin-induced GLUT4 translocation and membrane ruffle formation. Mol. Cell. Biol. 23: 1209– 1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ijuin T, et al. 2008. Increased insulin action in SKIP heterozygous knockout mice. Mol. Cell. Biol. 28: 5184– 5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishii K, et al. 1994. Epidermal growth factor triggers the translocation of insulin-responsive glucose transporter (GLUT4). Biochem. Biophys. Res. Commun. 205: 857– 863 [DOI] [PubMed] [Google Scholar]
- 17. Jacobs C, Onnockx S, Vandenbroere I, Pirson I. 2004. Endogenous SHIP2 does not localize in lipid rafts in 3T3-L1 adipocytes. FEBS Lett. 565: 70– 74 [DOI] [PubMed] [Google Scholar]
- 18. Kamohara S, et al. 1995. Platelet-derived growth factor triggers translocation of the insulin-regulatable glucose transporter (type 4) predominantly through phosphatidylinositol 3-kinase binding sites on the receptor. Proc. Natl. Acad. Sci. U. S. A. 92: 1077– 1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komander D, et al. 2004. Structural insights into the regulation of PDK1 by phosphoinositides and inositol phosphates. EMBO J. 23: 3918– 3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei M, et al. 2000. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102: 387– 397 [DOI] [PubMed] [Google Scholar]
- 21. Parrini MC, Lei M, Harrison SC, Mayer BJ. 2002. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell 9: 73– 83 [DOI] [PubMed] [Google Scholar]
- 22. Pieper GM, Meier DA, Hager SR. 1995. Endothelial dysfunction in a model of hyperglycemia and hyperinsulinemia. Am. J. Physiol. 269: H845– H850 [DOI] [PubMed] [Google Scholar]
- 23. Pilling C, Landgraf KE, Falke JJ. 2011. The GRP1 PH domain, like the AKT1 PH domain, possesses a sentry glutamate residue essential for specific targeting to plasma membrane PI(3,4,5)P(3). Biochemistry 50: 9845– 9856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saltiel AR. 2001. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104: 517– 529 [DOI] [PubMed] [Google Scholar]
- 25. Sarbassov DD, et al. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14: 1296– 1302 [DOI] [PubMed] [Google Scholar]
- 26. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098– 1101 [DOI] [PubMed] [Google Scholar]
- 27. Sleeman MW, et al. 2005. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat. Med. 11: 199– 205 [DOI] [PubMed] [Google Scholar]
- 28. Sowers JR, et al. 1993. Hyperinsulinemia, insulin resistance, and hyperglycemia: contributing factors in the pathogenesis of hypertension and atherosclerosis. Am. J. Hypertens. 6: 260S– 270S [DOI] [PubMed] [Google Scholar]
- 29. Török D, et al. 2004. Insulin but not PDGF relies on actin remodeling and on VAMP2 for GLUT4 translocation in myoblasts. J. Cell Sci. 117: 5447– 5455 [DOI] [PubMed] [Google Scholar]
- 30. Tsakiridis T, Taha C, Grinstein S, Klip A. 1996. Insulin activates a p21-activated kinase in muscle cells via phosphatidylinositol 3-kinase. J. Biol. Chem. 271: 19664– 19667 [DOI] [PubMed] [Google Scholar]
- 31. Ueda S, et al. 2010. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J. 24: 2254– 2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whiteman EL, Chen JJ, Birnbaum MJ. 2003. Platelet-derived growth factor (PDGF) stimulates glucose transport in 3T3-L1 adipocytes overexpressing PDGF receptor by a pathway independent of insulin receptor substrates. Endocrinology 144: 3811– 3820 [DOI] [PubMed] [Google Scholar]
- 33. Wijesekara N, et al. 2005. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol. Cell. Biol. 25: 1135– 1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuasa T, et al. 2004. Platelet-derived growth factor stimulates glucose transport in skeletal muscles of transgenic mice specifically expressing platelet-derived growth factor receptor in the muscle, but it does not affect blood glucose levels. Diabetes 53: 2776– 2786 [DOI] [PubMed] [Google Scholar]
- 35. Zenke FT, King CC, Bohl BP, Bokoch GM. 1999. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem. 274: 32565– 32573 [DOI] [PubMed] [Google Scholar]
- 36. Zhou QL, et al. 2004. Analysis of insulin signalling by RNAi-based gene silencing. Biochem. Soc. Trans. 32: 817– 821 [DOI] [PubMed] [Google Scholar]