Abstract
Multiple Fibroblast growth factor (Fgf) ligands are expressed in the forebrain and facial ectoderm, and Vascular Endothelial Growth Factor (VEGF) is expressed in the facial ectoderm. Both pathways activate the MAP kinase cascade and can be suppressed by SU5402. We placed a bead soaked in SU5402 into the brain after emigration of neural crest cells was complete. Within 24 hours we observed reduced pMEK and pERK staining that persisted for at least 48 hours. This was accompanied by significant apoptosis in the face. By day 15 the upper beaks were truncated. Molecular changes in the FNP were also apparent. Normally, Shh is expressed in the Frontonasal Ectodermal Zone and controls patterned growth of the upper jaw. In treated embryos Shh expression was reduced. Both the structural and molecular deficits were mitigated after transplantation of FNP-derived mesenchymal cells. Thus, mesenchymal cells actively participate in signaling interactions of the face, and the absence of neural crest cells in neurocristopathies may not be merely structural.
Keywords: Shh, Fgf, VEGF, quail-chick chimera, neurocristopathies
Introduction
Molecular signaling among tissues regulates the complex processes of vertebrate embryogenesis. During development of the upper jaw, the forebrain, neural crest mesenchyme, and facial ectoderm control the cellular behaviors that pattern the skeleton. SHH signaling within the forebrain helps establish the Frontonasal Ectodermal Zone (FEZ) in the surface cephalic ectoderm (Marcucio et al., 2005). This signaling center regulates proximodistal extension and dorsoventral polarity of the upper jaw (Hu et al., 2003; Depew and Simpson, 2006; Depew and Compagnucci, 2008; Hu and Marcucio, 2009a), in part by regulating gene expression patterns and controlling the rate and location of cell proliferation in the mesenchyme (Hu and Marcucio, 2009b). Much of our previous work focused on the role of SHH in mediating signaling from the brain. Here we expand our understanding of these interactions by blocking signaling through receptors that activate the MAP kinase pathway after neural crest cells have emigrated from the neural tube.
FGF signaling is required for normal development of the upper and lower jaw (Crossley and Martin, 1995; Tucker et al., 1999; Mina et al., 2002; Mina et al., 2007). Multiple FGF receptors and ligands are expressed throughout the developing face (Matovinovic and Richman, 1997; Wilke et al., 1997; Bachler and Neubuser, 2001; Hu et al., 2003). In the developing upper jaw region, Fgf8 is initially expressed in a continuous domain that spans the anterior neural epithelium and surface cephalic ectoderm that will cover the Frontonasal process (FNP). Upon closure of the anterior neuropore this domain segregates into distinct telencephalic and ectodermal domains. When neural crest cells arrive in the FNP around Hamburger Hamilton stage 20 (HH20) (Hamburger, 1951) Shh expression is induced in the cephalic ectoderm and forms a boundary with the Fgf8-expressing cells (Hu et al., 2003, Hu, 2009b). Soon after the onset of Shh expression, Fgf8 transcripts are down-regulated in the midline and become restricted to the nasal pits where it is co-expressed with Fgf9, Fgf10, and Fgf18 (Karabagli et al., 2002; Havens et al., 2006). However, disrupting production of a single ligand, Fgf8, produces severe facial malformations in mice suggesting this is a major factor that contributes to the control of facial morphogenesis (e.g., (Storm et al., 2003; Storm et al., 2006)). Fgf receptors are present on mesenchymal cells, including the neural crest of the FNP beginning at least by HH10 and persisting at least through growth of the FNP (Wilke et al., 1997), and FGF signaling regulates expression of a variety of genes within the mesenchyme of the FNP (Firnberg and Neubuser, 2002; Szabo-Rogers et al., 2008; Higashihori et al., 2010).
Altered FGF signaling creates malformations during development of the face. Blocking FGF signaling in the facial mesenchyme during periods of outgrowth leads to hypoplasia of the maxillary process derivatives (Szabo-Rogers et al., 2008; Hu and Marcucio, 2009a; Szabo-Rogers et al., 2009). Similarly in mice, systematic reduction in signaling by Fgf8 correlates with cell survival and graded defects in the forebrain (Storm et al., 2003; Storm et al., 2006). Mutations in FGF ligands and receptors are associated with cleft lip and palate in humans (Riley and Murray, 2007). Finally, these data, combined with the importance of FGF signaling in development, has led to the idea that mutations or disruptions to FGF signaling could contribute to neurocristopathies (Etchevers et al., 2006).
Our goal here was to block activation of Fgf signaling using a small molecule inhibitor, SU5402. In addition to blocking Fgf signaling, SU5402 also blocks activation of VEGF (Flk) receptors equally well (Sun et al., 1999), and both pathways activate the MAP kinase cascade (Takahashi et al., 1999; Ribisi et al., 2000). While Fgf signaling has been well-studied, less is known about the role of signaling by Vascular Endothelial Growth Factor (VEGF) during facial development. A recent report indicates that VEGF is expressed in the surface cephalic ectoderm and signals to adjacent mesenchymal cells through the receptor Neuropilin-1 to regulate migration of neural crest cells into the facial prominences (McLennan et al., 2009). Further, VEGF signaling is required for formation of the vasculature via the receptors Flt and Flk in mice (Gerber et al., 1999).
In this work we disrupted Fgf and VEGF signaling and examined the effects on development of the Frontonasal Process (FNP) and its derivatives by placing a bead soaked in SU5402 into the brain and then examining the effects on neural crest migration into the FNP, vasculature development, facial morphology, and the associated molecular changes. We then assessed the ability of exogenous FNP-derived mesenchymal cells to rescue the molecular and morphological defects. Our results reveal that reduction of mesenchymal cells is only one component to the generation of malformations, and that the ensuing molecular changes may compound the production of disease phenotypes in the head.
Results
Blockade of FGF signaling alters facial morphology
Multiple Fgf ligands and VEGF are expressed in the surface ectoderm of the head and they signal to the adjacent mesenchymal cells (Firnberg and Neubuser, 2002; Karabagli et al., 2002; Szabo-Rogers et al., 2008; McLennan et al., 2009). To assess the extent to which signaling by the FGF pathway and VEGF regulates facial development, we placed a bead that was soaked in SU5402 (10mM) into the forebrain at HH11 and then examined a variety of outcomes to define the relationship between MAP kinase activity, cellular function, and morphology.
Signaling by FGF and VEGF ligands activate the MAP kinase signaling pathway and leads to phosphorylation of ERK and MEK (Takahashi et al., 1999; Ribisi et al., 2000). Phosphorylated forms of ERK and MEK were broadly detectable throughout the craniofacial complex at 24 hours after placing a control bead soaked in PBS. At this time large region of the neural tube, the facial mesenchyme, and the cephalic ectoderm were stained positively for pERK (Fig. 1A) and pMEK (Fig. 1C). However, within 24 hours of implanting a bead soaked in SU5402 into the brain both pERK (Fig. 1B) and pMEK (Fig. 1D) were nearly undetectable in the brain, mesenchyme, and ectoderm of the head indicating that the MAP kinase pathway was significantly reduced in the treated embryos compared to controls. At 48 hours pERK and pMEK remained at low levels (not shown).
Figure 1. Effect of SU5402 on cell signaling and survival.
(A) In control embryos that were sectioned sagitally (n=6), immunohistochemistry reveals the presence of pERK throughout the face and brain at HH 15. (B) At this time, embryos treated with SU5402 exhibit decreased pERK staining (n=6). (C) Similarly, control embryos exhibit high levels of pMEK (n=6), and (D) treated embryos have reduced expression levels (n=6). (E) In medial sections, TUNEL staining revealed apoptotic cells (bright green dots) in the brain of control embryos (n=8) and (F) treated embryos (n=12) at HH15. (G) Cell death in lateral regions of control embryos was not apparent (n=8), but (H) in treated embryos large regions of apoptotic mesenchyme was evident (n=12). Scale bars: 200μm.
Previously, investigators determined that genetic reduction in Fgf8 expression leads to increased apoptosis in the head (Storm et al., 2003; Szabo-Rogers et al., 2008). Therefore, we examined apoptosis in treated embryos using a TUNEL assay. Twenty-four hours after treatment apoptosis was evident in the neural tube of control embryos (Fig. 1E), but there was little evidence of cell death in the mesenchyme of these embryos at this time (Fig. 1G). In contrast, large numbers of apoptotic mesenchymal cells were present in the upper jaw anlagen of embryos that were treated with SU5402 (Fig. 1E, F).
Next, we performed a morphologic analysis on embryos treated with SU5402. At 24, 48, 72 and 96 hours and 13 days after treatment. Gross analysis revealed severe malformations that resembled neurocristopathies where there is a reduction in the number of neural crest precursor cells (Fig. 2). At 24 and 48 hours after bead placement, neural crest cells are normally migrating to form the facial primordia (Fig. 2A, C). During this period of time there was a reduction in the population of neural crest cells destined for the FNP (Figs 2, 4). The surface ectoderm covering the face was tightly confined to the contour of the developing brain, and no apparent neural crest cells were intervening. Beginning at this time, treated embryos had small eyes (Fig. 2B, D) and there was a marked reduction in perfusion of the head (Supplemental Fig. 2). At 72 hours, treated embryos were severely malformed. However, the defects were confined primarily to the brain and middle part of the upper jaw. The forebrain had not expanded, embryos were anophthalmic, and the reduction in the population of neural crest cells led to the lack of an FNP. In contrast, the maxillary processes were well-formed and the mandibular primordia appeared unaltered in treated embryos (Figs 2E, F). By 96 hours, treated embryos continued to grow. The brain, the eyes, and the FNP were hypoplastic. However, at this time there was evidence of growth within the FNP suggesting that some neural crest cells survived treatment and had begun to form the middle part of the upper jaw (Fig. 2G, H). Some of the neural crest cells may have been spared from apoptosis, or other populations of neural crest cells may have contributed to the FNP population, but distinguishing among the multitude of possibilities was beyond the scope of this work. However, the maxillary and mandibular processes appeared to be spared by SU5402-treatment suggesting that expansion of either of these continuous populations could account for the presence of neural crest at these time points. Nonetheless, in treated embryos, the neural crest cells found in the FNP at this time were insufficient to develop into an upper jaw. At day 15, treated embryos had a severe reduction in the upper beak (Fig. 2I, K) due to the absence of the skeletal elements (Fig. 2J, L). Hence, treating embryos with SU5402 created a phenotype that resembled neurocristopathies that affect the developing face in humans (Etchevers et al., 2006).
Figure 2. Morphologic and skeletal defects after application of SU5402.
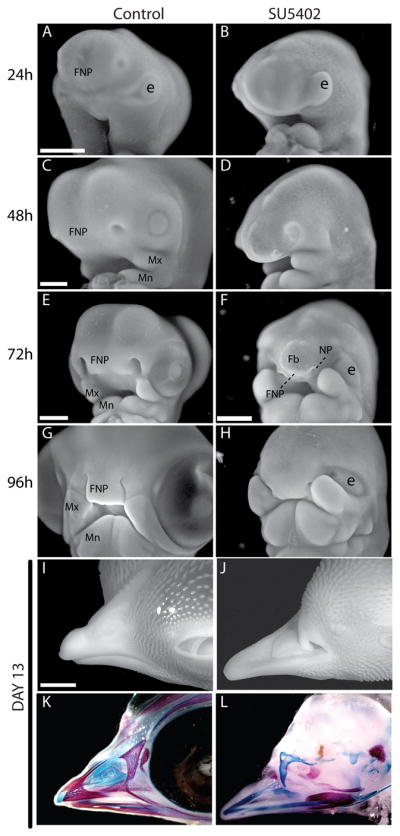
(A) A control embryo 24 hrs after treatment (n=12) illustrating normal morphology. (B) Treated embryos do not appear different from controls at this time (n=12), but the reduction in neural crest cells can be appreciated by the protruding eye (e) which is normally surrounded by ingressing neural crest cells. (C) By 48 hrs after treatment control embryos had grown, and the FNP, maxillary (Mx), and mandibular (Mn) processes were large and well-defined (n=10). (D) In treated embryos the forebrain was reduced in size (Fb), the FNP was ill-defined, but the maxillary and manidbular processes were growing (n=18). (E) By 72 hrs control embryos continued to increase in size and the facial primordial were all well-established (n=8). (F) However, in treated embryos, the forebrain was rudimentary (Fb), the FNP (FNP) was not populated by many neural crest cells, and the nasal pits (NP) were not well developed (n=17). (G) By 96 hours after treatment the FNP and Mx were apposed and nearing fusion while the mandible was greatly increased in size (n=10). (H) Treated embryos had large Mx and Mn prcoesses. However, the FNP was rudimentary and did not appose the Mx (n=12). (I) External morphology and (J) bone and cartilage staining with alizarin red and alcian blue illustrate the normal morphology of the upper jaw in controls (n=6). (K) Treated embryos were anophthalmic and had truncations of the upper jaw (n=12). (L) Alizarin red and alcian blue staining of treated embryos demonstrated extreme hypoplasia of the skeletal elements that comprise the upper jaw. Rudimentary bones and cartilages with no obvious pattern were present in the upper jaw (n=12). A–D: 500μm, E–H: 1mm, I–L:2.5 mm.
Figure 4. Gene expression patterns are restored in treated embryos by quail neural crest cells.
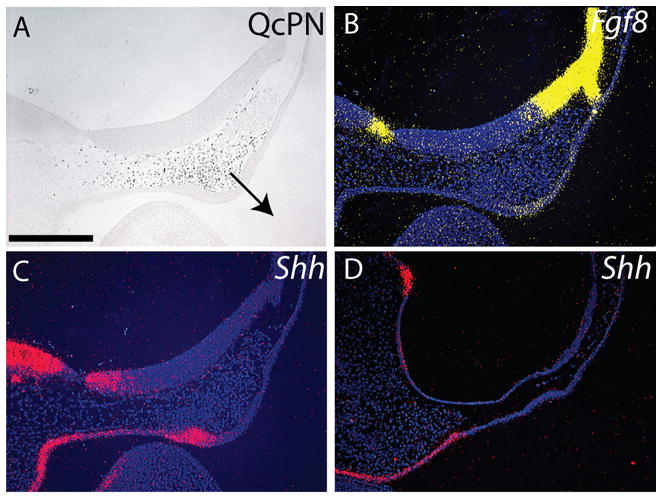
(A) Immunohistochemistry was used to assess quail mesenchyme at 24 hours after engraftment in chimeras. In this embryo, quail cells are located adjacent to the forebrain and are apposing the presumptive FEZ. The FNP exhibits signs of outgrowth (n=12, arrow=direction of growth). (B) Expression of Fgf8 in chimeric embryos (n=8). (C) The addition of quail cells to the FNP of treated embryos restored expression of Shh in the FEZ (n=8), (D) while embryos treated with SU5402 have no neural crest cells and no Shh expression in the ectoderm below the forebrain at HH22 (n=8). Scale bar: 250μm.
Molecular changes in embryos after addition of SU5402
The molecular changes within the face that occur in the absence of neural crest cells are not well known. First, we examined expression of Fgf8. We discovered that at 48hrs after treatment, the forebrain was thinner in treated compared to control embryos, and concomitantly Fgf8 expression was reduced in the anterior part of the forebrain and was not apparent in the optic recess (Fig. 3A, B). At 72 hours, Fgf8 was down-regulated in the FEZ of control embryos (Fig. 3C), but in treated embryos the Fgf8 expression domain was maintained and had shifted proximally (Fig. 3D). This agrees with other data suggesting that Shh signaling antagonizes Fgf8 expression in the FEZ (Cordero et al., 2004; Abzhanov et al., 2007).
Figure 3. SU5402 alters gene expression patterns in the FEZ.

(A) At 48 hrs after application of DMSO-soaked beads, Fgf8 transcripts were detected in the optic recess (OR), the anterior telencephalon (arrowhead), and the FEZ (arrow) of control embryos (n=6). (B) At this time embryos treated with SU5402 expressed Fgf8 in the anterior forebrain and FEZ, but the domain of expression in the optic recess was absent (n=8). (C) In control embryos at 72 hrs after treatment Fgf8 expression remained in the optic recess and anterior telencephalon but was absent from the midline of the FEZ (n=6). (D) After treatment with SU5402 the Fgf8 domain in |FEZ was still present and this domain (arrow) and the domain of expression in the forebrain (arrowhead) were shifted proximally (n=8). (E) In control embryos at 48 hrs (HH 19/20) after bead implantation Shh transcripts were detected in the diencephalon (Di), telencephalon (Te) and the FEZ (arrow; n=6), (F) but in treated embryos a small domain of Shh was detected in the brain (arrowhead; n=12). (G, I) At 72 hours (HH 23/24) Shh expression had expanded to include all the ectoderm encasing the roof of the mouth (arrow; n=5). (H, J) However, in embryos treated with SU5402 Shh expression in the brain was disrupted. In the surface ectoderm, the Shh domain (arrow) was only apparent in regions that were adjacent to neural crest mesenchyme (dotted line; n=8). (K) Normally, at 96 hrs after bead implantation, Shh expression extended across the mediolateral axis of the FNP during outgrowth of the upper jaw (n=6). (L) However, at this time in embryos treated with SU5402 the FEZ was split into two and Shh expression was apparent in lateral domains of the FNP (n=10). Scale bars: A–H: 500μm, I–L: 1mm.
We observed similar alterations in Shh expression in treated embryos. At 48 hours, 2 domains of Shh expression were present in the anterior forebrain and expression in the FEZ had begun (Fig. 3E). However, in treated embryos there was a single Shh expression domain in the brain and there was no evidence of Shh expression in the FEZ (Fig. 3F). By 72 hours, the Shh expression domain in the ectoderm of control embryos had expanded to cover the roof of the stomodeum (Fig. 3G, I), but was still small in treated embryos (Fig. 4H, J). In each treated embryo we observed expression of Shh in ectoderm that was adjacent to neural crest mesenchyme (Fig. 3H). By 96 hrs the FNP of control embryos exhibited significant growth and elongation that was associated with a single domain of Shh expression that spanned the midline of the FNP (Fig. 3K). In treated embryos, the Shh expression domain was split and was associated with growth occurring in lateral regions of the FNP of treated embryos (Fig. 3L).
Role of neural crest cells in rescuing defects caused by addition of SU5402
Results of our analysis suggested that the morphologic changes occurring in embryos treated with SU5402 resulted from reduced survival of neural crest cells as well as from the direct effects of reduced FGF and VEGF signaling. In order to begin distinguishing these possibilities, we repeated the above experiment, and at 48 hours after treatment we collected neural crest cells from the FNP of quail embryos and transplanted them into the treated chicks. Within 24hrs of transplantation the quail cells had stimulated thickening of the facial ectoderm, and the FNP exhibited signs of outgrowth (Fig. 4A). In these chimeric embryos, Fgf8 was expressed in the dorsal ectoderm of the FNP (Fig. 4B) and Shh expression was stimulated in the ectoderm comprising the ventral FNP (Fig. 4C, D).
When we allowed control, treated, and treated-chimeric embryos to develop to days 10 13 we observed differences in morphology. Normally, the upper beak projects just beyond the tip of the lower beak (Fig. 5A). In treated embryos, the upper beak was significantly truncated and the eyes were small or absent (Figs 2K and 5B). In treated-chimeric embryos growth of the upper beak was restored, but the eyes were still significantly malformed (Fig. 5C, n=6/11). At day 10, the prenasal cartilage extended beyond the rostrum in chimeras that appeared “rescued” (4/8), while at day 13 bone and cartilage formed the upper beak in chimeras (3/4 were rescued, 1/4 was severely malformed). These morphologies corresponded to dramatic differences in proliferation in each group of embryo. The control embryos exhibited BrDU incorporation throughout the upper beak, and there were higher densities of proliferating cells near the distal tip (Fig. 5D, G). While embryos treated with SU5402 did show signs of cell proliferation there was no evidence of the high densities of BrDU incorporation that was apparent in the controls (Fig. 5E, H). After engraftment of quail neural crest cells there were abundant cells that had incorporated BrDU in the upper jaw (Fig. 5F, I) indicating that growth had been restored to the upper jaw anlagen. However, the increased proliferation was apparent in the chick cells and not in the quail donors. A similar heterogeneity in cell proliferation has been shown to shape the beaks of different birds (Wu et al., 2004; Wu et al., 2006), but here, large regions of quail derived cells were largely devoid of BrDU incorporation suggesting that these cells did not respond to local signals that stimulate proliferation, possibly because the donor cells were derived from older embryos (Fig. 5F inset). Along with this observation quail cells appeared to participate in skeletogenesis in small regions of the chimeras. At day 10, quail cells were present as chondrocytes and undifferentiated mesenchyme (Fig. 5J, K) and endothelial cells (Fig. 5L). At day 13 small regions of bone and cartilage were derived from the donor tissue, but the vast majority of the rescued skeleton was host-derived (not shown).
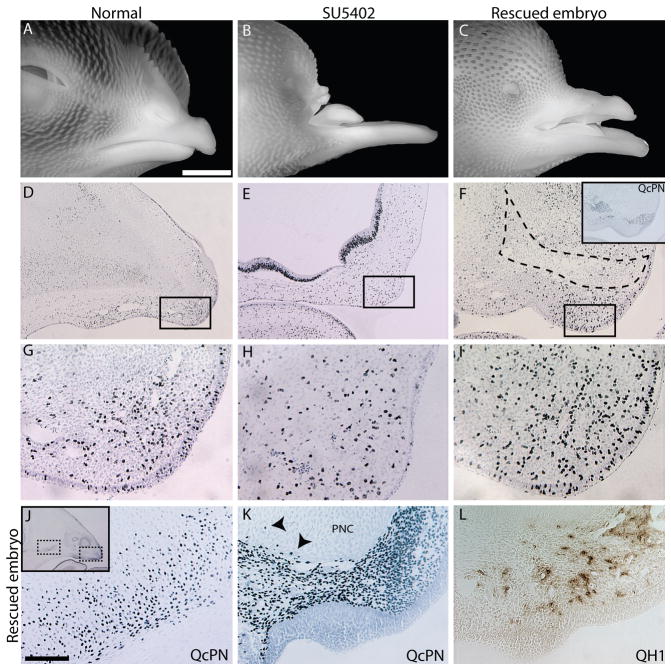
Figure 5. Morphology of the upper jaw is rescued after engraftment of quail neural crest cells.
(A) A lateral view of a control embryo illustrating normal morphology of the avian face at day 15 of development (n=8). (B) Embryos treated with SU5402 have anophthalmia and truncations of the upper jaw (n=12). (C) When quail neural crest cells were grafted into treated embryos, the upper jaw was normalized, but the embryos still exhibited anophthalmia (n=8). (D) Low magnification view of the tip of the upper jaw in day 10 control (n=8), (E) SU5402-treated (n=8), (F) and rescued (n=6) embryos at day 10 that were labeled and stained for BrDU incorporation. The inset in F shows the distribution of quail neural crest cells detected by QcPN immunostaining on an adjacent section. This region of quail cells is outlined with a black dotted line and does not exhibit a large number of BrDU-positive cells. The box in each panel illustrates the regions of high magnification in G–I. (G) In control embryos a high density of proliferating cells is evident at the tip of the upper jaw. (H) Cells in this same region of treated embryos exhibit reduced proliferation, and (I) in rescued embryos the surviving host mesenchyme has increased proliferation that resemble normal embryos. (J) Chimerism in rescued embryos was examined by QcPN immunostaining. In this case, the donor cells were located at the distal tip of the upper jaw. In this chimera, the medial portion of the prenasal cartilage (PNC) was derived from a mixture of donor and host cells. (Boxed areas in inset shown in J (left) and K (right). (K) Higher magnification of boxed region in J (right box) shows a high density of quail cells at the distal tip of the upper jaw, but only a few donor cells were integrated into the rescued PNC in this region (arrows). The ectoderm was derived exclusively from the host. (L) Immunohistochemistry using an anti-quail endothelial cell antibody (QH1) revealed that quail cells contributed to blood vessels throughout the developing upper jaw of chimeras. Scale bars: A–C: 5mm, D–F, J: 500μm, G–I, I, K, L: 100μm.
Discussion
Application of SU5402-soaked beads to the brain during early stages of facial development created malformations of the upper jaw. Our treatment likely blocked Fgf and VEGF signaling from both the neural and surface ectoderm of the FNP. In this experiment, neural crest cells migrating into the FNP underwent apoptosis en masse. In this experiment, neural crest cells migrating into the FNP, but not the maxillary or mandibular processes, underwent apoptosis en masse. Additionally, the vasculature was severely disrupted in treated embryos which likely created a localized region of ischemia in the head. Indeed, in our chimeric embryos, we observed a large contribution of quail donor cells to the vasculature of the head suggesting that restoration of blood flow may have played a role in rescuing the treated embryos. However, we did not explore this in detail in this work.
The morphological changes resulting from SU5402 treatment were evident beginning within the first 24 hours of treatment. However, apoptosis did not completely eliminate the neural crest population from the FNP. By 96 hours after treatment (~HH22) growth of the FNP became evident as surviving or regenerated neural crest cells populated this region. Nonetheless, the upper jaw was severely truncated and the skeleton was nearly absent at day 15 which suggested to us that the malformations were not simply the result of reduced progenitor cell numbers. Indeed, the FEZ did not form correctly in treated embryos; Shh expression was altered, and the combination of reduced cell number and the abnormal FEZ created the severe upper jaw malformations in these embryos.
Effect of FGF and VEGF Signaling During Jaw Development
FGF and VEGF signaling is required in the neural crest during migration into the FNP and pharyngeal arches. Reducing FGF signaling in murine embryos leads to extensive apoptosis of the forebrain and adjacent neural crest cells (Storm et al., 2003). The role of FGF signaling is more than as a survival factor; FGF8 participates in regulating migration of neural crest cells into the FNP. Application of FGF-soaked beads to embryos after ablation of neural crest progenitors rescued deficits produced in embryos by stimulating regeneration and migration of neural crest cells into the FNP (Creuzet et al., 2004). Signaling by FGFs also regulate proliferation and differentiation of neural crest mesenchyme. FGF signaling from the nasal pit controls survival and proliferation of neural crest mesenchyme that will form the lateral aspects of the upper jaw (Song et al., 2004; Szabo-Rogers et al., 2008; Szabo-Rogers et al., 2009). A recent report illustrates the role that VEGF plays during facial development. Application of ectopic VEGF to the face of embryos acted as a chemoattractant for neural crest cells emigrating from the neural tube (McLennan et al., 2009). In our experiments we cannot distinguish the role of FGF and VEGF from each other, because both pathways should have been completely blocked by our treatment. However, the phenotypes we observed appeared to result from massive apoptosis and not altered migration suggesting that Fgf signaling was significantly disrupted in these experiments.
Autonomous vs. Induced Molecular Properties of the FEZ
We initially defined the FEZ based on the juxtaposed expression domains of Shh and Fgf8 (Hu et al., 2003). However, there are a series of signaling molecules that are likely to mediate the function of this tissue including: Bmp2, Bmp4, Bmp7, and Wnt9b among others (Wall and Hogan, 1995; Barlow and Francis-West, 1997; Ashique et al., 2002; Lan, 2006; Foppiano et al., 2007; Merrill et al., 2008). Expression of these molecules, and of Fgf8 appear to be autonomous in the cephalic ectoderm (e.g., (Shigetani et al., 2000)). We did not detect major changes in the expression patterns of these molecules after treating embryos with SU5402, although Fgf8 was maintained for longer periods of time in the FEZ of SU5402-treated embryos possibly due to the absence of an antagonizing signal from Shh. Similarly, the expression of these molecules was unaffected after blocking or activating SHH signaling within the brain (Marcucio et al., 2005; Hu and Marcucio, 2009a). These data illustrate that there is a basic blueprint that is intrinsic to the surface ectoderm of the face.
In contrast, expression of Shh requires signals from multiple sources during development of the upper jaw. SHH signaling from the brain to the ectoderm prior to the arrival of neural crest cells within the FNP is required for Shh expression in the FEZ ((Marcucio et al., 2005; Eberhart et al., 2006), data not shown). Previous work has suggested that an additional signal is supplied by the neural crest cells (Eames and Schneider, 2005; Marcucio et al., 2005; Eberhart et al., 2008). In our current work two pieces of data prove this assertion. First, Shh expression was only observed in ectoderm that was located adjacent to neural crest cells in embryos treated with SU5402. Second, transplantation of neural crest cells from untreated embryos stimulated Shh expression in the FEZ and rescued malformations of the upper jaw. These data provide unequivocal evidence that the neural crest cells participate in the induction of Shh expression in the FEZ.
Understanding the intrinsic and non-cell autonomous properties of the ectoderm is relatively straightforward. However, defining the roles of each molecule during development of the upper jaw is more difficult. Our work (Foppiano et al., 2007; Hu and Marcucio, 2009b; Hu and Marcucio, 2009a) and that of others (Jeong et al., 2004; Welsh and O’Brien, 2009) suggest that SHH signaling from the ectoderm is a key regulatory factor that controls growth and morphogenesis of the upper jaw. During development of the secondary palate, Shh expression patterns correspond to the length of the upper jaw. In this work, the authors demonstrate that the ruggae covering the palatal shelves act as signaling centers and Shh expressed within the ruggae is the key signal that regulates proximodistal extension (Welsh and O’Brien, 2009). These data agree with our previous work. After blocking SHH signaling within the brain we observed significant morphological alterations of the upper jaw (Marcucio et al., 2005). However the only significant molecular change that we observed was the absence of Shh expression in the FEZ. Further, blocking the ability of neural crest cells to respond to SHH signals produce similar malformations (Jeong et al., 2004). Thus, SHH from the ectoderm appears to be critical signal that regulates morphogenesis of the upper jaw.
Role of Neural Crest Cells in Controlling Morphogenesis of the Face
Our results reveal a fundamental mechanism by which neural crest cells may control morphogenesis of the face. Neural crest cells participate in patterning the cephalic ectoderm that regulates growth and patterning of the upper jaw. We propose a two-step model for induction of Shh expression in the FEZ. First, SHH signaling from the forebrain stimulates the ability of the ectoderm to express Shh. This signal may establish regions that are competent to express Shh (Hu and Marcucio, 2009a). Then, as neural crest cells arrive in the FNP they induce Shh expression in the FEZ. We have demonstrated that BMP signaling within the neural crest is required for the onset of Shh expression in the FEZ (Foppiano et al., 2007). Whether this is a direct result of BMP signaling from the neural crest to the ectoderm is unknown. Nonetheless, once Shh expression begins in the FEZ, proximodistal extension of the upper jaw is initiated by controlling expression patterns of genes that are responsible for growth and development of the skeleton in the upper jaw (Hu and Marcucio, 2009b).
Differences in the spatial organization of Shh expression in the FEZ corresponds to different growth characteristics observed in avian and mammalian embryos. In birds, Shh expression spans the entire mediolateral width of the FNP, but in mammalian embryos Shh expression is restricted to lateral regions (Hu and Marcucio, 2009b). Signals from the brain participate in establishing the correct spatial pattern of Shh expression in the FEZ. When SHH signaling in the avian brain was activated, the FEZ was split into two domains and growth resembled that observed in mammals. Interestingly, in embryos treated with SU5402 the Shh expression domain in the FEZ was often split into two lateral domains and the faces appeared mammalian-like. This appearance resulted from an abnormal distribution of neural crest cells in treated chicks. Larger numbers of neural crest cells were located in the lateral regions compared to medial regions of the upper jaw in the treated chicks, and the ectoderm adjacent to these neural crest cells expressed Shh. This same organization is apparent in mammalian embryos, and the regionalization of neural crest cells may help regulate the unique expression pattern of Shh that distinguishes the mammalian and avian face. From these observations we conclude that hierarchical signaling events among the brain, the neural crest and facial ectoderm act to generate morphologic diversification of the face. By altering these signaling interactions phenotypic diversification that is observed during either evolution or disease can be produced.
Experimental Procedures
Treatment with SU5402
Fertilized chick eggs were prepared as described (Hu and Marcucio, 2009a). Ion exchange beads (AG1-X2, 100–200 mesh, and 106–180 mm diameter; BioRad) were soaked in SU5402 10mM in dimethylsulfoxide (DMSO; Sigma) or in DMSO as the control. One Bead was positioned inside the forebrain of chick embryos at HH 11.
Creation of quail-chick chimeras
To test the ability of neural crest cells to restore morphology to the face of treated embryos, we transplanted FNP-derived mesenchymal cells from quails into the FNP of treated chicks (Fig. 6). Quail embryos were incubated to HH22 and the FNP (Fig. 6, boxed line) was dissected. FNP tissues were placed in DMEM and digested with Dispase (2.4 units/ml, on ice for 20 minutes) to facilitate separation of mesenchyme from ectoderm and neuroectoderm. The mesenchyme was dissociated by incubation in 0.25%Trypsin-EDTA (Invitrogen Corporation) for 20min at 37°C, and then cells were collected via centrifugation. After resuspension the isolated mesenchymal cells were injected into the presumptive FNP of treated embryos that had reached HH18.
Figure 6. Creating Quail-Chick chimeras.
Schematic showing isolation and transplantation of FNP-derived mesenchymal cells. ectoderm (ect) and neuroectoderm (NE).
Embryo processing
Embryos were fixed in 4% paraformaldehyde at 4°C overnight, transferred to PBS containing 0.01% ethidium bromide and photographed using a Leica MFLZIII dissecting microscope equipped with epifluorescent illumination. Embryos were dehydrated and embedded in paraffin. Sections, 8 μm sagittal, were then prepared.
Immunohistochemistry
QCPN
To detect quail cells, chimeric embryos were fixed in Serra’s fixative, dehydrated, embedded in paraffin and cut into 10μm sagittal sections. Immunodetection of quail cells used QCPN, the quail-specific monoclonal antibody (Developmental Studies Hybridoma Bank), followed by incubation with a second antibody conjugated to Horseradish peroxidase (HRP). Diaminobenzidine (DAB, Sigma) was used to detect HRP. Sections were imaged using brightfield optics (Leica DMRB).
pMEK, pERK
Immunostaining was performed to detect expression of phosphorylated forms of ERK, MEK (Santa Cruz Biotechnology, Inc.) on tissue sections. Briefly, Sections were incubated with primary antibodies (1:100 dilution for pMEK, pERK) at 4°C overnight or at room temperature for 2 hrs. Sections were then incubated with a second antibody conjugated to Horseradish peroxidase (HRP). Diaminobenzidine (DAB, Sigma) was used to detect HRP. Sections were imaged using brightfield optics.
In situ hybridization
In situ hybridization was performed on paraffin wax embedded sections as previously described (Hu and Marcucio, 2009b). Subclones of Shh and Fgf8 were linearized for transcription of 35S-labeled anti-sense riboprobes. After hybridization sections were incubated with emulsion (Kodak) and were allowed to expose for 10 days, then sections were counterstained with bis-benzimide. Images are superimpositions of the fluorescent and pseudo-colored darkfield image. Color was applied linearly to the entire image using Adobe Photoshop.
Alcian Blue/Alizarin Red Staining
Embryos were processed for cartilage and bone staining with Alcian blue and alizarin red (Wassersug, 1976; Hu et al., 2008).
BrdU labeling and TUNEL analysis
Twenty minutes prior to sacrifice, 1μl of BrdU labeling reagent (Zymed, South San Francisco, CA) was injected into the vitelline vein. Embryos were fixed, embedded and sectioned as described. BrdU labeled cells were visualized on paraffin sections; detection of BrdU incorporation was assessed by immunohistochemistry and detection with diaminobenzidine (DAB) followed by counterstaining all nuclei with hematoxylin following the manufacturer’s instructions (Zymed). DNA fragmentation was examined using a TUNEL kit following the manufacturer’s instructions (Apoptag Plus, Intergen).
Supplementary Material
Ion exchange beads were soaked in concentrations of SU5402 10mM/ml dissolved in dimethylsulfoxide (DMSO) or beads were soaked in DMSO alone as the control for 1 hour at RT and then placed into the forebrain after emigration of neural crest cells was complete. The bead is labeled and outlined with a dotted white line. The embryo is outlined with a solid black line. The midbrain (M) and Hindbrain (H) are labeled. (scale bar: 500μm).
(A) Blood flow in a control embryo illustrates complete perfusion of the head (arrows; n=5). (B) After blockade of FGF signaling blood flow to the head is absent (n=8). The avascular region of the developing head is outlined with a dotted line. Scale bar: 1mm.
Acknowledgments
We would like to thank members of the Laboratory for Skeletal Regeneration at the Orthopaedic Trauma Institute for support, and we are especially grateful for the effort of Gina Baldoza. We would like to thank Benedikt Halgrimsson and Richard Schneider for discussions about this work and Stan “the Eggman” Keena of Petaluma farms for providing farm fresh eggs. This work was funded by the NIH (NIDCR: R01-DE018234) to R.M.
References
- Abzhanov A, Cordero DR, Sen J, Tabin CJ, Helms JA. Cross-regulatory interactions between Fgf8 and Shh in the avian frontonasal prominence. Congenital anomalies. 2007;47:136–148. doi: 10.1111/j.1741-4520.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Bachler M, Neubuser A. Expression of members of the Fgf family and their receptors during midfacial development. Mech Dev. 2001;100:313–316. doi: 10.1016/s0925-4773(00)00518-9. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci U S A. 2004;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Compagnucci C. Tweaking the hinge and caps: testing a model of the organization of jaws. J Exp Zoolog B Mol Dev Evol. 2008;310:315–335. doi: 10.1002/jez.b.21205. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA. 21st century neontology and the comparative development of the vertebrate skull. Dev Dyn. 2006;235:1256–1291. doi: 10.1002/dvdy.20796. [DOI] [PubMed] [Google Scholar]
- Eames BF, Schneider RA. Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development. 2005;132:1499–1509. doi: 10.1242/dev.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, He X, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet. 2008;40:290–298. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–1077. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Amiel J, Lyonnet S. Molecular bases of human neurocristopathies. Adv Exp Med Biol. 2006;589:213–234. doi: 10.1007/978-0-387-46954-6_14. [DOI] [PubMed] [Google Scholar]
- Firnberg N, Neubuser A. FGF Signaling Regulates Expression of Tbx2, Erm, Pea3, and Pax3 in the Early Nasal Region. Developmental Biology. 2002;247:237–250. doi: 10.1006/dbio.2002.0696. [DOI] [PubMed] [Google Scholar]
- Foppiano S, Hu D, Marcucio RS. Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Dev Biol. 2007;312:103–114. doi: 10.1016/j.ydbio.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of developmental stages in development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Higashihori N, Buchtova M, Richman JM. The function and regulation of TBX22 in avian frontonasal morphogenesis. Dev Dyn. 2010;239:458–473. doi: 10.1002/dvdy.22182. [DOI] [PubMed] [Google Scholar]
- Hu D, Colnot C, Marcucio RS. Effect of bone morphogenetic protein signaling on development of the jaw skeleton. Dev Dyn. 2008;237:3727–3737. doi: 10.1002/dvdy.21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio R, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009a;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev Biol. 2009b;325:200–210. doi: 10.1016/j.ydbio.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Ryan RC, Zhang Z, Bullard SA, Bush JO, Maltby KM, Lidral AC, Jiang R. Expression of Wnt9b and Activation of Canonical Wnt Signaling During Midfacial Morphogenesis in Mice. Dev Dyn. 2006;235:1448–1454. doi: 10.1002/dvdy.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Matovinovic E, Richman JM. Epithelium is required for maintaining FGFR-2 expression levels in facial mesenchyme of the developing chick embryo. Dev Dyn. 1997;210:407–416. doi: 10.1002/(SICI)1097-0177(199712)210:4<407::AID-AJA5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McLennan R, Teddy JM, Kasemeier-Kulesa JC, Romine MH, Kulesa PM. Vascular endothelial growth factor (VEGF) regulates cranial neural crest migration in vivo. Dev Biol. 2009;339:114–125. doi: 10.1016/j.ydbio.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AE, Eames BF, Weston SJ, Heath T, Schneider RA. Mesenchyme-dependent BMP signaling directs the timing of mandibular osteogenesis. Development. 2008;135:1223–1234. doi: 10.1242/dev.015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina M, Havens B, Velonis DA. FGF signaling in mandibular skeletogenesis. Orthod Craniofac Res. 2007;10:59–66. doi: 10.1111/j.1601-6343.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Mina M, Wang YH, Ivanisevic AM, Upholt WB, Rodgers B. Region- and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev Dyn. 2002;223:333–352. doi: 10.1002/dvdy.10056. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribisi S, Jr, Mariani FV, Aamar E, Lamb TM, Frank D, Harland RM. Ras-mediated FGF signaling is required for the formation of posterior but not anterior neural tissue in Xenopus laevis. Dev Biol. 2000;227:183–196. doi: 10.1006/dbio.2000.9889. [DOI] [PubMed] [Google Scholar]
- Riley BM, Murray JC. Sequence evaluation of FGF and FGFR gene conserved non-coding elements in non-syndromic cleft lip and palate cases. Am J Med Genet A. 2007;143A:3228–3234. doi: 10.1002/ajmg.a.31965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetani Y, Nobusada Y, Kuratani S. Ectodermally derived FGF8 defines the maxillomandibular region in the early chick embryo: epithelial-mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev Biol. 2000;228:73–85. doi: 10.1006/dbio.2000.9932. [DOI] [PubMed] [Google Scholar]
- Song Y, Hui JN, Fu KK, Richman JM. Control of retinoic acid synthesis and FGF expression in the nasal pit is required to pattern the craniofacial skeleton. Developmental Biology. 2004;276:313–329. doi: 10.1016/j.ydbio.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Storm EE, Rubenstein JL, Martin GR. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proc Natl Acad Sci U S A. 2003;100:1757–1762. doi: 10.1073/pnas.0337736100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Tran N, Liang C, Tang F, Rice A, Schreck R, Waltz K, Shawver LK, McMahon G, Tang C. Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl)methylidenyl]indolin-2-ones as inhibitors of VEGF, FGF, and PDGF receptor tyrosine kinases. J Med Chem. 1999;42:5120–5130. doi: 10.1021/jm9904295. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Whiting CJ, Nimmagadda S, Fu K, Richman JM. Novel skeletogenic patterning roles for the olfactory pit. Development. 2009;136:219–229. doi: 10.1242/dev.023978. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Yamada G, Grigoriou M, Pachnis V, Sharpe PT. Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development. 1999;126:51–61. doi: 10.1242/dev.126.1.51. [DOI] [PubMed] [Google Scholar]
- Wall NA, Hogan BL. Expression of bone morphogenetic protein-4 (BMP-4), bone morphogenetic protein-7 (BMP-7), fibroblast growth factor-8 (FGF-8) and sonic hedgehog (SHH) during branchial arch development in the chick. Mech Dev. 1995;53:383–392. doi: 10.1016/0925-4773(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Wassersug R. A procedure for differential staining of cartilage and bone in whole formalin-fixed vertebrates. Stain Technology. 1976;51:131–134. doi: 10.3109/10520297609116684. [DOI] [PubMed] [Google Scholar]
- Welsh IC, O’Brien TP. Signaling integration in the rugae growth zone directs sequential SHH signaling center formation during the rostral outgrowth of the palate. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke TA, Gubbels S, Schwartz J, Richman JM. Expression of fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3) in the developing head and face. Developmental Dynamics. 1997;210:41–52. doi: 10.1002/(SICI)1097-0177(199709)210:1<41::AID-AJA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Shen JY, Widelitz RB, Chuong CM. Morphoregulation of avian beaks: comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235:1400–1412. doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ion exchange beads were soaked in concentrations of SU5402 10mM/ml dissolved in dimethylsulfoxide (DMSO) or beads were soaked in DMSO alone as the control for 1 hour at RT and then placed into the forebrain after emigration of neural crest cells was complete. The bead is labeled and outlined with a dotted white line. The embryo is outlined with a solid black line. The midbrain (M) and Hindbrain (H) are labeled. (scale bar: 500μm).
(A) Blood flow in a control embryo illustrates complete perfusion of the head (arrows; n=5). (B) After blockade of FGF signaling blood flow to the head is absent (n=8). The avascular region of the developing head is outlined with a dotted line. Scale bar: 1mm.