Abstract
Sepsis is denoted as a complex syndrome that results from a serious infection followed by amplified and dysregulated inflammatory response. The complex immune response associated with sepsis results in a high rate of morbidity and mortality, despite substantial basic science and clinical advances. Recently, accumulating evidence have demonstrated that regulatory T cells (Tregs) play important roles in suppression of immune response, as demonstrated by the number increase and functional enhancement following the onset of severe sepsis or septic shock. This article reviews recent advances in understanding the potential role of Tregs in the pathophysiology of septic response, as well as implications in the development of novel therapeutic strategies for improving the clinical outcome of patients with severe injury and subsequent septic complications.
Introduction
Sepsis, as a leading cause of the high morbidity and mortality in critical illnesses, represents a complex clinical syndrome that results from a serious infection followed by an amplified and dysregulated inflammatory response (Yao and others 2009). It is important to understand its pathogenic mechanism to develop novel preventative and treatment strategies. Increasing data have revealed the importance of immune function disorder in sepsis and septic shock (Yao and others 2007). It is well known that resistance to infection and its outcome is influenced by a complex interplay between the host, the microbe, and the environment. Severe sepsis occurs as a result of abnormalities in the host response to infection (Yende and Kellum 2009). Recent studies have proved that regulatory T cells (Tregs) play a pivotal role in the pathogenesis of septic complications with considerable effect on suppressing the adaptive immune response. This article reviews the current literature regarding the role of CD4+CD25+ Tregs in the development of sepsis, highlighting the results of preliminary studies in human beings, followed by a discussion of its potential implication in the research for patient management.
CD4+CD25+ Regulatory T Cells
Tregs are one of the T cell subsets that have strong immune-suppressive activity. Tregs have been demonstrated to be able to maintain peripheral immune tolerance by a complicated mechanism to control the extent of cell-mediated immunity and prevent the development of excessive immune-induced tissue damage (Xu and others 2008). Along with the development of molecular biology, Tregs have been shown to be of importance in regulating the host immune responses in autoimmunity, tumor immunity, allergy, infectious diseases, and transplant rejection in animal models as well as in humans (Sakaguchi and others 1995; Shevach 2000; Gallimore and Sakaguchi 2002; Wood and Sakaguchi 2003; Suvas and others 2004; Chatila 2005; Baecher-Allan and Anderson 2006).
Tregs can be classified as natural Tregs and adaptive Tregs based on their cellular characteristics and mechanism of activation/action (Mills 2004) (Fig. 1). CD4+CD25+ Tregs can either arise directly in the thymus (the so-called naturally occurring subsets) or be induced by antigen in the periphery. The former is a group of naturally arising CD4+CD25+Foxp3+ T cells that are derived from the thymus (Bluestone and Abbas 2003; Cozzo and others 2003; Fehervari and Sakaguchi 2004). Moreover, the thymic ontogeny of CD4+CD25+ Tregs has been identified to occur during the process of maturation of CD4+CD8+CD3high thymocytes (Cupedo and others 2005; Darrasse-Jeze and others 2005). The second group comprises a group of Tregs that develop from the activation and differentiation of mature CD4+CD25− T cells in the periphery; these so-called adaptive CD4+CD25+ Tregs express Foxp3 and exhibit typical markers of the natural CD4+CD25+ Tregs (Chen and others 2003). In addition to natural and adaptive CD4+CD25+ Tregs, type-1 T regulatory (Tr1) and T helper 3 (Th3) are other Treg populations that play an important role in maintaining peripheral tolerance (Chatila 2005). Tr1 and Th3 cells belong to adaptive Tregs that have been identified to produce interleukin (IL)-10 and transforming growth factor (TGF)-β, respectively (Weiner 2001; Levings and Roncarolo 2005). Foxp3 is also expressed in a small population of CD8+ T cells. In addition to these well-defined populations of CD4+ Tregs, there is also evidence for an immunosuppressive function of Foxp3+CD8+ Tregs that secrete either IL-10 or TGF-β (Haynes and others 2000; Garba and others 2002). However, because the lack of specific cell surface markers for each population, it is often hard to reconcile, determine, and/or separate the roles of these cells (Venet and others 2008).
FIG. 1.
Natural and induced Treg populations. It is well known that natural Tregs can express the cell surface marker CD25 and the transcriptional factor FOXP3. In the periphery the majority of natural Tregs constitutively express high levels of CD25, but a significant minority express low levels of CD25 (Fontenot and others 2005; Wan and Flavell 2005). Both populations are immunosuppressive, and both express Foxp3. Other populations of antigen-specific Tregs can be induced from induced CD4+CD25− or CD8+CD25− T cells in the periphery under the influence of semimature dendritic cells, IL-10, TGF-β, and possibly IFN-α. These inducible populations of Tregs include distinct subtypes of CD4+ T cell: CD4+CD25+ Tr, TH3, and Tr1 cells. Additionally, Foxp3+CD8+ T cells or a subtype of these cells can produce IL-10 and have been called CD8+ T regulatory cells (Mills 2004). Treg, regulatory T cell; IL-10, interleukin-10; TGF-β, transforming growth factor-β; IFN-α, interferon-α; TH3, T helper 3; Tr1, T regulatory 1.
Although several studies have suggested that only the CD4+ T cell subsets that express the highest levels of CD25 have in vitro suppressive activity (Takahashi and others 1998; Baecher-Allan and others 2001), CD25 is not a definitive marker of natural Tregs. CD25 is an activation marker for T cells and is therefore also expressed by effector Th1 and Th2 cells, and suppressive function of CD25− T cells has also been documented (Sakaguchi 2000; Shevach 2002). Further, Tregs are best recognized by the expression of the forkhead/winged helix, transcription factor p3 (Foxp3 in mouse and FOXP3 in humans), a member of the well-known and diverse forkhead transcription factor family, which appears to serve as a master switch gene for Tregs development and function (Fontenot and others 2003; Hori and others 2003; Khattri and others 2003; Ramsdell 2003). In addition, glucocorticoid-induced tumor necrosis factor (TNF) receptor family-related gene (GITR) is a member of the TNF receptor superfamily that is found normally at high levels on CD4+CD25+ Tregs. Nevertheless, GITR expression is also found in low levels on T effector cells and increases further on T cells following activation (Shimizu and others 2002; Kohm and others 2004; Ronchetti and others 2004). Finally, the cytotoxic T lymphocyte-associated antigen (CTLA)-4, which has been reported to be expressed on Tregs, is also expressed on potent effector T cells (Kingsley and others 2002).
One significant advance in the study of murine and human Tregs has been the discovery of the IL-7 receptor CD127 as a novel surface marker of Tregs. Two recent reports have indicated that CD127 is downregulated on a subset of T cells in peripheral blood (Banham 2006; Liu and others 2006). Moreover, they demonstrated that with the use of a combination of CD4, CD25, and CD127, a highly purified population of Tregs was isolated, and their number exceeded previous identification based on other cell surface markers. Likely, they found that CD127 could be used to quantitate Treg subsets in individuals with type 1 diabetes, and it upheld the use of CD127 as a biomarker for human Tregs (Liu and others 2006). At present, it has been confirmed that the Treg's special surface symbol is CD4+CD25+CD127−FOXP3+.
It has been documented the essential role of Tregs in controlling both adaptive and innate immune responses. These cells, typically hyporesponsive to antigenic stimulation in vitro, can proliferate in vivo and downregulate effector activities mediated by CD4+ T and CD8+ T cells and also natural killer (NK) cells (Nishijima and others 1986; Hoyt and others 1988; Lin and others 1993) (Fig. 2). Evidence are also emerging to indicate that Tregs can suppress the proliferation and function of other cell types, such as dendritic cells (DCs) and B cells (Hotchkiss and others 2001, 2002; Jonuleit and others 2001). Suppressor function of Tregs is supposed to be dependent on immunosuppressive cytokines—including IL-10, TGF-β, IL-4, and interferon-γ—or through mechanism of cell–cell contact via CTLA-4, GITR, or cell-associated TGF-β expression. In addition, Tregs may regulate production of tryptophan or induce cell apoptosis in target cells, contributing to their suppressor function (Itoh and others 1999; Fallarino and others 2003; Nakamura and others 2004; Zhang and others 2004).
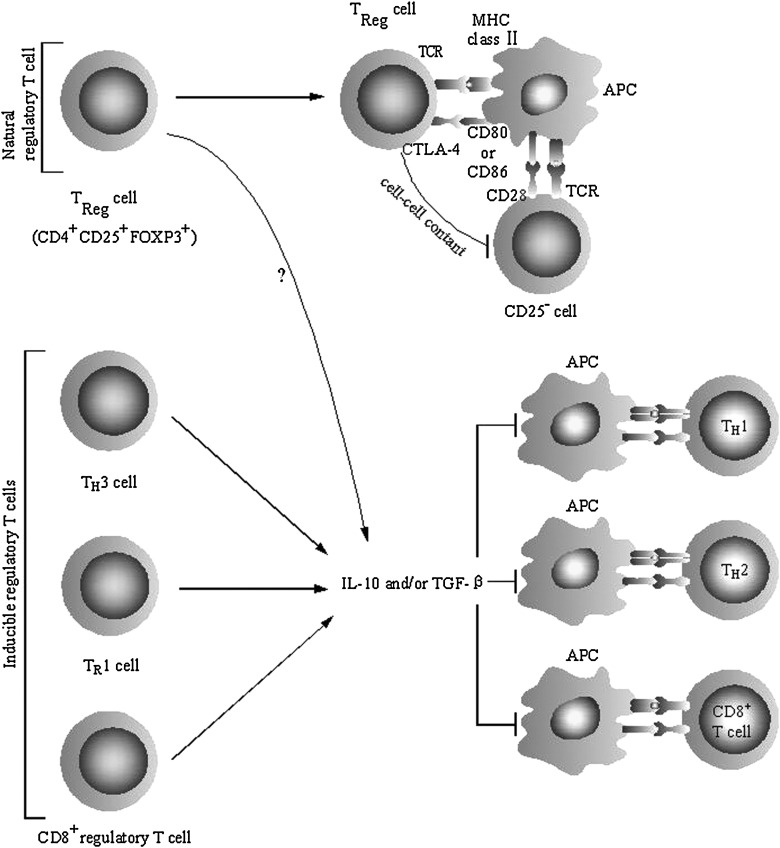
FIG. 2.
Targets of Tregs and mechanisms underlying immunosuppression. TReg cells (natural Tregs) inhibit the proliferation of CD25− T cells by multifactorial mechanisms including cell–cell contact. Natural Tregs express CTLA-4, which interacts with CD80 and/or CD86 on the surface of antigen-presenting cells, and this interaction delivers a negative signal for T cell activation. In addition, it has been demonstrated that secreted or cell surface TGF-β or IL-10 might play role in immunosuppression mediated by natural Tregs. Inducible populations of Tregs include TR1 cells, TH3 cells, and CD8+ Tregs, produce IL-10 and/or TGF-β. These immunosuppressive cytokines could directly inhibit the proliferation and cytokine formation of effector T cells, including TH1 cells, TH2 cells, and CD8+ cytotoxic T lymphocytes. Immunosuppressive function of these cytokines also performed through inhibiting the maturation and activation of antigen-presenting cells (Mills 2004). CTLA-4, cytotoxic T-lymphocyte antigen 4; TR1, T regulatory 1; MHC, major histocompatibility complex; APC, antigen presenting cell; TCR, T cell receptor.
The Role of Tregs in T Lymphocyte Anergy During Sepsis
The occurrence of a state of lymphocyte anergy has been described in patients with major trauma or burns, associated with mortality rate and with the development of secondary septic complications (Hotchkiss and Karl 2003; Monneret and others 2008). Anergy of T cells is a state of failure to proliferate or secrete cytokines and nonresponsiveness to their specific antigen. A study conducted by Choileain and others (2006) demonstrated that Tregs from burn-injured mice showed greater suppression of in vitro T cell proliferation and greater suppression of TH1-type cytokine production than Tregs from sham-injured animals. However, this study lacked sufficient power, at that time, to determine with an acceptable level of confidence the relationship between Treg activity and these later complications. Soon after, clinical evidence from the same laboratory provided that there was an injury-induced increase in the percentage of human Tregs. It was first demonstrated that Tregs inhibited T cell proliferation after injury in trauma patients and disrupted protective TH1-type cytokine production (MacConmara and others 2006). Therefore, the important observations showed that acute injury could induce or amplify CD4+CD25+ Treg function and that CD4+CD25+ Tregs might contribute to the development of postinjury immunosuppression.
Recently, Venet and others (2009) investigated whether Tregs were involved in the induction of lymphocyte anergy after sepsis. Observed in a total of 30 septic shock patients, the study reported an increased percentage of CD4+CD25+CD127− cells among the patients 3–7 days after onset of the septic shock when compared with healthy volunteers (12.0%±1.0% versus 6.8%±0.3%, P<0.001), closely correlated with a decrease in the number of CD4+ lymphocytes (410±54 versus 779±63 cells/μL, P<0.001). In those septic patients, the proliferative response of peripheral blood mononuclear cells after stimulation with mitogens was also markedly decreased in comparison with that in healthy individuals. Moreover, the increased proportion of CD25+ subset among CD4+ T cells was not due to a proliferation of Tregs but rather to a selective depletion of CD4+CD25− T cells (Monneret and others 2003; Venet and others 2004).
It was confirmed in an animal study that the percentages of CD4+CD25+Foxp3+ and CD4+CD25+CD127− cells were significantly increased 24 h after cecal ligation and puncture (CLP) in comparison with sham animals. Further, the proliferative response to mitogens of lymphocytes was restored when cocultured with Tregs of which Foxp3 expression was downregulated using ex vivo transfection with Foxp3-targeting siRNA. Therefore, based on these results of animal studies we deduced that the decreased proliferative response of lymphocytes observed in septic patients might be associated with the increased percentage of Tregs (Venet and others 2009).
From a methodological point of view, the measurement of percentage of Tregs might represent a simple and valuable surrogate marker of lymphocyte anergy usable on a routine basis for the monitoring of sepsis-induced immune dysfunction. The expression of FOXP3 is a normal consequence of CD4+ T cell activation (Allan and others 2007; Taylor and Llewelyn 2010). Consequently, simple analysis of FOXP3 expression can no longer be used as a surrogate marker of Tregs in humans (Gavin and others 2006; Pillai and others 2007; Wang and others 2007). Rather, like CD25, FOXP3 appears to be strictly associated with the unique phenotype and function of Tregs only when it is expressed constitutively and at high levels. However, the activation-induced expression of FOXP3 in effector T cells is not sufficient to uniformly suppress CD127 expression, while high and constitutive FOXP3 expression in Tregs is correlated with their characteristic CD127low/− phenotype. In view of the recent findings (Banham 2006; Liu and others 2006), the CD127 was downregulated on a subset of CD4+ T cells in peripheral blood. It was found that the majority of these cells were FoxP3+, including those that expressed low levels or no CD25. It is deduced that the low expression of CD127 might be a better cell surface marker for Tregs (Banham 2006). Later, strong expression of CD4 and CD25 in combination with the weak expression of CD127 was confirmed to be adequate for the characterization of circulating Tregs in blood samples from either healthy volunteers or patients with septic shock (Venet and others 2009). Also, the application of combined measurement of CD25 and CD127 has been recently proposed for accurate identification of Tregs in human whole blood and as a biomarker for human Tregs in various diseases (Liu and others 2006; Seddiki and others 2006; Codarri and others 2007). Finally, it was suggested that the determination of CD127 in addition to CD4 and CD25 may allow for a more rapid and standardizable estimation of Treg numbers, applicable in multicentered clinical trials, and should be of importance in better understanding the potential role of these cells in the development of severe sepsis (Venet and others 2009).
The Clinical Implication of Tregs in Sepsis
The aforementioned studies demonstrate that Tregs, as an important regulatory T cell subset, are closely correlated to the extent of immunosuppression during sepsis. At present, our understanding of the role of Tregs in patients during sepsis is still incomplete and there are several important questions that remain unanswered. However, our knowledge is accumulating in the past few years, and several studies, mainly concerning septic patients, have provided evidence that Tregs may be critically involved in the regulation of host response in the setting of serious infection (Table 1). For this reason, it is hoped that a better understanding of Tregs and its physiological action will help develop new therapeutical approaches aiming at modulating the immune response in conditions such as severe sepsis as well as septic shock.
Table 1.
CD4+CD25+ Regulatory T Cells in Patients with Sepsis
| Patients | Samples | Methods | Results | |
|---|---|---|---|---|
| Venet and others (2009) | Patients with septic shock | Peripheral blood | Investigate whether Treg participate in the induction of lymphocyte anergy | The increased circulating Treg percentage correlated with a decreased lymphoproliferative response |
| Saito and others (2008) | Septic patients | Peripheral blood | Measured the population of circulating Tregs and soluble CD25 in the plasma | Percentage of circulating CD4+CD25+ Tregs among the CD4+T cells↑ |
| Level of soluble CD25↑ | ||||
| Hein and others (2010) | Patients with shock | Peripheral blood | Measured cytokines and determined the lymphocyte count | The percentage of Tregs↑ |
| The absolute number of Tregs↑. The time course of Tregs was similar between survivors and nonsurvivors. No relation between Tregs and cytokine was found. |
||||
| Huang and others (2010) | Patients with major burns | Periphery blood | CTLA4 and FOXP3 were analyzed by flow cytometry; IL-10 and TGF-β were determined by ELISA | Expression of activation markers of Tregs↑ |
| Cytokines produced by Treg↑. The persistence of a pronounced immunoparalysis induced by Tregs is associated with a poor outcome after burns |
↑, increase;↓, decrease; Tregs, regulatory T cells; CTLA, cytotoxic T lymphocyte–associated antigen; IL-10, interleukin 10; TGF-β, transforming growth factor beta; ELISA, enzyme-linked immunosorbent assay.
In 2003, Monneret and others firstly demonstrated that the proportion of circulating Tregs in blood was increased at the outset of sepsis in a small group of patients with septic shock. Even more significant, they found that nonsurvivors presented higher percentage of CD4+CD25+ T-suppressor T cells. Likely, in some septic patients, it might induce a prolonged and severe immunoparalysis that was associated with poor prognosis.
Recently, 106 patients with extensive burn injury were included in our study. We found a higher expression of FOXP3 and CTLA-4 on the surface of CD4+CD25+ Tregs isolated from burn patients on postburn days 1–21 when compared with that of normal controls (P<0.05 or P<0.01) (Huang and others 2010). Additionally, the gene/expression of IL-10 and TGF-β1 in CD4+CD25+ Tregs from burn patients was augmented in comparison with normal controls, and there were marked differences among patients with different extents of burn injury (P<0.05 or P<0.01) (Huang and others 2010). Likely, among septic patients, the expression of these parameters in the survival group was markedly lower than those with fatal outcome on postburn days 3–21 (P<0.05 or P<0.01) (Huang and others 2010). Taken together, it could be assumed that severe burn injury per se could result in activation and maturation of CD4+CD25+ Tregs, invoking their immunodepressive activity to the full extent, finally leading to immunosuppression. Therefore, our studies indicate that elevated levels of cytokines produced by CD4+CD25+ Tregs and activation markers on CD4+CD25+ Tregs surface might be involved in the development of sepsis. In addition, it has been demonstrated that the persistence of a pronounced immunoparalysis induced by Tregs after severe sepsis is associated with a poor outcome in patients with major burns (Huang and others 2010).
Meantime, Hein and others (2010) reported that the percentage of Tregs increased as early as 3 days after the onset of shock, including septic and nonseptic shock. In the study, 43 patients suffering from shock (26 septic and 17 nonseptic) and 7 healthy volunteers were included. It was revealed that the absolute number of Tregs remained lower than in healthy volunteers and the percentage of Tregs was inversely correlated with severity at admission. But it was also noted that the time course of Tregs was similar between survivors and nonsurvivors and that no relation was found between Tregs and cytokine concentration.
Paradoxically, our human data did not seem in accordance with those reported by Hein and others. There seemed to have important differences between these 2 studies. First, the case of samples investigated by Hein and others was limited; second, there was a marked nonuniformity of patients in intensive care unit (ICU). Nevertheless, the percentages and counts of Tregs in peripheral blood, which were confirmed by our own as well as other results, are closely correlated with the prognosis of patients complicated by severe sepsis (P<0.05 or P<0.01) (Huang and others 2010). Therefore, the sequential monitoring of Tregs in peripheral blood from septic patients might be helpful to appraise the patient's condition and to judge the prognosis.
Early diagnosis of infection in systemic inflammatory response syndrome (SIRS) of ICU patients improves their outcome (Celis and others 1998). Although SIRS is an integral component of sepsis, 71.5% of critically ill patients with SIRS are not infected (Sprung and others 2006). With this in mind, it is important to discriminate infectious SIRS (ie, sepsis) from SIRS, for early diagnosis and treatment. In recent clinical studies, it was indicated that the percentages of circulating CD4+CD25+ Tregs among CD4+ T cells and the levels of soluble CD25 are significantly higher in septic patients in comparison to those of SIRS patients without infection. Therefore, it was concluded that the monitoring of the circulating CD4+CD25+ Tregs and soluble CD25 may be useful for predicting an increased risk for infectious complications in SIRS patients (Saito and others 2008).
The Interventional Strategy of Tregs for Sepsis
Significant advancements in our understanding of sepsis pathophysiology and immune dysfunction have occurred during the past 20 years, predominantly focusing on the roles of inflammation and activation of the innate immune system. Unfortunately, mortality from severe sepsis has only declined modestly, and several anti-inflammatory approaches have failed in clinical trials (Martin and others 2003; Weycker and others 2003). Accumulating clinical and experimental evidence indicate that sepsis causes a relative elevation in Treg number and increases its suppressive function. The problem of how manipulation of Tregs might improve sepsis outcome has received only minor attention, limited to animal studies.
In a recent report, Heuer and others (2005) documented that the adoptive transfer of in vitro–stimulated Tregs, but not CD4+CD25− cells, significantly improved survival in a mouse CLP model. It is known that the proper T cell function plays an important role in survival following CLP. This is the first one to demonstrate that Tregs improved sepsis survival through host T cells. It is also addressed that the effect on survival was accompanied by improved peritoneal bacterial clearance, enhanced peritoneal mast cell recruitment, and TNF-α production. In addition, another group of investigators observed that the number of Tregs significantly increased in wild-type mice, but not in TNF receptor type 2 (TNFR2)–deficient mice, following CLP. It was shown that Tregs expressed TNFR2 in mice and that TNF-α together with IL-2 was able to induce Treg proliferation and to enhance their suppressive function. This study reported that the depletion of Tregs decreased mortality following CLP, which suggested that elevated levels of TNF-α by interacting with TNFR2 predominantly expressed by Tregs, together with the sepsis-induced expansion of Tregs, might contribute to the postseptic immunosuppression and fatal consequences (Chen and others 2007). The findings were consistent with those of Choileain and others (2006) who observed that injured mice, which normally demonstrate suppressed Th1-type immunity, showed normal Th1 responses when depleted of Tregs.
In another report, Scumpia and others (2006) used monoclonal antibody against CD4 and CD25 to deplete both of these cell types before sepsis. Surprisingly, depletion of CD4+ or CD4+CD25+ Tregs did not alter mortality rate of sepsis. Similar results were reported by Wisnoski that a marked elevation in CD4+CD25+ Tregs and their percentages occurred after sepsis in septic (CLP) mice. In addition, in vivo depletion of CD25+ cells prior to CLP markedly restored proliferative capacity and Th1 cytokine release, while it did not alter plasma proinflammatory cytokine levels. Interestingly, when animals were pretreated with antibodies that depleted the CD25+ cell population, no effect on septic survival was revealed (Wisnoski and others 2007). The aforementioned studies demonstrate that the depletion of CD4+CD25+T cells does not alter mortality rate of sepsis. However, the problem could not be considered to be so simplistic, since CD4+CD25+ Tregs are not the only cells that express the CD25 receptor. Likely, such a depletion approach might have removed both the detrimental effects of CD4+CD25+ Tregs as well as CD25+ non-Tregs, for example, most activated T cells, which might be beneficial to a functional innate/adaptive immune response to septic challenge (Scumpia and others 2006; Wisnoski and others 2007). Thus, it was indicated that the ablation of CD25+ cells did not give rise to survival advantage or disadvantage.
GITR is expressed on both CD4+CD25+ Tregs and CD4+CD25− effector T cells (Shimizu and others 2002). One year later, the same group examined which subset of T cells was necessary for the survival benefit of anti-GITR treatment in a septic mice model. It was indicated that CD4+ T cells but not CD4+CD25+ Tregs were necessary for the survival benefit offered by anti-GITR treatment. In the study, the beneficial effects of anti-GITR treatment on T cell function led to a CD4+ T-cell-dependent improvement in survival, as the improvement was abolished in mice that were previously depletion of CD4+ T cells, but not CD25+ Tregs, which eliminated indirectly the survival benefit of anti-GITR treatment (Scumpia and others 2007).
It is acknowledged that the suppressive function of Tregs is amplified following sepsis or acute insults, demonstrating enhanced regulatory capacity of Tregs after injury. However, perhaps depending on the methodology used for experiment or the timing of Tregs depletion after injury, resultant effects of Tregs on mortality (beneficial or deleterious) and their potential role in injury-induced immune dysfunction remain unresolved (Table 2) (Venet and others 2008). As Tregs have been shown to affect both adaptive and innate immunity, the relationship between changes in their numbers or potency and outcome of septic patients is very complex. However, according to their known properties and the results of various studies in mice, the vital role of Tregs in the decrease of Th1 immune responsiveness and in the downregulation of proinflammatory cytokine formation secondary to severe insults is established.
Table 2.
CD4+CD25+ Regulatory T Cells in Murine Models of Injury
| Mouse model | Methods | Results | Effect of Treg on mortality | |
|---|---|---|---|---|
| Choileain and others (2006) | Burn injury | Anti-CD25 Antibody-mediated depletion | Prevent↓ | No effect |
| ↓Thl immune response | ||||
| ↓Mortality | ||||
| Heuer and others (2005) | Polymicrobial sepsis | Adoptive transfer | ↓Thl immune response | Protective |
| Chen and others (2007) | Polymicrobial sepsis | Anti-CD25 Antibody-mediated depletion | ↑Early survival | Deleterious |
| Scumpia and others (2006) | Polymicrobial sepsis | Anti-CD25 Antibody-mediated depletion | No effect on mortality | No effect |
| Wisnoski and others (2007) | Polymicrobial sepsis | Anti-CD25 Antibody-mediated depletion | Prevent↓ | No effect |
| Thl immune response | ||||
| No effect on mortality |
↑, Increase;↓, decrease.
Summary
An increased understanding of the alterations in human Treg function in patients during sepsis, together with controlled animal studies addressing mechanisms underlying this phenomenon, will help clarify the potential role of Tregs in patient's outcome after sepsis. While accumulating clinical and experimental evidence indicate that the manipulation of Tregs may provide a promising strategy for septic patients, the contradictory data as presented in this article suggest that more work is needed to further elucidate the conditions under which such a treatment may be effective (Kessel and others 2009). We believe that a better understanding with regard to the mechanism of regulatory function of Tregs, which are found to exert such a profound effect on the immune response, may help improve our clinical ability not only in diagnosis but also in treatment for the critically ill septic individuals.
Future Prospective
Taken together, the available data of these observations suggest that the manipulation of Tregs might provide a novel treatment modality for septic response. Nevertheless, the inconsistent results noted in the article implicate that further studies will be needed to clarify whether patients with sepsis will benefit from such a therapeutic strategy.
Acknowledgments
This work was supported, in part, by grants from the National Natural Science Foundation (Nos. 30971192, 81130035, 81071545, and 81121004), and the National Basic Research Program of China (No. 2012CB518102).
Author Disclosure Statement
The authors declare that they have no competing interests.
References
- Allan SE. Crome SQ. Crellin NK. Passerini L. Steiner TS. Bacchetta R. Roncarolo MG. Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C. Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C. Brown JA. Freeman GJ. Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3+ regulatory T cells. Trends Immunol. 2006;27:541–544. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Bluestone JA. Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Celis R. Torres A. Gatell JM. Almela M. Rodriquez-Roisin R. Agusti-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1998;93:318–324. doi: 10.1378/chest.93.2.318. [DOI] [PubMed] [Google Scholar]
- Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–959. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Chen W. Jin W. Hardegen N. Lei KJ. Li L. Marinos N. McGrady G. Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. Baumel M. Mannel DN. Howard Z. Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- Choileain NN. MacConmara M. Zang Y. Murphy TJ. Mannick JA. Lederer JA. Enhanced regulatory T cell activity is an element of the response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- Codarri L. Vallotton L. Ciuffreda D. Venetz JP. Garcia M. Hadaya K. Buhler L. Rotman S. Pascual M. Pantaleo G. Expansion and tissue infiltration of an allospecific CD4+CD25+CD45RO+IL-7Ralphahigh cell population in solid organ transplant recipients. J Exp Med. 2007;204:1533–1541. doi: 10.1084/jem.20062120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzo C. Larkin J. Caton AJ. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678–5682. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- Cupedo T. Nagasawa M. Weijer K. Blom B. Spits H. Development and activation of regulatory T cells in the human fetus. Eur J Immunol. 2005;35:383–390. doi: 10.1002/eji.200425763. [DOI] [PubMed] [Google Scholar]
- Darrasse-Jeze G. Marodon G. Salomon BL. Catala M. Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- Fallarino F. Grohmann U. Hwang KW. Orabona C. Vacca C. Bianchi R. Belladonna ML. Fioretti MC. Alegre ML. Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Fehervari Z. Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Fontenot JD. Gavin MA. Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD. Rasmussen JP. Williams LM. Dooley JL. Farr AG. Rudensky AY. Regulatory T cell lineage specification by the fork-head transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gallimore A. Sakaguchi S. Regulation of tumour immunity by CD25+ T cells. Immunology. 2002;107:5–9. doi: 10.1046/j.1365-2567.2002.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garba ML. Pilcher CD. Bingham AL. Eron J. Frelinger JA. HIV antigens can induce TGF-β1-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- Gavin MA. Torgerson TR. Houston E. DeRoos P. Ho WY. Stray-Pedersen A. Ocheltree EL. Greenberg PD. Ochs HD. Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LM. Vanderlugt CL. Dal Canto MC. Melvold RW. Miller SD. CD8(+) T cells from Theiler's virus-resistant BALB/cByJ mice downregulate pathogenic virus-specific CD4(+) T cells. J Neuro Immunol. 2000;106:43–52. doi: 10.1016/s0165-5728(00)00212-5. [DOI] [PubMed] [Google Scholar]
- Hein F. Massin F. Carvoisy-Popovic A. Barraud D. Levy B. Bollaert PE. Gibot S. The relationship between CD4+CD25+CD127- regulatory T cells and inflammatory response and outcome during shock states. Crit Care. 2010;14:R19. doi: 10.1186/cc8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer JG. Zhang T. Zhao J. Ding C. Cramer M. Justen KL. Vonderfecht SL. Na S. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- Hori S. Nomura T. Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS. Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS. Tinsley KW. Swanson PE. Grayson MH. Osborne DF. Wagner TH. Cobb JP. Coopersmith C. Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS. Tinsley KW. Swanson PE. Schmieg RE., Jr. Hui JJ. Chang KC. Osborne DF. Freeman BD. Cobb JP. Buchman TG. Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- Hoyt DB. Ozkan AN. Ninnemann JL. Hansbrough JF. Pinney E. Wormsley S. Trauma peptide induction of lymphocyte changes predictive of sepsis. J Surg Res. 1988;45:342–348. doi: 10.1016/0022-4804(88)90129-1. [DOI] [PubMed] [Google Scholar]
- Huang LF. Yao YM. Dong N. Yu Y. He LX. Sheng ZY. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care. 2010;14:R3. doi: 10.1186/cc8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M. Takahashi T. Sakaguchi N. Kuniyasu Y. Shimizu J. Otsuka F. Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Jonuleit H. Schmitt E. Steinbrink K. Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- Kessel A. Bamberger E. Masalha M. Toubi E. The role of T regulatory cells in human sepsis. J Autoimmun. 2009;32:211–215. doi: 10.1016/j.jaut.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Khattri R. Cox T. Yasayko SA. Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kingsley CI. Karim M. Bushell AR. Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4 and IL-10 dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- Kohm AP. Williams JS. Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- Levings MK. Roncarolo MG. Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr Top Microbiol Immunol. 2005;293:303–326. doi: 10.1007/3-540-27702-1_14. [DOI] [PubMed] [Google Scholar]
- Lin RY. Astiz ME. Saxon JC. Rackow EC. Altered leukocyte immunophenotypes in septic shock: studies of HLA-DR, CD11b, CD14, and IL-2 R epression. Chest. 1993;104:847–853. doi: 10.1378/chest.104.3.847. [DOI] [PubMed] [Google Scholar]
- Liu W. Putnam AL. Zhou XY. Szot GL. Lee MR. Zhu S. Gottlieb P. Kapranov P. Gingeras TR. Groth BFdS. Clayberger C. Soper DM. Ziegler SF. Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConmara MP. Maung AA. Fujimi S. McKenna AM. Delisle A. Lapchak PH. Rogers S. Lederer JA. Mannick JA. Increased CD4+CD25+ T Regulatory Cell Activity in Trauma Patients Depresses Protective Th1 Immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS. Mannino DM. Eaton S. Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Mills KHG. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- Monneret G. Debard AL. Venet F. Bohe J. Hequet O. Bienvenu J. Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- Monneret G. Venet F. Pachot A. Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. Kitani A. Fuss I. Pedersen A. Harada N. Nawata H. Strober W. TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- Nishijima MK. Takezawa J. Hosotsubo KK. Takahashi H. Shimada Y. Yoshiya I. Serial changes in cellular immunity of septic patients with multiple organ-system failure. Crit Care Med. 1986;14:87–91. doi: 10.1097/00003246-198602000-00002. [DOI] [PubMed] [Google Scholar]
- Pillai V. Ortega SB. Wang CK. Karandikar NJ. Transient regulatory T-cells:a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Ronchetti S. Zollo O. Bruscoli S. Agostini M. Bianchini R. Nocentini G. Ayroldi E. Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- Saito K. Wagatsuma T. Toyama H. Ejima Y. Hoahi K. Shibusawa M. Kato M. Klrosawa S. Sepsis is characterized by the increases in percentages of circulating CD4+CD25+ regulatory T cells and plasma level of soluble CD25. Tohoku J Exp Med. 2008;216:61–68. doi: 10.1620/tjem.216.61. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Sakaguchi N. Asano M. Toda M. Immunologic self-tolerance maintained by activated T cellsexpressing IL-2 receptor alpha-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Scumpia PO. Delano MJ. Kelly KM. O'Malley KA. McAuliffe PF. Brusko T. Ungaro R. Barker T. Wynn JL. Atkinson MA. Reeves WH. Salzler MJ. Moldawer LL. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–7949. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- Scumpia PO. Delano MJ. Kelly-Scumpia KM. Weinstein JS. Wynn JL. Winfield RD. Xia C. Chung CS. Ayala A. Atkinson MA. Reeves WH. Clare-Salzler MJ. Moldawer LL. Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood. 2007;110:3673–3681. doi: 10.1182/blood-2007-04-087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N. Santner-Nanan B. Martinson J. Zaunders J. Sasson S. Landay A. Solomon M. Selby W. Alexander SI. Nanan R. Kelleher A. Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–429. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+CD25+ suppressor T cells:more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Shimizu J. Yamazaki S. Takahashi T. Ishida Y. Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–140. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- Sprung CL. Sakr Y. Vincent JL. Le Gall JR. Reinhart K. Ranieri VM. Gerlach H. Fielden J. Groba CB. Payen D. An evaluation of systemic inflammatory response syndrome signs in the sepsis occurrence in acutely ill patients (SOAP) study. Intensive Care Med. 2006;32:421–427. doi: 10.1007/s00134-005-0039-8. [DOI] [PubMed] [Google Scholar]
- Suvas S. Azkur AK. Kim BS. Kumaraquru U. Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Kuniyasu Y. Toda M. Sakaguchi N. M. Itoh M. Iwata M. Shimizu J. Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Taylor AL. Llewelyn MJ. Superantigen-induced proliferation of human CD4+CD25- T cells is followed by a switch to a functional regulatory phenotype. J Immunol. 2010;185:6591–6598. doi: 10.4049/jimmunol.1002416. [DOI] [PubMed] [Google Scholar]
- Venet F. Chuang CS. Kherouf H. Geeraert A. Malcus C. Poitevin F. Bohe J. Lepape A. Ayala A. Monneret G. Increased circulating regulatory T cells (CD4+CD25+CD127-) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–686. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet F. Chung CS. Monneret G. Huang X. Horner B. Garber M. Ayala A. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- Venet F. Pachot A. Debard AL. Bohe J. Bienvenu J. Lepape A. Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25- lymphocytes. Crit Care Med. 2004;32:2329–2331. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- Wan YY. Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Ioan-Facsinay A. vander Voort EI. Huizinga TW. Toes RE. Transient expression of FOXP3 in human activated nonregulatory. Eur J Immunol. 2007;37:21–23. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta -secreting regulatory cells. Microbes Infect. 2001;3:947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- Weycker D. Akhras KS. Edelsberg J. Angus DC. Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- Wisnoski N. Chung CS. Chen Y. Huang X. Ayala A. The contribution of CD4+CD25+T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–257. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KJ. Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- Xu CT. Li WM. Yao YM. Regulation mechanism of regulatory T cells in immunoregulatory responses. Int J Pathol Clin Med. 2008;28:199–204. [Google Scholar]
- Yao YM. Huang LF. Lin HY. Improvement in understanding of potential mechanism and immunomodulatory strategy for sepsis. Trauma Surg. 2007;9:4–7. [Google Scholar]
- Yao YM. Sheng ZY. Huang LF. The effect of a novel cytokine, high mobility group box-1 protein, on the development of traumatic sepsis. Chin J Integr Med. 2009;15:13–15. doi: 10.1007/s11655-009-0013-0. [DOI] [PubMed] [Google Scholar]
- Yende S. Kellum JA. Understangding genetics of sepsis: will new technology help? Crit Care. 2009;13:141–142. doi: 10.1186/cc7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Koldzic DN. Izikson L. Reddy J. Nazareno RF. Sakaguchi S. Kuchroo VK. Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]