Abstract
While patients with advanced cancer experience a wide range of symptoms, no work has been done to determine an optimal cutpoint for a low versus a high number of symptoms. Analytic approaches that established clinically meaningful cutpoints for the severity of cancer pain and fatigue provided the foundation for this study. The purpose of this study was to determine the optimal cutpoint for low and high numbers of symptoms using a range of potential cutpoints and to determine if those cutpoints distinguished between the two symptom groups on demographic and clinical characteristics and depression, anxiety, and quality of life (QOL). Patients with advanced cancer (n=110) completed a symptom assessment scale, and measures of depression, anxiety, and QOL. Combinations of cutpoints were tested to yield one- and two-cutpoint solutions. Using analysis of variance for QOL scores, the F-ratio that indicated the highest between-group difference was determined to be the optimal cutpoint between low and high number of symptoms. A cutpoint of ≤12 symptoms (i.e., 0–12 is low, 13–32 is high) was the optimal cutpoint for total number of symptoms. Significant differences in depression, anxiety, and QOL scores validated this cutpoint. Psychological symptoms had higher occurrence rates in the high symptom group. Findings suggest that a threshold exists between a low and a high number of symptoms in patients with advanced cancer. Psychological symptoms were significantly different between patients in the low versus high symptom groups and may play an important role in QOL outcomes in patients with advanced cancer.
Introduction
Serlin and colleagues provided evidence to support the establishment of clinically meaningful cutpoints for mild, moderate, and severe pain in oncology patients.1 Since 1995, several studies refined these cutpoints for acute,2,3 chronic,4 and cancer5 pain and established cutpoints for cancer-related fatigue.6 Cutpoints were created based on the idea that within the symptom experience, symptom severity comprised the internal sensory dimension and interference caused by symptoms comprised the external reactive dimension.1 The nonlinear relationship between severity and interference with function was demonstrated by a statistically significant “jump” in interference scores as symptom severity went from mild to moderate or moderate to severe.1–5
Clinically meaningful cutpoints have served as a foundation for treatment guidelines. For example, the National Comprehesive Cancer Network used these cutpoints to establish treatment algorithms for cancer pain7 and fatigue management.8 In addition, cutpoints can be used to determine if management strategies are effective.7
Findings from recent reviews suggest that patients with advanced cancer experience numerous concurrent symptoms.9,10 Across 46 studies, 24 different symptoms occurred in ≥20% of the pooled samples. While total number of symptoms was not examined as a factor that contributes to significant decrements in quality of life (QOL), various components of this concept of symptom burden11,12 (e.g., symptom severity13,14 and symptom distress15,16) were associated with significant decreases in functional status and QOL. In addition, symptom assessment is a clinically meaningful proxy for QOL and an extremely reliable patient reported outcome.11,17 Therefore, it is reasonable to suggest that QOL could be used as an outcome to evaluate clinically meaningful cutpoints for low and high number of symptoms in patients with advanced cancer. In addition, given that patients with advanced cancer report more symptoms than patients with earlier-stage disease,9,18 and comprehensive, multidimensional symptom assessment tools may be burdensome for patients and clinicians, the determination of a cutpoint for number of symptoms might have some clinical utility. Routine screening with a symptom checklist could determine a patient's total number of symptoms. Then clinicians could use a low/high cutpoint to identify high-risk patients who warrant more-in-depth assessments and/or more-aggressive symptom management interventions.
Expanding on Serlin and colleagues' idea,1 in this study total number of symptoms was viewed as the sensory dimension of the symptom experience, and QOL was viewed as the reactive dimension. If total number of symptoms has a nonlinear relationship with QOL (as does pain severity and interference), then a significant jump in QOL scores would occur as the total number of symptoms goes from low to high. Said another way, clinically meaningful differences in total number of symptoms would be associated with significant differences in QOL. This hypothesis is supported by clinical observations in which patients with advanced cancer manage relatively well with a “low” number of symptoms. However, when the total number of symptoms crosses some threshold between low and high, various QOL domains are impaired and symptom management interventions are not effective. Therefore, the purposes of this study, in a sample of patients with advanced cancer, were to determine the optimal cutpoint for low and high number of symptoms using a range of potential cutpoints and to determine if those cutpoints distinguished between the symptom groups in any demographic and clinical characteristics as well as in depression, anxiety, and QOL.
Methods
Design and sample
This descriptive study of 110 patients is part of an ongoing randomized clinical trial that will determine the efficacy of two different doses of a psychoeducational intervention to improve cancer pain management. Patients were included if they: were adult oncology outpatients (≥18 years of age) experiencing cancer pain of somatic or visceral origin; were able to read, write, and understand English; agreed to participate and provided written informed consent; had a Karnofsky Performance Status (KPS) score of ≥50; had an average pain intensity score of ≥3.0 on a 0 to 10 numeric rating scale (NRS); had a life expectancy of ≥6 months; and had a telephone line. Patients were excluded if they: had neuropathic cancer pain; had a documented previous or current psychiatric disorder; or were receiving hospice care, in order not to interfere with the pain management program provided by hospice.
Settings
Patients were recruited from a comprehensive cancer center, two veterans' affairs hospitals, and four community-based oncology clinics. They were asked by a clinician at the site about study participation. If the patient was interested, the clinician informed the research nurse, who discussed the study and obtained written informed consent. Questionnaires were completed in patients' homes prior to the onset of the intervention.
Instruments
The Patient Information Questionnaire obtained demographic characteristics and KPS score.19,20 The valid and reliable Self-Administered Comorbidity Questionnaire (SCQ), with scores that range from 0 to 39, provided information on comorbidities.21,22 Medical records were reviewed to obtain information on cancer diagnosis, current treatments, reason for current treatments, and metastatic sites.
The Memorial Symptom Assessment Scale (MSAS) contains a list of 32 physical and psychological symptoms that occur as a result of cancer or its treatment. Patients were asked to indicate whether they had experienced each symptom in the past week (i.e., symptom occurrence). If they had experienced the symptom, they were asked to rate its frequency of occurrence, severity, and distress. MSAS was developed for use with oncology patients23,24 and has established validity in palliative care patients.25
Multidimensional Quality of Life Scale–Cancer Version 2 (MQOLS-CA2) is a 33-item instrument that measures five dimensions of QOL (i.e., psychological well-being, general physical well-being, nutrition, symptom distress, and interpersonal well-being).26,27 Each item is rated on a 0 to 10 NRS with higher scores indicating a better QOL. MQOLS-CA2 has well established validity and reliability.26,27
Measures used to validate cutpoints
The Center for Epidemiologic Studies–Depression (CES-D) scale consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. Higher scores indicate higher levels of depression. CES-D has well established concurrent and construct validity.28,29
Spielberger State–Trait Anxiety Inventories (STAI-S and STAI-T) each consist of 20 items. Scores can range from 20 to 80. Higher scores indicate greater anxiety. STAI-T measures an individual's predisposition to anxiety determined by his or her personality and estimates how a person generally feels. STAI-S measures an individual's transitory emotional response to a stressful situation. Both inventories have well established validity and reliability.30,31
The Medical Outcomes Study–Short Form (MOS-SF36), a 36-item instrument, is a generic measure of QOL. It consists of eight subscales and two component scores that evaluate important health concepts. Higher scores indicate better QOL.32,33
Data analysis
Data were analyzed using SPSS 18.0 (SPSS Inc., Chicago, IL). Descriptive statistics were used to characterize the sample and the study variables. Symptom occurrence rates were generated for each of the symptoms evaluated on the MSAS. Total number of symptoms was calculated by summing the number of symptoms based on a response on any one of the four dimensions—occurrence, frequency, severity, and distress.
A cutpoint that divided the sample into low and high number of symptoms was created using the analytic strategy of Serlin and colleagues.1 Twelve categorical variables that represented dichotomizing the number of symptoms into low and high using the twelve possible cutpoints between 5 and 16 were created (e.g., 0–5=low, 6–32=high, 0–6=low, 7–32=high, and so on up to 0–16=low, 17–32=high) and related to the five MQOLS-CA2 subscales using multivariate analysis of variance (MANOVA) and to the MQOLS-CA2 total score using analysis of variance (ANOVA). Combinations of cutpoints were tested to yield two cutpoints (three groups) as well as one cutpoint (two groups) solutions. The criterion used to determine the optimal cutpoint groups was the F-ratio for the between-group effect for both the MANOVA and the ANOVA (Table 1). While attempts were made to establish cutpoints for low, medium, and high total number of symptoms, a clear cutpoint between medium and high was not identified. Therefore, the analysis proceeded to determine a single cutpoint solution. Five symptoms was selected as the lower limit and 16 symptoms as the upper limit of testable cutpoints, because no patient reported fewer than 5 symptoms, and 16 symptoms was approximately equal to the mean number of symptoms for the sample.
Table 1.
Results of the Cutpoint Analyses for Total Number of Symptoms Using the Total and Subscale Scores from the Multidimensional Quality of Life Scale–Cancer Version 2
| |
Analysis of variance |
Multivariate analysis of variance |
||
|---|---|---|---|---|
| Cutpoints for number of symptoms per group | Rank | F | Rank | F |
| Low 0–5 High 6–32 |
10 | 3.385 | 12 | 1.384 |
| Low 0–6 High 7–32 |
8 | 6.986 | 11 | 2.349 |
| Low 0–7 High 8–32 |
11 | 2.696 | 4 | 4.479 |
| Low 0–8 High 9–32 |
12 | 1.324 | 9 | 3.475 |
| Low 0–9 High 10–32 |
7 | 9.196 | 5 | 4.478 |
| Low 0–10 High 11–32 |
6 | 9.401 | 7 | 4.053 |
| Low 0–11 High 12–32 |
9 | 5.716 | 10 | 3.439 |
|
Low 0–12 High 13–32 |
1 | 13.548 | 1 | 5.176 |
| Low 0–13 High 14–32 |
5 | 9.815 | 8 | 3.752 |
| Low 0–14 High 15–32 |
3 | 11.543 | 3 | 4.524 |
| Low 0–15 High 16–32 |
2 | 11.831 | 2 | 4.893 |
| Low 0–16 High 17–32 |
4 | 10.360 | 6 | 4.277 |
To determine if the optimal cutpoint for the total number of symptoms differed between the low and high symptom groups on demographic and clinical characteristics, independent sample t-tests and χ2 and Kendall's tau analyses were used. Preliminary analyses revealed significant between-groups differences for age and living arrangements. Because only age is associated with depression,34,35 anxiety36 and/or QOL37,38 analyses of covariance (ANCOVA), controlling for age, were used to evaluate for between-group differences in these measures. All calculations used actual values. Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest set of complete data between the groups. A p-value of<0.05 was considered statistically significant.
Results
Cutpoint calculations
As shown in Table 1, a cutpoint of ≤12 symptoms (i.e., 0–12 symptoms is low and 13–32 symptoms is high) was the optimal cutpoint, in that it had the largest between-group F-ratios for both the MQOLS-CA2 subscale and total scores. Using ≤12 symptoms as the cutpoint, 60.9% of the sample (n=67) was classified as having a high number of symptoms.
Differences in demographic and clinical characteristics
Consistent with the American Cancer Society's definition of advanced cancer,39 the majority of the patients had metastatic disease (∼90%) and their goals of treatment were control or palliation (∼96%). Except for age and living arrangements, no differences were found between the low and high symptom groups in any demographic or clinical characteristics (Table 2). Patients in the high symptom group were significantly younger and were less likely to live alone.
Table 2.
Differences in Demographic and Clinical Characteristics between the Low (n=43) and High (n=67) Total Number of Symptom Groups
| Characteristic | Low symptom group n=43 Mean (SD) | High symptom group n=67 Mean (SD) | Statistics t-test; p-value |
|---|---|---|---|
| Age (years) | 63.6 (10.1) | 57.4 (13.1) | 2.7; .009 |
| Education (years) | 15.5 (3.2) | 15.6 (2.5) | −0.02; .985 |
| KPS score | 72.9 (12.9) | 68.2 (11.5) | 1.89; .06 |
| Total number of symptoms | 9.7 (2.0) | 17.8 (4.0) | −14.10; <.0001 |
| SCQ score | 8.0 (3.7) | 8.9 (3.7) | −1.20; .235 |
| Number of metastatic sites | 1.7 (1.8) | 1.8 (1.2) | −.325; .746 |
| % (n) | % (n) | Fisher's exact test | |
| Male gender | 55.8 (24) | 40.3 (27) | .122 |
| Lives alone | 34.9 (15) | 13.6 (9) | .017 |
| White | 81.4 (35) | 71.9 (46) | .358 |
| Married/partnered | 62.8 (27) | 67.2 (43) | .684 |
| Not employed | 76.7 (33) | 74.6 (50) | 1.000 |
| Cancer diagnosis* | |||
| Breast | 34.9 (15) | 35.8 (24) | 1.000 |
| Colon | 0.0 (0) | 3.0 (2) | .519 |
| Lung | 9.3 (4) | 9.0 (6) | 1.000 |
| Melanoma | 2.3 (1) | 1.5 (1) | 1.000 |
| Prostate | 23.3 (10) | 25.4 (17) | 1.000 |
| Leukemia | 0.0 (0) | 1.5 (1) | 1.000 |
| Non-Hodgkin's lymphoma | 0.0 (0) | 1.5 (1) | 1.000 |
| Ovarian | 0.0 (0) | 3.0 (2) | .519 |
| Other | 32.6 (14) | 28.4 (19) | .674 |
| Two primary cancers | 2.3 (1) | 9.0 (6) | .243 |
| Current treatments* | |||
| Radiation therapy | 7.1 (3) | 9.0 (6) | 1.000 |
| Chemotherapy | 61.9 (26) | 49.3 (33) | 2.38 |
| Biotherapy | 4.8 (2) | 13.4 (9) | 1.98 |
| Hormonal therapy | 35.7 (15) | 31.3 (21) | .679 |
| Number of cancer treatments | |||
| 0 treatments | 16.7 (7) | 17.9 (12) | |
| 1 treatment | 57.1 (24) | 62.7 (42) | Kendall's tau=−.057; .538 |
| 2 treatments | 26.2 (11) | 17.9 (12) | |
| 3 treatments | 0.0 (0) | 1.5 (1) | |
| Metastatic sites | |||
| 0 | 9.3 (4) | 10.4 (7) | |
| 1 | 39.5 (17) | 35.8 (24) | |
| 2 | 32.6 (14) | 28.4 (19) | Kendall's tau=.030; .724 |
| 3 | 7.0 (3) | 16.4 (11) | |
| 4 | 9.3 (4) | 4.5 (3) | |
| 5 | 2.3 (1) | 4.5 (3) | |
| Reason for current treatment | |||
| Cure | 0.0 (0) | 3.6 (2) | |
| Control | 81.6 (31) | 76.4 (42) | χ2=1.49; .475 |
| Palliation | 18.4 (7) | 20.0 (11) | |
KPS, Karnofsky Performance Status; SCQ, Self-administered Comorbidity Questionnaire; SD, Standard deviation.
Percentage totals exceed 100% because patients may have more than one type of cancer or treatment.
Differences in symptom occurrence rates
As shown in Table 3, differences were found in the occurrence rates and rank order of the various MSAS symptoms. Pain, lack of energy, feeling drowsy, difficulty sleeping, constipation, lack of appetite, worrying, feeling sad, and difficulty concentrating ranked within the top 12 for both the low and high symptom groups. Numbness/tingling in hands/feet, changes in the way food tastes, and dry mouth were among the top 12 symptoms for the low but not for the high symptom group. In contrast, feeling nervous, feeling irritable, and nausea were in the top 12 for the high but not for the low symptom group. Of note, all four of the psychological symptoms (i.e., feeling sad, worrying, feeling nervous, feeling irritable) were among the top 12 symptoms in the high symptom group.
Table 3.
Differences in the Rank Order of Symptom Occurrence Rates in the Low and High Symptom Groups
| |
Low symptom group (n=43) |
High symptom group (n=67) |
||
|---|---|---|---|---|
| Rank | Symptom | Occurrence rate% | Symptom | Occurrence rate% |
| 1 | Pain | 97.6 | Lack of energy | 97.0 |
| 2 | Lack of energy | 92.7 | Pain | 96.9 |
| 3 | Feeling drowsy | 73.2 | Feeling sad | 90.9 |
| 4 | Difficulty sleeping | 58.5 | Feeling drowsy | 89.4 |
| 5 | Constipation | 51.2 | Worrying | 84.6 |
| 6 | Numbness/tingling in hands/feet | 48.8 | Difficulty concentrating | 81.8 |
| 7 | Lack of appetite | 45.2 | Difficulty sleeping | 81.8 |
| 8 | Worrying | 42.9 | Lack of appetite | 77.3 |
| 8 | Feeling sad | 42.9 | Feeling irritable | 76.1 |
| 10 | Difficulty concentrating | 39.0 | Feeling nervous | 72.3 |
| 11 | Change in the way food tastes | 37.2 | Constipation | 68.2 |
| 12 | Dry mouth | 36.6 | Nausea | 66.7 |
| 13 | Problems with sexual interest or activity | 28.2 | Problems with sexual interest or activity | 60.7 |
| 14 | Feeling irritable | 27.9 | Dry mouth | 60.6 |
| 15 | Nausea | 24.4 | Numbness/tingling in hands/feet | 60.0 |
| 16 | Itching | 21.4 | Itching | 57.6 |
| 17 | Dizziness | 20.9 | Sweats | 56.9 |
| 18 | Cough | 19.5 | “I don't look like myself” | 48.5 |
| 19 | Sweats | 19.0 | Feeling bloated | 47.7 |
| 19 | Problems with urination | 19.0 | Changes in skin | 46.3 |
| 19 | Feeling bloated | 19.0 | Change in the way food tastes | 44.8 |
| 22 | Weight loss | 18.6 | Dizziness | 44.8 |
| 23 | Feeling nervous | 17.1 | Shortness of breath | 40.9 |
| 24 | Shortness of breath | 16.7 | Cough | 38.5 |
| 24 | Diarrhea | 16.7 | Swelling of arms or legs | 37.3 |
| 26 | Vomiting | 14.3 | Weight loss | 37.3 |
| 26 | Swelling of arms or legs | 14.3 | Hair loss | 31.3 |
| 28 | Hair loss | 11.6 | Vomiting | 27.3 |
| 29 | “I don't look like myself” | 9.5 | Difficulty swallowing | 26.9 |
| 30 | Mouth sores | 9.3 | Problems with urination | 24.6 |
| 31 | Difficulty swallowing | 7.1 | Diarrhea | 22.7 |
| 31 | Changes in skin | 7.1 | Mouth sores | 19.7 |
With regard to occurrence rates, pain and lack of energy had similar occurrence rates in both symptom groups. For the low symptom group, occurrence rates for the next ten symptoms ranged from as high as 73.2% for feeling drowsy to 36.6% for dry mouth. For the high symptom group, the next 10 highest ranked symptoms had much higher occurrence rates (i.e., 90.9% for feeling sad to 66.7% for nausea).
Differences in depression and anxiety scores
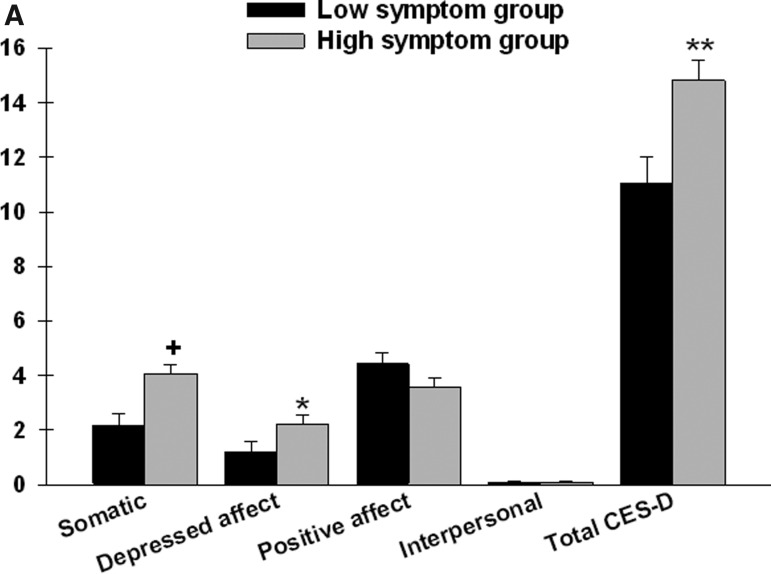
After controlling for age, significant between-group differences were found in two of the four CES-D subscales (somatic and depressed affect) as well as in the total CES-D score (Figure 1A). Patients in the high symptom group reported a higher somatic and depressed affect subscale scores as well as total CES-D score.
FIG. 1A.
Differences in Center for Epidemiologic Studies–Depression (CES-D) subscale and total scores between patients in the low (n=43) and high (n=67) symptom groups.
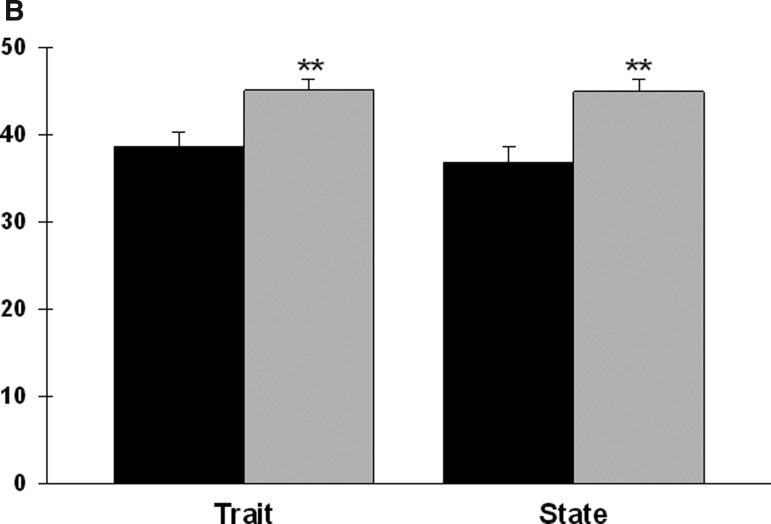
After controlling for age, significant between-group differences were found in both anxiety scores (Figure 1B). Patients in the high symptom group reported significantly higher state and trait anxiety scores.
FIG. 1B.
Differences in Spielberger State–Trait Anxiety Inventories (STAI) scores between patients in the low (n=43) and high (n=67) symptom groups. All values are plotted as means±standard error of the means after controlling for age (p values, *<.05, **≤.001, +<.0001).
Differences in QOL scores
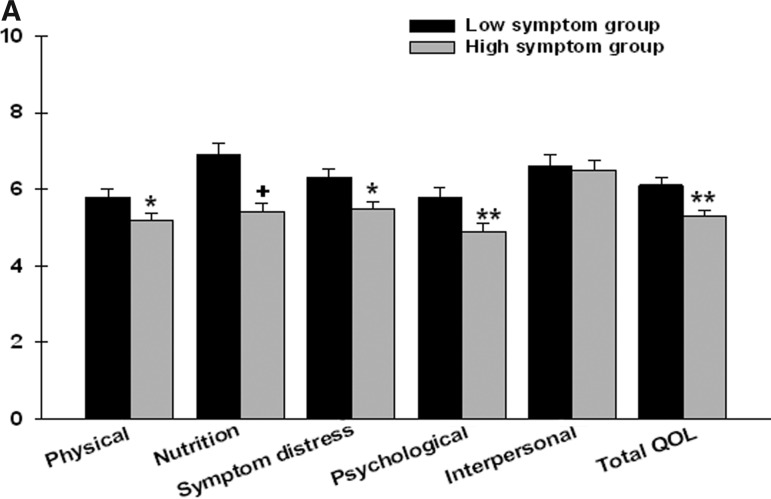
As expected, after controlling for age, significant between-group differences were found in the total MQOLS-CA2 score as well as in four of the five MQOLS-CA2 subscale (physical, nutrition, symptom distress, and psychological) scores (Figure 2A). Patients in the high symptom group had lower subscale and total MQOLS-CA2 scores.
FIG. 2A.
Differences in Multidimensional Quality of Life Scale–Cancer 2 (MQOLS-CA2) subscale and total quality of life (QOL) scores between patients in the low (n=43) and high (n=67) symptom groups.
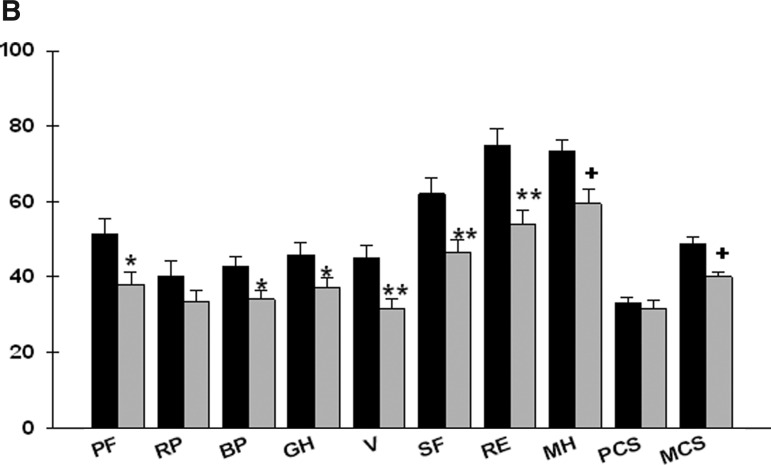
After controlling for age, significant between-group differences were found for seven of eight MOS-SF36 subscale scores (physical functioning, bodily pain, general health, vitality, social functioning, role limitations–emotional, and mental health), as well as in the mental component score (Figure 2B). Patients in the high symptom group reported significantly lower MOS-SF36 scores.
FIG. 2B.
Differences in Medical Outcomes Study–Short Form 36 (MOS-SF36) subscale (PF=physical functioning, RP=role limitations–physical, BP=bodily pain, GH=general health, V=vitality, SF=social functioning, RE=role limitations–emotional, MH=mental health) and component (PCS=physical component score, MCS=mental component score) scores between patients in the low (n=43) and high (n=67) symptom groups. All values are plotted as means±standard error of the means after controlling for age (p values, *<.05, .**≤.01, +<.0001).
Discussion
This study is the first to determine the optimal cutpoint for total number of symptoms in patients with advanced cancer. This finding suggests that the concept of a clinically meaningful cutpoint for symptom severity scores is transferable to total number of symptoms. In this sample, the cutpoint of 12 symptoms successfully differentiated between patients with advanced cancer based on a significant jump in both MQOL-CA2 subscale and total scores.
Validation of 12 symptoms as the optimal cutpoint was supported by significant between-group differences in depression and anxiety scores and in a generic measure of QOL. As shown in Table 4, between-group differences in these scores equate with medium to large effect sizes.40 Previous research suggests that an effect size of 0.2 to 0.5 is considered a minimally important difference and a clinically meaningful difference in QOL measures.41–43 Cinical significance goes beyond statistical significance to identify whether a change is large enough to be noticed by the patient.44–47 Our findings suggest that when a patient crosses the threshold from 12 to 13 symptoms he or she may notice a decrease in various aspects of QOL.
Table 4.
Effects Sizes for Between-Group Differences (High-Low) in Subscale and Total Scores for the Validation Scales for Depression, Anxiety, and Quality of Life
| Instrument | Effect size |
|---|---|
| Center for Epidemiologic Study–Depression (CES-D) Scale | |
| Somatic | .70 |
| Depressed affect | .42 |
| Positive affect | −.32 |
| Interpersonal | .04 |
| Total CES-D score | .58 |
| Spielberger State–Trait Anxiety Inventories | |
| Trait anxiety score | .59 |
| State anxiety score | .64 |
| Medical Outcomes Study–Short Form 36 | |
| Physical functioning | −.50 |
| Role limitations, physical | −.26 |
| Bodily pain | −.46 |
| General health | −.41 |
| Vitality | −.62 |
| Social functioning | −.57 |
| Role limitations, emotional | −.68 |
| Mental health | −.71 |
| Physical component score | −.17 |
| Mental component score | −.75 |
MQOLS-CA2 total scores in this study were similar to those reported in previous studies of oncology patients.48,49 Overall, patients with advanced cancer appear to have moderate decrements in QOL. However, further research is needed to determine the generalizability of these findings and whether response shifts occur in evaluations of QOL in these patients.50,51
The cutpoint that differentiated between low and high number of symptoms was validated by between-group differences in the rank order of the psychological symptoms on the MSAS. All four psychological symptoms (worrying, feeling sad, feeling nervous, feeling irritable) were among the top 12 symptoms in the high symptom group. In contrast, in the low symptom group, each psychological symptom had a lower overall rank and occurrence rate, and only worrying and feeling sad were in the top 12 symptoms.
CES-D scores for the low and high symptom groups in this study were similar to scores reported by patients with advanced head and neck cancer.52,53 In contrast, higher total CES-D scores were reported by patients with advanced stages of ovarian54 and prostate55 cancer. These inconsistent findings may be attributed to heterogeneity in cancer diagnoses, treatment regimens, and timing of assessments.
Mean state and trait anxiety scores in this study are similar to previous reports of patients with advanced cancer.56–58 State anxiety increases in response to physical danger and psychological stress, whereas higher trait anxiety is associated with diagnoses of psycho-neuroticism and/or depression.31,59 The consistent levels of anxiety across studies suggest that patients with advanced cancer may experience acute anxiety from a variety of physical and emotional stressors as well as chronic anxiety associated with depressive symptoms.
In this study, mean MOS-SF36 subscale and component scores for the total sample ranged from 32.1 (±8.8) for the physical component score to 64.8 (±19.8) for the mental health subscale. These scores are similar to those reported in one study,60 lower than scores reported in three studies of patients with advanced cancer,61–63 and higher than scores reported in another study64 of patients with advanced cancer. Reasons for these inconsistencies may include differences in studies' definition of advanced cancer, their inclusion and exclusion criteria, and timing of the assessments.
Differences in patients' reports of symptom occurrence and the rank order of the most common symptoms support the between-group differences found for the depression, anxiety, and psychological/mental health domains of the MOS-SF36. The largest effect sizes were found for the mental component and subscale scores related to psychological status (social functioning, vitality, role limitations–emotional, mental health). Emerging evidence suggests that psychological symptoms contribute to decrements in QOL in patients with advanced cancer.65–68 For example, higher depression scores were associated with higher symptom severity scores.69 In addition, in a study of cancer patients in their last year of life,68 higher levels of depressive symptoms at enrollment were associated with a worse symptom experience over time. It is not clear if psychological symptoms result in more symptoms or if length of time since diagnosis produces psychological “wear and tear” that results in more psychological and physical symptoms. Furthermore, it is not known if psychological and existential distress increase in patients with advanced cancer as they approach the end of life.70
Several study limitations need to be acknowledged. In this relatively small sample, only one optimal cutpoint for total number of symptoms was found. However, studies with larger samples may identify additional cutpoints. The fairly homogeneous sample of white and well-educated adults, all of whom had pain, limits the generalizability of the findings. However, given that pain was a highly prevalent symptom in both symptom groups, it is unlikely that the results of this study could be attributed only to pain. Finally, it is not possible to separate the effects of cancer and its treatment (e.g., side effects of medications) and the effects of chronic medical conditions on the patients' symptom experience.
Study findings suggest that a threshold exists between low and high number of symptoms in patients with advanced cancer. Additional research is needed to confirm these results and determine if additional cutpoints can be identified. In addition, studies need to determine if the use of cutpoints for total number of symptoms leads to improvements in clinical assessments and more tailored interventions for this vulnerable population. With the movement in health care toward systematizing best practices in an efficient manner, a need exists to develop screening criteria which, even if automated, would assure that the maximum number of patients who are at greatest risk for worse symptom outcomes are recognized with the minimum amount of effort. In the meantime, clinicians can administer a symptom checklist like the MSAS on a routine basis. Patients who report the occurrence of ≥13 symptoms warrant more detailed evaluation and more aggressive symptom management.
Acknowledgments
This work was supported by a grant (CA 116423) from the National Cancer Institute and the National Institute of Nursing Research. Dr. Miaskowski receives support from the American Cancer Society as a Clinical Research Professor. Dr. Aouizerat was funded through National Institutes of Health Roadmap for Medical Research Grant KL2 RR624130. The authors gratefully acknowledge the patients and family caregivers who participated in this research and our many colleagues who assisted with participant recruitment.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Serlin RC. Mendoza TR. Nakamura Y. Edwards KR. Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MP. Smith DG. Ehde DM. Robinsin LR. Pain site and the effects of amputation pain: Further clarification of the meaning of mild, moderate, and severe pain. Pain. 2001;91(3):317–322. doi: 10.1016/S0304-3959(00)00459-0. [DOI] [PubMed] [Google Scholar]
- 3.Dihle A. Helseth S. Paul SM. Miaskowski C. The exploration of the establishment of cutpoints to categorize the severity of acute postoperative pain. Clin J Pain. 2006;22(7):617–624. doi: 10.1097/01.ajp.0000210905.57546.c1. [DOI] [PubMed] [Google Scholar]
- 4.Zelman DC. Dukes E. Brandenburg N. Bostrom A. Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005;115(1–2):29–36. doi: 10.1016/j.pain.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Paul SM. Zelman DC. Smith M. Miaskowski C. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain. 2005;113(1–2):37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza TR. Wang XS. Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Swarm R. Abernethy AP. Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw. 2010;8(9):1046–1086. doi: 10.6004/jnccn.2010.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger AM. Abernethy AP. Atkinson A, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010;8(8):904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 9.Teunissen SC. Wesker W. Kruitwagen C. de Haes HC. Voest EE. de Graeff A. Symptom prevalence in patients with incurable cancer: A systematic review. J Pain Symptom Manage. 2007;34(1):94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Gilbertson-White S. Aouizerat BE. Jahan T. Miaskowski C. A review of the literature on multiple symptoms, their predictors, and associated outcomes in patients with advanced cancer. Palliat Support Care. 2011;9(1):81–102. doi: 10.1017/S147895151000057X. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS. Symptom burden: multiple symptoms, their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;(37):16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 12.Gapstur RL. Symptom burden: A concept analysis and implications for oncology nurses. Oncol Nurs Forum. 2007;34(3):673–680. doi: 10.1188/07.ONF.673-680. [DOI] [PubMed] [Google Scholar]
- 13.Peters L. Sellick K. Quality of life of cancer patients receiving inpatient and home-based palliative care. J Adv Nurs. 2006;53(5):524–533. doi: 10.1111/j.1365-2648.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- 14.Stromgren AS. Sjogren P. Goldschmidt D, et al. A longitudinal study of palliative care: Patient-evaluated outcome and impact of attrition. Cancer. 2005;103(8):1747–1755. doi: 10.1002/cncr.20958. [DOI] [PubMed] [Google Scholar]
- 15.McMillan SC. Small BJ. Symptom distress and quality of life in patients with cancer newly admitted to hospice home care. Oncol Nurs Forum. 2002;29(10):1421–1428. doi: 10.1188/02.ONF.1421-1428. [DOI] [PubMed] [Google Scholar]
- 16.Kutner JS. Bryant LL. Beaty BL. Fairclough DL. Time course and characteristics of symptom distress and quality of life at the end of life. J Pain Symptom Manage. 2007;34(3):227–236. doi: 10.1016/j.jpainsymman.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS. Farrar JT. Hausheer FH. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist. 2010;15(S2):13–18. doi: 10.1634/theoncologist.2009-S501. [DOI] [PubMed] [Google Scholar]
- 18.Molassiotis A. Zheng Y. Denton-Cardew L. Swindell R. Brunton L. Symptoms experienced by cancer patients during the first year from diagnosis: Patient and informal caregiver ratings and agreement. Palliat Support Care. 2010;8(3):313–324. doi: 10.1017/S1478951510000118. [DOI] [PubMed] [Google Scholar]
- 19.Karnofsky D. Performance Scale. New York: Plenum Press; 1977. [Google Scholar]
- 20.Karnofsky D. Abelmann WH. Craver LV. Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 21.MacLean CD. Littenberg B. Kennedy AG. Limitations of diabetes pharmacotherapy: Results from the Vermont Diabetes Information System study. BMC Fam Pract. 2006;7:50. doi: 10.1186/1471-2296-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SK. Zimmerman S. Williams CS. Zebrack BJ. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009;115(14):3312–3323. doi: 10.1002/cncr.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portenoy RK. Kornblith AB. Wong G, et al. Pain in ovarian cancer patients: Prevalence, characteristics, and associated symptoms. Cancer. 1994;74(3):907–915. doi: 10.1002/1097-0142(19940801)74:3<907::aid-cncr2820740318>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Portenoy RK. Thaler HT. Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3(3):183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 25.Tranmer JE. Heyland D. Dudgeon D. Groll D. Squires-Graham M. Coulson K. Measuring the symptom experience of seriously ill cancer and noncancer hospitalized patients near the end of life with the memorial symptom assessment scale. J Pain Symptom Manage. 2003;25(5):420–429. doi: 10.1016/s0885-3924(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 26.Padilla GV. Validity of health-related quality of life subscales. Prog Cardiovasc Nurs. 1992;7(1):13–20. [PubMed] [Google Scholar]
- 27.Padilla GV. Ferrell B. Grant MM. Rhiner M. Defining the content domain of quality of life for cancer patients with pain. Cancer Nurs. 1990;13(2):108–115. [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 29.Sheehan TJ. Fifield J. Reisine S. Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64(3):507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 30.Spielberger CG. Gorsuch RL. Suchene R. Vagg PR. Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 31.Kennedy BL. Schwab JJ. Morris RL. Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72(3):263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE., Jr. SF-36 health survey update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE., Jr. Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 34.Kurtz ME. Kurtz JC. Stommel M. Given CW. Given B. Physical functioning and depression among older persons with cancer. Cancer Pract. 2001;9(1):11–18. doi: 10.1046/j.1523-5394.2001.91004.x. [DOI] [PubMed] [Google Scholar]
- 35.Kurtz ME. Kurtz JC. Stommel M. Given CW. Given B. Predictors of depressive symptomatology of geriatric patients with colorectal cancer: A longitudinal view. Support Care Cancer. 2002;10(6):494–501. doi: 10.1007/s00520-001-0338-8. [DOI] [PubMed] [Google Scholar]
- 36.Kirkova J. Walsh D. Rybicki L, et al. Symptom severity and distress in advanced cancer. Palliat Med. 2010;24(3):330–339. doi: 10.1177/0269216309356380. [DOI] [PubMed] [Google Scholar]
- 37.Dodd MJ. Miaskowski C. Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- 38.Wedding U. Rohrig B. Klippstein A. Brix C. Pientka L. Hoffken K. Co-morbidity and functional deficits independently contribute to quality of life before chemotherapy in elderly cancer patients. Support Care Cancer. 2007;15(9):1097–1104. doi: 10.1007/s00520-007-0228-9. [DOI] [PubMed] [Google Scholar]
- 39.American Cancer Society: Advanced Cancer. 2011. www.cancer.org/Cancer/AdvanceCancer/DetailedGuide/advanced-cancer-advanced-cancer-intro. [Jan 3;2012 ]. www.cancer.org/Cancer/AdvanceCancer/DetailedGuide/advanced-cancer-advanced-cancer-intro
- 40.Cohen J. Cohen P. West S. Aiken L. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd. Hillsdale, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 41.Guyatt GH. Osoba D. Wu AW. Wyrwich KW. Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 42.Norman GR. Sloan JA. Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 43.Osoba D. Rodrigues G. Myles J. Zee B. Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 44.Sloan JA. Aaronson N. Cappelleri JC. Fairclough DL. Varricchio C Clinical Significance Consensus Meeting Group. Assessing the clinical significance of single items relative to summated scores. Mayo Clin Proc. 2002;77(5):479–487. [PubMed] [Google Scholar]
- 45.Sloan JA. Frost MH. Berzon R, et al. The clinical significance of quality of life assessments in oncology: A summary for clinicians. Support Care Cancer. 2006;14(10):988–998. doi: 10.1007/s00520-006-0085-y. [DOI] [PubMed] [Google Scholar]
- 46.Cella D. Bullinger M. Scott C. Barofsky I. Clinical Significance Consensus Meeting G: Group vs individual approaches to understanding the clinical significance of differences or changes in quality of life. Mayo Clin Proc. 2002;77(4):384–392. doi: 10.4065/77.4.384. [DOI] [PubMed] [Google Scholar]
- 47.Frost MH. Bonomi AE. Ferrans CE. Wong GY. Hays RD. Clinical Significance Consensus Meeting G: Patient, clinician, and population perspectives on determining the clinical significance of quality-of-life scores. Mayo Clin Proc. 2002;77(5):488–494. [PubMed] [Google Scholar]
- 48.Pud D. Ben Ami S. Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35(2):162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Miaskowski C. Cooper BA. Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis. Oncol Nurs Forum. 2006;33(5):E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz CE. Applications of response shift theory and methods to participation measurement: A brief history of a young field. Arch Phys Med Rehabil. 2010;91(9 Suppl):S38–S43. doi: 10.1016/j.apmr.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 51.Rapkin BD. Schwartz CE. Toward a theoretical model of quality-of-life appraisal: Implications of findings from studies of response shift. Health Qual Life Outcomes. 2004;2:14. doi: 10.1186/1477-7525-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Graeff A. de Leeuw JR. Ros WJ. Hordijk GJ. Blijham GH. Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37(3):332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 53.Derks W. de Leeuw JR. Hordijk GJ. Winnubst JA. Reasons for non-standard treatment in elderly patients with advanced head and neck cancer. Eur Arch Otorhinolaryngol. 2005;262(1):21–26. doi: 10.1007/s00405-004-0744-x. [DOI] [PubMed] [Google Scholar]
- 54.Bodurka-Bevers D. Basen-Engquist K. Carmack CL, et al. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78(3 Pt 1):302–308. doi: 10.1006/gyno.2000.5908. [DOI] [PubMed] [Google Scholar]
- 55.Ahles TA. Herndon JE., 2nd Small EJ, et al. Quality of life impact of three different doses of suramin in patients with metastatic hormone-refractory prostate carcinoma: Results of Intergroup O159/Cancer and Leukemia Group B 9480. Cancer. 2004;101(10):2202–2208. doi: 10.1002/cncr.20655. [DOI] [PubMed] [Google Scholar]
- 56.Bruera E. Willey J. Cohen M. Palmer JL. Expressive writing in patients receiving palliative care: A feasibility study. J Palliat Med. 2008;11(1):15–19. doi: 10.1089/jpm.2007.0112. [DOI] [PubMed] [Google Scholar]
- 57.Mystakidou K. Tsilika E. Parpa E. Gogou P. Theodorakis P. Vlahos L. Self-efficacy beliefs and levels of anxiety in advanced cancer patients. Eur J Cancer Care (Engl) 2010;19(2):205–211. doi: 10.1111/j.1365-2354.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- 58.Mystakidou K. Tsilika E. Parpa E. Papageorgiou C. Georgaki S. Vlahos L. Investigating the effects of TTS-fentanyl for cancer pain on the psychological status of patients naive to strong opioids: An open label study. Cancer Nurs. 2004;27(2):127–133. doi: 10.1097/00002820-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Bieling PJ. Antony MM. Swinson RP. The State-Trait Anxiety Inventory, Trait version: Structure and content re-examined. Behav Res Ther. 1998;36(7–8):777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 60.Miaskowski C. Dodd M. West C, et al. The use of a responder analysis to identify differences in patient outcomes following a self-care intervention to improve cancer pain management. Pain. 2007;129(1–2):55–63. doi: 10.1016/j.pain.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terrell JE. Ronis DL. Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130(4):401–408. doi: 10.1001/archotol.130.4.401. [DOI] [PubMed] [Google Scholar]
- 62.Popa-Velea O. Cernat B. Tambu A. Influence of personalized therapeutic approach on quality of life and psychiatric comorbidity in patients with advanced colonic cancer requiring palliative care. J Med Life. 2010;3(3):343–347. [PMC free article] [PubMed] [Google Scholar]
- 63.Adamsen L. Quist M. Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson DW., Jr. Eisenberg DF. Cella D. Zhao N. de Boer C. DeWitte M. The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. J Support Oncol. 2008;6(6):283–290. [PubMed] [Google Scholar]
- 65.Walsh D. Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer. 2006;14(8):831–836. doi: 10.1007/s00520-005-0899-z. [DOI] [PubMed] [Google Scholar]
- 66.Cheung WY. Le LW. Zimmermann C. Symptom clusters in patients with advanced cancers. Support Care Cancer. 2009;17(9):1223–1230. doi: 10.1007/s00520-009-0577-7. [DOI] [PubMed] [Google Scholar]
- 67.Francoeur RB. The relationship of cancer symptom clusters to depressive affect in the initial phase of palliative radiation. J Pain Symptom Manage. 2005;29(2):130–155. doi: 10.1016/j.jpainsymman.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doorenbos AZ. Given CW. Given B. Verbitsky N. Symptom experience in the last year of life among individuals with cancer. J Pain Symptom Manage. 2006;32(5):403–412. doi: 10.1016/j.jpainsymman.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Gruenigen VE. Hutchins JR. Reidy AM, et al. Gynecologic oncology patients' satisfaction and symptom severity during palliative chemotherapy. Health Qual Life Outcomes. 2006;4:84. doi: 10.1186/1477-7525-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lichtenthal WG. Nilsson M. Zhang B, et al. Do rates of mental disorders and existential distress among advanced stage cancer patients increase as death approaches? Psychooncology. 2009;18(1):50–61. doi: 10.1002/pon.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]