Abstract
The safety and immunogenicity of the MRK adenovirus type 5 (Ad5) HIV-1 clade B gag vaccine was assessed in an international Phase I trial. Three-hundred and sixty healthy HIV-uninfected adults were enrolled on five continents. Subjects received placebo or 1 × 109 or 1 × 1010 viral particles (vp) per dose of the MRKAd5 HIV-1 gag vaccine at day 1, week 4, and week 26. Immunogenicity was evaluated using an IFN-γ ELISPOT gag 15-mer assay with positive responses defined as ≥55 SFC/106 PBMCs and ≥4-fold over mock control. The vaccine was well tolerated. The most common adverse events were injection site reactions, headache, pyrexia, diarrhea, fatigue, and myalgia. At week 30, geometric mean ELISPOT responses were 24, 114, and 226 SFC/106 PBMCs in the placebo, 1 × 109 vp/dose, and 1 × 1010 vp/dose groups, respectively. Overall, responses to 1 × 1010 vp were 85% and 68% in subjects with low (≤200) and high (>200) baseline Ad5 titers, respectively. The MRKAd5 HIV-1 gag vaccine was immunogenic in diverse geographic regions. Gag ELISPOT responses were greater in the 1 × 1010 vp/dose groups than in the 1 × 109 vp/dose groups. Data from this first international study indicate that adenovirus-vectored vaccines are well tolerated and may be immunogenic in subjects from regions with high prevalence of preexisting Ad5 immunity.
Introduction
At the end of 2008, UNAIDS estimated that there were 33.4 million people living with AIDS worldwide. Approximately 2.7 million people were newly infected in 2008 alone. In parts of southern Africa, HIV prevalence among adults 15–49 years of age is over 30%.1 HIV care and treatment programs in resource-limited settings are constrained in their capacity to provide antiretroviral therapy to all individuals who may need it.2–5 In addition, as treatment rollout continues in resource-limited settings, viral resistance to the limited available therapy will become an increasing problem,6–10 further underscoring the need for effective HIV prevention. Although some efforts, including condom use and reduction in the number of sexual partners, have provided meaningful results in terms of HIV prevention, there are limited data in the prevention literature to support long-term behavioral change.11–20 In addition, although randomized trials of male circumcision have demonstrated efficacy rates as high as 60% in preventing HIV infection, obstacles remain to the widespread uptake of circumcision.21–26 A recent randomized controlled trial demonstrating that the use of coitally related tenofovir gel in women could reduce HIV acquisition by up to 54% in the most adherent women is encouraging but requires corroboration in a larger study.27 Given the expanding epidemic and the limitations of available approaches to prevention, an effective HIV vaccine is urgently needed. Several studies have shown that even a partially effective vaccine could have a considerable public health impact.28–30
Despite the public health importance of an effective HIV vaccine, approaches to vaccine development have been met with challenges. There is no known immune correlate of protection for HIV,31 and the virus is able to escape the host immune response. Moreover, there is broad antigenic variability among circulating viral clades worldwide that make the prospects of developing a vaccine effective in populations across the globe seem unlikely.32–40 Moreover, efforts to identify or induce broadly neutralizing anti-HIV antibodies have been unsuccessful.41,42 Despite these challenges, some studies have suggested the possibility of eliciting cross-clade cell-mediated immunologic responses.43–49 While various approaches to elicit cell-mediated immunity have included canarypox virus-vectored designs and DNA prime boost regimens,50–61 adenoviral-vectored HIV vaccines have elicited the most robust immune responses to date.62–66 Despite this fact, a proof of concept study (known as the STEP study) employing the trivalent (gag, pol, nef ), MRKAd5 HIV vaccine proved to be nonefficacious.67,68 The results of the STEP study underscore the complexity of HIV vaccine development. In that trial, while cell-mediated immune responses were robust, they failed to provide the same benefit of decreasing viral load demonstrated in animal studies.64–66 Although the mechanism of protection remains unclear, the recent report of a modest decrease in HIV-1 acquisition with a canarypox prime/gp120 boost regimen in the Thai RV144 trial, followed by the new discovery of broadly neutralizing HIV antibodies in an HIV-infected individual, provided encouragement to the field.69,70
We describe the first large multinational study designed to examine the safety and immunogenicity of an adenovirus-vectored HIV vaccine designed to elicit cell-mediated immunity in diverse global populations, which began before STEP study results became available. Our trial aimed to evaluate the safety and immunogenicity of the MRKAd5 HIV-1 gag vaccine in globally diverse participants with and without preexisting Ad5 immunity and from regions of the world with and without a predominance of circulating clade B HIV. The vaccine used in our study was a monovalent construct containing a single antigen encoded by an HIV-1 gag gene based on near-consensus clade B sequences in the replication-incompetent MRKAd5 vector that had previously been found to be safe and immunogenic in a North American population.71 The primary objective of this study was to assess the safety, tolerability, and immunogenicity of a three-dose regimen of the MRKAd5 HIV-1 gag vaccine at 1 × 109 and 1 × 1010 viral particles (vp) per dose administered at day 1, week 4, and week 26. A secondary objective was to compare immune responses to the vaccine as a function of baseline Ad5 titer and participants' geographic region.
Materials and Methods
Study design and procedures
Three hundred and sixty healthy, HIV-negative subjects from 24 sites, encompassing five geographic regions (North and South America, the Caribbean, South Africa, and Southeast Asia), were randomized into a double-blind, placebo-controlled study to receive a three-dose regimen of placebo or MRKAd5 HIV-1 gag vaccine at doses of 1 × 109 vp/dose or 1 × 1010 vp/dose on day 1, week 4, and week 26 in approximately a 1:3:3 ratio. The protocol was approved by ethics committees at each site, and written informed consent was obtained from all participants. Subjects were between 18 and 50 years of age and self-identified as being at low risk for HIV infection as determined at a site level. Behavioral HIV risks were reviewed and documented at the first screening visit, at weeks 18, 34, 43, 52, 78, 104, 156, 208, and 260, and at any other time point at the discretion of the investigators. Subjects were required to be in good health as assessed by medical history, physical examination, and laboratory tests including liver function tests, complete blood count, coagulation panel, urinalysis, and absence of HIV-1, HTLV-1, or hepatitis B or C virus infection. Exclusion criteria included recent receipt of immune globulin or blood products, recent vaccination with a live vaccine, clinically significant chronic medical conditions, major psychiatric illness, and a history of malignancies. Use of concomitant medications and nutritional supplements that can cause liver function abnormalities or bone marrow injury was not permitted within 1 month of study vaccine administration. Dual methods of contraception were required to be utilized during the first 52 weeks of the study. Ongoing risk assessments and risk reduction counseling were offered to subjects during the trial.
Subjects were vaccinated as per protocol, and injection site reactions were followed for 5 days after each vaccination. Subjects recorded the largest diameter of injection site induration and erythema on the patient diary card. Subjects also recorded daily temperatures for 5 days and systemic complaints for 29 days after each dose of vaccine. Blood samples were drawn for immunogenicity assays at multiple time points including 4 weeks after the second vaccination (study week 8) and at both 4 weeks (study week 30) and 1 year (study week 78) after the third vaccination.
Measures
Safety
Specific safety measures included the rate of (1) injection-site reactions from day 1 through day 5 following each vaccination, (2) systemic adverse experiences (AEs), including fever (defined as temperature >100°F/37.7°C) from day 1 through day 29 following each vaccination, and (3) vaccine-related serious adverse experiences (SAEs) throughout the duration of the study (5 years). Safety laboratories were collected at weeks 1, 2, 5, 6, 27, and 28.
Immunogenicity
Immunogenicity was measured in unfractionated peripheral blood mononuclear cells (PBMCs) by a gag-specific interferon (IFN)-γ ELISPOT assay using 15-mer gag peptides as previously described.72,73 Responders were defined as having a value of ≥55 spot-forming cells (SFCs)/106 PBMCs and ≥4-fold above mock antigen control. The primary endpoint was the ELISPOT response at week 30. Ad5 titer was measured using the adenovirus 5 neutralization assay, a validated quantitative assay for the measurement of adenovirus type 5 neutralizing antibodies (Nab) present in human serum.74 A titer of <18 is considered negative in the assay. Although baseline Ad5 titer was measured, assignment of subjects to study group was not stratified according to these titers. For all analyses, baseline Ad5 titer was stratified by low and high Ad5 neutralization titer (≤200 and >200).
HIV testing
An HIV envelope-based immunoassay was performed at weeks 18, 34, and 52. HIV whole virus immunoassays were performed at week 78 and during the long-term safety follow-up phase at weeks 104, 156, 208, and 260. If any HIV testing was positive, a Western blot and plasma HIV-1 RNA PCR were performed to confirm infection.
Statistical analyses
Safety
All subjects receiving ≥1 dose of MRKAd5 HIV-1 gag vaccine were included in the safety analysis. Adverse events were evaluated according to baseline Ad5 titers of ≤200 and >200 as well as combined for both baseline Ad5 titer groups. The proportions of subjects with injection-site reactions and systemic adverse events within 29 days following any of the three doses were summarized by treatment group separately for each baseline Ad5 stratum and for the combined strata. For the combined strata, summary statistics were calculated using a weighted average of the observed stratum-specific percentages, with weights proportional to the overall observed stratum sizes. The frequencies of specific types of injection-site reactions and systemic adverse events in the vaccine dose groups were compared to the corresponding frequency in the placebo group by the Miettinen and Nurminen method.75 One-tailed p-values <0.025 (without any multiplicity adjustment) were considered statistically significant. Small sample sizes precluded comparisons within baseline Ad5 strata.
Immunogenicity
Only subjects receiving all three scheduled doses of vaccine or placebo and without major protocol violations were included in the per protocol analysis of immunogenicity. ELISPOT results were summarized by region and treatment arm at each time point in a manner analogous to that used for the safety analyses. For the combined strata, summary statistics were calculated using a weighted average of the observed stratum-specific percentages, with weights based on observed stratum sizes at randomization within each region (see Table 1 for weights). Summary statistics included the proportions of ELISPOT responders and the geometric means of the quantitative ELISPOT responses. Differences in the frequencies of week 30 ELISPOT responders between a given vaccine dose group and the placebo group as well as between the two vaccine dose groups in the combined strata were analyzed using the Miettinen and Nurminen method.75 One-tailed p-values <0.025 were considered statistically significant. An analogous testing approach was used for comparison of geometric mean ELISPOTs. The effect of baseline Ad5 titers on ELISPOT responses was explored using standard linear regression techniques. Testing for a statistical interaction between the baseline Ad5 stratum and region in terms of difference in ELISPOT response rates was conducted using the Mehrotra method.76 In secondary analyses, data from the HIV-1 gag-specific IFN-γ ELISPOT assay obtained at 4 weeks after the second immunization (week 8) and 1 year after the third immunization (week 78) were summarized.
Table 1.
Observed Ad5 Sero-prevalence at Baseline by Study Region (Weight Table)
| Region | Na | Base Ad5 ≤200 | Base Ad5 >200 |
|---|---|---|---|
| North America | 86 | 63% | 37% |
| South America | 87 | 53% | 47% |
| Asia | 87 | 26% | 74% |
| Caribbean | 75 | 49% | 51% |
| South Africa | 22 | 36% | 64% |
| All regions pooled | 357 | 47% | 53% |
N, number of subjects randomized in each region with available baseline Ad5 titer.
Results
Demographics and safety
The study enrolled 360 healthy adults across five geographic regions of the world. The southern Africa cohort was not fully enrolled due to slow accrual within the region and incomplete enrollment prior to expiration of the study vaccine. Demographic data and baseline Ad5 titers across treatment groups are presented in Table 2. Overall, 53% of the subjects had baseline Ad5 titers >200 (Table 2). Ad5 titers at baseline were generally low in North American subjects and high in Asian and southern African subjects. In general, there was a relatively equal proportion of subjects with low and high Ad5 titers enrolled in South America and the Caribbean. Of the 360 randomized subjects, 296 received at least one dose of vaccine (1 × 109 or 1 × 1010 vp/dose) and 64 subjects received at least one dose of placebo (Fig. 1). In the vaccine groups, there were 16 subjects who did not receive all three vaccinations; one subject received MRKAd5 1 × 109 at day 1 and week 4 per protocol but was erroneously administered a single dose of placebo at week 26 and was excluded from the per-protocol analysis.
Table 2.
Demographics
| Placebo (N = 64) n (%) | 1 × 109vp/d MRK Ad5 HIV gag (N = 147) n (%) | 1 × 1010vp/d MRK Ad5 HIV-1 gag (N = 149) n (%) | Total (N = 360) n (%) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 39 (61%) | 73 (50%) | 86 (58%) | 198 (55%) |
| Female | 25 (39%) | 74 (50%) | 63 (42%) | 162 (45%) |
| Age | ||||
| Mean | 32.1 | 31.1 | 31.4 | 31.4 |
| SD | 8.33 | 8.07 | 8.29 | 8.19 |
| Median | 31.5 | 30 | 30 | 30 |
| Range | 18–50 | 18–50 | 18–50 | 18–50 |
| Race/ethnicity | ||||
| Asian | 15 (23%) | 36 (24%) | 38 | 89 (25%) |
| Black | 17 (27%) | 27 (18%) | 42 (28%) | 86 (24%) |
| White | 15 (23%) | 44 (30%) | 38 (26%) | 97 (27%) |
| Hispanic | 15 (23%) | 31 (21%) | 24 (16%) | 70 (19%) |
| Multiethnic | 2 (3%) | 7 (5%) | 7 (5%) | 16 (4%) |
| Native American | 0 (0%) | 2 (1%) | 0 (0%) | 2 (1%) |
| Baseline Ad5 titer | ||||
| ≤18 | 15 (23%) | 27 (18%) | 35 (23%) | 77 (21%) |
| 19–200 | 13 (20%) | 35 (24%) | 43 (29%) | 91 (25%) |
| >200 | 36 (56%) | 85 (58%) | 68 (46%) | 189 (53%) |
| Missing | 0 (0%) | 0 (0%) | 3 (2%) | 3 (1%) |
| Region | ||||
| North America | 15 (23%) | 36 (24%) | 36 (24%) | 87 (24%) |
| South America | 15 (23%) | 36 (24%) | 36 (24%) | 87 (24%) |
| Asia | 15 (23%) | 36 (24%) | 36 (24%) | 87 (24%) |
| Caribbean | 13 (20%) | 30 (20%) | 32 (21%) | 75 (21%) |
| South Africa | 6 (9%) | 9 (6%) | 9 (6%) | 24 (7%) |
FIG. 1.
Subject accounting. A total of 32 vaccinated subjects (30 in the vaccine groups and two in the placebo group) were excluded from the primary immunogenicity analysis. Reasons for exclusion are provided.
Frequencies of individual adverse events stratified by baseline Ad5 titer and vaccine dose are presented in Table 3. The most common adverse events were injection site reactions, headache, pyrexia, diarrhea, fatigue, and myalgia. Among those with low baseline Ad5 titer, fever and myalgia appeared to occur more frequently among recipients of the 1 × 1010 dose than among placebo recipients, although these differences did not reach statistical significance. When Ad5 titers were combined, only injection site adverse reactions (injection site pain and swelling) occurred more commonly in the 1 × 1010 treatment group than placebo (p < 0.025, one-tailed). Rates of adverse events in the 1 × 109 group were not significantly different from placebo (Table 3). Laboratory abnormalities (data not shown) did not differ significantly between active vaccine and placebo recipients. Four subjects discontinued treatment due to AEs. One of the AEs causing discontinuation of vaccination was serious (benign intracranial hypertension, which occurred 153 days after the second dose of MRKAd5 1 × 109), but this was not deemed related to the study vaccine. The other AEs, pruritic rash, diarrhea, urticaria, increased alanine aminotransferase, and decreased hemoglobin, were vaccine related, but not serious. No serious vaccine-related adverse events occurred in any treatment group. There were eight pregnancies reported during the first 78 weeks of the study. Pregnancy outcomes included four healthy full-term fetuses delivered vaginally, one elective abortion, two spontaneous abortions, and one unknown outcome. There were no deaths during the first 78 weeks of follow-up.
Table 3.
| |
Events during the 29 days following any dose |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Baseline Ad5 titer ≤200 |
Baseline Ad5 titer >200 |
Combinedc |
||||||
| Placebo (N = 28) | 1 × 109(N = 62) | 1 × 1010(N = 79) | Placebo (N = 36) | 1 × 109(N = 85) | 1 × 1010(N = 70) | Placebo | 1 × 109 | 1 × 1010 | |
| Local | |||||||||
| Injection site pain | 25 | 27 | 62 | 25 | 45 | 52 | 3 | 37 | 57d |
| Injection site erythema | 0 | 3 | 13 | 6 | 7 | 6 | 3 | 5 | 9 |
| Injection site swelling | 0 | 0 | 17 | 3 | 5 | 9 | 2 | 2 | 12d |
| Systemic | |||||||||
| Headache | 36 | 40 | 42 | 33 | 41 | 29 | 34 | 41 | 35 |
| Pyrexia | 21 | 21 | 44 | 25 | 21 | 26 | 23 | 21 | 35 |
| Diarrhea | 21 | 11 | 20 | 22 | 19 | 10 | 22 | 15 | 15 |
| Fatigue | 18 | 18 | 25 | 6 | 17 | 10 | 11 | 17 | 17 |
| Myalgia | 7 | 10 | 23 | 17 | 17 | 9 | 12 | 13 | 15 |
| Upper respiratory tract infection | 21 | 8 | 13 | 17 | 13 | 7 | 19 | 11 | 10 |
| Nasopharyngitis | 7 | 10 | 14 | 3 | 7 | 10 | 5 | 8 | 12 |
| Pharyngolaryngeal pain | 4 | 11 | 11 | 3 | 12 | 7 | 3 | 12 | 9 |
| Cough | 11 | 8 | 10 | 6 | 6 | 7 | 8 | 7 | 9 |
| Nasal congestion | 14 | 8 | 4 | 8 | 9 | 7 | 11 | 9 | 6 |
| Nausea | 7 | 10 | 5 | 3 | 12 | 6 | 5 | 11 | 5 |
| Dizziness | 14 | 7 | 8 | 11 | 6 | 3 | 13 | 6 | 5 |
| Back pain | 7 | 2 | 8 | 6 | 6 | 4 | 6 | 4 | 6 |
| Rhinorrhea | 0 | 5 | 4 | 3 | 6 | 7 | 2 | 5 | 6 |
| Arthralgia | 7 | 3 | 4 | 5 | 4 | 6 | 6 | 3 | 5 |
| Malaise | 4 | 0 | 6 | 0 | 9 | 3 | 2 | 5 | 5 |
| Abdominal pain | 4 | 2 | 5 | 3 | 4 | 5 | 3 | 3 | 5 |
| Influenza | 11 | 3 | 0 | 0 | 2 | 4 | 5 | 3 | 2 |
Adverse events with combined incidence ≥5% for at least one of the treatment groups are displayed.
N, number of subjects with follow-up. Three subjects missing baseline Ad5 titer were assigned to their region's most prevalent stratum.
Combined across baseline Ad5 strata using a weighted average based on observed stratum sizes at randomization (47% Ad5 ≤200, 53% Ad5 >200).
One-tailed p-value <0.025 for given dose vs. placebo based on the Miettinen and Nurminen method.
Immunogenicity
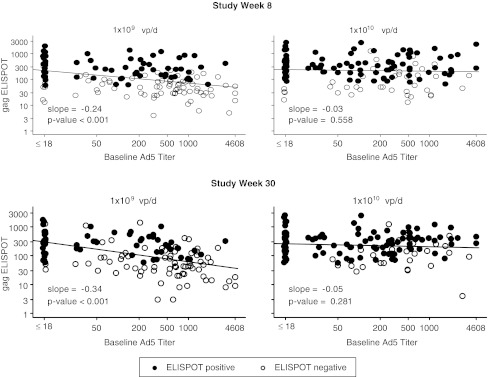
At the primary immunogenicity endpoint (week 30, 4 weeks after the third dose of study vaccine), the response rates and geometric mean (GM) ELISPOT responses were significantly higher for recipients of both active vaccine doses compared to placebo in all regions except southern Africa where only 18 subjects were vaccinated and data were available on 14 subjects (Table 4). When all regions and baseline Ad5 titers were combined, response rates were significantly higher for recipients of the 1 × 1010dose as compared to the 1 × 109dose. The GM ELISPOT response was 9-fold higher for recipients of the 1 × 1010dose as compared to placebo. Figure 2 shows the impact of baseline Ad5 titer on ELISPOT by dose level at study week 8 and week 30. Although there was a significant decline in ELISPOT with increasing Ad5 titer at the 1 × 109dose, no decrease in ELISPOT response was seen at the 1 × 1010 dose.
Table 4.
MRKAd5 gag 15mer Elispot Response at Week 30 (Per-Protocol Immunogenicity Population)a
| |
Baseline Ad5 ≤200 |
Baseline Ad5 >200 |
Combinedb |
|||||
|---|---|---|---|---|---|---|---|---|
| Dose (vp/d) | N | Response %c | GMd | N | Response %c | GMd | Response %c | GMd |
| North America | ||||||||
| Placebo | 8 | 0% | 19 | 6 | 0% | 11 | 0% | 16 |
| 1 × 109 | 17 | 77% | 206 | 13 | 23% | 60 | 57%e | 130e |
| 1 × 1010 | 23 | 87% | 227 | 7 | 29% | 192 | 65%e | 213e |
| South America | ||||||||
| Placebo | 8 | 0% | 18 | 7 | 0% | 24 | 0% | 21 |
| 1 × 109 | 12 | 42% | 149 | 21 | 38% | 105 | 40%e | 126e |
| 1 × 1010 | 22 | 87% | 280 | 13 | 77% | 134 | 82%e,f | 198e |
| Asia | ||||||||
| Placebo | 4 | 0% | 11 | 11 | 0% | 30 | 0% | 23 |
| 1 × 109 | 8 | 100% | 267 | 24 | 33% | 72 | 51%e | 101e |
| 1 × 1010 | 10 | 70% | 283 | 26 | 89% | 330 | 84%e,f | 316e,f |
| Caribbean | ||||||||
| Placebo | 4 | 0% | 41 | 8 | 0% | 61 | 0% | 50 |
| 1 × 109 | 14 | 43% | 248 | 13 | 31% | 52 | 37%e | 112e |
| 1 × 1010 | 17 | 88% | 200 | 12 | 58% | 153 | 73%e,f | 175e |
| S. Africa | ||||||||
| Placebo | 4 | 0% | 22 | 2 | 0% | 49 | 0% | 36 |
| 1 × 109 | 2 | 0% | 67 | 6 | 0% | 35 | 0% | 44 |
| 1 × 1010 | 2 | 100% | 81 | 4 | 0% | 263 | 36%f | 172f |
| All Regions Pooled | ||||||||
| Placebo | 28 | 0% | 20 | 34 | 0% | 29 | 0% | 24 |
| 1 × 109 | 53 | 60% | 206 | 77 | 30% | 69 | 44%e | 114e |
| 1 × 1010 | 74 | 85% | 235 | 62 | 68% | 218 | 76%e,f | 226e,f |
Vaccination regimen: day 1, week 4, and week 26.vp/d, viral particles per dose; N, number of subjects with evaluable immunogenicity data.
Combined across baseline Ad5 strata using a weighted average based on observed stratum sizes at randomization within each region (see the weight table).
ELISPOT responder: ≥55 SFC/106 PBMCs and ≥4-fold over media control.
GM, geometric mean ELISPOT (SFC/106 PBMCs) responses.
One-tailed p-value <0.025 for given dose versus placebo.
One-tailed p-value <0.025 for 1 × 109 versus 1 × 1010.
FIG. 2.
MRKAd5 gag ELISPOT response (SFC/106 PBMC) versus baseline anti-Ad5 antibody titer by dose level (all regions pooled). The figure demonstrates a significant decline in ELISPOT with increasing Ad5 titer at the 1 × 109 dose, but not at the 1 × 1010 dose. Filled circles (•) indicate participants who were ELISPOT positive or seroresponders. Open circles (○) indicate participants who were ELISPOT negative or nonresponders.
The effect of baseline Ad5 on ELISPOT response rate appeared to differ by region. For example, at the 1 × 1010 dose, the response rates at week 30 among North American subjects with Ad5 titer ≤200, and >200 were 87.0% and 28.6%, respectively, compared to 70.0% and 88.5%, respectively, for participants from Asia. The p-values for testing the interaction between baseline Ad5 status (≤200, >200) and region were significant for both the 1 × 1010 dose group (p = 0.045 including Africa and p = 0.025 excluding Africa) and the 1 × 109 dose group (p = 0.102 including Africa and p = 0.032 excluding Africa). These analyses were conducted both including and excluding Africa because of the small number of randomized study participants from that region. When all regions were pooled, the response rate was significantly higher in the low Ad5 stratum compared to the high Ad5 stratum (Table 4). However, at the 1 × 1010 dose, the response rate was over 68% even in the high Ad5 stratum (Table 4). Immunogenicity data obtained 1 year after the third dose of vaccine (week 78) persisted at levels near that achieved at week 30 in both dose groups regardless of baseline Ad5 antibody titer (Fig. 3).
FIG. 3.
Gag ELISPOT response over time by dose level and baseline anti-Ad5 antibody strata (all regions pooled): critical time points only with confidence intervals. Antibody titers persisted through week 78 at levels near that achieved at week 30.
HIV testing and HIV infections
Approximately 17% (59/340) of study participants had a positive HIV test result on the whole virus immunoassay performed at week 78 but negative HIV-1 RNA PCR tests. Four male participants (two enrolled in South America and two in South Africa) were found to have incident HIV infection during the course of the study to date; all HIV-infected subjects received three doses of active vaccine. Table 5 shows the baseline Ad5 titers, the number of days postvaccination that the first positive HIV result was obtained, and the initial HIV RNA levels of the four participants. Each case was identified through routine HIV serology testing per protocol, with reflex testing by Western blot and HIV RNA viral load. However, in the second case a report of high-risk behavior likely prompted an HIV RNA viral load test despite the fact that there was a negative ELISA (Table 5).
Table 5.
Summary of Incident Cases of HIV Infection
| Location | Treatment group | Baseline Ad5 titer | Number of doses received | Days postdose 3 first positive HIV test obtained | HIV RNA viral load at diagnosis (copies/ml) | Number of days post last negative HIV test |
|---|---|---|---|---|---|---|
| South America | HIV-1 gag MRKAd5 1 × 1010 | 46.7 | 3 | 183 | 648,003 | 69 |
| South America | HIV-1 gag MRKAd5 1 × 109 | 81.3 | 3 | 183 | >75,000a | 71 |
| South Africa | HIV-1 gag MRKAd5 1 × 109 | 340.4 | 3 | 538 | 34,724 | 153 |
| South Africa | HIV-1 gag MRKAd5 1 × 1010 | 480.6 | 3 | 548 | 10,266 | 365 |
At the time this HIV RNA sample was collected, an initial HIV rapid test (UNI-GOLD Recombigen HIV) was nonreactive, suggesting acute infection.
Discussion
This study represents the first truly global study of an adenovirus vector-based HIV vaccine designed to induce cell-mediated immunity. The vaccine was generally well tolerated among all subjects from all regions. Although local adverse events appeared to be dose dependent, local adverse events were not significantly affected by baseline Ad5 titer. There was an observed trend toward higher rates of fever in subjects with low baseline Ad5 titers who received the 1 × 1010 vp dose, as was observed in an earlier study of the of MRKAd5 HIV-1 gag vaccine. However, the small sample size of the low and high Ad5 titer groups limits the ability to conclude that these systemic AEs occur with greater frequency in subjects with low preexisting immunity to Ad5. There were no vaccine-related serious adverse events.
The MRKAd5 HIV-1 gag vaccine was immunogenic across a diverse global population. While preexisting immunity to Ad5 dampened the immunogenicity of the vaccine in some regions, increasing the vaccine dose from 1 × 109 to 1 × 1010 vp/dose generally appeared to mitigate this effect. In some cases, most notably in Caribbean and southern Africa regions, GM ELISPOT responses for low-dose vaccine recipients did not exceed that of placebo recipients, suggesting that a higher dose may be needed for a sufficiently immunogenic vaccine. The effect of baseline Ad5 titer on ELISPOT responses differed according to region as demonstrated by a statistically significant interaction between baseline Ad5 titer and region. Although in North America those with baseline Ad5 titer >200 had a lower ELISPOT response rate than those with Ad5 titer ≤200, this did not hold true for participants from Asia. There are several possible explanations for this finding. First, PBMC samples from international sites such as Asia were handled differently from those in North America. For international sites, PBMCs were isolated and frozen within 12 h of collection as opposed to U.S. sites where samples were transported at 4°C overnight and processed the following day. Data suggest that the magnitude of IFN-γ ELISPOT responses is significantly higher when PBMCs are isolated and frozen within 12 h of collection.73 Second, the geographic regions differ by human lymphocyte antigen (HLA) allele distribution, a factor known to influence disease progression in HIV infection,77 and may have an impact on response to HIV vaccination. Additional work on mapping epitope responses across the populations studied would be needed to determine whether greater ELISPOT responses were related to HLA type.
Approximately 17% of study participants had positive whole virus-based immunoassay results at week 78 but negative HIV PCR tests. Data from other phase 1 MRKAd5-vectored vaccines demonstrated that 41% of evaluable vaccine recipients had a positive ELISA but negative PCR results by week 78. In these studies, seroconversion was directly related to vaccine dose and inversely related to baseline Ad5 titer. Furthermore, among those subjects with positive ELISA results at week 78, ELISA positivity persisted through week 156 for the majority of evaluable participants who received doses containing ≥1 × 1010 gag-containing Ad5 particles/dose.78 Where possible, enzyme immunoassays (such as envelope-based assays) targeting proteins not expressed in the vaccine should be the first choice for screening vaccine recipients.
There were four incident HIV infections in the current study population of low-risk adults; all had received three doses of vaccine. The proof-of-concept STEP study of the MRKAd5 trivalent vaccine showed that men who were uncircumcised and who had high levels of preexisting Ad5 neutralizing antibodyAd5 titers were more likely to become HIV infected if they had received the trivalent vaccine as opposed to placebo.67,68 In the current study four men, all of whom were in the vaccine group, became HIV infected. There was over four times the number of participants in the vaccine as compared to the placebo group (296 vs. 64). Therefore, although more infections occurred among vaccine recipients, the rate of infection in vaccinees did not differ from that in placebo recipients. Because the number of infections that occurred in this study was small and no circumcision data were collected, it is not possible to draw conclusions about increased susceptibility among vaccine recipients based on baseline Ad5 titer or circumcision. Viral loads were not followed as part of the study. Therefore, there are no data on how vaccination may have impacted viral load among study participants. Data from the STEP study showed that vaccination had no impact on viral load among vaccine as compared to placebo recipients.67,68
The data from this study provide important information on the safety and immunogenicity of an adenovirus-vectored vaccine in a diverse population including those with high baseline rates of Ad5 seropositivity. A proof-of-concept study employing the trivalent (gag, pol, nef ) MRKAd5 HIV vaccine conducted in parallel showed the trivalent vaccine to be nonefficacious. These data emphasize the challenges of developing an efficacious HIV vaccine as cell-mediated immunity did not correlate with protection from infection or lowering of the viral load in those who became infected.67,68
The results of these studies have implications for the development of other Ad5-vectored HIV vaccine candidates under development. Ad5-vectored vaccines have provided the most robust immune responses of any approach to HIV vaccination to date. Preexisting immunity to Ad5 may be partially overcome with an increase in vaccine dose and hence may not be a barrier to developing such responses; however, more data are needed to fully understand the role that preexisting immunity may have in enhancing infection. In additional, more data are needed to understand the role of alternative adenoviral vectors in HIV vaccine development.
Our results indicate that the Ad5 vector is well tolerated and immunogenic in diverse global populations and, therefore, may be a good candidate for other cell-mediated vaccine strategies. The safety of these vectors should be explored in preclinical studies and small clinical proof-of-concept studies in select populations before assessing their role in large global studies.
Acknowledgments
We thank the study participants and staff at the study sites for their dedication to the development of an HIV-1 vaccine. We immensely appreciate the invaluable contributions and leadership of Scott Thaler (deceased), Robin Isaacs, and Randi Leavitt. We also appreciate the assistance of Nicole Frahm, Liza Noonan, Michael Keefer, Carmen Zorilla, Joseph Chiu, Massimo Cardinali, Nina Russell, Priya Kulkarni, Barbara Meyer, Karyn Davis, and Christine Lotz. Merck Research Laboratories, a Division of Merck & Company, Inc., funded the studies analyzed herein.
Author Disclosure Statement
O.N., F.D., X.S., E.Q., M.M., M.N.R., and D.V.M. are or were paid employees of Merck and potentially own Merck stock and/or have Merck stock options. G.G., P.P., J.K., and S.M.H. have all served as investigators on Merck-funded research.
References
- 1.UNAIDS The Joint United Nation Programme on HIV/AIDS: 2008 report on the global AIDS epidemic. Executive summary. 2008 [Google Scholar]
- 2.Bennett DE. The requirement for surveillance of HIV drug resistance within antiretroviral rollout in the developing world. Curr Opin Infect Dis. 2006;19:607–614. doi: 10.1097/QCO.0b013e3280109ff1. [DOI] [PubMed] [Google Scholar]
- 3.Laurent C. Meilo H. Guiard-Schmid J-B, et al. Antiretroviral therapy in public and private routine health care clinics in Cameroon: Lessons from the Douala Antiretroviral (DARVIR) Initiative. Clin Infect Dis. 2005;41:108–111. doi: 10.1086/430712. [DOI] [PubMed] [Google Scholar]
- 4.Muula AS. Chipeta J. Siziya S. Rudatsikira E. Mataya RH. Kataika E. Human resources requirements for highly active antiretroviral therapy scale-up in Malawi. BMC Health Serv Res. 2007;7:208. doi: 10.1186/1472-6963-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekelle P. Maglione M. Geotz MB. Wagner G. Wang Z. Hilton L, et al. AHRQ Publication No. 07-E014. Agency for Healthcare Research and Quality; Rockville, MD: 2007. Antiretroviral (ARV) drug resistance in the developing world. Evidence Report/Technology Assessment No. 156. (Prepared by Southern California Evidence-based Practice Center, Contract No. 290-02-0003.) [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzo CM. Germinario EAP. Bassani L, et al. Antiretroviral resistance mutations in untreated pregnant women with HIV infection in Uganda and Rwanda [letter to the editor] AIDS Res Hum Retroviruses. 2007;23:1449–1451. doi: 10.1089/aid.2007.0109. [DOI] [PubMed] [Google Scholar]
- 7.Marconi VC. Sunpath H. Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46:1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinson NA. Morris L. Gray G, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44:148–153. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 9.Ndembi N. Abraha A. Pilch H, et al. Molecular characterization of human immunodeficiency virus type 1 (HIV-1) and HIV-2 in Yaounde, Cameroon: Evidence of major drug resistance mutations in newly diagnosed patients infected with subtypes other than subtype B. J Clin Microbiol. 2008;46:177–184. doi: 10.1128/JCM.00428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toni TD. Masquelier B. Minga A, et al. HIV-1 antiretroviral drug resistance in recently infected patients in Abidjan, Côte d'Ivoire: A 4-year survey, 2002–2006. AIDS Res Hum Retroviruses. 2007;23:1155–1160. doi: 10.1089/aid.2007.0072. [DOI] [PubMed] [Google Scholar]
- 11.Lyles CM. Kay LS. Crepaz N, et al. Best-evidence interventions: Findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. Am J Public Health. 2007;97:133–143. doi: 10.2105/AJPH.2005.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson KG. Beutel AM. Maughan-Brown B. HIV risk perceptions and first sexual intercourse among youth in Cape Town, South Africa. Int Fam Plan Perspect. 2007;33:98–105. doi: 10.1363/ifpp.33.098.07. [DOI] [PubMed] [Google Scholar]
- 13.Dancy BL. Kaponda CPN. Kachingwe SI. Norr KF. Risky sexual behaviors of adolescents in rural Malawi: Evidence from focus groups. J Natl Black Nurses Assoc. 2006;17:22–28. [PubMed] [Google Scholar]
- 14.Holtgrave D. Evidence-based efforts to prevent HIV infection: An overview of current status and future challenges. Clin Infect Dis. 2007;45:S293–S299. doi: 10.1086/522553. [DOI] [PubMed] [Google Scholar]
- 15.Holtgrave DR. Curran JW. What works, and what remains to be done, in HIV prevention in the United States. Annu Rev Public Health. 2006;27:261–275. doi: 10.1146/annurev.publhealth.26.021304.144454. [DOI] [PubMed] [Google Scholar]
- 16.Ickovics JR. “Bundling” HIV prevention: Integrating services to promote synergistic gain. Prev Med. 2008;46:222–225. doi: 10.1016/j.ypmed.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy NC. Miksad RA. Fein OT. From treatment to prevention: The interplay between HIV/AIDS treatment availability and HIV/AIDS prevention programming in Khayelitsha, South Africa. J Urban Health. 2005;82:498–509. doi: 10.1093/jurban/jti090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lurie M. Pronyk P. de Moor E, et al. Sexual behavior and reproductive health among HIV-infected patients in urban and rural South Africa. J Acquir Immune Defic Syndr. 2008;47:484–493. doi: 10.1097/QAI.0b013e3181648de8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maganja RK. Maman S. Groves A. Mbwambo JK. Skinning the goat and pulling the load: Transactional sex among youth in Dar es Salaam, Tanzania. AIDS Care. 2007;19:974–981. doi: 10.1080/09540120701294286. [DOI] [PubMed] [Google Scholar]
- 20.Nkosana J. Rosenthal D. The dynamics of intergeneraltional sexual relationships: The experience of schoolgirls in Botswana. Sex Health. 2007;4:181–187. doi: 10.1071/sh06070. [DOI] [PubMed] [Google Scholar]
- 21.Nagelkerke NJD. Moses S. de Vlas SJ. Bailey RC. Modelling the public health impact of male circumcision for HIV prevention in high prevalence area in Africa. BMC Infect Dis. 2007;7:16. doi: 10.1186/1471-2334-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn TC. Circumcision and HIV transmission. Curr Opin Infect Dis. 2007;20:33–38. doi: 10.1097/QCO.0b013e328012c5bc. [DOI] [PubMed] [Google Scholar]
- 23.Sahasrabuddhe VV. Vermund SH. The future of HIV prevention: Control of sexually transmitted infections and circumcision interventions. Infect Dis Clin North Am. 2007;21:241–257. doi: 10.1016/j.idc.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams BG. Lloyd-Smith JO. Gouws E, et al. The potential impact of male circumcision on HIV in sub-Saharan Africa. PLoS Med. 2006;3:1032–1040. doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey RC. Moses S. Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 26.Gray RH. Kigozi G. Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: A randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 27.Karim Q. Karim S. Frohlich J, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amirfar S. Hollenberg JP. Abdool Karim SS. Modeling the impact of a partially effective HIV vaccine on HIV infection and death among women and infants in South Africa. J Acquir Immune Defic Syndr. 2006;43:219–225. doi: 10.1097/01.qai.0000230526.79341.83. [DOI] [PubMed] [Google Scholar]
- 29.Andersson KM. Owens DK. Vardas E. Gray GE. McIntyre JA. Paltiel AD. Predicting the impact of a partially effective HIV vaccine and subsequent risk behavior change on the heterosexual HIV epidemic in low- and middle-income countries: A South African example. J Acquir Immune Defic Syndr. 2007;46:78–90. doi: 10.1097/QAI.0b013e31812506fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blower SM. Bodine EN. Grovit-Ferbas K. Predicting the potential public health impact of disease-modifying HIV vaccines in South Africa: The problem of subtypes. Curr Drug Targets. 2005;5:179–192. doi: 10.2174/1568005054201616. [DOI] [PubMed] [Google Scholar]
- 31.Pantaleo G. Koup RA. Correlates of immune protection in HIV-1 infection: What we know, what we don't know, what we should know. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 32.Draenert R. Le Gall S. Pfafferott KJ, et al. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J Exp Med. 2004;199:905–915. doi: 10.1084/jem.20031982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokomaku Y. Miura H. Tomiyama H, et al. Impaired processing and presentation of cytotoxic-T-lymphocyte (CTL) epitopes are major escape mechanisms from CTL immune pressure in human immunodeficiency virus type 1 Infection. J Virol. 2004;78:1324–1332. doi: 10.1128/JVI.78.3.1324-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen TM. Altfeld M. Geer SC, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol. 2005;79:13239–13249. doi: 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ammaranond P. Zaunders J. Satchell C. Van Bockel D. Cooper DA. Kelleher AD. A new variant cytotoxic T lymphocyte escape mutation in HLA-B27-positive individuals infected with HIV type 1. AIDS Res Hum Retroviruses. 2005;21:395–397. doi: 10.1089/aid.2005.21.395. [DOI] [PubMed] [Google Scholar]
- 36.Pillay T. Zhang H-T. Drijfhout JW, et al. Unique acquisition of cytotoxic T-lymphocyte escape mutants in infant human immunodeficiency virus type 1 infection. J Virol. 2005;79:12100–12105. doi: 10.1128/JVI.79.18.12100-12105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ávila-Ríos S. Reyes-Terán G. Espinosa E. Cornering HIV: Taking advantage of interactions between selective pressures. Med Hypotheses. 2007;69:422–431. doi: 10.1016/j.mehy.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Seaman MS. Xu L. Beaudry K, et al. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;79:2956–2963. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson MM. Pérez-Ávarez L. Nájera R. Molecular epidemiology of HIV-1 genetic forms and its significance for vaccine development and therapy. Lancet Infect Dis. 2002;2:461–471. doi: 10.1016/s1473-3099(02)00343-2. [DOI] [PubMed] [Google Scholar]
- 40.van der Groen G. Nyambi PN. Beirnaert E, et al. Genetic variation of HIV type 1: Relevance of interclade variation to vaccine development. AIDS Res Hum Retroviruses. 1998;14:S-211–S-221. [PubMed] [Google Scholar]
- 41.The rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 42.Pitisuttithum P. Gilbert P. Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari G. Humphrey W. McElrath MJ, et al. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buseyne F. Chaix ML. Fleury B, et al. Cross-clade-specific cytotoxic T lymphocytes in HIV-1-infected children. Virology. 1998;250:316–324. doi: 10.1006/viro.1998.9373. [DOI] [PubMed] [Google Scholar]
- 45.Ferrari G. Kostyu DD. Cox J, et al. Identification of highly conserved and broadly cross-reactive HIV type 1 cytotoxic T lymphocyte epitopes as candidate immunogens for inclusion in Mycobacterium bovis BCG-vectored HIV vaccines. AIDS Res Hum Retroviruses. 2000;16:1433–1443. doi: 10.1089/08892220050140982. [DOI] [PubMed] [Google Scholar]
- 46.Fukada K. Tomiyama H. Wasi C, et al. Cytotoxic T-cell recognition of HIV-1 cross-clade and clade-specific epitopes in HIV-1-infected Thai and Japanese patients. AIDS. 2002;16:701–711. doi: 10.1097/00002030-200203290-00005. [DOI] [PubMed] [Google Scholar]
- 47.Lynch JA. deSouza M. Robb MD, et al. Cross-clade cytotoxic T cell response to human immunodeficiency virus type 1 proteins among HLA disparate North Americans and Thais. J Infect Dis. 1998;178:1040–1046. doi: 10.1086/515652. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SE. Pedersen SL. Kunich JC, et al. Cross-clade envelope glycoprotein 160-specific CD8+ cytotoxic T lymphocyte responses in early HIV type 1 clade B infection. AIDS Res Hum Retroviruses. 1998;14:925–937. doi: 10.1089/aid.1998.14.925. [DOI] [PubMed] [Google Scholar]
- 49.Coplan PM. Gupta SB. Dubey SA, et al. Cross-reactivity of anti-HIV-1 T cell immune responses among the major HIV-1 clades in HIV-1-positive individuals from 4 continents. J Infect Dis. 2005;191:1427–1434. doi: 10.1086/428450. [DOI] [PubMed] [Google Scholar]
- 50.Barouch DH. Kunstman J. Glowczwskie J, et al. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J Virol. 2003;77:7367–7375. doi: 10.1128/JVI.77.13.7367-7375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barouch DH. Santra S. Kuroda MJ, et al. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J Virol. 2001;75:5151–5158. doi: 10.1128/JVI.75.11.5151-5158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belshe RB. Stevens C. Gorse GJ, et al. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus type 1 vaccine with or without gp120: A phase 2 study in higher- and lower-risk volunteers. J Infect Dis. 2001;183:1343–1352. doi: 10.1086/319863. [DOI] [PubMed] [Google Scholar]
- 53.Evans TG. Keefer MC. Weinhold KJ, et al. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J Infect Dis. 1999;180:290–298. doi: 10.1086/314895. [DOI] [PubMed] [Google Scholar]
- 54.Fang Z-Y. Kuli-Zade I. Spearman P. Efficient human immunodeficiency virus (HIV)-1 gag-env pseudovirion formation elicited from mammalian cells by a canarypox HIV vaccine candidate. J Infect Dis. 1999;180:1122–1132. doi: 10.1086/315028. [DOI] [PubMed] [Google Scholar]
- 55.Fleury B. Janvier G. Pialoux G, et al. Memory cytotoxic T lymphocyte responses in human immunodeficiency virus type 1 (HIV-1)––negative volunteers immunized with a recombinant canarypox expressing gp160 of HIV-1 and boosted with a recombinant gp160. J Infect Dis. 1996;174:734–738. doi: 10.1093/infdis/174.4.734. [DOI] [PubMed] [Google Scholar]
- 56.Goepfert PA. Horton H. McElrath MJ, et al. High-dose recombinant canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. J Infect Dis. 2005;192:1249–1259. doi: 10.1086/432915. [DOI] [PubMed] [Google Scholar]
- 57.Gupta K. Hudgens M. Corey L, et al. Safety and immunogenicity of a high-titered canarypox vaccine in combination with rgp120 in a diverse population of HIV-1-uninfected adults: AIDS vaccine evaluation group protocol 022A. J Acquir Immune Defic Syndr. 2002;29:254–261. doi: 10.1097/00126334-200203010-00005. [DOI] [PubMed] [Google Scholar]
- 58.Hanke T. Samuel RV. Blanchard TJ, et al. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vacccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hel Z. Nacsa J. Tryniszewska E, et al. Containment of simian immunodeficiency virus infection in vaccinated macaques: Correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol. 2002;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 60.Hel Z. Tsai WP. Thornton A, et al. Potentiation of simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J Immunol. 2001;167:7180–7191. doi: 10.4049/jimmunol.167.12.7180. [DOI] [PubMed] [Google Scholar]
- 61.Shen L. Chen ZW. Miller MD, et al. Recombinant virus vaccine-induced SIV-specific CD8+ cytotoxic T lymphocytes. Science. 1991;252:440–443. doi: 10.1126/science.1708168. [DOI] [PubMed] [Google Scholar]
- 62.Catanzaro AT. Koup RA. Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casimiro DR. Wang F. Schleif WA, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing gag. J Virol. 2005;79:15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casimiro DR. Chen L. Fu T-M, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shiver JW. Fu T-M. Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity [letter] Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 66.Santra S. Seaman MS. Xu L, et al. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79:6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson M. Mehrotra D. Fitzgerald D, et al. Efficacy results from the STEP Study (Merck V520 Protocol 023/HVTN 502): A phase II test-of-concept trial of the MRKAd5 HIV-1 Gag/Pol/Nef Trivalent Vaccine. 15th Conference on Retroviruses and Opportunistic Infections; 2008. Abstract 88LB. [Google Scholar]
- 68.Buchbinder SP. Mehrotra DV. Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rerks-Ngarm S. Pitisuttithum P. Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 70.Wu X. Yang ZY. Liang Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harro CD. Robertson MN. Lally MA, et al. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res Hum Retroviruses. 2009;25:1–12. doi: 10.1089/aid.2008.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubey S. Clair J. Fu T-M, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr. 2007;45:20–27. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 73.Kierstead LS. Dubey S. Meyer B, et al. Enhanced rates and magnitude of immune responses detected against an HIV vaccine: Effect of using an optimized process for isolating PBMC. AIDS Res Hum Retroviruses. 2007;23:86–92. doi: 10.1089/aid.2006.0129. [DOI] [PubMed] [Google Scholar]
- 74.Aste-Amézaga M. Bett AJ. Wang F, et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: Application in epidemiologic studies in the design of adenovector vaccines. Hum Gene Ther. 2004;15:293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
- 75.Miettinen O. Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 76.Mehrotra DV. Heyse JF. Use of the false discovery rate for evaluating clinical safety data. Stat Methods Med Res. 2004;13:227–238. doi: 10.1191/0962280204sm363ra. [DOI] [PubMed] [Google Scholar]
- 77.Kaslow RA. Carrington M. Apple R, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 78.Quirk EK. Mogg R. Brown DD, et al. HIV seroconversion without infection after receipt of adenovirus-vectored HIV type 1 vaccine. Clin Infect Dis. 2008;47:1593–1599. doi: 10.1086/593313. [DOI] [PubMed] [Google Scholar]