Abstract
The aim of this study was to analyze the predictive value of CXCL9, CXCL10, and CXCL11 concentrations before and after 4 and 12 weeks of treatment with pegylated interferon-α2b and ribavirin in patients with chronic hepatitis C infected with the hepatitis C virus genotype 1. The study included 46 adult patients (29 women and 17 men). Chemokine quantification in the serum was performed at baseline and after 1, 3, and 6 months of treatment by enzyme immunoassay. Chemokine responses were compared in patients achieving a sustained virological response to treatment (SVR, n=26) and the non-SVR group (n=20). The differences in the CXCL9 and CXCL10 concentrations between the SVR and non-SVR groups were statistically significant. A multivariant analysis showed a significant association between treatment failure and higher concentrations of CXCL10. A higher predictive value of CXCL10 concentrations after 4 weeks of treatment compared to pretreatment values has been found (area under the curve 0.9288 and 0.7942, respectively, P=0.016). CXCL10 concentrations above 250 pg/mL 4 weeks after the start of treatment were independently associated with non-SVR. In conclusion, the results of this study have shown that CXCL10 concentrations at the time of a rapid viral response (4 weeks) are better predictors of achieving SVR compared to baseline levels. Additionally, this study suggests an important role of CXCL9 as a biomarker of SVR in patients with chronic hepatitis C.
Introduction
Hepatitis C virus (HCV) is a hepatotropic virus that has developed multiple strategies for evasion of effector mechanisms of both innate and specific immunity, resulting in the establishment of chronic infection in the majority of infected individuals (Irshad and others 2008). In the absence of treatment, chronic hepatitis C is associated with cirrhosis, hepatocellular carcinoma, and liver failure in a subset of infected patients (Gonzalez and Keeffe 2011). Molecular mechanisms (both immunological and genetic) responsible for hepatic cell injury and rate of hepatic fibrogenesis in chronic hepatitis C are at present incompletely understood (Irshad and others 2008).
Clearance of HCV infection and effective control of the virus depend on the presence of HCV-specific T-cells and their successful migration from the sites of antigen recognition into the liver (Larrubia and others 2008; Diepolder 2009). However, HCV-specific immunity often fails to eradicate the virus. Additionally, a concomitant recruitment of T-cells specific for other antigens into the liver can be observed in patients with chronic infection C (Neumann-Haefelin and others 2008; Kasprowicz and others 2010). The presence of this nonspecific inflammatory infiltrate consisting of CD8+ T-cells that are not specific for HCV is considered responsible for liver damage (Larrubia and others 2008).
The recruitment of T-cells (both specific for HCV and not specific for the virus) into the liver is mediated by the interaction between chemokines secreted by infected cells and corresponding chemokine receptors on migrating leukocytes (Charo and Ransohoff 2006; Heydtmann and Adams 2009). Chemokines are a heterogeneous group of chemotactic cytokines responsible for the recruitment of leukocytes to sites of inflammation as well as for a variety of other biological activities relevant for development, angiogenesis, maintenance of homeostasis, and pathogenesis of various diseases (Charo and Ransohoff 2006). Chemokines and their receptors are proposed to have a dual role in the pathogenesis of chronic hepatitis C contributing both to the viral clearance as well as development of chronic liver inflammation (Larrubia and others 2008).
A number of studies investigated the role of various chemokines as possible biological markers of hepatocellular damage and treatment-induced viral clearance in patients with chronic hepatitis C.
Several studies have shown that pretreatment levels of CXCL10 [interferon-γ-inducible protein (IP-10)] are predictive of failure to treatment with pegylated interferon alpha 2 (PEG IFN-α2) and ribavirin (Butera and others 2005; Diago and others 2006; Lagging and others 2006; Romero and others 2006; Askarieh and others 2010; Lee and others 2010; Vargas and others 2010). Additionally, serum CXCL10 concentrations in patients with chronic hepatitis C correlated with the HCV viral load, alanine aminotransferase levels, the hepatic inflammatory activity, and the fibrosis stage (Reiberger and others 2008; Zeremski and others 2009).
Casrouge and others (2011) recently showed that CXCL10 in the plasma of patients with chronic hepatitis C exists in a form of an antagonist that is capable of binding to the CXCR3 receptor, but fails to induce signaling. These findings helped to explain why CXCL10, for example, a proinflammatory chemokine that induces lymphocyte trafficking into the liver represents a negative prognostic marker of treatment outcome.
The aim of this study was to analyze the predictive value of CXCL9, CXCL10, and CXCL11 concentrations at baseline and after 4 and 12 weeks of treatment with PEG IFN-α2b and ribavirin in patients with chronic hepatitis C infected with the HCV genotype 1.
Materials and Methods
Patients and samples
The prospective study included 46 treatment-naive adult patients (29 women and 17 men) with chronic hepatitis C receiving clinical care at the Croatian Reference Center for Viral Hepatitis, Zagreb, Croatia. The patients were enrolled consecutively upon signing the informed consent.
All patients included in the study were infected with the HCV genotype 1. All patients underwent a liver biopsy as a part of pretreatment evaluation. The patients were treated with a combination of pegylated interferon alpha 2b (PEG IFN-α2b) 1.5 μg/kg and ribavirin (weight-based regimen) for 48 weeks. Serum samples for HCV RNA quantification were collected before treatment and after weeks 4 [rapid viral response (RVR)], 12 [early viral response (EVR)], and 48 [end of treatment response (ETR)] of treatment as well as at week 72 [24 weeks after the treatment was completed, sustained viral response (SVR)]. The treatment was discontinued if the viral load decreased by less than 2 log HCV RNA copies/mL at week 12 compared with baseline values or if HCV RNA was still detectable at week 24. SVR was defined as undetectable HCV RNA at 24 weeks after completion of therapy.
Chemokine quantification in the serum was performed before treatment and after 4, 12, and 24 weeks of treatment. The samples for chemokine quantification were stored at −80°C in 2 aliquots to avoid repeated freeze and thaw cycles.
The patients were classified into 2 groups according to the virological response at week 24 after treatment (SVR); responders, and nonresponders.
Methods
HCV RNA in the serum of patients before and during treatment was quantified by using the COBAS AmpliPrep/COBAS TaqMan HCV real-time polymerase chain reaction (PCR) assay with the COBAS AmpliPrep Instrument and the COBAS TaqMan 48 Analyzer (Roche Molecular Systems, Inc., Pleasanton, CA). HCV genotyping was performed by using the Versant HCV Genotyping assay (LiPA, Bayer Diagnostics, Puteaux, Cedex, France) based on PCR and reverse hybridization.
Concentrations of CXCL9, CXCL10, and CXCL11 in the serum of patients with chronic hepatitis C were determined by using Quantikine Human CXCL9, Quantikine Human CXCL10, and Quantikine Human CXCL11 ELISA assays (R&D Systems, Minneapolis, MN). Molecular and immunological assays were performed at the Department of Molecular Diagnostics, the University Hospital for Infectious Diseases (UHID), Zagreb, Croatia.
Liver biopsy was performed at the Department of Viral Hepatitis (UHID) and the histological analysis was performed by a skilled pathologist at the Zagreb University Hospital. ISHAK scoring system was used as the indicator of the histological activity.
Data collection
Selected clinical and laboratory data on the patients were extracted from patient records of the Department of Viral Hepatitis at UHID. Results of virological and immunological assays were extracted from the database of the Department of Molecular Diagnostics (UHID). The Ethics Committee of UHID approved this study.
Statistical analysis
Categorical data were expressed in frequencies and relevant percentage. The significance of the observed differences in frequencies between relevant subgroups was tested with the chi-square test or the Fisher's exact test when appropriate. For continuous variables, we calculated mean values with standard deviations or median values with 25th and 75th percentile depending on distribution. Differences between subgroups of interest were tested with the Wilcoxon rank-sum test. We conducted the longitudinal data analysis of cytokine concentrations comparing responders and nonresponders. A generalized linear mixed effects model was used to better estimate factors independently associated with cytokine levels in various time intervals from the start of treatment. We included into the model baseline variables, which significantly differed between responders and nonresponders after the univariate analysis. To assess predictive utility of cytokine levels before and 1 month after the start of treatment on patients' outcome, a receiver operating characteristic (ROC) curve was constructed. The area under the curve (AUC), the positive predictive value and the negative predictive value were determined from the ROC curve for assessing the predictive efficacy of serum cytokines. After identifying the optimal threshold value, the logistic regression analysis was done to estimate the significance of association between the estimated threshold and treatment outcome. P-value less than 0.05 was considered significant. All analyses were performed using SAS for Windows, version 9.2. SAS Institute, Inc.
Results
Patients characteristics
Concentrations of CXCL9, CXCL10, and CXCL11 in patients with chronic hepatitis C were analyzed in 46 Caucasian patients infected with a genotype 1 of HCV. Selected demographic parameters of the patients are presented in Table 1. Mean age of enrolled patients was 41.5 years (SD±12.4 years), whereas median weight was 68.5 kg (range 50–97 kg). Median fibrosis index in enrolled patients was 3 (range 2–6), whereas median HAI was 8 (range 4–17). The univariate analysis was done to compare SVR versus non-SVR and groups differed in several variables: age (median 35.0 versus 50.5, P=0.001) and number of RNA copies (median 266,045 versus 727,084, P=0.036). The incidence of higher fibrosis index (4–6) was also lower in the SVR group (11.5% versus 45%, P=0.010). The groups did not differ regarding body mass index, gender, and HAI index (data not presented).
Table 1.
Baseline Characteristics of Patients with Chronic Hepatitis C According to Treatment Response
| |
Median (25–75 percentiles) |
|
||
|---|---|---|---|---|
| Patient characteristics | Non-SVR (n=20) | SVR (n=26) | Total patient group (n=46) | P value |
| Age (years) | 50.5 (39–59) | 35 (28–40) | 39.5 (30–52) | 0.0012 |
| Sex (n, %) | 0.391 | |||
| Male | 6 (30%) | 11 (42.3%) | 17 (37%) | |
| Female | 14 (45%) | 15 (57.7%) | 29 (63%) | |
| Liver biopsy (ISHAK score) | ||||
| Fibrosis (n, %) | 0.010 | |||
| Stage 1–3 | 11 (55%) | 23 (88.5%) | 34 (73.9%) | |
| Stage 4–6 | 9 (45%) | 3 (11.5%) | 12 (26.1%) | |
| Histology activity index (HAI, n, %) | 0.364 | |||
| HAI 0–6 | 6 (30%) | 7 (26.9%) | 13 (28.3%) | |
| HAI 7–12 | 11 (55%) | 18 (69.2%) | 29 (63%) | |
| HAI 13–18 | 3 (15%) | 1 (3–8%) | 4 (8.7%) | |
| HCV RNA (IU/mL) before treatment | 727,084 (280,140–1,700,000) | 266,045 (93,900–694,000) | 354,132 (165,000–1,243,560) | 0.036 |
| Body mass index | 23.9 (22–25.5) | 23.7 (21–24.9) | 23.7 (21.9–25) | 0.527 |
HCV, hepatitis C virus; SVR, sustained viral response.
Virological response
Median HCV RNA levels before treatment were 354 132 IU/mL (range 1,505–9,470,000 IU/mL). The majority of patients (24 of 46, 52%) were infected with the HCV genotype 1b, while the genotype 1a was detected in 9 of 46 (19%) patients (in 13 patients subgenotypes 1a and 1b were not determined due to limitation of the molecular assay).
Following treatment with a combination of PEG IFN-α2b and ribavirin, RVR was achieved in 18 of 46 (39%) patients and EVR was achieved in 37 of 46 patients (80.4%). ETR was achieved in 35 of 46 (76%) patients and subsequently 26 of 46 (56.5%) patients achieved SVR.
Chemokine responses were compared in patients achieving SVR (n=26) and the non-SVR group (n=20).
Chemokine analysis
Concentrations of CXCL9, CXCL10, and CXCL11 were measured before treatment and after 4, 12, and 24 weeks of treatment (Table 2).
Table 2.
Concentrations of CXCL9, CXCL10, and CXCL11 in the Plasma of Patients with Chronic Hepatitis C During Treatment with Pegylated Interferon Alpha 2a and Ribavirin Based on the Virological Response
| |
Concentrations of plasma chemokines in patients with chronic hepatitis C based on the response to treatment with PEG IFN-α2a and ribavirin (pg/mL, median, range) |
|
|---|---|---|
| Chemokines | SVR, n=26 patients | Non-SVR, n=20 |
| CXCL9 | ||
| Baseline | 171.5 (52–485) | 200 (113–442) |
| 4 weeks | 118.5 (36–399) | 176 (126–1,990) |
| 12 weeks | 153.5 (35–425) | 214 (92–290) |
| 24 weeks | 191.5 (30–510) | 298 (84–583) |
| CXCL10 | ||
| Baseline | 185 (63–518) | 395.5 (111–926) |
| 4 weeks | 156 (92–396) | 424 (90–815) |
| 12 weeks | 199 (105–780) | 277.5 (108–674) |
| 24 weeks | 202 (80–650) | 272 (76–514) |
| CXCL11 | ||
| Baseline | 109 (0–499) | 127.5 (77–341) |
| 4 weeks | 96.5 (0–211) | 135.5 (94–304) |
| 12 weeks | 97.5 (5–267) | 120.5 (81–167) |
| 24 weeks | 114 (82–267) | 127 (81–203) |
Differences in the CXCL9 and CXCL10 concentrations between SVR and non-SVR groups were statistically significant (P=0.002 and P<0.0001, respectively).
The difference in the CXCL11 concentration between SVR and non-SVR groups was not statistically significant (P=0.143).
PEG IFN-α2a, pegylated interferon alpha 2a.
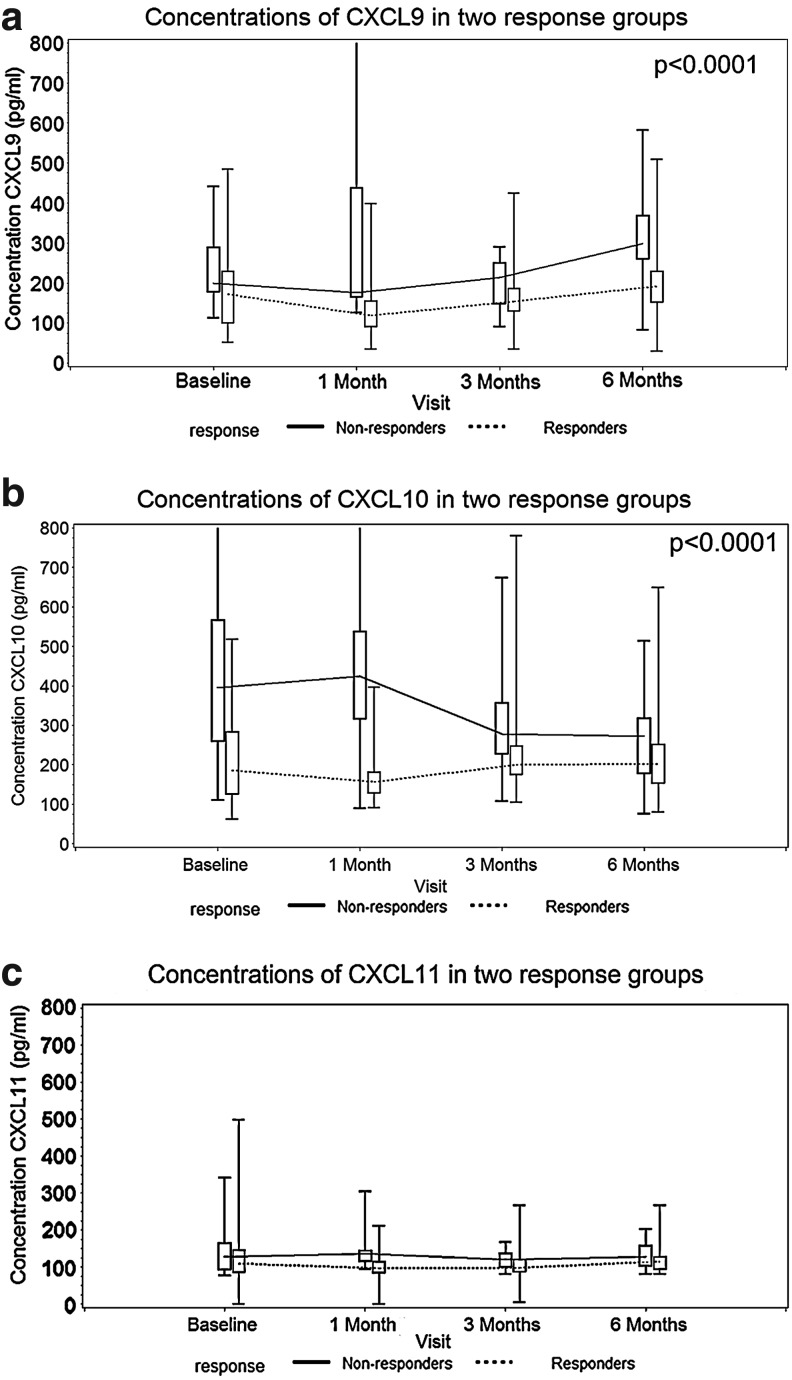
Concentration of CXCL9 decreased from baseline values in both SVR and non-SVR groups (median 171.5 and 200 pg/mL, respectively), to 118.5 and 176 pg/mL after 1 month of treatment, but after that a gradual increase followed, more pronounced in the non-SVR group (Table 2 and Fig. 1a). The highest CXCL9 concentrations were observed in the non-SVR patient group after 6 months of treatment (298 pg/mL) (Table 2). The longitudinal data analysis showed significant differences in the CXCL9 concentrations between the SVR and non-SVR groups (P=0.002, Fig. 1a).
FIG. 1.
(a–c) Concentrations of CXCL9, CXCL10, and CXCL11 in the serum of patients with chronic hepatitis C treated with pegylated interferon alpha 2b and ribavirin based on the virological response.
CXCL10 concentrations in the plasma of patients in the SVR and non-SVR groups showed different patterns (Fig. 1b). In the SVR group, CXCL10 concentrations decreased after 1 month of treatment (median 185 pg/mL at baseline versus 156 pg/mL after 1 month) (Table 2). Contrary to this finding, CXCL10 concentrations in non-SVR groups increased from a median baseline value of 395.5–424 pg/mL after 1 month of treatment (Table 2). CXCL10 concentrations decreased after 3 and 6 months of treatment in both patient groups (Table 2 and Fig. 1b). The longitudinal data analysis also showed significant differences in the CXCL10 concentrations between the SVR and non-SVR groups (P<0.0001). The difference in CXCL10 levels was most pronounced 1 month after treatment.
Median concentrations of CXCL11 in the SVR and non-SVR group at baseline were 109 and 127.5 pg/mL, respectively (Table 2 and Fig. 1c). After 6 months of treatment with PEG IFN-α2a and ribavirin, median CXCL11 concentration in the SVR group was 114 and 127 pg/mL in the non-SVR group (Table 2 and Fig. 1c). Contrary to the CXCL9 and CXCL10 concentrations, the longitudinal data analysis did not show significant differences in the CXCL11 concentrations between the SVR and non-SVR groups (P=0.143).
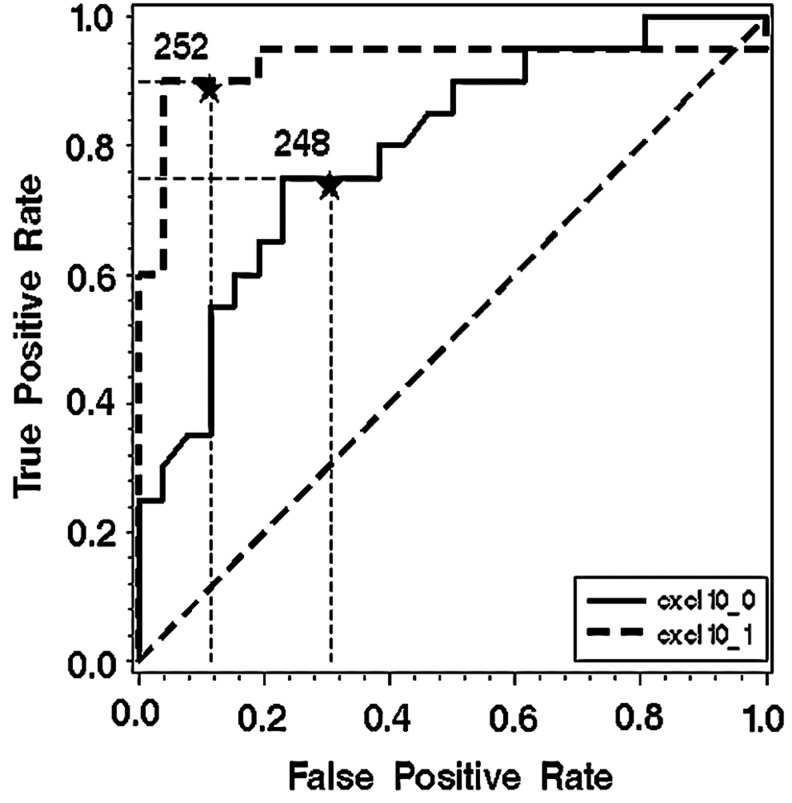
To estimate independent association of the response on treatment and chemokine CXCL10 concentrations throughout of study, the multivariate longitudinal data analysis (generalized linear mixed model) was done. The analysis showed a significant association between treatment failure and higher concentrations of CXCL10 after adjustment for HCV RNA levels, fibrosis levels, and HAI (Fig. 2 and Table 3).
FIG. 2.
Logistic regression analysis assessing the prognostic value of chemokine CXCL10 concentration after 4 weeks of treatment.
Table 3.
Multivariant Analysis of Parameters Associated with CXCL10 Levels During Treatment of Chronic Hepatitis C (Generalized Linear Model)
|
Type III test of fixed effects | ||||
|---|---|---|---|---|
| Effect | No. of DF | Den DF | F value | Pr<F |
| Control | 3 | 135 | 2.66 | 0.0506 |
| Response (SVR) | 1 | 40 | 16.28 | 0.0002 |
| Histology activity index | 2 | 40 | 1.15 | 0.3278 |
| Fibrosis levels | 1 | 40 | 0.31 | 0.5790 |
| HCV RNA before treatment | 1 | 40 | 0.81 | 0.3745 |
DF, degrees of freedom.
After observation that response groups differed mostly in chemokine CXCL10 concentrations, a receiver under the operation curve was constructed to assess the predictive value of this chemokine concentrations on the treatment response. A higher predictive value of CXCL10 concentrations after 4 weeks of treatment compared to pretreatment values has been found (AUC 0.9288 versus 0.7942, respectively=0.018). Our analysis also suggested that the breaking point concentration of about 250 pg/mL could be used as a predictor value. To test this hypothesis, the logistic regression analysis was performed to assess if chemokine CXCL10 levels after 4 weeks of treatment could be used as a prognostic parameter of treatment response after adjustment for other most important parameters associated with non-SVR, age, and the grade of fibrosis, presented as a dichotomous variable (fibrosis 1–3 and 4–6). CXCL10 concentration higher than 250 pg/mL was associated with a 40-fold risk of non-SVR (OR=40.743, 95% CI 5.598 to 296.534), while age and the fibrosis grade did not show significant association (OR=1.080, 95% CI 0.992 to 1.175 and OR=0.330, 95% CI 0.028 to 3.849, respectively). Other variables like number of copies did not enter the model to avoid overcapacitation of the model.
Discussion
This study found significant differences in plasma CXCL9 and CXCL10 concentrations between patients with chronic hepatitis C (genotype 1) who achieved SVR to PEG IFN-α2b and ribavirin compared to non-SVR patients. Higher CXCL10 concentrations were associated with treatment failure. Importantly, CXCL10 concentrations after 4 weeks of treatment (RVR) had a higher predictive value for achieving SVR compared to pretreatment concentrations of this chemokine. To our knowledge, this is the first study showing that CXCL10 concentrations above 250 pg/mL 4 weeks after treatment initiation were independently associated with non-SVR. Although CXCL10 levels after 4 weeks of treatment are associated with a 40-fold greater risk of non-SVR after adjustment for age and stage of fibrosis, these data should be interpreted cautiously because of the small number of patients.
Several studies have shown CXCL10 to be a negative predictive marker of SVR to standard treatment of chronic hepatitis C (PEG IFN-α2 and ribavirin) in patients with genotypes 1 and 4. The majority of the studies so far focused on the predictive value of CXCL10 concentrations before treatment as possible markers of SVR.
Diago and others (2006) have shown that, in patients with HCV genotype 1 infection, pretreatment CXCL10 concentrations predict SVR to treatment with PEG IFN-α2 and ribavirin.
Romero and others (2006) analyzed an association between plasma CXCL10 concentrations and liver histological results and the virological response and treatment outcome in patients with HCV genotypes 1–4. Low plasma CXCL10 levels were found to be independent predictors of RVR and SVR. A separate analysis of patients infected with the HCV genotype 1 showed that using cutoff CXCL10 concentrations of 150 and 600 pg/mL for predicting SVR correspond to specificity and sensitivity of 81% and 95%, respectively.
Lagging and others (2006) also showed that pretreatment CXCL10 concentrations predict RVR and SVR in patients with the HCV genotype 1. A baseline cutoff CXCL10 concentration of 600 pg/mL yielded a negative predictive value of 79% for achieving SVR in that study.
The results of our study have also shown that higher concentrations of CXCL10 in the plasma are associated with failure to achieve SVR. Due to the fact that the above-mentioned studies did not measure CXCL10 concentration at the time of RVR (4 weeks of treatment), a comparison with our findings on the higher predictive value for achieving SVR at week 4 compared with pretreatment values is not possible.
Butera and others (2005) showed elevated levels of all CXCR3 ligands (CXCL9, CXCL10, and CXCL11) in HCV-infected patients with the genotype 1. However, only CXCL10 concentrations at baseline were significantly higher in patients who subsequently become nonresponders to treatment, whereas CXCL9 and CXCL11 levels failed to show significant association with the treatment outcome. Contrary to these findings, our study has shown significant differences in plasma CXCL9 concentrations between patients achieving SVR and nonresponders. To the best of our knowledge, this is the first study demonstrating the role of CXCL9 in identifying patients with chronic hepatitis C who subsequently fail to respond to treatment.
The value of CXCL10 concentrations in the plasma at baseline for predicting the virological response to treatment of chronic hepatitis C with PEG IFN-α2 and ribavirin has been also shown in patients with HIV/HCV coinfection (HCV genotype 1) (Reiberger and others 2008; Vargas and others 2010). Reiberger and others (2008) have also shown that a CXCL10 cutoff value of 400 pg/mL might serve as a useful predictive marker for treatment of chronic hepatitis C in patients with HIV/HCV coinfection because it predicted SVR.
A biological foundation for a negative prognostic value of CXCL10 in the context of treatment response has been recently explained by a study showing that CXCL10 in the plasma of patients with chronic hepatitis C exists in an antagonist form (Casrouge and others 2011). The CXCL10 antagonist is formed via in situ aminoterminal truncation of the protein that is mediated by dipeptidyl peptidase IV (DPP4 or CD26). The antagonist form of CXCL10 successfully binds to the CXCR3 receptor, but is unable to initiate signal transduction, therefore abrogating the biological effect of the chemokine itself. This finding helped to explain an apparent paradox of why a chemokine responsible for recruitment of activated Th1 type lymphocytes in the liver represents a negative marker of treatment response.
The possible value of other soluble biomarkers as predictors of treatment response in chronic hepatitis C has also been investigated. For example, Lee and others (2010) correlated a number of soluble markers (sCD30, interleukin-13 receptor alpha 2, total and active transforming growth factor beta-1, interleukin-18, and CXCL10) with severity of fibrosis and treatment outcome in patients with chronic hepatitis C. The results of this study showed that higher TGF-β1 and low CXCL10 are associated with failure to treatment. These results suggest that attempts to identify other biomarkers of treatment response in chronic hepatitis C should not be limited to chemokines only.
An association between CXCL10 concentrations not only in plasma, but also in liver biopsies with HCV RNA kinetics during chronic hepatitis C treatment was recently investigated by Askarieh and others (2010). Low levels of intrahepatic and plasma CXCL10 were found to be predictors of favorable first-phase decline (24 h and 4 days) of HCV RNA during treatment with PEG IFN-α2 and ribavirin. However, pretreatment concentrations of CXCL10 in the liver or plasma did not influence HCV RNA decline during the second phase (days 8 and 29) or at later time points.
Zeremski and others (2011) recently analyzed plasma levels of CXCL9-11 in patients with acute HCV infection showing that chemokine synthesis in vivo begins between 38 and 53 days after virus aquisition and peaks between days 73 and 83 of infection. Week-to-week variations in chemokine concentrations as well as HCV RNA and ALT levels were interpreted as repeated cycles of gain and loss of immune control during acute hepatitis C. Further analysis on the kinetics of CXCR3 ligands in acute infection as well as characterization of their molecular forms (antagonists or not) is warranted.
In conclusion, the results of this study have shown that CXCL10 concentrations at the time of RVR (4 weeks) are better predictors of achieving SVR compared to baseline levels. Additionally, these results suggest an important role of CXCL9 as a biomarker of SVR in patients with chronic hepatitis C.
Acknowledgments
This study was in part supported by grants from the Croatian Ministry of Science, Education and Sports to A. Vince and S. Zidovec Lepej (Grant Nos. 143-0000000-0117 and 143-1080116-0097).
Author Disclosure Statement
No competing financial interests exist.
References
- Askarieh G. Alsiö A. Pugnale P. Negro F. Ferrari C. Neumann AU. Pawlotsky JM. Schalm SW. Zeuzem S. Norkrans G. Westin J. Söderholm J. Hellstrand K. Lagging M DITTO-HCV and NORDynamIC Study Groups. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51(5):1523–1530. doi: 10.1002/hep.23509. [DOI] [PubMed] [Google Scholar]
- Butera D. Marukian S. Iwamaye AE. Hembrador E. Chambers TJ. Di Bisceglie AM. Charles ED. Talal AH. Jacobson IM. Rice CM. Dustin LB. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106(4):1175–1182. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A. Decalf J. Ahloulay M. Lababidi C. Mansour H. Vallet-Pichard A. Mallet V. Mottez E. Mapes J. Fontanet A. Pol S. Albert ML. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest. 2011;121(1):308–317. doi: 10.1172/JCI40594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF. Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Diago M. Castellano G. García-Samaniego J. Pérez C. Fernández I. Romero M. Iacono OL. García-Monzón C. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55(3):374–379. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepolder HM. New insights into the immunopathogenesis of chronic hepatitis C. Antiviral Res. 2009;82(3):103–109. doi: 10.1016/j.antiviral.2009.02.203. [DOI] [PubMed] [Google Scholar]
- Gonzalez SA. Keeffe EB. Chronic viral hepatitis: epidemiology, molecular biology, and antiviral therapy. Front Biosci. 2011;16:225–250. doi: 10.2741/3685. [DOI] [PubMed] [Google Scholar]
- Heydtmann M. Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology. 2009;49(2):676–688. doi: 10.1002/hep.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad M. Khushboo I. Singh S. Singh S. Hepatitis C virus (HCV): a review of immunological aspects. Int Rev Immunol. 2008;27(6):497–517. doi: 10.1080/08830180802432178. [DOI] [PubMed] [Google Scholar]
- Kasprowicz V. Kang YH. Lucas M. Schulze zur Wiesch J. Kuntzen T. Fleming V. Nolan BE. Longworth S. Berical A. Bengsch B. Thimme R. Lewis-Ximenez L. Allen TM. Kim AY. Klenerman P. Lauer GM. Hepatitis C virus (HCV) sequence variation induces an HCV-specific T-cell phenotype analogous to spontaneous resolution. J Virol. 2010;84(3):1656–1663. doi: 10.1128/JVI.01499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagging M. Romero AI. Westin J. Norkrans G. Dhillon AP. Pawlotsky J-M. Zeuzem S. von Wagner M. Negro F. Schalm SW. Haagmans BL. Ferrari C. Missale G. Neumann AU. Verheij-Hart E. Hellstrand K. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006;44(6):1617–1625. doi: 10.1002/hep.21407. [DOI] [PubMed] [Google Scholar]
- Larrubia JR. Benito-Martínez S. Calvino M. Sanz-de-Villalobos E. Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14(47):7149–7159. doi: 10.3748/wjg.14.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Varano J. Flexman JP. Cheng W. Watson MW. Rossi E. Adams LA. Bulsara M. Price P. Decreased IP-10 and elevated TGFbeta1 levels are associated with viral clearance following therapy in patients with hepatitis C virus. Dis Markers. 2010;28(5):273–280. doi: 10.3233/DMA-2010-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin C. Timm J. Spangenberg HC. Wischniowski N. Nazarova N. Kersting N. Roggendorf M. Allen TM. Blum HE. Thimme R. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology. 2008;47(6):1824–1836. doi: 10.1002/hep.22242. [DOI] [PubMed] [Google Scholar]
- Reiberger T. Aberle JH. Kundi M. Kohrgruber N. Rieger A. Gangl A. Holzmann H. Peck-Radosavljevic M. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir Ther. 2008;13(8):969–976. [PubMed] [Google Scholar]
- Romero AI. Lagging M. Westin J. Dhillon AP. Dustin LB. Pawlotsky JM. Neumann AU. Ferrari C. Missale G. Haagmans BL. Schalm SW. Zeuzem S. Negro F. Verheij-Hart E. Hellstrand K DITTO-HCV Study Group. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194(7):895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- Vargas A. Berenguer J. Ryan P. Catalán P. López JC. Cosín J. Miralles P. Resino S. Plasma interferon-gamma-inducible protein-10 can predict virologic response to hepatitis C virus therapy in HIV/HCV-coinfected patients with HCV genotype 1. J Acquir Immune Defic Syndr. 2010;54(2):219–220. doi: 10.1097/QAI.0b013e3181d01d05. [DOI] [PubMed] [Google Scholar]
- Zeremski M. Dimova R. Brown Q. Jacobson IM. Markatou M. Talal AH. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis. 2009;200(11):1774–1780. doi: 10.1086/646614. [DOI] [PubMed] [Google Scholar]
- Zeremski M. Dimova R. Astemborski J. Thomas DL. Talal AH. CXCL9 and CXCL10 chemokines as predictors of liver fibrosis in a cohort of primarily African-American injection drug users with chronic hepatitis C. J Infect Dis. 2011;204(6):832–836. doi: 10.1093/infdis/jir424. [DOI] [PMC free article] [PubMed] [Google Scholar]