Abstract
Objective:
Stem cells are of great interest for regenerating disturbed tissues and organs. These cells are commonly isolated from the bone marrow, but there has been interest in other tissues in the recent years. In this study, we evaluated the possibility of isolation of stem cells from oral connective tissue and investigated their characteristics.
Materials and Methods:
In this experimental study, sampling from the bone marrow and oral connective tissue of a beagle dog was performed under general anesthesia. Bone marrow stem cell isolation was performed according to the established protocols. The samples obtained from oral soft tissue were broken to small pieces and after adding collagenase I, the samples were incubated for 45 minutes in 37°C. Other processes were similar to the processes which were carried out on bone marrow cells. Then cell properties were compared to evaluate if the cells from the connective tissue were stem cells.
Results:
The cells from the bone marrow and connective tissue had the same morphology. The result of colony forming unit assay was relatively similar. Population doubling time was similar too. In addition, both cell groups differentiated to osteoblasts in osteogenic media.
Conclusion:
The cells isolated from the oral connective tissue had the characteristics of stem cells, including fibroblastoid morphology, self renewal properties, high proliferation rate and differentiation potential.
Keywords: Oral, Connective Tissue, Stem Cell
INTRODUCTION
Stem cells, which have high growth and differentiation potential, have been of great interest in the recent years. Their properties, culture condition and their efficacy to regenerate injured tissues have been evaluated by many researchers. These cells are defined with two distinct characteristics for the first time; self renewal (producing daughter cells) and the capacity to differentiate to many cell lines [1].
Mesenchymal stem cells have the potential to differentiate to chondrocytes, osteoblasts, fibroblasts, adipocytes and many mesenchymal tissues. These cells are present in many tissues and organs such as the muscles and bone marrow [2, 3]. The aim of this study was to obtain stem cells by means of an easier and more comfortable method, with no more tissue loss, trauma to tissues and postoperative complications. So we decided to use oral soft tissue, which is always available, has fewer complications after surgery and also has good blood supply.
MATERIALS AND METHODS
In this experimental study, bone marrow and soft tissue samples were isolated from an adult healthy dog. The procedure was conducted under general anesthesia. At the beginning, 10 ml bone marrow sample was harvested from the dog’s iliac crest. The sample was diluted with the same amount of PBS and centrifuged at 400gr for 20 minutes. The mononuclear cell layer was then collected and rinsed with PBS solution. The cells were expanded with DMEM containing 10% FBS in flasks. After 24hours, non-adherent cells were removed and new culture medium was added to the adherent cells. This medium was changed every 3 days. The samples which were obtained from the oral soft tissue were broken to small pieces. After adding collagenase I, the samples were incubated for 45 minutes in 37°C. The other processes were just the same as the processes carried out on bone marrow cells. When the confluence of adherent cells (bone marrow and oral soft tissue cells) reached the amount of 80–90%, they were washed twice with PBS solution and released from culture surface by using 0.25% trypsin-EDTA solution and plated in tissue culture polystyrene flasks. Primary cultures of cells proliferated until reaching a confluent growth-arrested condition. The rate of cell proliferation was calculated by measuring the population doubling time after passage. Then the medium was changed to osteogenic medium (DMEM containing 10% FBS, 10−7 M dexamethasone, 69 nM insulin and 0.2 mM indomethacin.)
Twenty one days after osteogenic differentiation, Alizarin red staining was conducted for assessing differentiation. Oil red staining test evaluated adipocytes differentiation after 31 days. To evaluate colony forming cells, 100 cells were plated in 10cm dishes containing DMEM and 10% FBS. Culture medium was changed every 3 days. The samples were then stained, making it possible to count the number of colonies. This test was repeated three times for each cell group. Five days after the beginning of osteogenic differentiation, the genes; namely, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), TBP (TATA box binding protein), ALP and Osteocalcin) were evaluated by RT-PCT. Details of temperatures, times and cycle numbers are shown in Table 1.
Table 1.
Temperatures, Times and Number of Cycles in Real-Time PCR
| Function | Cycle | Time | Temperature | Number |
|---|---|---|---|---|
| Initial denaturation | 1 | 3 minutes | 94°C | 1 |
| Cycle denaturation | 30 seconds | 94°C | 2 | |
| Primer annealing | 45 seconds | 60°C | ||
| Extension | 35 | 45 seconds | 72°C | |
| Substrate clearance | 1 | 7 minutes | 72°C | 3 |
RESULTS
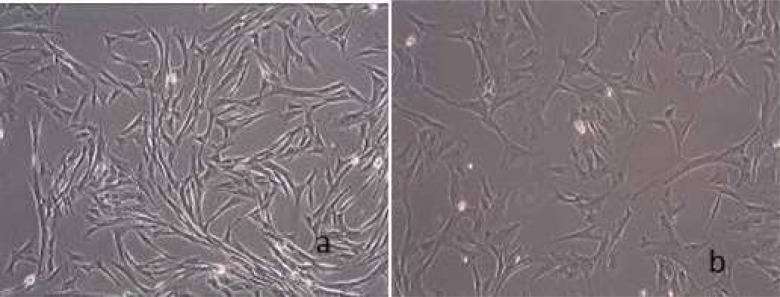
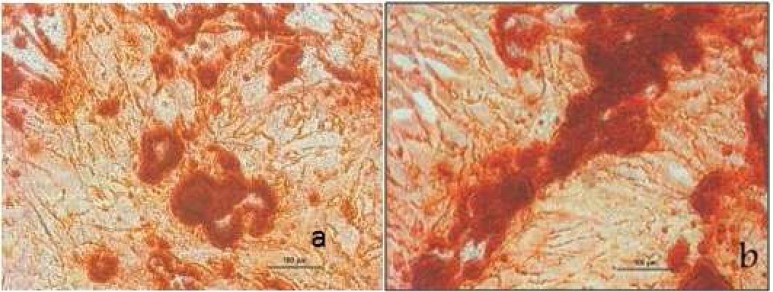
In this study, cell populations derived from dog bone marrow and oral soft tissue were morphologically similar. They were visible as adherent spindle-shaped and fibroblast like cells at the bottom of the plates. Figure 1 shows these cells after second passage. Alizarin Red staining on the 21st day showed that the observed deposits are calcified in both groups. Bone nodules stained with alizarin red are shown in Fig 2.
Fig 1.
Cells after second passage A. Bone marrow cells B. Connective tissue cells
Fig 2.
Deposits in mineralized nodules stained with Alizarin Red A. Bone marrow cells B. Connective tissue cells
Oil Red staining on the 31st day did not show any adipocyte differentiation in the cell groups.
Colony counting in hematoxylin stained samples demonstrated that oral tissue colonies occupy 36% of the plate surface which was more than that of the bone marrow colonies occupying 29% of the plate surface.
Cell proliferation evaluated by Population Doubling Time (PDT) test was more in the bone marrow group in comparison to the oral tissue group with a PDT of 24 hours and 30 hours, respectively. The results of assessing GAPDH, TBP, Osteocalcin and ALP genes are demonstrated in Table 2.
Table 2.
Results of Assessing GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase), TBP (TATA Box Bining Protein), Osteocalcin and ALP (Alkaline phosphatase) Genes in Bone Marrow and Oral Connective Tissue Cells
| PRIMER | Bone Marrow | Oral Soft Tissue | Size | |
|---|---|---|---|---|
| Total RNA | 65.1 | 69.2 | μg/μl | |
| OD | 260/280 | 1.81 | 1.82 | |
| 260 | 1.628 | 1.730 | ||
| 1 | D.GAPDH | ++ | ++ | 113 |
| 2 | D.TBP | + | + | 109 |
| 3 | D.Osteocalcin | + | - | 135 |
| 4 | D.ALP | ++ | + | 162 |
PCR results described in Fig 3 indicated that osteocalcin gene was only present in bone marrow cells and ALP gene was present in both groups; however, it was more significant in bone marrow cells.
Fig 3.
A. Real Time-PCR results for GAPDH, TBP and Osteocalcin genes B. Real Time-PCR results for ALP gene
DISCUSSION
Mesenchymal stem cells have been conventionally isolated from the bone marrow and more recently from some other tissues such as the deciduous and permanent dental pulp tissue, PDL, impacted teeth and follicles. [4–8]. Cells isolated from these tissues are used clinically in regenerating injured tissues and organs such as bone defects and other clinically compromised conditions such as sinus elevating surgeries [9].
This study was designed to investigate the applicability of mesenchymal stem cells derived from the oral palatal connective tissue using the same conditions established for the cultivation of bone marrow derived mesenchymal stem cells. Tooth extraction is essential for isolating stem cells from the pulp, PDL and follicle.
On the other hand, obtaining bone marrow derived stem cells would be absolutely painful and causing various complications. So we suggest that palatal tissue would be suitable for isolating stem cells in a more available and convenient way, while no more tissue loss, trauma and complications would occur.
Lindolfo et al (2006) found that long term mesenchymal stem cell cultures may be established from all organs and tissues irrespective of their embryonic origin. They suggested that the cell populations obtained could be practically defined as mesenchymal stem cells since they exhibit the capacity of prolonged self-renewal and differentiation to mesenchymal cell lineages.
Morphology and surface markers were similar in cells, while differentiation potentials were quite different. They suggested that presence of mesenchymal stem cells in all tissues attributes to their presence around blood vessels [10].
In our study the cultured cells were adherent and fibroblastoid shape, just the same as bone marrow stem cells isolated. The results of the colony forming assay showed that colonies have been formed in both cell groups and the percentage of colonies in the connective cell plate was more than that of the colonies in the bone marrow plates. So we concluded that self-renewal potential in connective tissue cells was more in comparison with cultured bone marrow cells.
In many studies, colony forming potential is evaluated by this test. In a study which was carried out by Digirolamo et al in 1999, it was concluded that cell proliferation potential may be evaluated by colony forming assay when cells are plated in low density [11]. Seo et al (2004) and Nagatomo et al (2006) evaluated PDL stem cell colonies and compared their properties with bone marrow stem cells [12, 13]. In a study conducted by Gronthos et al, evaluating dental pulp stem cells, they suggested that this property is not strong enough in these cells [14]. Baghaban Eslaminejad et al compared the proliferation potential of stem cells from the dental pulp of deciduous and permanent teeth using multiple cell growth indices as PDT (Population doubling time) and colonogenic activity [15].
High proliferation rate is a special characteristic of stem cells. “Population Doubling Time Test” showed that both cell groups had a high proliferation rate; however, the rate was higher in bone marrow cells. Population Doubling Time is different in various stem cells depending on their origin. For example this time in embryonic stem cells is 36 hours which is a bit more than the time in oral connective tissue cells [16]. Miura et al, using this test compared the proliferation rate of stem cells by using this test. They demonstrated that this rate in deciduous pulp stem cells was more than that of bone marrow and permanent dental pulp stem cells [17].
We evaluated osteogenesis and adipogenesis to assess the multipotency of cells. After Alizarin red staining on the 21st day, calcified deposits were seen in the plates of both cell groups. Then the genes (GAPDH, TBP, Osteocalcin and ALP) were assessed by conducting Real time-PCR on the cells. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and TBP (TATA box binding protein) are expressed in all cells and we checked them for defining PCR process validity. Osteocalcin, a non-collagenous protein of extracellular matrix, is just produced by osteoblasts and it is a marker of osteogenesis. ALP (Alkaline phosphatase) is a product of osteoblast activation and its raise is the result of active bone formation. ALP and Osteocalcin genes regulate protein production.
In this study Osteocalcin gene was expressed only in bone marrow cells; however, ALP was present in both cell groups.
Negative result obtained from Oil Red staining (on the 31st day) demonstrated that both cell groups (even bone marrow stem cells) were not able to differentiate to adipocytes with the protocol we used, suggesting inclusion of minor changes in adipogenic protocol for dog cells.
CONCLUSION
In this study we cultured oral connective tissue cells. Based on the fact that their morphology, self-renewal capacity, high proliferation rate and osteogenic differentiation potential was the same as bone marrow stem cells, we concluded that these cells have the special properties of stem cells.
Ultimately, we suggest using flow cytometry in later studies in order to evaluate cell surface markers.
REFERENCES
- 1.Lakshmipathy U, Verfaillie C. Stem cell plasticity. Blood Rev. 2005 Jan;19(1):29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997 Jul;15(4):546–57. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. PNAS Proc Natl Acad Sci USA. 2000 Dec;97(25):13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, et al. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2–3):406–8. doi: 10.1080/03008200290001023. [DOI] [PubMed] [Google Scholar]
- 6.Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. J Periodont Res. 2006 Dec;41(6):547–53. doi: 10.1111/j.1600-0765.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 7.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008 Feb;34(2):166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007 Aug;10(3):149–60. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 9.Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, Ahmadbeigi N. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Aug;106(2):203–9. doi: 10.1016/j.tripleo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Science. 2006 Jun 1;119(Pt 11):2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 11.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999 Nov;107(2):275–81. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagatomo K, Komaki M, Sekiya I, Sakaguchi Y, Noguchi K, Oda S, et al. Stem cell properties of human periodontal ligament cells. J Periodontal Res. 2006 Aug;41(4):303–10. doi: 10.1111/j.1600-0765.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- 13.Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005 Oct;84(10):907–12. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- 14.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002 Aug;81(8):531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 15.Eslamineja MB, Vahabi S, Shariati M, Nazarian H. In vitro growth and characterization of stem cells from human dental pulp of deciduous versus permanent teeth. J Dent (Tehran) 2010 Fall;7(4):185–95. [PMC free article] [PubMed] [Google Scholar]
- 16.Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell. 4th ed. New York: Garland Science; 2002. pp. 1308–12. [Google Scholar]
- 17.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003 May 13;100(10):5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]