Abstract
Objective:
Both anorganic bovine bone (ABB) and β-tricalcium phosphate (β-TCP) are used in clinical practice as bone substitute materials, but there is limited data comparing these two materials in standardized defects.
The aim of this study was to histologically evaluate the effectiveness of ABB and β-TCP in the healing of experimentally induced bone defects.
Materials and Methods:
Eighteen bone defects were created on the calvaria of six rabbits. In each animal, one defect was left untreated and the other two were filled with ABB and β-TCP. After one month, histological sections were prepared. Type and vitality of newly formed bone, percentage of new bone formation and residual material, thickness of trabeculae, inflammation and foreign body reaction were assessed.
Results:
The newly formed osseous tissue was vital in all defects and consisted of woven and lamellar bone. Mean percentages of new bone formation were 30.83±14.29%, 16.83±11.07% and 14.00±8.17% in β-TCP, ABB and control groups, respectively and the mean percentages of residual biomaterial were 24.17±14.01% and 36.50±8.43% in β-TCP and ABB groups, respectively. However, the differences were not statistically significant (all ps>0.05). Inflammatory infiltration was statistically higher in β-TCP compared to the control group (p=0.025), but the difference was not significant between β-TCP and ABB groups (p=0.083). Trabeculation thickness and foreign body reaction were not statistically different between β-TCP and ABB groups.
Conclusion:
β-TCP and ABB were not different with regard to the quantity and quality of newly formed osseous tissue. However, inflammatory infiltration was higher in sites filled with β-TCP.
Keywords: Bone Regeneration, Wound Healing, Anorganic Bovine Bone, Beta Tricalcium Phosphate, Rabbits
INTRODUCTION
To replace a missing tooth with a dental implant, existence of the adequate bone is an essential prerequisite. Sometimes, there is a lack of available bone as a consequence of atrophy, trauma, failure to develop or surgical resection [1]. Several grafting materials including autografts, allografts, xenografts and alloplasts have been used for bone augmentation [2]. While autogenous bone is called as a gold standard [3], harvesting a sufficient amount of autogenous bone is not possible in most cases.
Anorganic bovine bone (ABB) has osteoconductive properties and is documented as a bio-compatible material. Although several clinical investigations have demonstrated its favorable results, [1, 4] histological studies have shown variable degrees of residual material even after 3 years [5].
β-tricalcium phosphate (β-TCP) is a synthetic calcium phosphate ceramic used in regenerative therapy as an alloplastic material. However, there is controversy regarding its resorptive and regenerative potential. A number of reports have indicated good biodegradability and biocompatibility, while others have shown rapid degradation and weak mechanical properties [6–10].
To the best of our knowledge, there is limited data comparing these two materials with regard to the amount of bone formation and graft resorption properties [11]. The aim of this study was to compare these two materials in experimentally induced defects in rabbit calvaria.
MATERIALS AND METHODS
This study was approved by the Animal Care and Use Committee of Tehran University of Medical Sciences and was performed strictly in accordance with the recommendations of the Helsinki convention for the use and care of animals.
Six white male New Zealand rabbits weighing 2.5–3.5 kg were maintained on standard laboratory chow with free access to water for 2 weeks prior to the first day of the experiment. The animals were anesthetized with intramuscular injections of 2% (5mg/kg) xylazine and 10% (40mg/kg) ketamin (Alafason, WOEDEN-HOLLAND). The surgical sites on the calvaria were shaved and scrubbed with 7% betadine for 5 minutes. After isolation, an anterior-posterior (craniocaudal) incision was made with a No. 15 surgical blade and dermal and subdermal tissues along with the periosteum were retracted with a periosteal elevator. Three identical holes (3.1 × 6 mm) were created on the frontal and parietal bones by a round bur. For standardization, anatomical landmarks including the occipital process and craniocaudal suture were used. These two landmarks cross in the middle and form a plus sign (+). The defects were oriented on the right anterior, right posterior and left posterior aspects of the intersection of these landmarks.
ABB (Bio-Oss®, Geistlich Biomaterials, Wolhusen, Switzerland; particle size: 250–1000 μm) and β-TCP (BioResorb®, Oraltronics, Bremen, Germany; particle size: 500–1000 μm) were used to fill two of the defects, leaving one hole unfilled which served as control. Then, the periosteum and skin were sutured. After transferring the rabbits to their cages, enrofloxacine (0.7 ml/day) and florbiprofen (0.3 ml/day) were injected subcutaneously for 5 to 7 days.
All animals were sacrificed after a 1-month healing period by an overdose of pentobarbital (100mg/kg), injected intravenously.
After sacrifice, dermal and mucosal tissues of the calvarium were separated with a #15 surgical blade and the entire calvarium was removed using a reciprocating saw. The specimens were fixed in 10% formalin for 14 days and immersed in 10% formic acid for another two weeks until decalcified.
All samples were retransferred to formalin for 48 hours before final preparation for sectioning.
Ten histologic sections with a thickness of 5 μm were prepared from each defect and stained with hematoxilin and eosin.
Inflammation, foreign body reaction, bone vitality, bone quality, thickness of bone trabeculae, percentage of residual biomaterial and newly formed bone were assessed in the bases of the defects by a light microscope (BX41, Olympus Co., Tokyo, Japan) at a magnification of ×40. Inflammation was graded using a five-tiered grading system as follows: 0, no inflammatory cells; I, little and scattered inflammation; II, focal inflammation with 5 to 10 inflammatory cells; III, focal inflammation with 10 to 50 inflammatory cells; and IV, focal inflammation with more than 50 inflammatory cells.
Foreign body reaction was determined by the presence of multinucleated giant cells in granulomatous response. Observation of osteocytes in trabecular lacunae confirmed bone vitality. Bone quality was recorded as woven bone alone, both woven and lamellar bone, and lamellar bone alone. An eye-piece micrometer was used to measure the thickness of bone trabeculae. Trabeculation thicknesses of >60 μm, 20–60 μm, and 1–20 μm were considered as grades I, II and III, respectively. Digital photograph was taken for each sample at a magnification of ×40 and the percentage of residual material and newly formed bone were calculated using Adobe Photoshop 7.0 software. Statistical analyses were performed using ANOVA, Friedman and dual competition post Hoc tests.
RESULTS
The newly formed osseous tissue was vital in all specimens. All specimens showed mild inflammatory infiltrations (grade I and II). All of negative controls, 75% of the ABB, and 16.7% of the β-TCP specimens demonstrated grade I inflammation and other samples showed grade II inflammation. Overall, β-TCP showed more inflammatory infiltration compared to the control group (p=0.025).
Foreign body reaction was positive in four out of 6 defects filled with β-TCP. However, this reaction was observed in 2 out of 6 defects of the ABB group and none of the control group as expected. The difference between β-TCP and negative control groups was statistically significant (p=0.046).
The newly formed osseous tissue was consisted of both woven and lamellar bone in all specimens.
Regarding the thickness of bone trabeculae, 16.7% and 83.3% of the negative controls were grade I and II, respectively; 33.3% and 66.6% of the ABB samples were grade I and II, respectively; and 50%, 33.3% and 16.7% of the β-TCP specimens were grade I, II and III, respectively. However, according to Friedman test the differences were not statistically significant (p=0.846).
According to repeated measure ANOVA test, the mean percentage of new bone formation was 16.83±11.07% in ABB group, 30.83±14.29% in β-TCP group, and 14.00±8.17% in the control group (Figure 1). No statistically significant differences were seen among groups in this regard (p>0.05).
Fig 1.

Means and standard deviations of the percentage of newly formed bone in ABB (Bio-Oss), β-TCP and negative control groups
The mean percentage of residual material in the defects filled with ABB and β-TCP was 36.50±8.43% and 24.17±14.01%, respectively. However, the difference between groups was not statistically significant (p>0.05) (Figure 2).
Fig 2.

Means and standard deviations of the percentage of residual biomaterial in ABB and β-TCP groups
DISCUSSION
This study was designed to compare bone healing after application of two osteoconductive graft materials (ABB and β-TCP) in experimentally induced bone defects in rabbit calvaria.
Since the embryonic process of development of both alveolar bone and calvaria vault are through intramembranous bone formation, calvaria of the rabbit was selected as the experimental site to make a more proper comparison. Biomaterials used in bone grafting procedures have been shown to influence modeling, remodeling and healing of osseous tissues.
These substances are also known to enhance and stimulate bone formation via osteogenesis, osteoconduction or osteoinduction [2]. The remodeling process lasts for 3 to 6 months in humans and 6 weeks in rabbits [12].
According to Schallhorn [13], the essential properties of an ideal grafting material is biologic acceptability, predictability, clinical feasibility, minimal operative and postoperative complications and patient acceptance.
The results obtained in the present investigation demonstrated no adverse inflammatory consequences following application of β-TCP and ABB. Although the inflammatory infiltration was more in defects filled with β-TCP, inflammatory reactions were slight in all specimens.
In this study, foreign body reaction was observed in 16.7% and 66.7% of the ABB and β-TCP cases, respectively. This was in accordance with the findings reported by Ogose et al. [9] who evaluated the histologic characteristics of β-TCP in the human femur and found osteoclast-like giant cells surrounding β-TCP particles. Degradation of graft materials may be considered as a positive characteristic if replaced by newly formed vital bone. Previous investigations have shown that β-TCP has prominent bioresorbable properties [9]. In the current study, the resorption and bone formation induced by β-TCP was slightly greater than ABB, but a significant difference was not found between the two materials. The results of this study are in accordance with the study of Tamimi et al [14].
The amount of newly formed bone in osseous defects is probably one of the most important factors for determining regeneration. Considering that both materials showed desirable healing properties, β-TCP and ABB seem to qualify as acceptable grafting biomaterials. Artzi et al. used ABB particles in fresh human extraction sockets and found 82.3% new bone formation [15]. Ogose et al [9] observed a considerable amount of newly formed bone on β-TCP particles, 4 weeks after grafting. They reported excellent osteoconductive and resorptive properties for β-TCP in human osseous tissues. Wada et al. [10] used fresh autogenous bone, allogenic decalcified freeze-dried bone, and β-TCP for the treatment of experimental furcation defects. Regeneration was more prominent following application of autogenous bone as compared to the other two substances.
CONCLUSION
According to the results of the present study, β-TCP and ABB may have similar osteoconductive properties and there are no significant differences between these two materials with regard to the quantity and quality of newly formed bone. However, inflammatory infiltration is higher in sites filled with β-TCP.
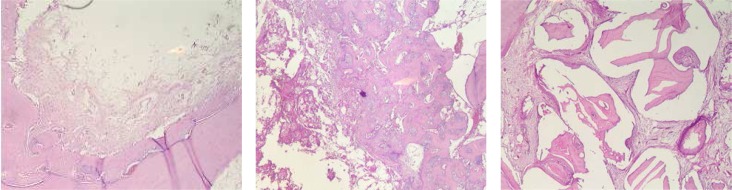
Fig 3.
Hematoxylin and Eosin-stained section of defects. A. Control, new bone formation is observed on the border and fibrous connective tissue is discernible in the center of the defect. B. β-TCP, abundant new bone formation is observed around β-TCP particles. C. ABB, residual particles are seen in the defect (original magnification ×40).
Acknowledgments
The authors would like to thank Hesarak Institute of serum production; School of Public Health and Institute of Public Health Researches; Tehran University of Medical Sciences and the Department of Oral and Maxillofacial Pathology; Faculty of Dentistry; Shahid Beheshti University of Medical Sciences for their assistance in this research.
REFERENCES
- 1.Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev. 2009;4:CD003607. doi: 10.1002/14651858.CD003607.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams RC, Cochran DL, Giannobile WV, Lynch SE. Tissue engineering: what does it mean? Why is it important? Compend Contin Educ Dent. 2005 Jan;26(1) 54, 6, 8 passim. [PubMed] [Google Scholar]
- 3.Wood RM, Moore DL. Grafting of the maxillary sinus with intraorally harvested autogenous bone prior to implant placement. Int J Oral Maxillofac Implants. 1988 Fall;3(3):209–14. [PubMed] [Google Scholar]
- 4.Cordaro L, Bosshardt DD, Palattella P, Rao W, Serino G, Chiapasco M. Maxillary sinus grafting with Bio-Oss or Straumann Bone Ceramic: histomorphometric results from a randomized controlled multicenter clinical trial. Clin Oral Implants Res. 2008 Aug;19(8):796–803. doi: 10.1111/j.1600-0501.2008.01565.x. [DOI] [PubMed] [Google Scholar]
- 5.Hallman M, Lundgren S, Sennerby L. Histologic analysis of clinical biopsies taken 6 months and 3 years after maxillary sinus floor augmentation with 80% bovine hydroxyapatite and 20% autogenous bone mixed with fibrin glue. Clin Implant Dent Relat Res. 2001;3(2):87–96. doi: 10.1111/j.1708-8208.2001.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 6.Aybar B, Bilir A, Akcakaya H, Ceyhan T. Effects of tricalcium phosphate bone graft materials on primary cultures of osteoblast cells in vitro. Clin Oral Implants Res. 2004 Feb;15(1):119–25. doi: 10.1111/j.1600-0501.2004.01002.x. [DOI] [PubMed] [Google Scholar]
- 7.Dong J, Uemura T, Shirasaki Y, Tateishi T. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials. 2002 Dec;23(23):4493–502. doi: 10.1016/s0142-9612(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 8.Gera I, Dori F, Keglevich T, Anton S, Szilagyi E, Windisch P. [Experience with the clinical use of beta-tri-calcium phosphate (Cerasorb) as a bone replacement graft material in human periodontal osseous defects] Fogorv Sz. 2002 Aug;95(4):143–7. [PubMed] [Google Scholar]
- 9.Ogose A, Hotta T, Hatano H, Kawashima H, Tokunaga K, Endo N, et al. Histological examination of beta-tricalcium phosphate graft in human femur. J Biomed Mater Res. 2002;63(5):601–4. doi: 10.1002/jbm.10380. [DOI] [PubMed] [Google Scholar]
- 10.Wada T, Wu CH, Sugita H, Sugita N, Katagiri S, Shimizu M, et al. Autogenous, allogenic, and beta-TCP grafts: comparative effectiveness in experimental bone furcation defects in dogs. J Oral Implantol. 1989;15(4):231–6. [PubMed] [Google Scholar]
- 11.Jensen SS, Broggini N, Hjorting-Hansen E, Schenk R, Buser D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res. 2006 Jun;17(3):237–43. doi: 10.1111/j.1600-0501.2005.01257.x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts WE, Smith RK, Zilberman Y, Mozsary PG, Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod. 1984 Aug;86(2):95–111. doi: 10.1016/0002-9416(84)90301-4. [DOI] [PubMed] [Google Scholar]
- 13.Schallhorn RG. Present status of osseous grafting procedures. J Periodontol. 1977 Sep;48(9):570–6. doi: 10.1902/jop.1977.48.9.570. [DOI] [PubMed] [Google Scholar]
- 14.Tamimi FM, Torres J, Tresguerres I, Clemente C, Lopez-Cabarcos E, Blanco LJ. Bone augmentation in rabbit calvariae: comparative study between Bio-Oss and a novel beta-TCP/DCPD granulate. J Clin Periodontol. 2006 Dec;33(12):922–8. doi: 10.1111/j.1600-051X.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 15.Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: histomorphometric evaluations at 9 months. J Periodontol. 2000 Jun;71(6):1015–23. doi: 10.1902/jop.2000.71.6.1015. [DOI] [PubMed] [Google Scholar]