Abstract
Objective:
NovaMin, a synthetic mineral composed of calcium, sodium, phosphorous and silica releases deposits of crystalline hydroxyl-carbonate apatite (HCA) structurally similar to tooth mineral composition. The aim of this investigation was to compare the potential remineralization effect of topical NovaMin and Sodium Fluoride gel on caries like lesions in permanent teeth.
Materials and Methods:
A total of 60 sound human freshly extracted teeth were subjected to a pH-cycling protocol. Specimens were randomly assigned to one of the two treatment groups with NovaMin contained dentifrice applied to group 1, while group 2 received a dentifrice containing 1.1% neutral Sodium Fluoride. Pastes were applied five times after the samples received a demineralization from an earlier cariogenic challenge. Specimens were then evaluated by a Surface Micro Hardness test (SMH, 25G, 5s). Post-treatment SMH measurements were conducted and Mann Whitney test was employed for statistical analysis.
Results:
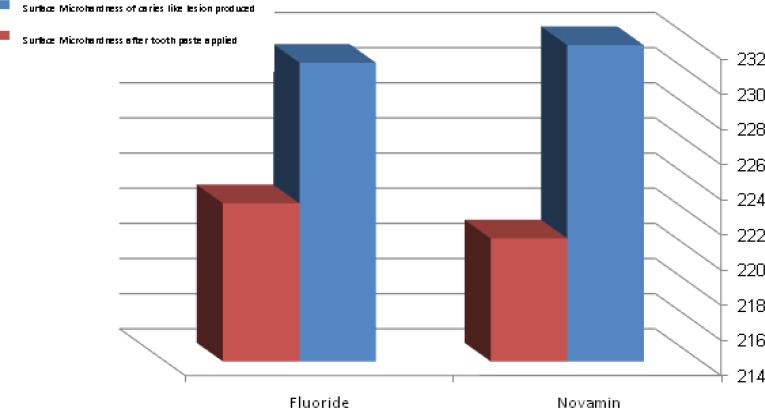
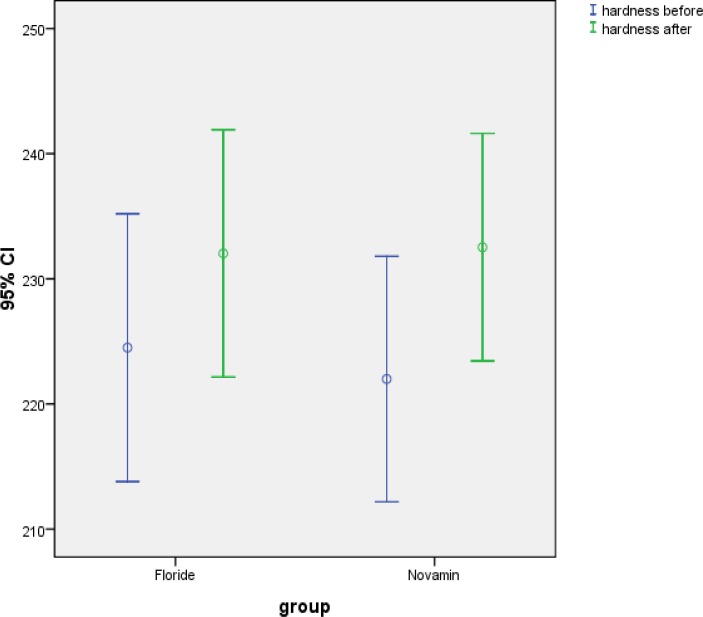
Mean post lesion SMH values were 221.99±26.27 and 224.50±28.64 for the first and second groups, respectively. Post treatment SMH values were 232.52±24.34 for NovaMin and 232.03 ±24.46 for the fluoride group. Two way ANOVA test showed a highly significant difference between the two different treatment protocols (p<0.001).
Conclusion:
NovaMin dentifrice appears to have a greater effect on remineralization of carious-like lesions when compared to that of fluoride containing dentifrice in permanent teeth.
Keywords: Remineralization, NovaMin, Sodium Fluoride, Surface Microhardness, Teeth
INTRODUCTION
For many years attempts were made to develop products capable of producing a chemical bond to the hard tooth structure in order to prevent them from any caries initiation processes in a manner similar to the natural tooth structure [1–3].
NovaMin is a known component made of bio-active glass particulates with a median size of less than 20 microns.
It has been tested for its effectiveness in remineralizing hard tooth structure while occluding dentinal tubules [4–6].
It is indicated that when NovaMin comes in contact with saliva or any aqueous media, its active ingredient, inorganic chemical calcium sodium phosphor silicate, binds to the tooth surface in order to initiate the remineralization process on the tooth enamel.
This is performed by providing silica, calcium, phosphorous and sodium ions to the tooth structure [7, 8]. A localized transient increase in pH occurs during the initial exposure of the mineral due to the release of sodium. This rise in pH helps the calcium and phosphate to form the NovaMin particles, followed by calcium and phosphorous found in saliva to form a calcium phosphate (ca-p) layer. As the particles’ reaction continues and deposition of calcium phosphate complex takes place, this layer crystalizes into a calcium hydroxyl apatite, also known as hydroxyl carbonate apatite [9, 10]. Likowski et al. [11] indicated that 2.5% and 7.0% w/w bioglass® containing dentifrices could significantly reduce the patient’s pain and sensitivity against stimuli through their daily use. Antibacterial effect of NovaMin tooth paste has beendocumented against several periodontal pathogens. Sodium Ion is released for several days providing a long term re-mineralization potential.[9, 12–15].
Antibacterial effect of NovaMin tooth paste has beendocumented against several periodontal pathogens. Sodium Ion is released for several days providing a long term re-mineralization potential.[9, 12–15].
The antibacterial effect of NovaMin is originated from its sodium and calcium contents followed by its effect on bacterial liquid balance [16–18]. The purpose of this study was to compare the remineralization potency of NovaMin and fluoride tooth pastes when applied on tooth enamel with artificial carious lesions when compared to the same effect from a fluoride gel of 1.1%.
MATERIAL AND METHODS
This in vitro investigation was carried out on 60 sound freshly extracted premolar teeth for orthodontic reason. Samples were stored in normal saline prior to the investigation and initial cleaning and drying process.
Teeth were then thoroughly covered by a layer of nail varnish (Max factor, France) leaving a window of 1x1 mm diameter at the buccal surface enamel open (Fig 2 and 3).
Fig 2.

1x1mm opening at the buccal surface
Fig 3.
Homogenized tooth paste oravive and fluoride solutions
Samples were drowned in 1.5 ml of demineralizing solution for one hour in order to induce an artificial carious lesion on the open surface. Demineralizing solution contained 1.4mM calcium, 0.9 mM phosphorous, 0.5 M acetate buffer and 0.03 ppm fluoride with a pH of 5.
Sample teeth were then assigned randomly to one of the two groups (29 each) after being removed from the demineralizing solution.
All dissolved samples were placed in a different remineralizing solution for 22 hours.
Group I was treated by Oravive revitalizing tooth paste containing calcium sodium phosphosilicate (NovaMin) with a remineralization potential (Fig 2).
Group II was treated with Topex take home care 1.1 neutral sodium fluoride gel (Sultan) containing 0.5% fluoride ion equal to 5000 ppm fluoride, distilled water, glycerin, FD&C Blue E#1 and sweeteners) (Fig 7).
Fig 7.
Mean surface hardness of teeth before and after fluoride and NovaMin have been applied
The demineralization and remineralization cycle was repeated for three times. Surface microhardness of caries like lesions (SMH) were measured using the Vicker’s Micro Hardness device (Stress Duramin, Germany) with the power of 25 grams for 25 seconds (Fig 5) (Metalography laboratory-research and science division, Islamic Azad University, Tehran, Iran).
Fig 5.
Comparing the mean Surface Microhardness between the two media
Samples with artificial carious lesion were covered by artificial saliva to simulate the mouth condition before treatment by reminera-lizing tooth paste.
Artificial saliva contained 2gr/lit methyl-p-hydroxybenzoat, 10gr/lit sodium carboxy methyl celulose, 625gr/lit potassium chloride, 0.059gr/lit Mg Cl2, 6H2O, 0.166gr/lit CaCl2-2H2O, 0.804gr/lit K2HPO4, 0.326gr/lit KH2PO4 in which the added KOH pH was set at 6.75 [11].
Both tooth pastes were prepared in the form of a solution with a mixture of 15 gram paste and 45 ml of deionized water homogenized using a magnetic mixer on a vibrating machine [11].
Samples were immersed in Oravive (Nova-Min) in group I, while teeth in group II were immersed in Topex solution.
Both sample groups were removed from their containers after two minutes in order to initiate the demineralization and remineralization cycles and repeated for 5 days [19].
At the end of the surface treatment, micro-hardness of all lesions in both groups were measured using Vicker’s mi crohardness device.
The distribution of different surface micro-hardness values in both groups were evaluated using Kolmogorov Smirnov test.
Mann Whitney test was then used to evaluate the differences between the mean of micro-hardness measurements. Mann_Whitney test was used to see the changes in the mean of the lesions’ microhardness in both groups. The level of significance was considered at α=0.05.
RESULTS
Based on the results of this in vitro investigation it appears that NovaMin has a higher capability to enhance enamel resistance against caries development by altering its surface microhardness when compared to Topex fluoride tooth paste.
The mean values obtained for post lesion SMH were 221.99±26.27μm and 224.50±28.64μm for group I and II, respectively. Post treatment SMH values were recorded at 232.52±24.34μm for NovaMin and 232.03 ±24.46μm for the fluoride group. Kolmogorov Smirnoff test indicated that both groups did not follow a normal distribution path before and after immersion in both remineralizing solutions (p=0.001 and p=0.031). However the differences in both solutions followed a normal distribution (p=0.200 and p=0.129). Mann Whitney test indicated that there was no significant difference between the surface microhardness measurements of teeth before and after NovaMin or fluoride applications (p=0.326 and p=0.877, respectively).
DISCUSSION
The remineralization effect of NovaMin on artificial caries-like lesions of the human enamel under oral condition could revolutionize many aspects of dental practice especially those related to the development of hard tissue defects and carious lesions.
The result of the current study revealed that a considerable amount of surface microhardness of the enamel is achieved after treatment with fluoride or NovaMin tooth paste. However, it appears that the effect of NovaMin was superior to that of 1.1 percent fluoride tooth paste. This could provide basic and potentially important information for its clinical ramification.
Although the overall result of this study shows increase in the surface hardness of all teeth, this increase was significantly higher in the NovaMin group (p< 0.001).
The pH cycling technique used in this investigation has been recommended to induce caries-like lesions as a routine procedure for demineralization and remineralization of the teeth.
In fact it has been extensively used to study the effect of various tooth pastes to strengthen hard tooth structure in the recent years [9,14,19–22]. Levis et al. (2001) indicated that the calcium containing dentifrices have significantly lower remineralization ability even in the presence of fluoride paste (NaF).
The difference with the current results could be due to the differences in the type of tooth paste used by Levis et al (Sooth RX) [23].
As an artificial carious lesion represents all the histological specifications and criteria of naturally developed caries while it is more homogeneous, it is considered quite reliable for demineralization and remineralization studies. Sound subsurface structure (layer) of artificial caries represents these lesions in a more favorable way for caries studies [14, 19, 21, 24].
Low concentration of fluoride added to demineralizing/remineralizing solutions simulate the oral environment and prevent severe enamel surface destruction [14–19].
Demineralization of the enamel depends on the pH and the amount of calcium and phosphate available in the saliva. Subsaturation accelerates dissolution of hydroxy apatite, therefore enhancing the release of calcium and phosphate ions towards the enamel surface.
Hypersaturation and then precipitation of hydroxyapatite, however, results in enamel surface remineralization [19, 25].
Microhardness evaluation is considered as a reliable and a widely used technique to evaluate remineralization changes of the tooth surface [9, 10]. Although the role of calcium and phosphate ions is well recognized on the demineralization/remineralization of the enamel surface, the inorganic content of saliva should also be considered as an effective factor in this process. Artificial saliva was used in order to simulate the oral condition for releasing the bioactive content of NovaMin into the environment. This condition helps the calcium sodium phosphosilicate bioactive glass to gradually substitute by hydrogen ions. As the process continues, a thick layer full of calcium and phosphate precipitates on the tooth surface with an increased pH of the environment preventing the demineralization process [22,25]. Several earlier studies have varying recommended timings of 90 seconds, 2 or 4 minutes brushing for a sufficiently long and effective tooth paste use. The positive result of this study on increasing surface microhardness of the enamel samples approves the effectiveness of 2 minutes brushing time affected by NovaMin and fluoride tooth paste in each pH cycling [21]. The remineralization effect of NovaMin and its significant increase in surface microhardness found in the present study was in line with several earlier studies when compared to this effect by fluoride tooth paste [5, 12, 13, 17, 20]. Based on the illustrated superior effect of NovaMin on remineralization of the enamel to fluoride tooth paste, NovaMin could be suggested as an effective tooth paste for controlling caries processes especially in caries-sensitive teeth or those suffering from enamel hypoplasia or hyposalivation [10, 18, 26, 27].
Although the result of the present study indicates that NovaMin has significantly increased surface microhardness, combination of both pastes (NovaMin and fluoride) may have a synergic effect on remineralization of the enamel [16,17].
Interestingly, one of the facts in favor of NovaMin is the lack of any hypoplastic spots found on young children’s teeth following the use of conventional tooth pastes [27].
This is usually due to swallowing the paste during daily tooth brushing.
This could be counted as one of the advantages of NovaMin over fluoride containing pastes making it recommendable and safe to be prescribed as a tooth paste of choice for young children.
CONCLUSION
Based on the results of this in vitro study both fluoride tooth paste (Sultan) and (Oravive) are effective in remineralization of caries-induced lesions in permanent teeth.
NovaMin tooth paste had more remineralization effect on artificial caries in permanent teeth.
The difference between the two tested tooth pastes was significant for increase in surface microhardness of the enamel by NovaMin (p=0.005).
Fig 1.
Sample teeth in coded pots for the study groups
Fig 4.

Vicker’s microhardness device
Fig 6.
Samples are drowned in tooth paste solutions
Table 1.
Mean surface micro-hardness measures following a caries like lesion initiation or Fluoride application on teeth surfaces
| Groups | No. of Samples | Mean | SD | |
|---|---|---|---|---|
| Primary Microhardness | NovaMin | 30 | 221.99 | 26.27 |
| Fluoride | 30 | 224.50 | 28.64 | |
| Secondary Hardness | NovaMin | 30 | 232.52 | 24.34 |
| Fluoride | 30 | 232.03 | 26.46 | |
| Differences | NovaMin | 30 | 10.53 | 4.64 |
| Fluoride | 30 | 7.52 | 3.61 |
Acknowledgments
Authors would like to thank Dr Kharrazi for his valuable statistical advises as well as the Science Lab Staff at the faculty of Medicine, ShahidBeheshti Medical University.
REFERENCES
- 1.Reynolds EC. Calcium phosphate-based remineralization system: scientific evidence? Aust Dent J. 2008 Sep;53(3):268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 2.Du Min Q, Bian Z, Jiang H, Greenspan DC, Burwell AK, Zhong J, et al. Clinical evaluation of a dentifrice containing calcium sodium phosphocilicate (novamin) for the treatment of dentin hypersensitivity. Am J Dent. 2008 Aug;21(4):210–4. [PubMed] [Google Scholar]
- 3.Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001 Dec;80(12):2066–70. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 4.La Torre G, Greenspan DC. The role of ionic release from Novamin (Calcium Sodium Phosphocilicate) in yubule occlusion: an exploratory invitro study using radio-labeled isotopes. J Clin Dent. 2010;21(3):72–6. [PubMed] [Google Scholar]
- 5.Burwell A, Jennings D, Muscle D, Greenspan DC. NovaMin and dentine hypersensitivity-invitro evidence of efficacy. J Clin Dent. 2010;21(3):66–71. [PubMed] [Google Scholar]
- 6.Cochrane NJ, Saranathan S, Cai F, Cross Kj, Reynolds EC. Enamel subsurface lesion remineralization with casein phosphopeptide stabilized solution of calcium, phosphate and fluoride. Caries Res. 2008;42(2):88–97. doi: 10.1159/000113161. [DOI] [PubMed] [Google Scholar]
- 7.Manton DJ, Walker GD, Cai F, Cochrane NJ, Shen P, Reynolds EC. Remineralization of enamel subsurface lesions in situ by the use of three commercially available sugar-free gums. Int J Paediatr Dent. 2008 Jul;18(4):284–90. doi: 10.1111/j.1365-263X.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 8.Lata S, Varghese NO, Varoughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phosphopeptide on enamel lesions. An invitro comparative evaluation. J Conserv Dent. 2010 Jan;13(1):42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White DJ. The application of in vitro models to research on demineralization and remineralization of teeth. Adv Dent Res. 1995 Nov;9(3):175–93. doi: 10.1177/08959374950090030101. [DOI] [PubMed] [Google Scholar]
- 10.Rehder Neto FC, Maeda FA, Turssi CP, Serra MC. Potential agents to control enamel caries-like lesions. J Dent. 2009 Oct;37(10):786–90. doi: 10.1016/j.jdent.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Litkowski L, Greenspan DC. A clinical study of the effect of calcium sodium phosphocilicate on dentin hypersensitivity –proof of principle. J Clin Dent. 2010;21(3):77–81. [PubMed] [Google Scholar]
- 12.Burwell AK, Greenspan DC. Potential for dentifrice protection against enamel erosion in an in vitro model. Caries Res. 2007;41(4):268–334. [Google Scholar]
- 13.Andersson OH, Kangasniemi I. Calcium phosphate formation at the surface of bioactive glass in vitro. J Biomed Mater Res. 1991 Aug;25(8):1019–30. doi: 10.1002/jbm.820250808. [DOI] [PubMed] [Google Scholar]
- 14.Casals E, Boukpessi T, McQueen CM, Eversole SL, Faller RV. Ant caries potential of commercial dentifrices as determined by fluoridation and remineralization efficiency. J Contemp Dent Pract. 2007 Nov 1;8(7):1–10. [PubMed] [Google Scholar]
- 15.Cross KJ, Hug NL, Palamara JE, Perich JW, Reynolds EC. Physicochemical characterization of casein phosphopeptide-amorphos calcium phosphate nanocomplexes. J Biol Chem. 2005 Apr;280(15):15362–9. doi: 10.1074/jbc.M413504200. [DOI] [PubMed] [Google Scholar]
- 16.Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (NovaMin): remineralization potential. Adv Dent Res. 2009;21(1):35–9. doi: 10.1177/0895937409335621. [DOI] [PubMed] [Google Scholar]
- 17.Azarpazhooh A, Limback H. Clinical efficacy of casein derivatives, a systemic review of literature. J Am Dent Assoc. 2008 Jul;139(7):915–24. doi: 10.14219/jada.archive.2008.0278. [DOI] [PubMed] [Google Scholar]
- 18.Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine-in vitro. J Dent. 2007 Aug;35(8):695–8. doi: 10.1016/j.jdent.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Quieroz CS, Hara AT, Paes Leme AF, Cury JA. PH-cycling models to evaluate the effect of low fluoride dentifrice on enamel demineralization and remineralization. Braz Dent J. 2008;19(1):21–7. doi: 10.1590/s0103-64402008000100004. [DOI] [PubMed] [Google Scholar]
- 20.Viera AE, Delbem AC, Sassaki KT, Rodrigues E, Cury JA, Cunha RF. Fluoride dose response in PH-cycling models using bovine enamel. Caries Res. 2005 Nov-Dec;39(8):514–20. doi: 10.1159/000088189. [DOI] [PubMed] [Google Scholar]
- 21.Oshiro M, Yamaguchi K, Takamizawa T, Inage H, Watanabe T, Irokawa A, et al. Effect of CPP-ACP paste on tooth mineralization: an FE-SEM study. J Oral Sci. 2007 Jun;49(2):115–20. doi: 10.2334/josnusd.49.115. [DOI] [PubMed] [Google Scholar]
- 22.Pulido MT, Wefel JS, Hermandez MM, Denehy GE, Guzman-Armstrong S, Chalmers JM, et al. The inhibitory effect of MI paste, fluoride and a combination of both on the progression of artificial caries-like lesions in enamel. Oper Dent. 2008 Sep-Oct;33(5):550–5. doi: 10.2341/07-136. [DOI] [PubMed] [Google Scholar]
- 23.Lewis RD, Marken BF, Smith SR. Anti-plaque efficacy of a chalk-based antimicrobial dentifrice. SADJ. 2001 Apr;56(4):178–81. [PubMed] [Google Scholar]
- 24.Argenta RM, Tabchoury CP, Cury JA. A modified pH-cycling model to evaluate fluoride effect on enamel demineralization. Pesqui Odontol. Bras. 2003 Jul-Sep;17(3):241–6. doi: 10.1590/s1517-74912003000300008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Zou J, Yang R, Zhou X. Remineralization effects of casein phosphopeptide-amorphous calcium phosphate crème on artificial early lesions of primary teeth. Int J Paediatr Dent. 2011 Sep;21(5):374–81. doi: 10.1111/j.1365-263X.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan N, Kavitha M, Loganathan SC. Comparison of the remineralization potential of CPP-ACP and CPP-ACP with 900 ppm fluoride on eroded human enamel: An in situ study. Arch Oral Biol. 2010 Jul;55(7):451–4. doi: 10.1016/j.archoralbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: an in vitro study. Aust Dent J. 2008 Mar;53(1):34–40. doi: 10.1111/j.1834-7819.2007.00006.x. [DOI] [PubMed] [Google Scholar]