Abstract
Background
Antidepressants can impair sexual arousal. Exercise increases genital arousal in healthy women, likely due to increasing sympathetic nervous system (SNS) activity.
Purpose
Test if exercise increases genital arousal in women taking antidepressants, including selective serotonin reuptake inhibitors (SSRIs), which suppress SNS activity, and selective serotonin and norepinephrine reuptake inhibitors (SNRIs), which suppress the SNS less.
Method
Women reporting antidepressant-related sexual arousal problems (N=47) participated in three counterbalanced sessions where they watched an erotic film while we recorded genital and SNS arousal. In two sessions, women exercised for 20 min, either 5 or 15 min prior to the films.
Results
During the no-exercise condition, women taking SSRIs showed significantly less genital response than women taking SNRIs. Exercise prior to sexual stimuli increased genital arousal in both groups. Women reporting greater sexual dysfunction had larger increases in genital arousal post-exercise. For women taking SSRIs, genital arousal was linked to SNS activity.
Conclusions
Exercise may improve antidepressant-related genital arousal problems.
Keywords: Antidepressant side effects, Sexual arousal functioning, Exercise, Sympathetic nervous system activity
Introduction
Antidepressant medications are the most common treatment for depression and anxiety in the USA [1]. Women present for treatment of depression more often than men and are twice as likely to be prescribed antidepressants for the same complaints [2, 3]; an estimated one in six American women has been prescribed an antidepressant [4]. About 85% of antidepressants prescribed are selective serotonin reuptake inhibitors (SSRIs) or selective serotonin and norepinephrine reuptake inhibitors (SNRIs) [4].
All antidepressants are associated with sexual side effects. Approximately 96% of women taking antidepressants report at least one sexual side effect [5] of which 20–50% qualifies as a distinct clinical problem [6]. Both SSRIs and SNRIs are associated with disruption of sexual desire, genital arousal, and delay or loss of orgasm. In addition to the negative impact on quality of life, sexual side effects are one of the biggest threats to treatment adherence [7]. Despite this, there are few empirically validated treatments for sexual side effects that do not impact therapeutic efficacy [8]. One of the most common treatments prescribed to women experiencing sexual side effects is sildenafil [9], which appears to improve orgasm latency but not sexual desire, mental sexual arousal, lubrication, or overall measures of sexual functioning [10]. Similarly, bupropion, another drug commonly prescribed to counteract sexual side effects, has shown mixed results in randomized clinical trials, with some reporting improvement [11] and others not [12]. Even in the few cases where augmentation is effective, each additional drug carries its own side effect burden and increases cost of care [13].
It has been suggested that genital arousal and orgasm side effects of SSRIs are linked to peripheral nervous system adrenergic pathways [14], particularly to changes in sympathetic nervous system (SNS) activity following antidepressant treatment [15]. Serotonin has an inhibitory effect on norepinephrine [16], particularly that of serotonergic 1A and 2 C receptor-linked inhibition of sympathetically controlled blood vessels [17] and other peripheral nervous system outputs [18, 19]. A number of studies have confirmed that SSRIs suppress SNS activity, particularly norepinephrine release [20] and sympathetic muscle and vascular nerve firing [21]. Both SSRIs and SNRIs have a facilitatory effect on serotonin, which in turn may inhibit norepinephrine and suppress SNS activity; however, SNRIs may counteract this inhibitory effect by directly facilitating norepinephrine availability [22]. As SNS activity mediates vaginal sexual arousal [23], this suggests one possible mechanism for genital arousal side effects. Specifically, there is a curvilinear relationship between levels of SNS activity and genital arousal in women [24]: Moderate SNS activation is associated with higher vaginal sexual arousal than very high or very low SNS activity. This may also help explain why SNRIs (which have less suppression of SNS activity) are associated with lower rates of genital arousal and orgasm side effects than SSRIs (which have greater suppression of SNS activity) [15].
If women are taking medications that suppress the SNS, it is possible that genital arousal would be similarly suppressed. This is not to say that sexual side effects are due solely to SNS suppression—there are many systems through which antidepressants may affect sexual functioning—but rather that SNS suppression may contribute to sexual side effects and thus be a focus for intervention. Moreover, targeting the SNS is less likely to interfere with the (presumably) central nervous system mechanisms responsible for antidepressant effects. Independently activating the SNS prior to sexual activity may be a relatively unobtrusive and inexpensive intervention for sexual side effects.
Only one prior study has attempted to use SNS activation as a means of treating sexual arousal side effects. In this study, 19 women receiving sertraline, paroxetine, or fluoxetine participated in an 8-week, randomized, crossover, placebo-controlled, at-home trial to determine if ephedrine, an adrenergic agonist, alleviated SSRI-induced sexual side effects [25]. In a reanalysis of the data, Ahrold and Meston [26] compared the effects of ephedrine on sexual side effects in the women taking either fluoxetine, which has secondary properties as a weak norepinephrine reuptake inhibitor [27–29] with the sexual side effects seen in the women taking sertraline or paroxetine, which do not act as norepinephrine reuptake inhibitors. The women taking sertraline or paroxetine had greater improvement of genital arousal and orgasm (relative to placebo) with ephedrine than did women taking fluoxetine.
In the present study, we replicated an exercise manipulation previously used in women not taking antidepressants to increase SNS activity and vaginal sexual arousal [30, 31] in order to further test the potential efficacy of SNS activation on vaginal sexual arousal side effects in women taking antidepressants. We chose to use exercise as our method of SNS activation as it has been shown to increase genital arousal in women and is an inexpensive, easily accessible intervention that would not be expected to interfere with the main effects of antidepressants or have significant adverse effects.
Meston and Gorzalka [30, 31] used 20 min of moderately intense exercise (stationary cycling) to induce SNS activity and measured genital sexual arousal under three conditions—5, 15, and 30 min post-exercise. Women’s genital arousal was significantly and marginally increased 15 and 30 min post-exercise, respectively, and marginally decreased at 5 min post-exercise. Based on the notion of a curvilinear relationship between SNS activation and genital arousal in women, the authors speculated that, for healthy women, the high level of SNS activation at 5 min was too high to facilitate sexual arousal [30]. In the current study, we hypothesized that, as women taking serotonergic antidepressants are likely to have suppressed SNS tone, their genital arousal would be greater at 5 min post-exercise than at 15 min. Moreover, women who have experienced a greater impact of antidepressants on genital arousal, that is, those reporting the greatest genital arousal dysfunction, would likely have the most room for improvement and thus demonstrate the largest effects of such an intervention. While previous reports have documented improved sexual function in depressed women using exercise over a period of several weeks [32], these studies did not investigate the immediate impact of exercise on sexual response, and as noted, exercise may have an acute effect.
In summary, we had the following hypotheses. We hypothesized that as in the Meston and Gorzalka studies, women’s genital sexual arousal would be greater post-exercise relative to a no-exercise control. In contrast to the Meston and Gorzalka studies, however, we hypothesized that the increases in SNS activity at 5-min post-exercise would be associated with greater genital arousal than those seen at 15-min post-exercise. As SSRIs may have a greater SNS suppression effect than do SNRIs, we expected women taking SNRIs would show beneficial effects of exercise on genital arousal but to a lesser extent than those taking SSRIs. Finally, we predicted that those women who reported higher levels of genital arousal dysfunction, regardless of medication class, would have a greater increase in genital arousal post-exercise than those who reported less severe problems. Although we measured mental (i.e., self-reported, subjectively experienced) sexual arousal, we did not make specific hypotheses regarding the effects of exercise on mental sexual arousal. While it is possible that the facilitation of genital arousal would translate into heightened mental sexual arousal, previous studies using this paradigm did not find significant increases in self-reported sexual arousal [30, 31], and laboratory studies of this nature show modest synchrony of measures of genital and self-reported sexual arousal [33].
Method
Recruitment
Participants were recruited from the community via advertisements posted online, in local newspapers and flyers that outlined the sexual nature of the experiment. Interested participants completed a phone screening for major inclusion and exclusion criteria. Inclusion criteria were: heterosexual or bisexual female aged 18 years or older, taking an SSRI or SNRI, and currently sexually active. Exclusion criteria were: use of other medications known to affect sexual or vascular functioning, with the exception of hormonal contraceptives; use of more than one antidepressant or any other psychoactive medications; body mass index (BMI) over 40 kg/m2; any injury or medical condition that would preclude exercising (e.g., untreated exercise-induced asthma); medical conditions likely to affect genital arousal (e.g., recent surgery to pelvic or reproductive organs); fewer than three menstrual periods in the last 6 months for any reason other than continuous use of hormonal contraceptives; current levels of clinically significant depression or anxiety; or distress due to history of sexual trauma. Participants also completed a clinical interview to confirm the presence of sexual side effects. Specifically, participants were asked about their sexual function in four areas (desire, mental and genital arousal, orgasm, and pain) with follow-up questions regarding the onset, severity, frequency, and distress or impairment associated with each side effect. Participants had to report that effects first appeared, or were significantly worsened, within no less than 1 week and no more than 12 weeks of starting an antidepressant. Although all participants reported genital arousal side effects, not all were distressed about these effects, and thus not all participants qualified for a DSM-IV-TR diagnosis of sexual dysfunction [34].
Each participant completed three counterbalanced experimental conditions (see “Procedures”). All participants were oriented to the study procedures during their first laboratory visit and gave informed consent. Participants then completed a fitness assessment in which we measured their height, weight, two measures of resting heart rate (HR) and blood pressure (which were averaged), and waist and hip circumference. From these measures, we calculated BMI and waist-to-hip ratio. To reduce the risk of injury during the exercise conditions, we excluded participants who had three or more of the following measurements: a BMI over 40 kg/m2, a waist-to-hip ratio over 0.9, average resting HR over 90, average systolic blood pressure over 140, or diastolic blood pressure over 95; these measures have been shown to significantly predict risk of adverse events during exercise [35]. Two women were excluded based on these criteria. From these data, we also calculated the participant’s maximum HR using the equation recommended by Gellish et al. [36].
Procedures
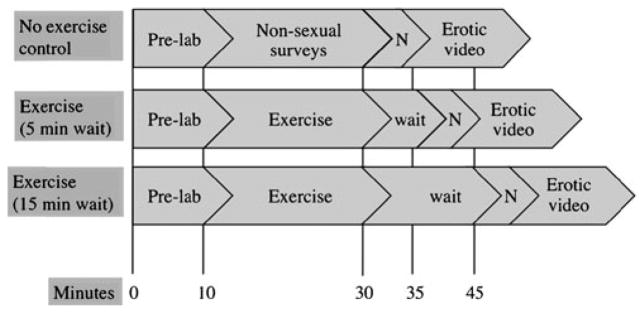
There were three experimental conditions, one no-exercise control condition and two exercise conditions (see Fig. 1). Sessions took place within a 2-week period, and no session took place during the participant’s menstrual period. All procedures took place in a private, internally locked room with an intercom between the researcher and participants. During the no-exercise condition, participants watched a neutral and then erotic film while their HR was recorded by electrocardiograph, genital arousal by vaginal photoplethysmograph, and mental sexual arousal by self-report questionnaire. In one of the exercise conditions, participants ran on a treadmill for 20 min at 80% of their maximum HR, waited 5 min, and then watched a similar neutral-erotic film sequence. The other exercise condition was identical with the exception that participants waited 15 min before viewing the films.
Fig. 1.
Experimental timeline. Note: “Pre-lab” time included informed consent procedures and fitness assessment (if that was the first time the participant was in the lab), or re-orientation to the procedures for that day (if they had attended a previous session). “N” indicates the 3-minute neutral video
Stimuli
Stimuli included three counterbalanced films: a 3-min neutral segment followed by one of three 10-min erotic films depicting a heterosexual couple engaging in foreplay, cunnilingus, and vaginal intercourse. To standardize epochs across stimuli types, physiological measures were calculated from responses to the entire 3-min neutral film and the last 3 min of the erotic films. Erotic segments were female-centered, commercially available films and have previously been shown to elicit genital arousal in women [37, 38].
Physiological Assessments
At the beginning of each experimental session, a researcher instructed the participants in how to position a vaginal photoplethysmograph and attach wires for an electrocardiograph, cleaned the electrode sites, and placed electrocardiograph electrodes for participants.
Physiological Sexual Arousal
Genital arousal was assessed using vaginal pulse amplitude, which was sampled continuously throughout the film sequences with a vaginal photoplethysmograph. Vaginal pulse amplitude reflects phasic changes in vaginal blood flow and is specific to sexual arousal [39]. Larger amplitudes reflect higher vaginal engorgement and thus greater sexual arousal; because there is no absolute zero point for this measure, each participant served as her own control. The vaginal photoplethysmograph signal was recorded using BIOPAC hardware and software (BIOPAC Systems, Inc., Santa Barbara, CA, USA) and was band-pass-filtered to remove noise. Visible movement artifacts were removed manually, as per previous studies of this nature [40–42]. Differences were computed between the peak and trough of each pulse and averaged separately for neutral and erotic segments. Percent change between the neutral and erotic segments was calculated for each condition.
Heart Rate Variability
As a manipulation check on SNS activation, we measured heart rate variability (HRV). The parasympathetic nervous system (PNS) and SNS interact to regulate HR. Respiration briefly interrupts PNS influences on HR, causing temporary fluctuations in the beat-to-beat intervals termed respiratory sinus arrhythmia. At rest, when the PNS is dominant, respiratory sinus arrhythmias are prominent in HR and can be easily detected. When the heart is put under stress, the PNS withdraws (and the SNS becomes more active) and respiratory sinus arrhythmia become less prominent. Variability in HR is thus thought to reflect the balance of PNS and SNS activity. Specifically, it is thought that low-frequency components of HR waveforms represent influences of both SNS and PNS, while high-frequency elements represent just the PNS. Thus, one index of SNS activity is the ratio of power in low and high-frequency bands of HR signal [43]. Higher values of this measure represent higher SNS activity (relative to PNS activity).
Heart rate was measured continuously during film presentations at a rate of 200 samples/s with a three-lead electrocardiograph with BIOPAC hardware and software. Beat-to-beat intervals were collected using a peak finder function, and the low frequency/high frequency ratio for the neutral and erotic segments were calculated using Kubios HRV Analysis Software (Biosignal Analysis and Medical Imagine Group, University of Kuopio, Kuopio, Finland). As with vaginal pulse amplitude, we calculated the percent change between the neutral and erotic segments for each condition.
Self-Report Assessments
Mental Sexual Arousal
Mental sexual arousal was measured before and after the films using the Film Scale, a 41-item questionnaire of mental sexual arousal, sexual desire, and positive and negative affects [44]. We conducted a factor analysis of scale items in the no-exercise control session. A three-factor solution was supported by a Scree plot and explained 66.1% of the total variance; these three factors appeared to assess mental sexual arousal/desire (25 items), negative emotion (11 items), and perception of non-sexual arousal such as increased HR (three items). With regard to the sexual desire/arousal factor, sexual medicine experts have recently argued that there is significant overlap between women’s mental sexual arousal and desire, noting that women use the terms interchangeably [45], and there is a high comorbidity of female desire and mental sexual arousal disorders [46]. For both the mental sexual arousal/desire and perception of non-sexual physical arousal factors, we used difference scores between pre- and post-film administrations.
Sexual Functioning
Global sexual functioning and genital arousal functioning were measured with the Female Sexual Function Index (I47), a validated 19-item questionnaire with six domains: desire, arousal, lubrication, orgasm, satisfaction, and sexual pain. We used the lubrication domain as our index of genital arousal function as lubrication is strongly correlated with vaginal vasocongestion [48]. The Female Sexual Function Index has been shown to differentiate between women with and without sexual arousal dysfunction [47]. A total score of 26.5 or below indicates clinically relevant sexual dysfunction [49]; cutoffs specific to genital arousal dysfunction have not yet been established.
Demographics
We assessed the participant’s age, ethnicity, type of antidepressant medication, level of education, and length of current sexual relationship.
Daily Screener
Participants filled out a questionnaire each day that they participated. This questionnaire assessed use of nicotine or non-antidepressant prescription drugs (e.g., allergy medications), time of last meal or vigorous exercise, and any significant stressor that day. Out of the total 141 sessions conducted, 25 included non-antidepressant drug use within an hour of the session, a meal within 2 h, or more than 5 min of vigorous exercise within 2 h of the session; no participant indicated a significant stressor experienced that day. Analyses were conducted excluding these 25 sessions; results did not significantly change, thus analyses reported include all data.
Results
Sample Characteristics
A total of 50 women were enrolled in the study; of these, three did not complete all conditions, leaving a final sample of 47 women. The SSRI group included women taking fluoxetine (8), paroxetine (2), sertraline (13), or citalopram (9). The SNRI group included women taking duloxetine (11) or venlafaxine (4). Participants had a mean age of 29.04 (SD=7.93 years) and were predominantly Caucasian (77%) (see Table 1 for further demographics).
Table 1.
Demographics
| SSRI (n=32) | SNRI (n=15) | Total (N=47) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Demographic | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 26.65 | 5.31 | 34.12 | 10.17 | 29.04 | 7.93 |
| Time on medication (years) | 2.34 | 2.37 | 3.36 | 3.02 | 2.66 | 2.61 |
| Body Mass Index | 22.92 | 3.15 | 24.77 | 3.18 | 23.52 | 3.25 |
| FSFI total score | 26.45 | 4.32 | 28.35 | 4.04 | 27.07 | 4.28 |
| FSFI lubrication subscore | 4.93 | 1.17 | 5.42 | 0.05 | 5.09 | 1.05 |
| Count | % | Count | % | Count | % | |
| Ethnicity | ||||||
| White | 24 | 75 | 12 | 80 | 36 | 77 |
| Hispanic | 2 | 6 | 3 | 20 | 5 | 11 |
| Black | 2 | 6 | 0 | 0 | 2 | 4 |
| Other | 1 | 3 | 0 | 0 | 1 | 2 |
| Relationship status | ||||||
| Single/dating casually | 7 | 22 | 3 | 20 | 10 | 21 |
| In a long-term relationship | 24 | 75 | 8 | 53 | 32 | 68 |
| Married | 1 | 3 | 4 | 27 | 5 | 11 |
| Reason for medication | ||||||
| Mood disorder | 8 | 25 | 7 | 47 | 15 | 32 |
| Anxiety disorder | 10 | 31 | 1 | 7 | 11 | 23 |
| Mood and anxiety disorder | 13 | 41 | 5 | 33 | 18 | 38 |
| Mood and other disorder | 1 | 3 | 1 | 7 | 2 | 4 |
| Chronic pain | 0 | 0 | 1 | 7 | 1 | 2 |
FSFI Female Sexual Function Index, SSRI selective serotonin reuptake inhibitor, SNRI selective serotonin and norepinephrine reuptake inhibitor taking SSRIs demonstrated less genital and SNS response than did those taking SNRIs.
Group Differences
Women taking SNRIs were significantly older than those taking SSRIs [1, 46], F=11.05, p<0.01, and thus age was used as a covariate for all group comparisons. No other demographic was significantly different between groups. Total sexual functioning (as measured by the Female Sexual Function Index) and lubrication domain scores were higher in the SNRI than SSRI group, although this difference was not statistically significant (see Table 1).
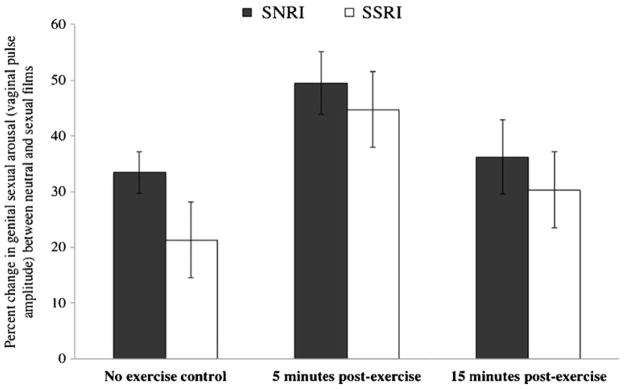
To compare the non-exercise level of genital arousal across groups, we conducted an ANOVA with percent change in vaginal pulse amplitude during the no-exercise condition as the dependent variable, group (SSRI, SNRI) as the independent variable, and age as a covariate. Women in the SSRI group had a significantly lower genital response (average change between neutral and sexual stimuli= 19.2%) than did women in the SNRI group (average change=37.9%), F(1, 44)=4.54, p<0.05. Similarly, women taking SSRIs had less change in HRV between neutral and sexual films (average change=54.0%) than did the women taking SNRIs (average change=78.9%) in the no-exercise control, F(1, 44)=11.05, p<0.05. In other words, women
Physiological Measures
SNS Activation
There was a significant effect of session on HRV, F(1, 45)= 6.38, p<0.05. Follow-up contrasts confirmed that participant’s change in HRV was significantly greater in the 5 min post-exercise condition than the 15 min post-exercise condition, which in turn was higher than the no-exercise control condition, indicating that our manipulation incrementally increased SNS activity.
For the SNRI group, there were no significant associations between HRV and genital arousal. However, for the SSRI group, there was a significant correlation between HRV and genital arousal for the 5 min post-exercise condition, r=−0.69, p<0.05, and the 15-min post-exercise condition, r=−0.46, p<0.05. (A negative percent change in the low frequency/high frequency ratio post-exercise would indicate higher SNS activity in the erotic segment than the neutral, as the neutral films were presented first.) Thus, increased SNS activity was associated with increased genital arousal for the SSRI group only, at both 5 and 15 min post-exercise.
Genital Arousal
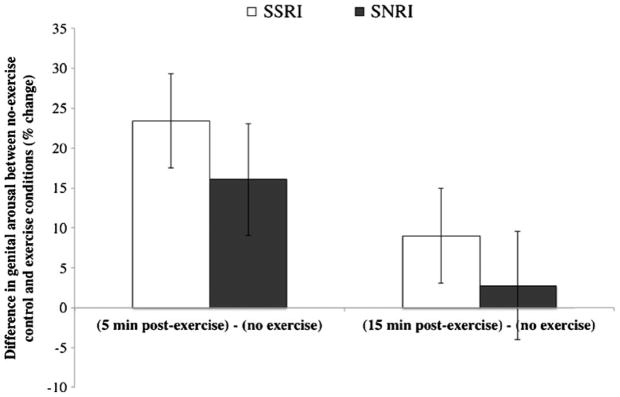
We first conducted a repeated-measures ANCOVA with genital sexual arousal (vaginal pulse amplitude) during each condition (no-exercise control, 5 min post-exercise, and 15 min post-exercise) as the repeated measure, age as a covariate, and group as a between-subjects variable. There was a significant main effect of condition on genital arousal, F(2, 42)=4.11, p<0.05 (see Fig. 2). Follow-up contrast tests revealed that genital arousal was significantly increased in the two exercise conditions relative to the no-exercise control, F(1, 43)=8.63, p<0.05; moreover, genital arousal was significantly higher 5 min post-exercise than 15 min post-exercise, F(1, 43)=3.20, p<0.05 (see Fig. 3). The interaction between condition and group was not significant, F(2, 43)=0.16, p=0.85.
Fig. 2.
Effects of exercise on women’s genital sexual arousal. Note: SSRI: selective serotonin reuptake inhibitor; SNRI: selective serotonin and norepinephrine reuptake inhibitor
Fig. 3.
Relative increase in women’s genital sexual arousal between no-exercise control condition and exercise conditions. Note: SSRI: selective serotonin reuptake inhibitor; SNRI: selective serotonin and norepinephrine reuptake inhibitor
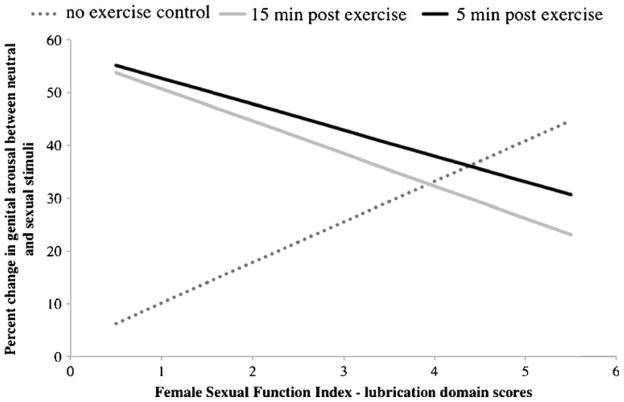
Following recommended procedures for testing interactions between covariates and repeated-measures effects [50], we then completed another ANCOVA, identical to the first, but including the Female Sexual Function Index lubrication domain factor as an additional covariate. There was a significant interaction between condition and lubrication function on genital arousal, F(1, 43)=7.06, p<0.05, such that these effects were amplified in women reporting higher levels of genital arousal dysfunction (see Fig. 4).
Fig. 4.
Interaction between sexual arousal functioning and genital arousal by condition
Psychological Measures
We used the same statistical design as with genital arousal measures for analyzing the effects of exercise on self-reported sexual arousal. There were no significant main effects or interactions. To test for the general relationships between dependent variables, we calculated correlations for each condition using the two Film Scale factors (mental sexual arousal and perception of non-sexual physical arousal) and the two physiological variables (vaginal pulse amplitude and HRV). We adjusted for potential type I errors using the Sidak alpha-level correction (i.e., only p values< 0.0102 were considered significant). The correlation between mental sexual arousal and vaginal pulse amplitude was significant in the no-exercise control and 15 min post-exercise conditions (r=0.26, p=0.01 and r=0.29, p<0.01, respectively), but not for the 5 min post-exercise condition, r=−0.09, p=ns. For the 5 min post-exercise condition only, there was a significant correlation between mental sexual arousal and perception of non-sexual physical arousal (r=−0.29, p<0.01) such that women who noticed more non-sexual physical arousal reported less mental sexual arousal to the film.
Discussion
This was the first study to document that acute exercise improves genital arousal in women taking serotonergic antidepressants. While preliminary, these findings suggest that exercise may help treat antidepressant-related female genital arousal dysfunction.
We found support for our hypothesis that SSRIs dampen both SNS activity and genital arousal more than SNRIs. The arterial vasculature that supports vaginal vasocongestion (i.e., genital arousal) is controlled largely by sympathetic innervation (for a review, see Carmeliet et al. [51]): Following stimulation of norepinephrine β2 receptors, vaginal arteries become relaxed, allowing blood to pool.1 In the peripheral nervous system, serotonin inhibits release of norepinephrine (for a review, see Gothert et al. [17]), and thus SSRIs may reduce vasocongestion, leading to lower genital arousal. Because SNRIs increase both free serotonin and norepinephrine, it is possible that there is less net effect on genital arousal. Exercise may act as an amplifier, increasing the synaptic availability of norepinephrine for subsequent genital response. For both groups, exercise increased genital arousal, as in women not taking antidepressants [30, 31]; however, women taking SSRIs had relatively suppressed arousal (both SNS and genital) during the no-exercise control condition. In other words, SNS activation increased genital arousal in women taking SSRIs to that of those taking SNRIs, suggesting that the effect was partially mediated by norepinephrine. The relationship between HRV, an objective measure of SNS activity, and genital arousal also suggests a meditational role for norepinephrine. For women taking SSRIs only, the increased genital arousal associated with exercise was correlated with SNS activity. The same was not true for women taking SNRIs, possibly because they did not need as much of an SNS boost. It should be noted that, without direct comparison, we cannot know with certainty if these gains are larger than those experienced by women not taking antidepressants.
There was a significant interaction between the effects of exercise and genital arousal functioning, such that those women who benefited most were those who reported the greatest genital arousal dysfunction. This suggests that clinically, exercise may be a useful prescription for vaginal arousal problems secondary to antidepressants. While exercise is known to improve sexual functioning by way of enhancing general physical health, body image [53], and positive mood [54], it appears there may be additional benefit of exercising directly before sexual activity in that SNS activation could boost subsequent genital arousal.
Women’s self-reported sexual arousal did not appear to increase with exercise. This implies that the effect of exercise on genital arousal was not due to misinterpretation of general, non-sexual arousal as sexual. In fact, 5-min post-exercise, mental sexual arousal was negatively correlated with self-reported non-sexual physical arousal, suggesting that participants’ attention may have been diverted from their genitals to non-sexual cues, thus diminishing the mental experience of sexual arousal. Women with sexual arousal problems are more likely to become distracted by non-sexual cues and disengage from physical sensations [55], a phenomenon identified by Masters and Johnson as “spectatoring” [56]. While the erotic film was a sufficient stimulus to trigger genital arousal, it may not have diverted attention from non-sexual cues. If so, we would expect that, given more intense stimuli (such as a sexual partner) or a more active role (such as engaging in sexual activity), the effects of exercise on genital arousal would translate into mental arousal as well. Given these results, we would recommend that a holistic treatment for sexual side effects would include not only the exercise intervention outlined here but also psychoeducation or therapy targeted specifically to mental sexual arousal concerns.
This study had some limitations. We tested only pre-menopausal women; there is evidence that post-menopausal women do not experience increases in genital arousal following SNS activation [57]. Similarly, we included women who were and were not taking hormonal contraceptives. While hormonal contraceptive use does not appear to impact vaginal pulse amplitude [38], our measure of genital arousal, nor HRV [58], it has been shown to impact sexual function in some women [59]. We did not control for non-SNS effects of exercise. Exercise may have an indirect impact through increasing positive mood [60]. However, this is unlikely, as we did not see significant positive effects of exercise on self-report measures. Similarly, it is possible that exercise acted through a non-SNS neurological mechanism. For example, exercise may modestly increase production of nitric oxide [61], a neurotransmitter associated with relaxation of genital blood vessels. While there was some evidence that SNS activity played a meditational role, other potential mechanisms cannot be ruled out. We did not distinguish between women taking different doses of the same medication in our analyses. It is likely that women taking higher doses of antidepressants would experience larger side effects, and thus larger gains in genital arousal post-exercise; however, our current sample is insufficiently powered to investigate this level of inquiry. Finally, translational studies will be needed to determine the effectiveness of acute exercise as an intervention in a clinical environment. In particular, the present study examined only women with adequate treatment response who had stabilized on their antidepressant dose and type. It would be helpful to know if this intervention works similarly well in women who are first starting antidepressants, when side effects are most frequently expressed.
We found that, for women taking antidepressants, exercise enhanced genital sexual arousal. There was some evidence that SNS activity mediated these effects, particularly for women taking SSRIs. During a no-exercise control, women taking SSRIs had lower genital arousal and SNS response to sexual stimuli than those taking SNRIs, suggesting that SSRIs may suppress SNS activity and thus genital arousal. We also found that exercise-induced increases in genital arousal were greatest for those women reporting the lowest sexual arousal functioning. Although preliminary, these findings support the prescription of exercise for antidepressant-related vaginal arousal dysfunction. In addition, this study supported a novel hypothesis as to a mechanism of sexual arousal side effects of serotonergic antidepressants: SSRIs may impair norepinephrine-mediated vaginal vasocongestion. Due to their action on norepinephrine, SNRIs may have a lesser net effect on women’s genital arousal.
Acknowledgments
This research was supported by Grant Number 1F31MH085416 from the National Institutes of Mental Health (NIMH) to Tierney Lorenz and, in part, by 1RO1 HD051676 from the National Institute of Child Health and Human Development (NICHD) to Cindy M. Meston. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
A preliminary report of data from this study was presented at the annual meeting of the International Society for the Study of Women’s Sexual Health (ISSWSH) in Scottsdale, AZ, February 2011.
Stimulation of other NE receptors, particularly the α1 receptors, generally causes vasoconstriction; however, there is evidence that in sacral nerves such as those innervating the genital vasculature, the effect of β2-related blood vessel dilation is far stronger than that ofα1-related constriction [52].
Conflict of Interest Statement The authors have no conflict of interest to disclose.
Contributor Information
Tierney A. Lorenz, Email: tierney.lorenz@gmail.com, Department of Psychology, University of Texas at Austin, 1 University Station A8000, Austin, TX 78712, USA
Cindy M. Meston, Department of Psychology, University of Texas at Austin, 1 University Station A8000, Austin, TX 78712, USA
References
- 1.Olfson M, Marcus S, Druss B, et al. National trends in the outpatient treatment of depression. JAMA. 2002;287:203–209. doi: 10.1001/jama.287.2.203. [DOI] [PubMed] [Google Scholar]
- 2.Ashton A, Rosen R. Accommodation to serotonin reuptake inhibitor-induced sexual dysfunction. J Sex Marital Ther. 1998;24:191–192. doi: 10.1080/00926239808404932. [DOI] [PubMed] [Google Scholar]
- 3.Thiels C, Linden M, Grieger F, Leonard J. Gender differences in routine treatment of depressed outpatients with the selective serotonin reuptake inhibitor sertraline. Int Clin Psychopharmacol. 2005;20:1–23. doi: 10.1097/00004850-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Paulose-Ram R, Safran M, Jonas B, Gu Q, Orwig D. Trends in psychotropic medication use among US adults. Pharmacoepidem Dr S. 2007;16:560–570. doi: 10.1002/pds.1367. [DOI] [PubMed] [Google Scholar]
- 5.Clayon A, Keller A, McGarvey EL. Burden of phase-specific sexual dysfunction with SSRIs. J Affect Disord. 2006;91:27–32. doi: 10.1016/j.jad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Rosen R, Lane R, Menza M. Effects of SSRIs on sexual function: A critical review. J Clin Psychopharmacol. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Werneke U, Northey S, Bhugra D. Antidepressants and sexual dysfunction. Acta Psychiatr Scand. 2006;114:384–397. doi: 10.1111/j.1600-0447.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor M, Rudkin L, Hawton K. Strategies for managing antidepressant-induced sexual dysfunction: Systematic review of randomised controlled trials. J Affect Disord. 2005;88:241–254. doi: 10.1016/j.jad.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Perlis R, Fava M, Nierenberg A, et al. Strategies for treatment of SSRI-associated sexual dysfunction: A survey of an academic psychopharmacology practice. Harv Rev Psychiatry. 2002;10:109–114. [PubMed] [Google Scholar]
- 10.Nurnberg H, Hensley P, Heiman J, et al. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: A randomized controlled trial. JAMA. 2008;300:395–404. doi: 10.1001/jama.300.4.395. [DOI] [PubMed] [Google Scholar]
- 11.Safarinejad MR. Reversal of SSRI-induced female sexual dysfunction by adjunctive bupropion in menstruating women: A double-blind, placebo-controlled and randomized study. J Psychopharmacol (Oxf) 2011;25:370–378. doi: 10.1177/0269881109351966. [DOI] [PubMed] [Google Scholar]
- 12.Masand PS, Ashton AK, Gupta S, Frank B. Sustained-release bupropion for selective serotonin reuptake inhibitor-induced sexual dysfunction: A randomized, double-blind, placebo-controlled, parallel-group study. A J Psychiatry. 2001;158:805–807. doi: 10.1176/appi.ajp.158.5.805. [DOI] [PubMed] [Google Scholar]
- 13.Souery D, Amsterdam J, De Montigny C, et al. Treatment resistant depression: Methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9:83–91. doi: 10.1016/s0924-977x(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 14.Montejo A. Are sexual disorders core symptoms of depression? Medicographia. 2008;30:24–29. [Google Scholar]
- 15.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: A meta-analysis. J Clin Psychopharmacol. 2009;29:259–266. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 16.Millan M, Lejeune F, Gobert A. Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic and noradrenergic transmission in the frontal cortex: Relevance to the actions of antidepressant agents. J Psychopharmacol (Oxf) 2000;14:114–138. doi: 10.1177/026988110001400202. [DOI] [PubMed] [Google Scholar]
- 17.Gothert M, Fink K, Frolich D, et al. Presynaptic 5-HT auto-and heteroreceptors in the human central and peripheral nervous system. Behav Brain Res. 1995;73:89–92. doi: 10.1016/0166-4328(96)00076-9. [DOI] [PubMed] [Google Scholar]
- 18.Enguix M, Sanchez L, Villazun M, et al. Differential regulation of rat peripheral 5-HT 2A and 5-HT 2B receptor systems: Influence of drug treatment. N-S Arch Pharmacol. 2003;368:79–90. doi: 10.1007/s00210-003-0775-7. [DOI] [PubMed] [Google Scholar]
- 19.Hull E, Muschamp J, Sato S. Dopamine and serotonin: Influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Shores M, Pascualy M, Lewis N, Flatness D, Veith R. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrino. 2001;26:433–439. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 21.Barton D, Dawood T, Lambert E, et al. Sympathetic activity in major depressive disorder: Identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 22.Licht C, Penninx B, de Geus E. Response to depression and blood pressure control: All antidepressants are not the same. Hypertension. 2009;54:e2. doi: 10.1161/HYPERTENSIONAHA.109.133272. [DOI] [PubMed] [Google Scholar]
- 23.Bradford A, Meston C. The Psychophysiology of Sex. Bloomington, Indiana: Indiana University Press; 2007. Autonomic nervous system influences: The role of the sympathetic nervous system in female sexual arousal; pp. 66–82. [Google Scholar]
- 24.Lorenz TK, Harte CB, Hamilton LD, Meston CM. Evidence for a curvilinear relationship between SNS activation and women’s sexual arousal. Psychophysiology. doi: 10.1111/j.1469-8986.2011.01285.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meston CM. A randomized, placebo-controlled, crossover study of ephedrine for SSRI-induced female sexual dysfunction. J Sex Marital Ther. 2004;30:57–68. doi: 10.1080/00926230490247093. [DOI] [PubMed] [Google Scholar]
- 26.Ahrold T, Meston C. Effects of SNS activation on SSRI-induced sexual side effects differ by SSRI. J Sex Marital Ther. 2009;35:311–319. doi: 10.1080/00926230902851322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bymaster FP, Zhang W, Carter PA, et al. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- 28.Perry K, Fuller R. Fluoxetine increases norepinephrine release in rat hypothalamus as measured by tissue levels of MHPG-SO 4 and microdialysis in conscious rats. J Neural Transm. 1997;104:953–966. doi: 10.1007/BF01285563. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol. 1999;19:467–489. doi: 10.1023/A:1006986824213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meston CM, Gorzalka BB. The effects of immediate, delayed, and residual sympathetic activation on sexual arousal in women. Behav Res Ther. 1996;34:143–148. doi: 10.1016/0005-7967(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 31.Meston CM, Gorzalka BB. Differential effects of sympathetic activation on sexual arousal in sexually dysfunctional and functional women. J Abnorm Psychol. 1996;105:582–591. doi: 10.1037//0021-843x.105.4.582. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman B, Babyak M, Sherwood A, et al. Effects of aerobic exercise on sexual functioning in depressed adults. Mental Health and Physical Activity. 2009;2:23–28. [Google Scholar]
- 33.Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of sef-reported and genital measures of sexual arousal in men and women: A meta-analysis. Arch Sex Behav. 2010;39:5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: Author; 2000. Revised. [Google Scholar]
- 35.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 36.Gellish RL, Goslin BR, Olson RE, et al. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc. 2007;39:822–829. doi: 10.1097/mss.0b013e31803349c6. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton LD, Fogle EA, Meston CM. The roles of testosterone and alpha-amylase in exercise-induced sexual arousal in women. Journal of Sexual Medicine. 2008;5:845–853. doi: 10.1111/j.1743-6109.2007.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laan E, Everaerd W, van Bellen G, Hanewald G. Women’s sexual and emotional responses to male- and female-produced erotica. Arch Sex Behav. 1994;23:153–169. doi: 10.1007/BF01542096. [DOI] [PubMed] [Google Scholar]
- 39.Laan E, Everaerd W, Evers A. Assessment of female sexual arousal: Response specificity and construct validity. Psychophysiology. 1995;32:476–485. doi: 10.1111/j.1469-8986.1995.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 40.Laan E, van Driel E, van Lunsen R. Genital responsiveness in healthy women with and without sexual arousal disorder. Journal of Sexual Medicine. 2008;5:1424–1435. doi: 10.1111/j.1743-6109.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 41.Harte C, Meston C. Gender comparisons in the concordance between physiological and subjective sexual arousal. International Society for the Study of Women’s Sexual Health; Orlando, FL. 2007. [Google Scholar]
- 42.Harte C, Meston C. The inhibitory effects of nicotine on physiological sexual arousal in nonsmoking women: Results from a randomized, double blind, placebo controlled, cross over trial. Journal of Sexual Medicine. 2008;5:1184–1197. doi: 10.1111/j.1743-6109.2008.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 44.Heiman J, Rowland D. Affective and physiological sexual response patterns: The effects of instructions on sexually functional and dysfunctional men. J Psychosom Res. 1983;27:105–116. doi: 10.1016/0022-3999(83)90086-7. [DOI] [PubMed] [Google Scholar]
- 45.Brotto L. The DSM diagnostic criteria for hypoactive sexual desire disorder in women. Arch Sex Behav. 2010;39:221–239. doi: 10.1007/s10508-009-9543-1. [DOI] [PubMed] [Google Scholar]
- 46.Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS) Menopause. 2006;13:46–56. doi: 10.1097/01.gme.0000172596.76272.06. [DOI] [PubMed] [Google Scholar]
- 47.Rosen RC, Brown C, Heiman JR, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 48.Ottesen B, Pedersen B, Nielsen J, et al. Vasoactive intestinal polypeptide (VIP) provokes vaginal lubrication in normal women. Peptides. 1987;8:797–800. doi: 10.1016/0196-9781(87)90061-1. [DOI] [PubMed] [Google Scholar]
- 49.Wiegel M, Meston CM, Rosen R. The female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 50.Thomas MSC, Annaz D, Ansari D, et al. Using developmental trajectories to understand developmental disorders. J Speech Lang Hear Res. 2009;52:336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- 51.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 52.von Heyden B, Riemer R, Nunes L, et al. Response of guinea pig smooth and striated urethral sphincter to cromakalim, prazosin, nifedipine, nitroprusside, and electrical stimulation. Neurourol Urodyn. 1995;14:153–168. doi: 10.1002/nau.1930140208. [DOI] [PubMed] [Google Scholar]
- 53.Davison T, McCabe M. Relationships between men’s and women’s body image and their psychological, social, and sexual functioning. Sex Roles. 2005;52:463–475. [Google Scholar]
- 54.Lawlor D, Hopker S. The effectiveness of exercise as an intervention in the management of depression: Systematic review and meta-regression analysis of randomised controlled trials. Br Med J. 2001;322:1–8. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dove NL, Wiederman MW. Cognitive distraction and women’s sexual functioning. J Sex Marital Ther. 2000;26:67–78. doi: 10.1080/009262300278650. [DOI] [PubMed] [Google Scholar]
- 56.Masters W, Johnson V. Human Sexual Inadequacy. Boston: Little Brown; 1970. [Google Scholar]
- 57.Brotto L, Gorzalka B. Genital and subjective sexual arousal in postmenopausal women: Influence of laboratory-induced hyper-ventilation. J Sex Marital Ther. 2002;28:39–53. doi: 10.1080/00926230252851186. [DOI] [PubMed] [Google Scholar]
- 58.Schueller PO, Feuring M, Sharkova Y, Grimm W, Christ M. Effects of synthetic progestagens on autonomic tone, neurohormones and C-reactive protein levels in young healthy females in reproductive age. Int J Cardiol. 2006;111:42–48. doi: 10.1016/j.ijcard.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 59.Sanders SA, Graham CA, Bass JL, Bancroft J. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception. 2001;64:51–58. doi: 10.1016/s0010-7824(01)00218-9. [DOI] [PubMed] [Google Scholar]
- 60.Blumenthal J, Babyak M, Moore K, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 61.Tschakovsky M, Joyner M. Nitric oxide and muscle blood flow in exercise. Appl Physiol Nutr Me. 2008;33:151–154. doi: 10.1139/H07-148. [DOI] [PubMed] [Google Scholar]