Abstract
Aims
Microdomain signalling mechanisms underlie key aspects of artery function and the modulation of intracellular calcium, with transient receptor potential (TRP) channels playing an integral role. This study determines the distribution and role of TRP canonical type 3 (C3) channels in the control of endothelium-derived hyperpolarization (EDH)-mediated vasodilator tone in rat mesenteric artery.
Methods and results
TRPC3 antibody specificity was verified using rat tissue, human embryonic kidney (HEK)-293 cells stably transfected with mouse TRPC3 cDNA, and TRPC3 knock-out (KO) mouse tissue using western blotting and confocal and ultrastructural immunohistochemistry. TRPC3-Pyr3 (ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate) specificity was verified using patch clamp of mouse mesenteric artery endothelial and TRPC3-transfected HEK cells, and TRPC3 KO and wild-type mouse aortic endothelial cell calcium imaging and mesenteric artery pressure myography. TRPC3 distribution, expression, and role in EDH-mediated function were examined in rat mesenteric artery using immunohistochemistry and western blotting, and pressure myography and endothelial cell membrane potential recordings. In rat mesenteric artery, TRPC3 was diffusely distributed in the endothelium, with approximately five-fold higher expression at potential myoendothelial microdomain contact sites, and immunoelectron microscopy confirmed TRPC3 at these sites. Western blotting and endothelial damage confirmed primary endothelial TRPC3 expression. In rat mesenteric artery endothelial cells, Pyr3 inhibited hyperpolarization generation, and with individual SKCa (apamin) or IKCa (TRAM-34) block, Pyr3 abolished the residual respective IKCa- and SKCa-dependent EDH-mediated vasodilation.
Conclusion
The spatial localization of TRPC3 and associated channels, receptors, and calcium stores are integral for myoendothelial microdomain function. TRPC3 facilitates endothelial SKCa and IKCa activation, as key components of EDH-mediated vasodilator activity and for regulating mesenteric artery tone.
Keywords: Endothelium, Calcium channel, Potassium channel, Signalling microdomain, Smooth muscle, Vasodilation
1. Introduction
The pathways that underlie endothelium-dependent vasodilation due to endothelium-derived hyperpolarization (EDH) are a topic of ongoing debate.1,2 The specific mechanisms underlying EDH vary within and between vascular beds and in disease, where alterations represent potential therapeutic targets for correction.3 In rat mesenteric artery, mechanisms intimately linked with EDH occur at specialized myoendothelial microdomain contact sites,4–7 where localized gap junction connexins (Cxs)37 and 40, intermediate-conductance calcium-activated potassium channels (IKCa), and endothelial endoplasmic reticulum (ER) inositol 1,4,5-trisphosphate receptors (IP3R) are present.5,7,8 Calcium influx across the membrane and Ca2+ release from stores underlie EDH generation.9 While EDH-related IP3R-mediated calcium release9,10 is the likely source of calcium for endothelial IKCa activation,7 the source of calcium underlying small conductance calcium-activated potassium channels (SKCa)-related EDH activity is less well characterized, but is also likely to involve an IP3R-dependent mechanism, perhaps via a pathway distinct to that associated with IKCa.
From a functional perspective, myoendothelial gap junction Cxs permits the direct transfer of an EDH current to the smooth muscle.3,7,11 In addition, in rat mesenteric artery, IKCa-dependent K+ release into the highly localized (approximately <30 nm) myoendothelial space between endothelial and smooth muscle cells facilitates diffusion-mediated signalling,5–7 and separately or synergistically, activation of low-level endothelial cell membrane SKca and IKCa8 may also contribute to EDH via their release of diffusible K+ as a ‘cloud’ across the ∼1 μm wide internal elastic lamina (IEL) ‘space’.12 Collectively, in rat mesenteric arteries, the SKCa and IKCa-dependent K+ release activates adjacent smooth muscle Na-K-ATPase,6,13 with the K+ outflow also interacting with closely associated inward rectifying potassium channels (Kir) on endothelial projections,6,13 with some activity also potentially occurring at more diffusely distributed Kir on the endothelial cell membrane. The net result of the myoendothelial gap junction and K+-mediated signalling is hyperpolarization of the adjacent smooth muscle, closure of smooth muscle voltage-dependent calcium channels, inhibition of phospholipase C,14 and vessel relaxation. In theory, there may be three types of myoendothelial signalling microdomains in rat mesenteric arteries: (i) those that facilitate current transfer (as myoendothelial gap junction/Cx-based sites only); (ii) K+-mediated signalling alone (as IKCa-IP3R and/or SKCa-based sites); or (iii) a combination of these two mechanisms. Regardless, a regenerative mechanism of refilling of the localized ER calcium store is necessary at specialized myoendothelial microdomain sites to maintain the local functional mechanisms involved in EDH generation.
Transient receptor potential (TRP) channels are a family of generally non-selective cation channels that are activated and regulated by a wide variety of stimuli15–18 and play significant roles in cellular calcium homeostasis. A role for several TRP channel subtypes in vascular function has been suggested,15,17–19 and in vascular EDH-type function, these include TRP ankyrin type 1 (A1)20,21, TRP canonical type 3 (C3)22,23, TRP vanilloid type 3 (V3)21, and TRPV4.24–26 In rat cerebral artery, TRPA1 has been suggested to play a role in myoendothelial signalling,20 with a similar role being proposed for TRPC3 in co-cultured mouse aortic endothelial and smooth muscle cells.22 In rat cerebral artery, TRPV3 has also been suggested to facilitate endothelial cell calcium influx for EDH-type vasodilator activity.21 In a similar manner, calcium entry through endothelial TRPV4 channels has been reported to trigger nitric oxide (NO)-dependent relaxation in rat carotid artery, and NO- and EDH-type relaxation in rat gracilis muscle arterioles.25 A role for TRPV4 has also been suggested in EDH-type relaxation in rat cerebral artery,24 with a potential role for such channels in NO- and EDH-type relaxation in mouse mesenteric artery.26 However, no direct relationship with myoendothelial microdomain activity was demonstrated in the above endothelial TRPV3 and 4 studies.
The basis for investigating the potential role of TRPC3 in rat mesenteric artery comes from the suggestion that TRPC3 are present in close proximity to IP3R22 at myoendothelial contact sites in co-cultured mouse aortic endothelial and smooth muscle cells. An interactive association of TRPC3 and IP3Rs has also been described in isolated uterine artery endothelial cells,23 although whether this association occurred at myoendothelial microdomains in intact arteries was not clarified. Regardless, direct interaction of TRPC3 and IP3R would be predicted to facilitate calcium entry at microdomain sites.
The present study determines the distribution and role of TRPC3 in rat mesenteric arteries, with the hypothesis that such channels are present at microdomains to facilitate ER calcium influx that underlies IKCa and SKCa activation and endothelial cell hyperpolarization, as an essential component of EDH-mediated vasodilation.
2. Methods
2.1. Animals and tissue
Adult male Sprague Dawley rats were anaesthetized with sodium pentathol (100 mg/kg; ip), and ∼300 μm diameter first- to third-order mesenteric arteries dissected in Krebs' solution containing (in mM): 112 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4.7H2O, 0.7 KH2PO4, 10 HEPES, 11.6 glucose, 2.5 CaCl2.2H2O; pH 7.3. Male 8–10-week-old TRPC3 knock-out (KO) mice27 generated on a 129SvEv/C57BL/6J mixed background and age-matched littermate WT controls were anaesthetized with isofluorane and the aorta dissected in Hank's balanced salt solution for imaging studies. Rat liver, and mouse liver, aorta, and mesenteric arteries were also dissected from anaesthetized (sodium pentathol; 100 mg/kg; ip) animals for reagent characterization studies.
All procedures conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996), and were approved by the Animal Ethics Committees of the University of New South Wales (09/43B), Monash University (SOBSA/P/2007/100), and Baylor College of Medicine (AN-4366). Respiration rate and tactile responses were monitored to indicate adequacy of anaesthesia.
2.2. Reagent characterization
TRPC3 antibody specificity was determined by immunohistochemistry using stably transfected human embryonic kidney (HEK)-293 cells expressing mouse TRPC3 (see Supplementary material online, Figure S1A–C), and by confocal immunohistochemistry (see Supplementary material online, Figures S1D–I and S2A) and western blotting using extracts of tissues from a rat (see Supplementary material online, Figure S3).
The specificity of the putative TRPC3 blocker Pyr3 (ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate) was validated by pressure myography in the mesenteric artery from KO and WT mice, patch clamp using freshly isolated mouse mesenteric artery endothelial cells (see Supplementary material online, Figure S4) and transfected HEK cells expressing TRPC3 (see Supplementary material online, Figure S5), and by calcium imaging using aortic endothelial cells obtained from KO and WT mice (see Supplementary material online, Figure S2B).
Full methods, including patch clamp and calcium imaging used for reagent characterization, as well as statistics, drug, and reagent details are available as Supplementary material online. Primary antibodies and primer characteristics are detailed in Supplementary material online, Tables S1 and S2.
2.3. Rat mesenteric artery TRPC3
The characteristics of TRPC3 channels in adult male Sprague Dawley rat mesenteric artery were determined using confocal and ultrastructural immunohistochemistry, western blotting, and pressure myography and endothelial cell membrane potential recordings (Figures 1 and 2).
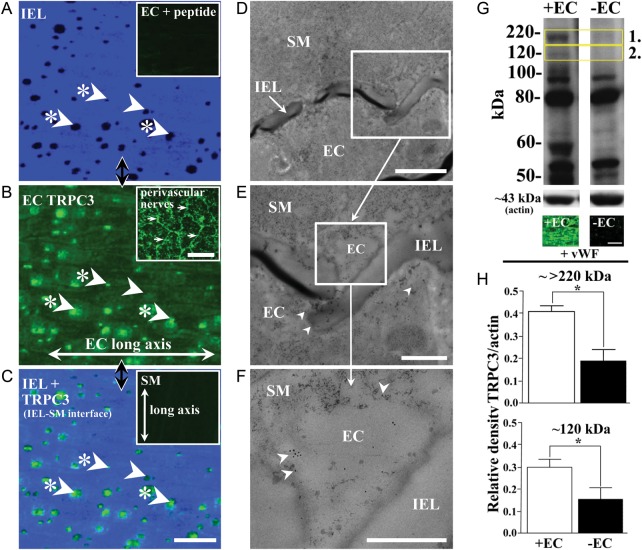
Figure 1.
TRPC3 localization and expression in rat mesenteric artery. Localized holes (dark spots; examples with arrows; A), are present in the internal elastic lamina (IEL; A) between the vascular endothelium and smooth muscle. TRPC3 presence in endothelial cells (EC; B and C) and apparent absence in smooth muscle cells (C, inset) was demonstrated using TRPC3 antibody batches AN-02, 03, and 07 (Supplementary material online, Table S1; AN-07, as example used here). Low-level diffuse TRPC3 is localized to the endothelial membrane (B), while overlay of IEL and TRPC3 label (C) shows strong TRPC3 expression at a proportion of IEL holes at the IEL-SM focal plane border (examples indicated with arrows with asterisks; see also Tables 1 and 2), as potential myoendothelial microdomain sites, noting that not all such sites have localized TRPC3 densities (example, arrow with no asterisk). Peptide block abolished staining (A, inset), with no labelling being present when incubated in the secondary antibody alone (data not shown). Perivascular nerve labelling (compare morphology with Milner et al.52) acts as a positive control (B, inset, examples indicated with arrows). The TRPC3 antibody (AN-07) conjugated to 10 nm colloidal gold (D–F) confirms TRPC3 localization to myoendothelial contact regions (F, examples arrowed), as potential myoendothelial microdomain signalling sites, as well as at other sites within the endothelium (B, examples indicated with arrows). Vessel region in (A–C) correspond. Longitudinal vessel axis (A–C), left to right, n= 6 and 3, each from a different animal (A–C and D–F, respectively). The characteristics of rat mesenteric artery TRPC3 and its primary endothelial expression were examined using western blotting and the TRPC3 antibody (ACC-016; AN-07 used here; Supplementary material online, Table S1). Monoglycosylated TRPC3 is present as a band at ∼120 kDa (G2, box; +EC; see also Goel et al.53), while the band at approximately >220 kDa (G1, box; see also Dietrich et al.54) probably represents an undissociated aggregate of the TRPC3 tetrameric channel complex. TRPC3 expression was normalized to actin (at ∼43 kDa; lower bands, G), with endothelial removal (−EC; G, upper right column) reducing TRPC3 expression at approximately 120 and >220 kDa by approximately two-fold each (H, upper and lower, respectively; *P< 0.05, significant; values being mean ± SEM). Two lanes per artery were run with tissue from three different rats, 12 in total, with 20 μg of protein per lane being loaded. von Willebrand factor (vWF) labelling verified endothelial removal in whole-mount preparations (G, lower panels). Scale bars, 25 μm (A–C and G), 50 μm (B, inset), 5 μm (D), 2 μm (E), 1 μm (F).
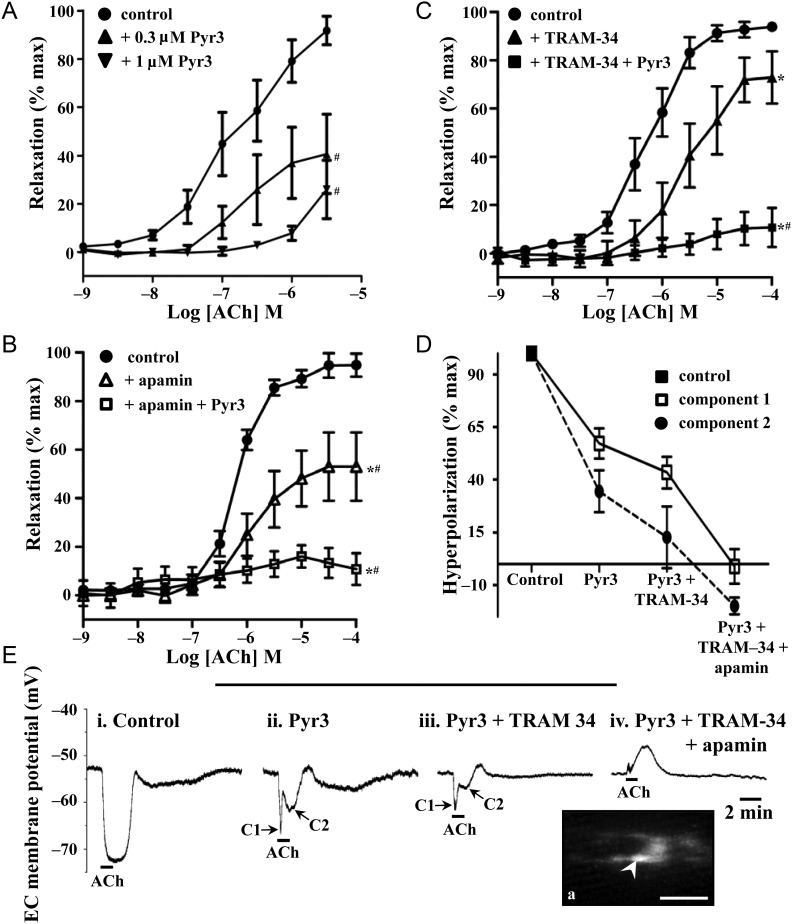
Figure 2.
TRPC3 in endothelium-dependent relaxation and endothelial cell hyperpolarization in rat mesenteric artery. In pressurized rat mesenteric arteries, relaxation to ACh (1 nM–100 μM) was examined in the presence and absence of Pyr3 (0.3 μM, A; 1 μM, A–C) with apamin (50 nM; B), or TRAM-34 (1 μM; C), or combined apamin and TRAM-34, to determine the relative contribution of TRPC3, SKCa, and IKCa. L-NAME (100 μM), ODQ (10 μM), and indomethacin (10 μM) were present in all experiments. n= 5–7 experiments, each from different animals; P< 0.05 indicates difference in *pEC50, or #Emax, relative to the ACh control (see Table 3 for drug characteristics). The endothelial cell hyperpolarization evoked by ACh [1 μM; 100%; n= 6; E(i)] in the presence of L-NAME (100 μM) and indomethacin (10 μM) was recorded in the presence of Pyr3 alone [1 μM; n= 6; E(ii)], Pyr3 and TRAM-34 [5 μM; n= 4; E(iii)], and Pyr3, TRAM-34, and apamin [100 nM, n= 4; D and E(iv)]. Example endothelial cell membrane potential recordings show hyperpolarization before and after the addition of Pyr3, TRAM-34, and apamin [E(ii–iv)]. Pyr3 (1 μM) reduced the hyperpolarization to ACh [D and E(ii–iv)], with the remaining hyperpolarization having a complex nature [D and E(ii–iv)], consisting of an initial rapid component [C1, with arrow in E(ii,iii)] followed by a second slower component [C2, with arrow in E(ii,iii)]. In the presence of all blockers, ACh evoked endothelial cell depolarization (E, far right) trace. Inset shows the example of dye-filled endothelial cells (Ea, inset, arrow) indicates cells from which recording was made. Scale bar, 50 μm, with the longitudinal vessel axis, left to right.
2.3.1. Histology
The distribution of TRPC3 was examined in rat mesenteric artery, and KO and WT mouse aorta and mesenteric artery, using conventional confocal immunohistochemistry as previously described.4,8 The distribution of the von Willebrand factor (vWF) was examined and arteries in which the endothelium had been disrupted compared with the intact artery, with methods as previously described.4,8,28
2.3.2. Western blotting
The specificity of the TRPC3 antibody and characteristics of TRPC3 channels in adult male Sprague Dawley rat mesenteric artery were determined using western blotting. Rat mesenteric arteries and liver and mouse liver were rapidly frozen, and stored in liquid nitrogen, with western blotting conducted with controls, as previously described.13,29 Of note, to verify potential endothelial or smooth muscle cell TRPC3 expression, samples were prepared for western blotting using rat mesenteric artery from which the endothelium had been disrupted.
2.3.3. TRPC3 and EDH-mediated vasodilation—pressure myography
The characteristics of TRPC3 channels in adult male Sprague Dawley rat and mouse mesenteric arteries were determined using pressure myography. Freshly dissected rat (internal diameter in 0 mM calcium, 287 ± 6 µm; n= 15) and mouse mesenteric arteries (internal diameter in 0 mM calcium, control, 175 ± 8 µm; n= 4; TRPC3 KO, 167 ± 8 µm; n= 6) were cannulated in a pressure myograph and continuously superfused with Krebs' solution (37°C) at a rate of 3 mL/min, bubbled with 5% CO2–95% N2. Arteries were pressurized to 80 mmHg with incremental increases over 80 min. Vessels were initially pre-constricted with superfused phenylephrine (PE) [1 μM; to 80% of maximum constriction, including with equal levels of pre-constriction in the presence of Pyr3, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34), and apamin], which was present in all experiments. Endothelium-dependent vasodilation in rat mesenteric arteries was evoked with increasing concentrations of acetylcholine (Ach) (1–30 μM) added cumulatively to the bath, while the response in mouse mesenteric artery was evoked using the PAR-2 agonist, SLIGRL, at 10 μM.30
Experiments were conducted in the presence of Nω-nitro-l-arginine methyl ester (L-NAME; 100 μM; NO synthase blocker), ODQ (1H-[1,2,4] oxadiazolo-[4,3-a]quinoxalin-1-one]; 10 μM; sGC blocker), and indomethacin (10 μM; cyclooxygenase blocker), as well as the putative TRPC3 blocker Pyr3 (1 μM for 20 min)31, apamin (15 min; 100 nM), and/or TRAM-34 (30 min; 1 μM), the latter two agents as defining blockers of EDH,1,12,32 prior to addition of ACh or SLIGRL. Additional experiments on rat mesenteric artery were also performed in the absence of L-NAME, ODQ, and indomethacin. Artery diameter changes are expressed as a percentage of the maximal dilation achieved by replacing the control Krebs' solution with calcium-free Krebs' solution at the end of the experiment.
2.3.4. TRPC3 and EDH—sharp electrode electrophysiology
The characteristics of TRPC3 channels in adult male Sprague Dawley rat mesenteric artery were determined using endothelial cell membrane potential recordings. Freshly dissected rat mesenteric arteries were cut along the longitudinal axis and pinned, endothelium uppermost, to the base of a recording chamber and continuously superfused as described previously.4 L-NAME (200 μM) and indomethacin (1 μM) were present in all experiments. The membrane potential of endothelial cells was recorded using intracellular glass microelectrodes containing 1 M KCl (100–140 MΩ resistance) and the tips were filled with Lucifer Yellow CH to allow unequivocal identification of every impaled cell.4 Endothelial cells were stimulated with 1 µM ACh for 1 min. Responses were recorded in the absence (control) and presence of Pyr3 (1 µM), TRAM-34 (5 µM), and apamin (100 nM). Responses recorded in the presence of blockers were expressed as a per cent of the control response in L-NAME and indomethacin.
3. Results
3.1. Reagent characterization
Specificity of the TRPC3 antibody was confirmed using confocal immunohistochemistry. TRPC3 was absent in both cell layers of TRPC3 KO mouse mesenteric artery (see Supplementary material online, Figure S1H and I). In WT mesenteric artery, TRPC3 was present in the endothelium, but there was an apparent absence in the smooth muscle (see Supplementary material online, Figure S1D–G). Under equivalent conditions, TRPC3 was detected in the endothelium and smooth muscle of WT aorta (see Supplementary material online, Figure S2A).
Additional specificity data for Pyr3 and the TRPC3 antibody are detailed in the Supplementary material online, Results and Discussion, and Supplementary material online, Figures S1–S5.
3.2. Mesenteric artery
3.2.1. TRPC3 protein localization and expression
The distribution of TRPC3 in rat mesenteric artery was determined using confocal immunohistochemistry of whole-mount tissue (Figure 1A–C), as well as high-resolution ultrastructural immunolocalization (Figure 1D–F). Semi-quantitative mean fluorescence density of TRPC3 over the endothelial cell membrane, exclusive of IEL hole sites, was 2558 ± 115, compared with 13 827 ± 788 arbitrary units for IEL holes sites exhibiting densities of TRPC3 labelling (Figure 1B). Thus, TRPC3 was present at a low level over the endothelial cell membrane at non-IEL hole sites, with a greater than five-fold higher fluorescence density at ∼73% of IEL hole sites (Figure 1A–D; Tables 1 and 2). The comparative frequency of TRPC3 at the IEL sites was approximately three-fold higher than that of myoendothelial gap junctions, which were present at ∼23% of IEL holes sites (Tables 1and 2). Confocal fluorescence density of TRPC3-labelled rat mesenteric artery smooth muscle was not significantly different from that of the negatively labelled TRPC3 KO mouse mesenteric artery smooth muscle (1187 ± 27, compared with 1225 ± 10 arbitrary units; P< 0.05; compare Figure 1C, inset, and Supplementary material online, Figure S1I, respectively). Ultrastructural immunohistochemistry confirmed TRPC3 expression at regions of close association of endothelial and smooth muscle cells of <30 nm (Figure 1D–F), as potential myoendothelial microdomain sites, as well as at the endothelial, but not the smooth muscle cell membrane and cytoplasm. TRPC3 was also expressed in the perivascular nerve plexus (Figure 1B, inset).
Table 1.
IEL hole, TRPC3, and myoendothelial gap junction (MEGJ) characteristics (per 104 μm2) in rat mesenteric artery
| IEL holes | IEL holes with TRPC3 plaques | IEL holes with no TRPC3 | Number of TRPC3 plaques | TRPC3 plaques not at IEL holes | MEGJ density |
|---|---|---|---|---|---|
| 71 ± 7 | 52 ± 6 | 19 ± 6 | 60 ± 7 | 8 ± 2 | 16.3 ± 1.8 |
For IEL and TRPC3 characteristics, n =4, each from a different rat; each n being the mean data from four different randomly selected 104 μm2 regions. For MEGJ characteristics, n= 3 series of 5 μm of serial sections, each from a different animal. Data are mean ± SEM.
Table 2.
Proportion of internal elastic lamina holes with localized channel densities in rat mesenteric artery
| TRPC3 | IKCaa | IP3Rb | MEGJs (EM) | Cx37a | Cx40a |
|---|---|---|---|---|---|
| ∼73% | ∼80% | ∼75% | ∼23% | ∼28% | ∼31% |
IKCa, intermediate-conductance calcium-activated potassium channel; IP3R, inositol 1,4,5-trisphosphate receptor. EM, from serial-section electron microscopy analysis. See also Supplementary material online, Figure S6.
aFrom Sandow et al.4
bFrom Sandow et al.7
Rat mesenteric artery TRPC3 protein expression was confirmed by western blotting, which recognized the monoglycosylated and complex forms of the TRPC3 protein as faint bands at approximately 120 and >220 kDa, respectively (Figure 1G and H; see Supplementary material online, Figure S3C and D), as well as a band at ∼60 kDa (Figure 1G; see Supplementary material online, Figure S3C and D), which is a likely breakdown product of the TRPC3 protein. Each of these bands was abolished by peptide block of the primary antibody. To verify potential endothelial or smooth muscle cell TRPC3 expression, western blots were run on endothelium-disrupted, compared with intact mesenteric arteries. The TRPC3 signal at approximately 120 and >220 kDa from intact vessels was approximately two-fold higher than from endothelium-disrupted vessels (Figure 1G and H), with the ∼60 kDa band being reduced in a similar manner (Figure 1G). Endothelium disruption of samples of the same vessel segments as used in the endothelium-disrupted western blot experiments was confirmed with vWF immunohistochemistry (Figure 1G, lower panels).
3.2.2. TRPC3 and EDH-mediated vasodilation—pressure myography
In rat mesenteric arteries, in the presence of L-NAME, ODQ, and indomethacin, ACh-induced vasodilation was significantly attenuated and blocked by respective 0.3 and 1 μM Pyr3 application (Figure 2A; Table 3). In the absence of L-NAME, ODQ, and indomethacin, maximum ACh-induced dilation was unaffected, although the sensitivity to ACh was increased (see Supplementary material online, Figure S6). Further, the absence of L-NAME, ODQ, and indomethacin had no effect on the maximum dilation in the presence of 1 μM Pyr3 (see Supplementary material online, Figure S6). Higher concentrations of Pyr3 (3 and 10 μM) were found to block PE-induced constriction (n= 3, each; data not shown), and thus 1 μM was used in subsequent experiments, for specific Pyr3 block. ACh-induced vasodilation was significantly attenuated by the SKCa blocker, apamin (100 nM), and abolished with the additional application of Pyr3 (1 μM; Figure 2B; Table 3) or the IKCa blocker, TRAM-34 (1 μM; Table 3). In a similar manner, ACh-induced vasodilation was significantly attenuated by TRAM-34, and abolished with the addition of Pyr3 (Figure 2C; Table 3). Thus, with individual SKCa or IKCa block, Pyr3 blocked the respective residual KCa-mediated components of EDH-mediated vasodilation.
Table 3.
Effect of drug interventions on endothelium-dependent vasodilation in rat mesenteric arteries
| pEC50 | Emax | n | |
|---|---|---|---|
| Vehicle | 6.4 ± 0.1 | 94.3 ± 2.1 | 14 |
| L-NAME (100 μM) + ODQ (10 μM) + indomethacin (10 μM) | 6.9 ± 0.2 | 86.1 ± 5.0 | 10 |
| L-NAME/ODQ + indomethacin + Pyr3 (0.3 M) | 6.8 ± 0.2 | 38.5 ± 16.6*,† | 6 |
| L-NAME/ODQ + indomethacin + Pyr3 (1 μM) | — | 26.0 ± 12.1*,† | 5 |
| L-NAME/ODQ + indomethacin + TRAM-34 (1 μM) | 5.4 ± 0.3*,† | 72.9 ± 10.8 | 7 |
| L-NAME/ODQ + indomethacin + TRAM-34 + Pyr3 (1 μM) | — | 10.7 ± 8.1*,†,‡ | 7 |
| L-NAME/ODQ + indomethacin + apamin (50 nM) | 5.6 ± 0.2*,† | 53.0 ± 11.8† | 7 |
| L-NAME/ODQ + indomethacin + apamin + TRAM-34 | — | 13.1 ± 5.6* | 3 |
| L-NAME/ODQ + indomethacin + apamin + Pyr3 (1 μM) | — | 10.8 ± 5.5*,†,§ | 7 |
| L-NAME/ODQ + indomethacin + apamin + TRAM-34 + Pyr3 | — | 1.3 ± 2.8* | 4 |
Data are mean ± SEM.
*P< 0.05, significant compared with vehicle.
†P< 0.05, significant compared with L-NAME + ODQ + indomethacin.
‡P< 0.05, significant compared with L-NAME + ODQ + indomethacin + TRAM-34.
§P< 0.05, significant compared with L-NAME + ODQ + indomethacin + apamin.
3.2.3. Sharp electrode electrophysiology—endothelial cell membrane potential
In the presence of L-NAME and indomethacin, ACh (1 μM) evoked a hyperpolarization of 23 ± 1 mV (n= 6) in rat mesenteric artery endothelial cells, with Pyr3 (1 μM) significantly reducing the hyperpolarization amplitude (Figure 2D and E). In the presence of Pyr3, the hyperpolarization had a complex nature, consisting of an initial rapid component of 13 ± 2 mV (n= 6, P< 0.05, compared with control; Figure 2D; C1 in Figure 2E) followed by a second slower component of 8 ± 3 mV (n= 6, P< 0.05, compared with control; Figure 2D; C2 in Figure 2E). Both components of this remaining hyperpolarization were abolished by the combination of TRAM-34 and apamin (Figure 2D and E).
4. Discussion
The present anatomical and functional data support a link between TRPC3 channels, and the KCa channel-mediated endothelial vasodilator function associated with the EDH mechanism in rat mesenteric arteries. TRPC3 probably facilitates calcium entry for close spatially associated IP3R-dependent ER calcium store refilling,7,9,10 and/or direct activation of SKCa and IKCa. Support for such a role of TRPC3 is illustrated in the present study by coincident TRPC3 and IKCa localization at a higher density at a proportion of IEL hole sites, as well as at the endothelial cell surface, where lower level SKCa and IKCa occur.6,8,13 Similar spatial localization of ER-IP3R occurs near the cell membrane in the subplasmalemmal compartment,33 with higher level localization at IEL hole sites7,34 (summarized in Figure 3; see also Supplementary material online, Figure S7). Further, functional Pyr3-mediated TRPC3 block selectively abolishes the remaining respective SKCa- and IKCa-mediated EDH activity. Collectively, these data support the presence of a functional signalling microdomain which is critical for EDH activity in rat mesenteric arteries.
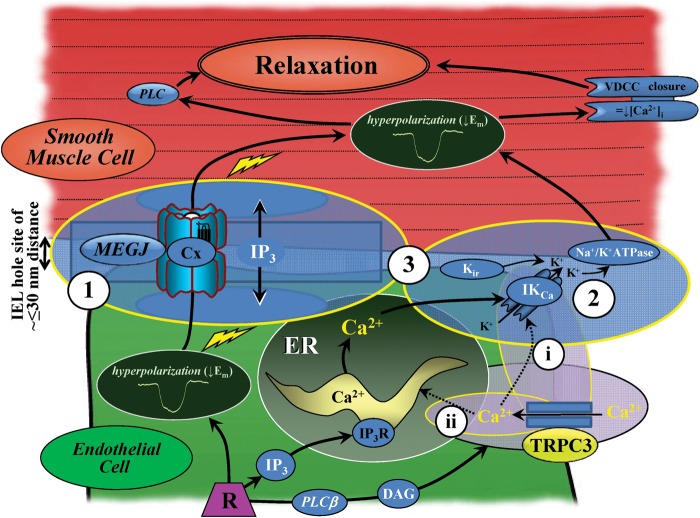
Figure 3.
The vasodilator signalling mechanism at myoendothelial contact sites in rat mesenteric artery. At sites of close contact between the endothelium and smooth muscle, localized gap junction connexins (Cx), endoplasmic reticulum (ER) 1,4,5-triphosphate receptors (IP3R), and intermediate-conductance calcium-activated potassium channels (IKCa) occur in close proximity to TRPC3. The localization and differential distribution of these channels and receptors suggests that these myoendothelial microdomains enable transfer of a connexin (Cx)-dependent endothelial hyperpolarizing current (1) and/or localized K+ activity (2 and 3), with the net effect being smooth muscle hyperpolarization and endothelium-dependent relaxation (modified from Sandow et al.;7,34 see also Figure 1 and Supplementary material online, FigureS7). TRPC3-dependent calcium influx may activate myoendothelial KCa directly (i), and/or refill the IP3R-mediated ER store (ii). Inward rectifying potassium channels (Kir) are exclusive to the endothelium6,13,55 and are activated by potassium in a feedback with KCa and Na+/K+-ATPase activity. DAG, diacylglycerol; Em, membrane potential; MEGJ, myoendothelial gap junction; PLCβ, phospholipase C-beta; VDCC, voltage-dependent calcium channel.
A functional and spatial association between TRPC3, IP3R, and KCa exists in a variety of cell types. For example, a functional interaction of IP3R and TRPC3 has been proposed in HEK-293 cells,35 cultured bovine pulmonary artery endothelial cells,36 and passage 3–4 bovine uterine artery endothelial cells,23 the latter being suggested to relate to NO-mediated activity.37 Further demonstration of such a relationship has also been shown in rat cerebral artery, and in isolated smooth muscle cells of these arteries,38 as well as in isolated rabbit coronary artery smooth muscle cells.39 Similar reports of a functional interaction of IP3R and large-conductance KCa have also been suggested in isolated basilar artery smooth muscle cells,40 and in cultured murine pancreatic β-cells,41 while IP3R and IKCa have been reported to functionally interact in isolated guinea pig gastric smooth muscle cells.42 Thus, the precedent for functional interaction of TRPC3, IP3R, and KCa exists, supporting the potential spatial-related functional activity of these channels and receptors in myoendothelial signalling in rat mesenteric arteries.
The present data show that in the mesenteric artery of adult male rats, TRPC3 is expressed in endothelial cells. Indeed, given that the endothelium constitutes a relatively small mass of the vessel wall compared with the smooth muscle (approximately <10%; see Figure 2 in Sandow and Hill43 and Figure 1A in Haddock et al.13 for examples), that TRPC3 expression is reduced by >50% with endothelial removal, and that TRPC3 is expressed in the perivascular nerve plexus (Figure 1B, inset), any smooth muscle TRPC3 expression in the intact rat mesenteric artery is negligible.
In rat mesenteric artery, 0.3–1 μM Pyr3 significantly attenuates EDH-mediated vasodilation, with this activity being verified in the mesenteric artery from KO and WT mice. At these concentrations, the actions of Pyr3 were selective for EDH-mediated vasodilation (see Supplementary material online, Figure S6) and did not influence the level of underlying vasoconstriction, with patch-clamp data supporting a lack of direct Pyr3 effect on SKCa or IKCa (see Supplementary material online, Figure S4). Furthermore, when either SKCa or IKCa were blocked, subsequent exposure to Pyr3 blocked the residual EDH-mediated vasodilator activity. This indicates that TRPC3 channels are integral to the function of SKCa and IKCa, and thus underpin key aspects of EDH activity in rat mesenteric artery. Consistent with this, Pyr3 inhibited the generation of hyperpolarization in mesenteric endothelial cells, which is the initial critical step for EDH activity. The transformation of endothelial cell hyperpolarization into a complex multi-component response in the presence of Pyr3 is reminiscent of changes seen in EDH-mediated smooth muscle responses following inhibition of the different sources of Ca2+ in rat mesenteric arteries.9 Thus, the actions of Pyr3 may reflect inhibition of Ca2+ influx pathways necessary for ER Ca2+ store refilling and for direct activation of SKCa and IKCa in rat mesenteric arteries (Figure 3). Notably, EDH actions extend from transient to more tonic effects on blood flow regulation in vivo,44–46 and the relative contribution of different EDH mechanisms to vasodilation can vary between isometric vs. isobaric conditions and in vivo.47 The role of TRPC3 in the generation of EDH under in vivo conditions will need to be elucidated in future studies.
The present data lend further support to the concept that there is heterogeneity in EDH-related myoendothelial signalling mechanisms in rat mesenteric arteries (Figure 3; see also4–10,13,34,48,49). Based on present and previously published data from this vessel on the function and distribution of TRPC3, SKCa, IKCa, IP3R, and myoendothelial gap junctions, three potential signalling pathways for EDH-related activity at myoendothelial contact sites exist in the normal rat mesenteric artery: (i) TRPC3–IKCa–IP3R functional microdomains at ∼70–80% of IEL hole sites, of which ∼25–30% are also associated with myoendothelial gap junction sites (Table 2), these latter sites also facilitating (ii) Cx-mediated transfer of current, with mechanism (iii) a combination of (i) and (ii). Indeed, these mechanisms may operate independently or in synergy, as suggested for similar mechanisms in the human mesenteric50 and saphenous artery of the obese rat.29
This study characterized the selectivity and potency of a putative TRPC3 blocker, Pyr3,31 in rat mesenteric artery, with additional characterization of the agent being carried out in transfected HEK cells expressing murine TRPC3 and in WT and KO mouse tissues. Pyr3 was found to attenuate the carbachol (CCh)-induced inward current in transfected HEK cells expressing TRPC3 (see Supplementary material online, Figure S5), as well as the CCh-induced calcium influx component in WT, but not KO-derived aortic endothelial cells (see Supplementary material online, Figure S2B), as well the ATP-induced K+ current in mouse mesenteric artery endothelial cells (see Supplementary material online, Figure S4). Interestingly, the Pyr3 response in the WT cell line does not completely mimic that in the KO cell line (see Supplementary material online, Figure S2B). The slight attenuation of the response in KO cells was limited to the early phase of the calcium response, as typically associated with release from internal stores,51 and suggests a degree of potential non-specific action at higher (10 μM) Pyr3 concentrations. Indeed, the concentration used in the present calcium imaging work is approximately three-fold higher than the 3 μM full Pyr3 block previously reported using HEK cells transfected with murine TRPC3.31
The smooth muscle layer of the rat mesenteric artery is the site of action for the α-adrenergic agonist and vasoconstrictor, PE. The absence of TRPC3 at this site (see present data and Hill et al.48) and the block of PE-induced constriction by 3 and 10 μM Pyr3 observed in this study suggests that Pyr3 may possess a non-specific action at these concentrations in the smooth muscle layer of intact vessels, consistent with the above potential early-phase non-specific Pyr3 action on calcium in isolated endothelial cells. Together, these findings suggest that Pyr3 is an effective inhibitor of TRPC3 channels, but at higher concentrations may have concentration-dependent non-selective effects possibly related to inhibition of calcium release from internal stores.
5. Concluding remarks
TPRC3 channel localization and function in rat mesenteric artery are consistent with their role at myoendothelial signalling sites associated with endothelial cell calcium dynamics and KCa-dependent EDH. In this role, TRPC3 channels probably promote activation of endothelial SKCa and IKCa through providing calcium directly to the channels and/or facilitating refilling of the IP3R-mediated ER calcium stores (Figure 3; see also4–10,13,34,48,49). Thus, TRPC3 have a fundamental role in EDH-mediated vasodilation and thus in the regulation of vascular tone in rat mesenteric artery.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from the National Health and Medical Research Council of Australia (ID401112 and 455243 to S.L.S., 510202 to P.B., and 546087 to M.T. and S.L.S.), the Australian Research Council (DP1097202 to G.D.H.), and the National Institutes of Health (project Z01-ES-101684 to L.B. and R01 HL088435 to S.P.M.).
Conflict of interest: none declared.
Supplementary Material
References
- 1.Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Kohler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int. 2007;72:145–150. doi: 10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- 3.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of EDHF. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 5.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks EDHF dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 6.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during EDHF signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandow SL, Haddock RE, Hill CE, Chadha PE, Kerr PM, Welsh DG, et al. What's where and why at a vascular myoendothelial microdomain signaling complex? Clin Exp Pharmacol Physiol. 2009;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Sources of Ca2+ in relation to generation of acetylcholine-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br J Pharmacol. 1997;120:1328–1334. doi: 10.1038/sj.bjp.0701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao YX, Zheng JP, He JY, Li J, Xu CB, Edvinsson L. Induces vasodilatation of rat mesenteric artery in vitro mainly by inhibiting receptor-mediated Ca2+-influx and Ca2+-release. Arch Pharmacol Res. 2005;28:709–715. doi: 10.1007/BF02969362. [DOI] [PubMed] [Google Scholar]
- 11.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 12.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 13.Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PloS One. 2011;6:e16423. doi: 10.1371/journal.pone.0016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh T, Seki N, Suzuki S, Ito S, Kajikuri J, Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of IP3 in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol. 2008;35:1116–1120. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vennekens R. Emerging concepts for the role of TRP channels in the cardiovascular system. J Physiol. 2011;589:1527–1534. doi: 10.1113/jphysiol.2010.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci. 2010;119:19–36. doi: 10.1042/CS20090641. [DOI] [PubMed] [Google Scholar]
- 19.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 20.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of TRPV3 elicits endothelium-dependent vasodilation. Mol Pharmacol. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isakson BE, Duling BR. Organization of IP3-R1 and TRPC3 at the myoendothelial junction may influence polarized calcium signaling. Exp Biol (Late Breaking Abstracts) 2006;786:783. [Google Scholar]
- 23.Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced store-operated Ca2+ channel function in uterine artery endothelial cells is associated with enhanced agonist-specific transient receptor potential channel 3-IP3R2 interaction. J Endocrinol. 2006;190:385–395. doi: 10.1677/joe.1.06773. [DOI] [PubMed] [Google Scholar]
- 24.Marrelli SP, O'Neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 25.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, et al. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, et al. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, et al. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins37 and 40. Am J Physiol. 2006;291:H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- 29.Chadha PS, Haddock RE, Howitt L, Morris MJ, Murphy TV, Grayson TH, et al. Obesity upregulates IKCa and myoendothelial gap junctions to maintain endothelial vasodilator function. J Pharmacol Exp Ther. 2010;335:284–293. doi: 10.1124/jpet.110.167593. [DOI] [PubMed] [Google Scholar]
- 30.Beleznai T, Takano H, Hamill C, Yarova P, Douglas G, Channon K, et al. Enhanced K+-channel-mediated endothelium-dependent local and conducted dilation of small mesenteric arteries from ApoE-/- mice. Cardiovasc Res. 2011;92:199–208. doi: 10.1093/cvr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci USA. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire JJ, Ding H, Triggle CR. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s) Can J Physiol Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- 33.Isshiki M, Mutoh A, Fujita T. Subcortical Ca2+ waves sneaking under the plasma membrane in endothelial cells. Circ Res. 2004;95:e11–e21. doi: 10.1161/01.RES.0000138447.81133.98. [DOI] [PubMed] [Google Scholar]
- 34.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions and microdomains: anatomical links to function? Microcirculation. 2012;19:403–415. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 35.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, et al. Modulation of Ca2+ entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamouchi M, Philipp S, Flockerzi V, Wissenbach U, Mamin A, Raeymaekers L, et al. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol. 1999;518:345–358. doi: 10.1111/j.1469-7793.1999.0345p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, et al. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod. 2010;82:66–75. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adebiyi A, Zhao G, Narayanan D, Thomas CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3R in smooth muscle cells regulates arterial contractility. Circ Res. 2010;106:1603–1612. doi: 10.1161/CIRCRESAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peppiatt-Wildman CM, Albert AP, Saleh SN, Large WA. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol. 2007;580:755–764. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CJ, Weir BK, Macdonald RL, Zhang H. Erythrocyte lysate releases Ca2+ from IP3-sensitive stores and activates Ca2+-dependent K+ channels in rat basilar smooth muscle cells. Neurol Res. 1998;20:23–30. doi: 10.1080/01616412.1998.11740480. [DOI] [PubMed] [Google Scholar]
- 41.Ammala C, Larsson O, Berggren PO, Bokvist K, Juntti-Berggren L, Kindmark H, et al. Inositol trisphosphate-dependent periodic activation of a Ca2+-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991;353:849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- 42.Yang M, Li XL, Xu HY, Sun JB, Mei B, Zheng HF, et al. Role of arachidonic acid in hyposmotic membrane stretch-induced increase in calcium-activated potassium currents in gastric myocytes. Acta Pharmacol Sin. 2005;26:1233–1242. doi: 10.1111/j.1745-7254.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 43.Sandow SL, Hill CE. The incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in EDHF-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 44.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 45.Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired EDHF-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 46.Milkau M, Kohler R, de Wit C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J. 2010;24:3572–3579. doi: 10.1096/fj.10-158956. [DOI] [PubMed] [Google Scholar]
- 47.Boettcher M, de Wit C. Distinct endothelium-derived hyperpolarizing factors emerge in vitro and in vivo and are mediated in part via connexin 40-dependent myoendothelial coupling. Hypertension. 2011;57:802–808. doi: 10.1161/HYPERTENSIONAHA.110.165894. [DOI] [PubMed] [Google Scholar]
- 48.Hill AJ, Hinton JM, Cheng H, Gao Z, Bates DO, Hancox JC, et al. A TRPC-like non-selective cation current activated by α1-adrenoceptors in rat mesenteric artery smooth muscle cells. Cell Calcium. 2006;40:29–40. doi: 10.1016/j.ceca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Sandow SL, Gzik DJ, Lee RMKW. Arterial internal elastic lamina holes: relationship to function? J Anat. 2009;214:258–266. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chadha PS, Lu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, et al. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions, IKCa and NO. J Pharmacol Exp Ther. 2011;336:701–708. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- 51.Adams DJ, Barakeh J, Laskey R, Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- 52.Milner P, Belai A, Tomlinson A, Hoyle CH, Sarner S, Burnstock G. Effects of long-term laxative treatment on neuropeptides in rat mesenteric vessels and caecum. J Pharm Pharmacol. 1992;44:777–779. doi: 10.1111/j.2042-7158.1992.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 53.Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- 54.Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem. 2003;278:47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- 55.Hill CE. Long distance conduction of vasodilation: A passive or regenerative process? Microcirculation. 2012;19:379–390. doi: 10.1111/j.1549-8719.2012.00169.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.