Abstract
Aims
Innate mechanisms of inter-organ protection underlie the phenomenon of remote ischaemic preconditioning (RPc) in which episode(s) of ischaemia and reperfusion in tissues remote from the heart reduce myocardial ischaemia/reperfusion injury. The uncertainty surrounding the mechanism(s) underlying RPc centres on whether humoral factor(s) produced during ischaemia/reperfusion of remote tissue and released into the systemic circulation mediate RPc, or whether a neural signal is required. While these two hypotheses may not be incompatible, one approach to clarify the potential role of a neural pathway requires targeted disruption or activation of discrete central nervous substrate(s).
Methods and results
Using a rat model of myocardial ischaemia/reperfusion injury in combination with viral gene transfer, pharmaco-, and optogenetics, we tested the hypothesis that RPc cardioprotection depends on the activity of vagal pre-ganglionic neurones and consequently an intact parasympathetic drive. For cell-specific silencing or activation, neurones of the brainstem dorsal motor nucleus of the vagus nerve (DVMN) were targeted using viral vectors to express a Drosophila allatostatin receptor (AlstR) or light-sensitive fast channelrhodopsin variant (ChIEF), respectively. RPc cardioprotection, elicited by ischaemia/reperfusion of the limbs, was abolished when DVMN neurones transduced to express AlstR were silenced by selective ligand allatostatin or in conditions of systemic muscarinic receptor blockade with atropine. In the absence of remote ischaemia/reperfusion, optogenetic activation of DVMN neurones transduced to express ChIEF reduced infarct size, mimicking the effect of RPc.
Conclusion
These data indicate a crucial dependence of RPc cardioprotection against ischaemia/reperfusion injury upon the activity of a distinct population of vagal pre-ganglionic neurones.
Keywords: Brain, Ischaemia/reperfusion injury, Preconditioning, Vagus nerve
1. Introduction
Our body is capable of recruiting powerful innate mechanisms that are highly effective in protecting tissues and organ function against ischaemia/reperfusion injury. A landmark study by Murry et al.1 demonstrated that the exposure of the myocardium to short non-lethal ischaemia-reperfusion episodes renders it more tolerant to subsequent severe, potentially lethal, periods of ischaemia, i.e. the heart becomes ‘preconditioned’. A similar level of protection can be achieved by remote ischaemic preconditioning (RPc)—a phenomenon in which episode(s) of ischaemia and reperfusion in tissues remote from the heart protect the myocardium against ischaemia/reperfusion injury. Although, promising results of recent trials in patients with acute myocardial infarction2 may facilitate the introduction of RPc procedure(s) into clinical practice, the mechanisms underlying RPc cardioprotection remain unclear.
The importance of the autonomic nervous system in mediating myocardial protection against ischaemia/reperfusion injury has been suggested by the demonstration that RPc is abolished under ganglionic blockade following systemic administration of hexamethonium,3 which blocks transmission in both sympathetic and parasympathetic ganglia. Vagus nerve stimulation reduces myocardial injury,4–6 while RPc cardioprotection was found to be abolished in conditions of bilateral vagotomy.7,8 These data suggest that an intact parasympathetic activity is important for RPc cardioprotection. However, the vagus is a mixed nerve containing both sensory and motor fibres. Therefore, to avoid confounding factors associated with complete surgical vagotomy or electrical stimulation of the whole nerve, selective vagal ‘de-efferentation’ or selective recruitment of vagal motor outflow require cell-specific targeting of vagal pre-ganglionic neurones. In this study, we used pharmaco- and optogenetic approaches to test the hypothesis that the functional integrity of the central nervous parasympathetic circuitry is required for myocardial protection established by ischaemia/reperfusion stimulus applied to a remote tissue.
2. Methods
All the experiments were performed in accordance with the European Commission Directive 86/609/EEC (European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes) and the UK Home Office (Scientific Procedures) Act (1986) with project approval from the Institutional Animal Care and Use Committee.
2.1. Animal preparation
Adult male Sprague-Dawley rats (280–340 g) were anaesthetised with pentobarbitone sodium (induction 60 mg kg−1 ip; maintenance 10–15 mg kg−1h−1 iv). Adequate anaesthesia was ensured by monitoring heart rate, arterial blood pressure, and the absence of a withdrawal response to a paw pinch. Animals were placed supine and a right carotid artery and left jugular vein cannulated for the measurement of arterial blood pressure and the administration of anaesthetic, respectively. The trachea was cannulated and animals were ventilated with room air using a positive pressure ventilator (Harvard Rodent Ventilator). A tidal volume of ∼8–10 mL kg−1 and a ventilator frequency similar to the normal respiratory frequency (∼60 strokes min−1) were used. Partial pressures of O2 and CO2 as well as pH of the arterial blood were measured every hour. The rate and volume of mechanical ventilation were adjusted to maintain blood gases within the physiological range. Standard lead II ECG was recorded throughout the experiment. Body temperature was maintained with a servo-controlled heating pad at 37.0 ± 0.2°C.
2.2. Induction of RPc
To establish RPc cardioprotection, the protocol described by Shahid et al.9 was used. Blood supply to the limbs was interrupted for 15 min by placing vessel clamps on both femoral arteries at the proximal level ∼1 cm below the inguinal ligament. The Sham-RPc procedure involved dissection of both femoral arteries without occlusion.
2.3. Myocardial ischaemia/reperfusion
The heart was exposed via a left thoracotomy. A 5-0 monofilament polypropylene suture was passed around the left anterior descending (LAD) coronary artery to induce a temporary occlusion. The animals were subjected to 30 min of LAD coronary artery ligation, followed by 120 min of reperfusion. Successful coronary artery occlusion was confirmed by elevation of the ST-segment in the ECG and an immediate 15–30 mmHg fall in arterial blood pressure.
2.4. Measurements of infarct size
At the end of the reperfusion period, the LAD artery was re-occluded and 1 mL of 1.5% Evans blue dye was injected into the jugular vein for the assessment of the area at risk. The animal was then given an anaesthetic overdose (pentobarbital 250 mg kg−1 iv), the heart was excised, left ventricle was isolated, frozen, and sectioned into five to six transverse slices from the apex to the base. The slices were weighed and photographed. The area at risk was demarcated by the absence of Evans blue staining. The slices were then incubated with 1% 2,3,5-triphenyltetrasodium chloride (TTC) in Tris buffer (pH 7.4) for 15 min at 37°C, fixed in 4% formalin for 24 h, and photographed again. Viable myocardium is stained red by TTC, whereas necrotic myocardium appears pale yellow. The area at risk and the necrotic area were determined by computerized planimetry, normalized to the weight of each slice, with degree of necrosis (i.e. infarct size) expressed as the percentage of area at risk.
2.5. Targeting vagal pre-ganglionic neurones with viral vectors
Cholinergic vagal pre-ganglionic neurones of the dorsal motor nucleus of the vagus nerve (DVMN) express the transcriptional factor, Phox2, and were targeted using an artificial Phox2-activated promoter—PRSx810—incorporated into lenti- and adenoviral vectors (AVs).11 Expression driven by PRSx8 is dependent on the activity of Phox2 and, therefore, expression in DVMN neurones is not surprising, given the putative role of Phox2 in the development of these neurones.12
The lentiviral construct used to express the Gi-protein-coupled Drosophila allatostatin receptor (AlstR) in DVMN neurones has been described previously.13,14 In brief, the plasmid pTYF-PRSx8-AlstR-IRES2-eGFP (enhanced green fluorescent protein) was used to generate the lentiviral vector (LV). The LV system used was HIV-1-derived and pseudotyped with the VSV-G envelope.15 Titres of PRSx8-AlstR-eGFP-LV and the control vector (PRSx8-eGFP-LV) were between 1 × 109 and 1 × 1010 transducing units mL−1. Viral concentration and titration were carried out as described in detail previously.15
To control the activity of DVMN neurones with high temporal resolution, we generated a new AV where a mutant of channelrhodopsin (ChR)—ChIEF—is fused with a red fluorescent protein—the tandem dimer Tomato (tdTomato) protein. The ChIEFtdTomato clone was kindly provided by Dr JY Lin (University of California). ChIEF is a chimeric ChR variant constructed from the N-terminal part of the ChR1 and the C-terminal part of the ChR2 and also incorporates an isoleucine 170 to valine mutation. ChIEF combines the reduced inactivation characteristics of ChR1 with more favourable cation permeability properties conferred by ChR2.16 The I170V mutation further improves the channel closure kinetics. Therefore, ChIEF allows a greater temporal control of neuronal activation by light pulses. Its spectral properties are similar to those of ChR2 but it shows more efficient membrane expression and trafficking in the mammalian cells.16
In this study, ChIEFtdTomato was engineered into an AV under the control of an enhanced17,18 PRSx8 promoter.10,11 AVs were produced as described previously.19,20
2.6. Viral gene transfer
Rats were anaesthetized [ketamine (60 mg kg−1; im) and medetomidine (250 μg kg−1, im)] and placed in a stereotaxic frame. Adequate surgical anaesthesia was ensured by the absence of a withdrawal response to a paw pinch. DVMN neurones were targeted with two microinjections per side (0.25 μL each, 0.05 μL min−1) of a viral suspension containing PRSx8-AlstR-eGFP-LV, PRSx8-eGFP-LV, PRSx8-ChIEFtdTomato-AV, or PRSx8-DsRed-AV (control for optogenetic experiments; this vector drives the expression of DsRed in the DVMN neurones). The PRSx8 promoter is also active in a population of noradrenergic neurones (A2 cell group) of the nucleus of the solitary tract located dorsal to the DVMN.21 To avoid transduction of A2 neurones, injections were placed immediately ventral to the DVMN using the following coordinates from calamus scriptorius (i) 0.5 mm rostral, 0.6 mm lateral, 0.8 mm ventral and (ii) 1.0 mm rostral, 0.8 mm lateral, 0.6 mm ventral. In our preliminary validation experiments conducted in six animals, along with widespread expression of the transgene in the DVMN, we only found occasional (2–5 per brainstem) neurones expressing ChIEFtdTomato and stained positive for the noradrenergic marker DBH. Similarly, in contrast to strong AlstR expression in almost the entire population of DVMN neurones, the majority of dorsally located noradrenergic neurones were not transduced (Figure 1A and B). Thus, precise stereotaxic targeting of the viral vector ensured specific transduction of DVMN neurones. Anaesthesia was reversed with atipemazole (1 mg kg−1) and no complications were observed after the surgery.
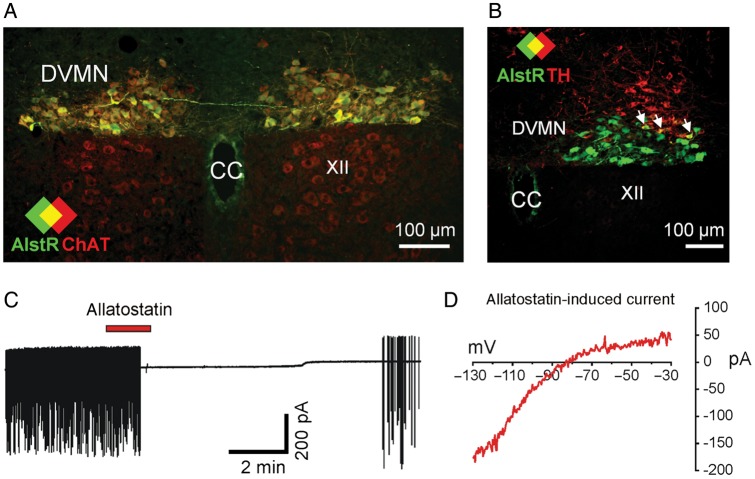
Figure 1.
Genetic targeting and silencing of vagal pre-ganglionic neurones in the dorsal motor nucleus of the vagus nerve (DVMN). (A) Confocal image of the coronal section of the rat brainstem targeted to express allatostatin receptor (AlstR) in the DVMN. Figure illustrates a representative example of the distribution of choline acetyltransferase (ChAT)-positive (i.e. cholinergic) (red) DVMN neurones transduced to express AlstR/eGFP (green). Colocalization appears yellow. Bregma level −14 mm. XII, hypoglossal motor nucleus (ChAT-positive, but not expressing eGFP). CC, central canal; (B) a representative example of the distribution of AlstR/eGFP-transduced DVMN neurones in relation to the location of A2 noradrenergic cells identified by tyrosine hydroxylase (TH) immunohistochemistry (red). Along with strong expression of AlstR/eGFP in the DVMN only occasional noradrenergic neurones were found to be transduced. Colocalization appears yellow (arrows). Bregma level −13.8 mm. (C) Representative cell-attached recording from an AlstR/eGFP-positive DVMN neurone illustrating its rapid and reversible silencing in response to allatostatin (0.5 µM); (D) current–voltage relationship (IV) of allatostatin-induced current. Transduced DVMN neurone was voltage-clamped to −30 mV and hyperpolarizing voltage ramps to −130 mV (700 ms duration) were applied before and during allatostatin application. The displayed IV was obtained by subtracting the whole-cell IV obtained under control conditions, from that obtained in the presence of allatostatin.
2.7. In vitro electrophysiology: verification of transgene functionality
Rapid inhibitory and readily reversible effects of allatostatin were previously reported to be selective for AlstR-expressing neurones.22 Binding of an allatostatin peptide to AlstR23 should lead to opening of inwardly rectifying K+ channels, neuronal hyperpolarization, and cessation of action potential firing.22,24 Naturally, AlstRs are only expressed in insects and allatostatin itself does not interfere with endogenous receptor complexes in mammals.22,23 Conversely, the activation of ChIEFtdTomato using blue light should lead to immediate neuronal depolarization and action potential firing. Visually guided patch-clamp recordings in the brainstem slice preparation were used to verify the efficacy of these approaches in silencing and activating DVMN neurones, respectively.
Seven to ten days after the delivery of PRSx8-AlstR-eGFP-LV or PRSx8-ChIEFtdTomato-AV into the DVMN, rats were sacrificed with halothane overdose and 200-µm coronal brainstem slices were cut and maintained as described previously.25 Patch pipettes (3–6 MΩ) were pulled from thin-walled borosilicate capillary glass (Clark Electromedical Instruments, Pangbourne, UK). Electrodes were filled with (in millimolar) 120 K-gluconate, 5 HEPES, 5 BAPTA, 1 NaCl, 1 MgCl2, 1 CaCl2, 2 K2ATP (pH 7.2). Recordings were carried out under an epifluorescence microscope (Zeiss Axioskop 2 FS, Zeiss, Germany) in artificial cerebrospinal fluid (aCSF, containing in millimolar; 118 NaCl, 3 KCl, 25 NaHCO3, 1 MgCl2, 2 CaCl2, and 10 glucose) saturated with 95% O2/5% CO2 (pH 7.4) at 28–32°C. The recording chamber (volume 2 mL) was perfused with aCSF at a rate of 4–5 mL min−1. Recordings were performed in cell attached configuration and whole-cell mode using an EPC-9 amplifier and Pulse/Pulsefit software (Heka Elektronik, Lambrecht, Germany). Currents or membrane potentials were filtered at 1 kHz and digitized at 3 kHz.
DVMN neurones expressing AlstR were identified by eGFP fluorescence. To test neuronal responses to activation of AlstR, allatostatin (0.5–1 µM; Ser-Arg-Pro-Tyr-Ser-Phe-Gly-Leu-NH2, Phoenix Pharmaceuticals, USA) was added to the perfusate. For recordings from the DVMN neurones expressing ChIEFtdTomato, cells were identified by red (tdTomato) fluorescence. A light fibre connected to a 445 nm laser (Omicon, Germany) was submerged into the recording chamber and pointed towards the slice. Duration and intensity of light pulses were controlled by laser software and triggered from the EPC-9 amplifier. Control recordings verified that neither allatostatin (n = 5) nor 445 nm light (n = 4) had an effect on electrical activity in untransduced DVMN neurones.
2.8. Immunohistochemistry: verification of transgene expression
At the end of the in vivo experiments, the rats transduced to express AlstR/eGFP or ChIEFtdTomato in the DVMN neurones were perfused through the ascending aorta with 0.9% saline solution followed by 4% phosphate-buffered (0.1 M, pH 7.4) paraformaldehyde. After 12 h of post-fixation and subsequent cryoprotection (30% sucrose), 30-μm-thick coronal sections were collected along the rostro-caudal extent of the medulla oblongata. Sections were processed for the immunohistochemical detection of choline acetyl-transferase (ChAT) or tyrosine hydroxylase (TH). Tissue was incubated in goat anti-ChAT (1:500, Chemicon) or sheep anti-TH (1:250, Abcam) followed by donkey anti-goat Alexa Fluor568 or biotinylated rabbit anti-sheep (1:500) amplified by AMCA avidin DCS (1:250, Vector laboratories). Identification of AlstR-expressing neurones was enhanced by eGFP immunostaining as described previously.13 All brainstem sections from this group, including those stained for ChAT and TH, were subsequently incubated in chicken anti-GFP antibody (1:250, Avés) for 48 h followed by goat anti-chicken Alexa Fluor488 (1:1000, Molecular Probes).
2.9. Experimental protocols
2.9.1. RPc in conditions of DVMN silencing
A small occipital craniotomy was performed and a miniature polyethylene catheter was slid around the caudal surface of the cerebellum and secured in place with dental impression material with the catheter tip placed in the cisterna magna. All rats transduced with either PRSx8-AlstR-eGFP-LV or PRSx8-eGFP-LV received slow continuous infusion of allatostatin (100 µM in aCSF; 4 µL h−1) starting 15 min prior to RPc or sham-RPc procedures and lasting till the end of the myocardial ischaemia/reperfusion period.
2.9.2. Optogenetic conditioning of the heart
Occipital craniotomy was performed and the cerebellum was partially removed to expose the dorsal part of the medulla oblongata overlaying dorsal vagal complex, including the DVMN. DVMN neurones were stimulated using blue light (445 nm, 10 ms pulses, 10 Hz) delivered via an optrode placed against the brainstem surface. Light stimulation commenced 25 min prior to myocardial ischaemia (to follow the timeline of the RPc protocol) and lasted 10 min into the reperfusion period. Atropine was administered (initial bolus dose 2 mg kg−1 iv 15 min prior to light onset; followed by infusion at a rate of 1 mg kg−1 h−1 iv) to separate groups of animals in order to determine the effect of systemic muscarinic receptor blockade on cardioprotection elicited by optogenetic stimulation of the DVMN neurones.
2.9.3. RPc in conditions of systemic muscarinic receptor blockade with atropine
The systemic muscarinic receptor blockade was achieved by iv infusion of atropine methyl nitrate 15 min prior to RPc or sham-RPc procedures (initial bolus dose 2 mg kg−1 iv; followed by infusion at a rate of 1 mg kg−1 h−1 iv).
2.10. Statistical analysis
Data are reported as mean ± SEM. Data were compared by Kruskal–Wallis ANOVA by ranks or Student's t-test, as appropriate. Values of P< 0.05 were considered to be significant.
3. Results
A LV bearing the PRSx8 promoter was highly efficient in driving the expression of AlstR/eGFP in DVMN neurones (Figure 1A and B). First, patch-clamp recordings in brainstem slices were used to confirm that activation of AlstR inhibits the activity of transduced neurones by opening inwardly rectifying K+ channels, hyperpolarization, and cessation of action potential generation.22 DVMN neurones expressing AlstR/eGFP recorded in cell-attached configuration had a mean firing rate of 1.7 ± 0.4 Hz (n = 6). Application of allatostatin reversibly abolished action potential firing within 2 min in five cells (Figure 1C) and decreased firing rate by 55% in one remaining cell. In whole-cell current clamp recording configuration, allatostatin induced a 5.3 ± 0.8 mV hyperpolarization and cessation of firing, caused by activation of an inwardly rectifying K+ conductance, as revealed in voltage clamp recordings (n = 4) (Figure 1D). Thus, targeted stereotaxic delivery of the viral vector (avoiding transduction of dorsally located catecholaminergic neurones, Figure 1B), the specificity of the PRSx8 promoter, and the effective silencing of transduced AlstR-expressing neurones by allatostatin allowed the accurate assessment of the functional role of DVMN vagal pre-ganglionic neurones with high temporal resolution.
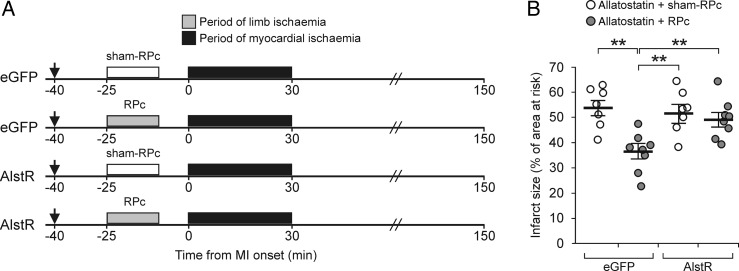
The efficacy of RPc cardioprotection was then assessed, while DVMN neurones were inhibited by allatostatin (100 µM) continuously infused (4 µL h−1) into the cisterna magna (Figure 2A). RPc induced by ischaemia/reperfusion of the limbs reduced myocardial ischaemia/reperfusion injury as evident from a significant (P < 0.01) reduction in the infarct size (Figure 2B). Selective DVMN silencing abolished RPc cardioprotection (P = 0.004) while having no effect on the infarct size in animals not subjected to RPc (Figure 2B). There were no differences in the areas at risk between groups of animals recruited into the experimental groups. There were also no differences in mean arterial blood pressure or heart rate between the groups of animals either before, or during myocardial ischaemia and reperfusion (Table 1). Thus, RPc cardioprotection appears to be crucially dependent on the activity of DVMN neurones.
Figure 2.
Remote preconditioning (RPc) cardioprotection critically depends on the activity of DVMN vagal pre-ganglionic neurones. (A) Illustration of the experimental protocols. RPc was induced by 15 min occlusion of both femoral arteries, followed by 10 min reperfusion. The Sham-RPc procedure involved dissection of both femoral arteries without occlusion. Arrow indicates the start of allatostatin infusion into the cisterna magna. eGFP, animals transduced to express eGFP in the DVMN. AlstR, animals transduced to express AlstR/eGFP in the DVMN. MI, myocardial ischaemia. (B) Silencing DVMN neurones abolishes RPc cardioprotection. The infarct size is presented as the percentage of area at risk. Individual data and means ± SEM are shown. **P< 0.01 (Kruskal–Wallis ANOVA by ranks).
Table 1.
Haemodynamic data
| Variable | Pre-ischaemia | End ischaemia | Reperfusion (min) |
|||
|---|---|---|---|---|---|---|
| 30 | 60 | 120 | ||||
| DVMN silencing experiment | ||||||
| DVMN neurones expressing eGFP | ||||||
| Allatostatin + sham-RPc (n = 7) | MAP | 106 ± 7 | 101 ± 7 | 96 ± 6 | 92 ± 5 | 91 ± 4 |
| HR | 408 ± 8 | 411 ± 9 | 389 ± 10 | 393 ± 10 | 405 ± 9 | |
| Allatostatin + RPc (n = 8) | MAP | 111 ± 11 | 108 ± 13 | 104 ± 8 | 101 ± 6 | 96 ± 7 |
| HR | 427 ± 8 | 429 ± 10 | 422 ± 8 | 427 ± 9 | 420 ± 10 | |
| DVMN neurones expressing AlstR | ||||||
| Allatostatin + sham-RPc (n = 7) | MAP | 115 ± 9 | 104 ± 10 | 99 ± 8 | 91 ± 7 | 82 ± 7 |
| HR | 401 ± 9 | 421 ± 13 | 397 ± 12 | 384 ± 12 | 387 ± 13 | |
| Allatostatin + RPc (n = 8) | MAP | 117 ± 9 | 103 ± 7 | 102 ± 5 | 101 ± 5 | 97 ± 6 |
| HR | 413 ± 15 | 430 ± 9 | 405 ± 7 | 407 ± 7 | 409 ± 10 | |
| Optogenetic preconditioning experiment | ||||||
| DsRed—DVMN blue light (n = 7) | MAP | 92 ± 9 | 82 ± 7 | 78 ± 3 | 77 ± 5 | 80 ± 5 |
| HR | 401 ± 12 | 403 ± 8 | 384 ± 5 | 380 ± 7 | 388 ± 8 | |
| CHIEFtdTomato—DVMN blue light (n = 6) | MAP | 99 ± 6 | 79 ± 4 | 78 ± 4 | 80 ± 5 | 83 ± 4 |
| HR | 420 ± 6 | 415 ± 8 | 402 ± 7 | 397 ± 9 | 393 ± 9 | |
| DsRed—DVMN blue light + atropine (n = 7) | MAP | 106 ± 11 | 85 ± 5 | 90 ± 4 | 90 ± 7 | 80 ± 7 |
| HR | 391 ± 17 | 392 ± 11 | 393 ± 10 | 410 ± 12 | 402 ± 11 | |
| CHIEFtdTomato—DVMN blue light + atropine (n = 8) | MAP | 99 ± 10 | 76 ± 6 | 84 ± 5 | 79 ± 4 | 71 ± 3 |
| HR | 379 ± 14 | 392 ± 15 | 389 ± 11 | 380 ± 20 | 384 ± 10 | |
AlstR, allatostatin receptor; DVMN, dorsal motor nucleus of the vagus nerve; eGFP, enhanced green fluorescent protein; HR, heart rate (min−1); MAP, mean arterial blood pressure (mmHg); RPc, remote preconditioning.
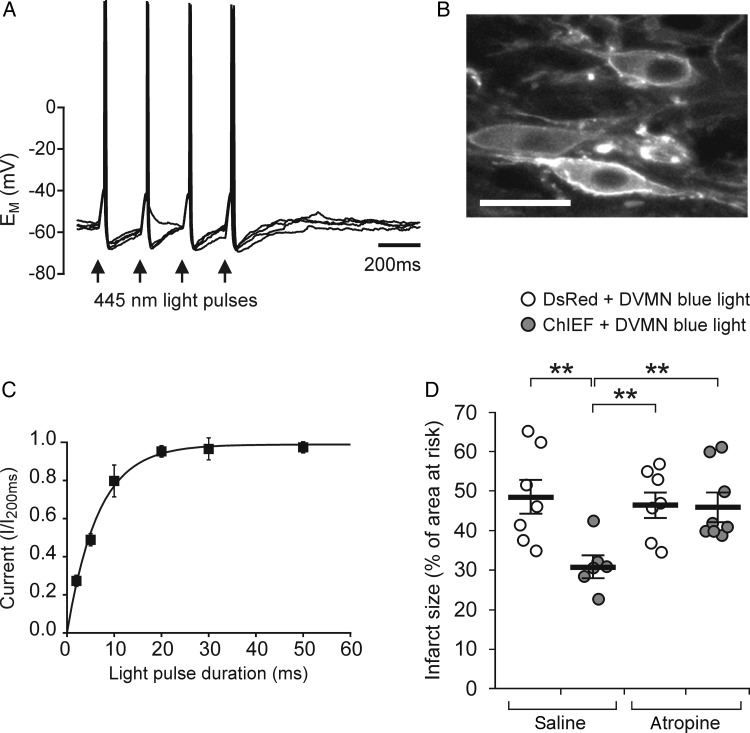
By extension, activation of DVMN neurones in the absence of remote ischaemia/reperfusion should result in cardioprotection. To test this hypothesis, we used optogenetics to increase DVMN neuronal activity with high resolution and specificity. DVMN neurones transduced to express ChIEFtdTomato (Figure 3A and B) displayed precisely timed depolarizations and action potential firing in response to flashes of blue (445 nm) light (Figure 3A). Voltage clamp analysis revealed that light stimulation induces an inward current with half maximal activation by a 5 ms light pulse (Figure 3C). Consequently, optogenetic stimulation of DVMN in vivo was performed with 10 ms pulse duration at a frequency of 10 Hz (a stimulation regime not associated with changes in heart rate or blood pressure; Table 1). Optogenetic stimulation of DVMN markedly reduced the infarct size (P = 0.008; Figure 3D), indicating that increased activity of this specific neuronal population is sufficient to limit ischaemia/reperfusion myocardial injury, effectively mimicking the effect of RPc. In conditions of systemic administration of atropine optogenetic stimulation of the DVMN neurones failed to establish cardioprotection (Figure 3D).
Figure 3.
Optogenetic stimulation of DVMN neurones mimics RPc cardioprotection. (A) Representative whole-cell current-clamp recording from CHIEFtdTomato-expressing DVMN neurone illustrating depolarization and action potential firing in response to blue light (20 ms pulses). Four consecutive traces are overlaid. Action potential was elicited in response to 15 out of 16 pulses; (B) DVMN neurones expressing CHIEFtdTomato. Scale bar = 30 µm. (C) The mean data from voltage-clamp recordings (n = 6) at a holding potential of −50 mV demonstrating the relationship between duration of the light stimulus and the normalized amplitude of inward current elicited by opening CHIEF channel; (D) optogenetic stimulation of DVMN neurones reduces myocardial ischaemia/reperfusion injury via a muscarinic receptor-mediated mechanism. Control animals were transduced to express DsRed in the DVMN. The infarct size is presented as the percentage of area at risk. Individual data and means ± SEM are shown. **P< 0.01 (Kruskal–Wallis ANOVA by ranks).
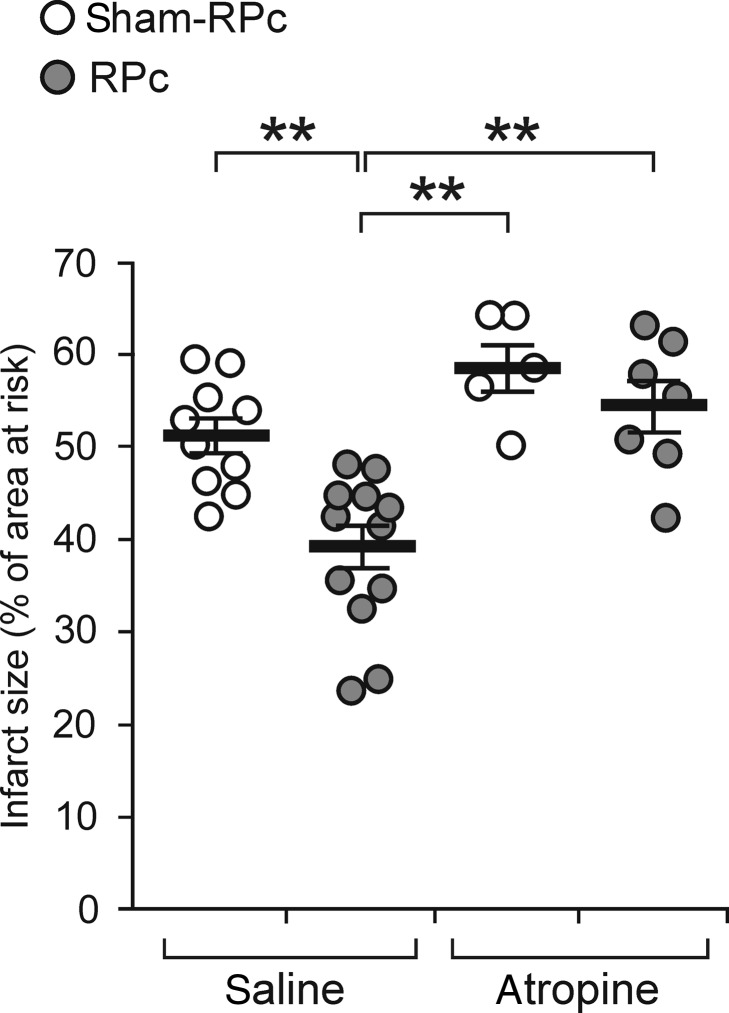
This observation prompted the experiment to determine whether a muscarinic receptor mechanism mediates RPc cardioprotection. RPc conferred no cardioprotection in conditions of the systemic muscarinic receptor blockade with atropine methyl nitrate (Figure 4) that has a limited ability to cross the blood–brain barrier. Atropine had no effect on heart rate in animals subjected to the RPc procedure (Table 2), consistent with the data showing that in rats, anaesthetized with pentobarbital, chronotropic vagal tone is significantly reduced.26
Figure 4.
Muscarinic receptor mechanisms mediate RPc cardioprotection. RPc was induced by 15 min occlusion of both femoral arteries, followed by 10 min reperfusion. The Sham-RPc procedure involved the dissection of both femoral arteries without occlusion. Atropine infusion commenced 15 min prior to RPc or sham-RPc. RPc conferred no cardioprotection in conditions of systemic muscarinic receptor blockade. The infarct size is presented as the percentage of area at risk. Individual data and means ± SEM are shown. **P< 0.01 (Kruskal–Wallis ANOVA by ranks).
Table 2.
Haemodynamic data
| Variable | Pre-ischaemia | End ischaemia | Reperfusion (min) |
|||
|---|---|---|---|---|---|---|
| 30 | 60 | 120 | ||||
| Experiment with systemic muscarinic receptor blockade | ||||||
| Sham-RPc (n = 10) | MAP | 98 ± 9 | 98 ± 7 | 100 ± 7 | 103 ± 6 | 106 ± 5 |
| HR | 416 ± 12 | 438 ± 11 | 421 ± 12 | 423 ± 9 | 419 ± 11 | |
| RPc (n = 12) | MAP | 103 ± 4 | 95 ± 5 | 97 ± 5 | 94 ± 4 | 90 ± 3 |
| HR | 427 ± 9 | 431 ± 7 | 437 ± 9 | 436 ± 7 | 434 ± 7 | |
| Atropine + sham-RPc (n = 5) | MAP | 105 ± 10 | 92 ± 5 | 104 ± 2 | 89 ± 10 | 81 ± 6 |
| HR | 412 ± 12 | 414 ± 18 | 410 ± 10 | 424 ± 11 | 409 ± 16 | |
| Atropine + RPc (n = 7) | MAP | 107 ± 7 | 95 ± 7 | 103 ± 3 | 103 ± 3 | 91 ± 5 |
| HR | 439 ± 22 | 437 ± 15 | 430 ± 11 | 424 ± 13 | 413 ± 18 | |
HR, heart rate (min−1); MAP, mean arterial blood pressure (mmHg); RPc, remote preconditioning.
4. Discussion
The present study reveals a crucial dependence of interorgan (limb-heart) protection against ischaemia/reperfusion injury upon the activity of a distinct population of vagal pre-ganglionic neurones residing in the DVMN. It also demonstrates for the first time that activation of a specific central nervous substrate can effectively protect the heart against lethal ischaemia/reperfusion injury.
Here, we tested the hypothesis that cardioprotection induced by ischaemia/reperfusion of the remote tissue requires functional integrity of the central nervous parasympathetic circuitry and, by extension, an intact parasympathetic activity. This hypothesis was based on the following earlier findings. First, RPc is abolished by systemic hexamethonium administration,3 which blocks transmission in both sympathetic and parasympathetic ganglia. Second, electrical stimulation of the vagus nerve is cardioprotective, limits myocardial ischaemia/reperfusion injury,5,6 and reduces the number of severe arrhythmias and overall lethality.4 Our recent observations also demonstrated that RPc cardioprotection is abolished by bilateral cervical vagotomy.7,8 However, while eliminating vagal efferent activity, cervical vagotomy also interrupts the transmission of sensory information from the heart (and other internal organs) to the CNS. To avoid confounding factors associated with complete surgical vagotomy, here we performed a selective (albeit partial) vagal ‘de-efferentation’ by cell-specific targeting and silencing of vagal pre-ganglionic neurones in the DVMN that contribute to tonic C-fibre-mediated innervation of the heart.27 In support of our hypothesis, we found that RPc fails to establish cardioprotection when DVMN neurones are silenced.
In this study, all the experiments were conducted in rats anaesthetized with pentobarbital which is known to suppress chronotropic vagal tone;26 hence the heart rate in animals subjected to RPc was not affected by either silencing or optogenetic activation of the DVMN neurones or in conditions of systemic muscarinic receptor blockade with atropine. This is consistent with the data demonstrating that the chronotropic control of the heart is provided predominantly by a population of vagal pre-ganglionic neurones located in the nucleus ambiguus.28 Collectively, these data indicate that while an intact vagal supply is essential for RPc cardioprotection, its beneficial effect is independent of heart rate modulation as demonstrated by prior studies involving electrical stimulation of the vagus nerve.5 In contrast to an earlier view still conveyed by the majority of the physiology textbooks, there is strong evidence that demonstrates functional vagal innervation of the ventricles (see for example Lewis et al.29). Here, we demonstrate that a muscarinic receptor mechanism mediates RPc cardioprotection and confirm the existence of a significant cholinergic supply to the right and left ventricles of the heart in a rat strain used in this study (Sprague-Dawley) (Supplementary material online, Figure). These results suggest that an increased efferent vagal outflow reduces myocardial injury through the potent cardioprotective effects of acetylcholine (which is as potent as adenosine in eliciting cardioprotection) reported previously.30–33
The mechanism of RPc-induced cardioprotection was suggested to involve humoral factor(s) produced during ischemia/reperfusion of the remote tissue and released into the systemic circulation,34–38 or a neural component,3,37,39–42 or both.37,43 Several studies clearly demonstrated that RPc cardioprotection requires intact sensory innervation of the peripheral ischaemic tissue.37,39,43 Based on those data, together with the results of our studies, we proposed the existence of a ‘remote preconditioning reflex’7 which involves sensory input from the remote ischaemic organ/tissue and protects the heart via recruitment of a distinct population of vagal pre-ganglionic neurones in the DVMN. Indeed, this study demonstrates that the key component of inter-organ remote protection against ischaemia/reperfusion injury is neural, since DVMN neurones are essential for RPc, while their activation (even in the absence of any humoral factor(s) released from the ischaemic limb) is sufficient to establish cardioprotection. These results do not exclude the involvement of humoral factor(s),37,43 but demonstrate that in order to establish cardioprotection an enigmatic ‘humoral pathway’ of RPc requires functional integrity of DVMN neurones. Several disparate experimental models indicate that direct myocardial preconditioning cardioprotection does not require intact connections between the heart and the central nervous system. Therefore, the parasympathetic pathway of myocardial protection described in this report may only be recruited under certain conditions, for example when RPc stimulus is applied to the tissue located away from the heart.
Ongoing trials of the efficacy of vagus nerve stimulation in chronic heart failure patients are expected to confirm the results of the preliminary report showing significant improvements in the quality of life and left ventricular function.44 Intermittent arm ischaemia in humans was recently reported to increase parasympathetic tone.45 The results of the present study obtained with optogenetic stimulation of DVMN suggest that timely application of the vagus nerve stimulation or other procedures/treatments which reflexly increase vagal activity may be highly effective in protecting the heart against acute lethal ischaemia/reperfusion injury and decreasing morbidity and mortality of patients with ischaemic heart disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This study was supported by The Wellcome Trust, British Heart Foundation and the British Council. G.L.A. is an Academy of Medical Sciences/Health Foundation Clinician-Scientist. A.V.G. is a Wellcome Trust Senior Research Fellow.
Supplementary Material
Acknowledgements
We thank Dr JY Lin (University of California) for providing ChIEFtdTomato clone and Dr Andrew Allen (University of Melbourne) for providing PRSx8-AlstR-eGFP-LV.
Conflict of interest: none declared.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 3.Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 4.Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- 5.Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, et al. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg. 2009;137:223–231. doi: 10.1016/j.jtcvs.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Calvillo L, Vanoli E, Andreoli E, Besana A, Omodeo E, Gnecchi M, et al. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 2011;58:500–507. doi: 10.1097/FJC.0b013e31822b7204. [DOI] [PubMed] [Google Scholar]
- 7.Gourine A, Gourine AV, Mastitskaya S, Ackland G. “Remote preconditioning reflex”—a neural pathway of cardioprotection during myocardial ischaemia and reperfusion induced by remote ischaemic preconditioning. Eur Heart J. 2010;31:319. [Google Scholar]
- 8.Basalay M, Barsukevich V, Mastitskaya S, Mrochek A, Pernow J, Sjoquist PO, et al. Remote ischaemic pre- and delayed postconditioning - similar degree of cardioprotection but distinct mechanisms. Exp Physiol. 2012 doi: 10.1113/expphysiol.2012.064923. doi:10.1113/expphysiol.2012.064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahid M, Tauseef M, Sharma KK, Fahim M. Brief femoral artery ischaemia provides protection against myocardial ischaemia-reperfusion injury in rats: the possible mechanisms. Exp Physiol. 2008;93:954–968. doi: 10.1113/expphysiol.2007.041442. [DOI] [PubMed] [Google Scholar]
- 10.Hwang DY, Carlezon WA, Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Human Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- 11.Lonergan T, Teschemacher AG, Hwang D-Y, Kim K-S, Kasparov S. Targeting brainstem centres of cardiovascular control using adenoviral vectors: impact of promoters on transgene expression. Physiol Genomics. 2005;20:165–172. doi: 10.1152/physiolgenomics.00120.2004. [DOI] [PubMed] [Google Scholar]
- 12.Tiveron MC, Hirsch MR, Brunet JF. The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci. 1996;16:7649–7660. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, et al. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marina N, Abdala AP, Korsak A, Simms AE, Allen AM, Paton JF, et al. Control of sympathetic vasomotor tone by catecholaminergic C1 neurones of the rostral ventrolateral medulla oblongata. Cardiovasc Res. 2011;91:703–710. doi: 10.1093/cvr/cvr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman JE, Huentelman MJ, Kasparov S, Metcalfe BL, Paton JFR, Katovich MJ, et al. Efficient large-scale production and concentration of HIV-1-based lentiviral vectors for use In vivo. Physiol Genomics. 2003;12:221–228. doi: 10.1152/physiolgenomics.00135.2002. [DOI] [PubMed] [Google Scholar]
- 16.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu BH, Yang Y, Paton JFR, Li F, Boulaire J, Kasparov S, et al. GAL4-NFkappaB fusion protein augments transgene expression from neuronal promoters in the rat brain. Mol Ther. 2006;14:872–882. doi: 10.1016/j.ymthe.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Benzekhroufa K, Liu BH, Teschemacher AG, Kasparov S. Targeting central serotonergic neurons with lentiviral vectors based on a transcriptional amplification strategy. Gene Ther. 2009;16:681–688. doi: 10.1038/gt.2009.7. [DOI] [PubMed] [Google Scholar]
- 19.Duale H, Kasparov S, Paton JFR, Teschemacher AG. Differences in transductional tropism of adenoviral and lentiviral vectors in the rat brainstem. Exp Physiol. 2005;90:71–78. doi: 10.1113/expphysiol.2004.029173. [DOI] [PubMed] [Google Scholar]
- 20.Teschemacher AG, Wang S, Lonergan T, Duale H, Waki H, Paton JFR, et al. Targeting specific neuronal populations in the brainstem using adeno- and lentiviral vectors: applications for imaging and studies of cell function. Exp Physiol. 2005;90:61–69. doi: 10.1113/expphysiol.2004.028191. [DOI] [PubMed] [Google Scholar]
- 21.Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, Paton JFR. Restraining infulence of A2 neurones in chronic control of blood pressure in SHR. Cardiovasc Res. 2007;76:184–193. doi: 10.1016/j.cardiores.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birgul N, Weise C, Kreienkamp HJ, Richter D. Reverse physiology in drosophila: identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to the mammalian somatostatin/galanin/opioid receptor family. EMBO J. 1999;18:5892–5900. doi: 10.1093/emboj/18.21.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Hopwood SE, Trapp S. TASK-like K+ channels mediate effects of 5-HT and extracellular pH in rat dorsal vagal neurones in vitro. J Physiol. 2005;568:145–154. doi: 10.1113/jphysiol.2005.093070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Leary DM, Jones JF. Discharge patterns of preganglionic neurones with axons in a cardiac vagal branch in the rat. Exp Physiol. 2003;88:711–723. doi: 10.1113/eph8802590. [DOI] [PubMed] [Google Scholar]
- 27.Jones JF. Vagal control of the rat heart. Exp Physiol. 2001;86:797–801. doi: 10.1111/j.1469-445x.2001.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 28.Spyer KM. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis ME, Al-Khalidi AH, Bonser RS, Clutton-Brock T, Morton D, Paterson D, et al. Vagus nerve stimulation decreases left ventricular contractility in vivo in the human and pig heart. J Physiol. 2001;534:547–552. doi: 10.1111/j.1469-7793.2001.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard V, Blanc T, Kaeffer N, Tron C, Thuillez C. Myocardial and coronary endothelial protective effects of acetylcholine after myocardial ischaemia and reperfusion in rats: role of nitric oxide. Br J Pharmacol. 1995;115:1532–1538. doi: 10.1111/j.1476-5381.1995.tb16647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian YZ, Levasseur JE, Yoshida K, Kukreja RC. KATP channels in rat heart: blockade of ischemic and acetylcholine-mediated preconditioning by glibenclamide. Am J Physiol. 1996;271:H23–H28. doi: 10.1152/ajpheart.1996.271.1.H23. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi F, Nasa Y, Yabe K, Ohba S, Hashizume Y, Ohaku H, et al. Activation of cardiac muscarinic receptor and ischemic preconditioning effects in in situ rat heart. Heart Vessels. 1997;12:74–83. doi: 10.1007/BF02820870. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. Circ Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- 34.Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a KATP channel-dependent mechanism. Transplantation. 2005;79:1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 35.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 37.Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- 38.Kingma JG, Jr, Simard D, Voisine P, Rouleau JR. Role of the autonomic nervous system in cardioprotection by remote preconditioning in isoflurane-anaesthetized dogs. Cardiovasc Res. 2011;89:384–391. doi: 10.1093/cvr/cvq306. [DOI] [PubMed] [Google Scholar]
- 39.Dong JH, Liu YX, Ji ES, He RR. Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats. Sheng Li Xue Bao. 2004;56:41–46. [PubMed] [Google Scholar]
- 40.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 41.Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, et al. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–S9. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, et al. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–H1603. doi: 10.1152/ajpheart.00396.2010. [DOI] [PubMed] [Google Scholar]
- 43.Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, et al. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol. 2012;107:1–10. doi: 10.1007/s00395-011-0241-5. [DOI] [PubMed] [Google Scholar]
- 44.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 45.Enko K, Nakamura K, Yunoki K, Miyoshi T, Akagi S, Yoshida M, et al. Intermittent arm ischemia induces vasodilatation of the contralateral upper limb. J Physiol Sci. 2011;61:507–513. doi: 10.1007/s12576-011-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.