Abstract
Aims
Na+/K+-ATPase (NKA) is essential in regulating [Na+]i, and thus cardiac myocyte Ca2+ and contractility via Na+/Ca2+ exchange. Different NKA-α subunit isoforms are present in the heart and may differ functionally, depending on specific membrane localization. In smooth muscle and astrocytes, NKA-α2 is located at the junctions with the endo(sarco)plasmic reticulum, where they could regulate local [Na+], and indirectly junctional cleft [Ca2+]. Whether this model holds for cardiac myocytes is unclear.
Methods and results
The ouabain-resistant NKA-α1 cannot be selectively blocked to assess its effect. To overcome this, we used mice in which NKA-α1 is ouabain sensitive and NKA-α2 is ouabain resistant (SWAP mice). We measured the effect of ouabain at low concentration on [Na+]i, Ca2+ transients, and the fractional sarcoplasmic reticulum (SR) Ca2+ release in cardiac myocytes from wild-type (WT; NKA-α2 inhibition) and SWAP mice (selective NKA-α1 block). At baseline, Na+ and Ca2+ regulations are similar in WT and SWAP mice. For equal levels of total NKA inhibition (∼25%), ouabain significantly increased Ca2+ transients (from ΔF/F0= 1.5 ± 0.1 to 1.8 ± 0.1), and fractional SR Ca2+ release (from 24 ± 3 to 29 ± 3%) in WT (NKA-α2 block) but not in SWAP myocytes (NKA-α1 block). This occurred despite a similar and modest increase in [Na+]i (∼2 mM) in both groups. The effect in WT mice was mediated specifically by NKA-α2 inhibition because at a similar concentration ouabain had no effect in transgenic mice where both NKA-α1 and NKA-α2 are ouabain resistant.
Conclusion
NKA-α2 has a more prominent role (vs. NKA-α1) in modulating cardiac myocyte SR Ca2+ release.
Keywords: Sarcolemma–SR junctions, Na+/K+-ATPase, Ouabain, Na+/Ca2+ exchanger
1. Introduction
Na+/K+-ATPase (NKA) is the main route for Na+ extrusion in cardiac myocytes and therefore is essential in regulating intracellular Na+ concentration ([Na+]i). Through the Na+/Ca2+ exchanger (NCX), [Na+]i, and NKA activity are tightly linked to cardiac myocyte Ca2+ and contractility.1 There are multiple NKA isoforms in the heart. NKA-α1 is the dominant, ubiquitous isoform, whereas NKA-α2 and NKA-α3 are present in smaller amounts and their expression differs significantly between species. For instance adult rodent hearts express NKA-α1 and NKA-α2,2 whereas dogs and macaques express NKA-α1 and NKA-α3.3 All three NKA-α isoforms are present in human hearts,3 with estimates of isoform distribution at the mRNA level ranging from relatively equal amounts of the three isoforms4 to NKA-α1 being dominant (62%) over NKA-α2 (15%), and NKA-α3 (23%).5
Different NKA isoforms may function differently, depending on specific membrane localization. In smooth muscle and astrocytes, NKA-α2 and NKA-α3 are located at the junctions with the endo(sarco)plasmic reticulum, where they regulate local [Na+]i, and, indirectly via NCX, junctional cleft [Ca2+]i.6 In contrast, NKA-α1 is ubiquitously distributed and may regulate bulk [Na+]i. It is unclear whether this model holds for cardiac myocytes. The functional density of NKA-α2 is about four times higher in the T-tubules (vs. external sarcolemma) in myocytes from both rats7,8 and mice,9 making this functionally plausible. However, its precise localization with respect to the junctions with the sarcoplasmic reticulum (SR), which occupy 40–48% of T-tubular membrane, is unknown. Hearts with genetically reduced NKA-α2 levels are hypercontractile as a result of larger Ca2+ transients, whereas hearts with reduced levels of NKA-α1 are hypocontractile.10 Moreover, elevated NKA-α2 expression results in smaller NCX current and Ca2+ transients.11 Selective NKA-α2 inhibition was found to increase rat myocyte contractility, despite unchanged global [Na+]i,8 and to slow Ca2+ extrusion through NCX.12 All these results suggest that NKA-α2 preferentially (vs. NKA-α1) regulates cardiac myocyte Ca2+ and contractility. However, other results challenge this. Moseley et al.13 found that NKA-α1+/−mice were severely compromised and Dostanic et al.14 showed that NKA-α1 also regulates cardiac contractility. Furthermore, two studies found that both NKA-α1 and NKA-α2 are physically and functionally associated with NCX in cardiac myocytes.14,15
Here, we used WT mice and mice that have NKA isoforms with swapped affinity for ouabain (NKA-α1 is ouabain sensitive and NKA-α2 is ouabain resistant; SWAP mice14) to directly assess the effect of selective NKA-α1/α2 blockade on [Na+]i, Ca2+ transients, and fractional SR Ca2+ release. Our results support the hypothesis that NKA-α2 has a more prominent role in regulating myocyte Ca2+ transients than NKA-α1.
2. Methods
2.1. Generation of SWAP mice and cardiac myocyte isolation
All animal protocols were approved by the animal welfare committees at the University of Cincinnati or University of California Davis and conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Mice expressing ouabain-sensitive NKA-α1 and ouabain-resistant NKA-α2 (SWAP) were developed by mating mice with ouabain-sensitive NKA-α1 isoform (α1SS α2SS)14 with mice having an ouabain-resistant NKA-α2 isoform (α1RR α2RR),16 as previously described.14 The ouabain-sensitive mouse NKA-α1 isoform was obtained by introducing the R111Q and D122N amino acid substitutions into the first extracellular domain of this isoform. Gln-111 and Asn-122 residues are naturally present in the high-affinity human and sheep NKA-α1 isoforms and were shown to confer sensitivity to cardiac glycosides.17,18 The ouabain-resistant mouse NKA-α2 isoform was obtained by introducing the L111R and N122D amino acid substitutions. The Arg-111 and Asp-122 residues are naturally present in the low-affinity rodent NKA-α1 isoforms. The L111R and N122D amino acid substitutions reduced 1000-fold the ouabain affinity of sheep NKA-α1 isoform without altering its enzymatic activity.17,18 The expression and tissue distribution of NKA-α1 and NKA-α2 isoforms are normal in SWAP animals and the mutations did not alter the enzymatic activity of the two isoforms.14 There are no differences in the haemodynamic parameters (both under basal conditions and during β-adrenergic stimulation) between WT and SWAP mice.14 Eleven SWAP, 10 WT, and 5 α1RR α2RR mice were used for this study.
Isolation of mouse ventricular myocytes was as previously described.19 Briefly, SWAP mice and age-matched WT littermates (3–4 months age) were anaesthetized in a gas chamber with 3–5% isoflurane (100% O2). Anaesthesia was confirmed by the complete lack of reflex to very firm toe pinch and/or touch of a suture thread to cornea of eye. Hearts were excised quickly, mounted on a gravity-driven Langendorff perfusion apparatus and digested by perfusion with 0.8 mg/mL collagenase (type B, Boehringer Mannheim, Indianapolis, IN, USA) in the presence of 20 μM Ca2+ and 1 mg/mL taurine. When the heart became flaccid (7–12 min), ventricular tissue was removed, dispersed, filtered, and myocyte suspensions were rinsed several times. Myocytes were used for experiments within 6h after isolation.
2.2. Simultaneous measurements of [Na+]i and Ca2+ transients
Myocytes were plated on glass-coverslips and incubated with 10 μM SBFI-AM in the presence of Pluronic F-127 (0.05% w/v) for 90 min at room temperature. Fluo-4 AM (10 μM) was added during the last 35 min. The dyes were allowed to further deesterify for 20 min in normal Tyrode's solution. Myocytes were placed on the stage of a fluorescence microscope and fluorescence was then alternatively excited at 340, 380 (for SBFI) and 480 nm (Fluo-4). For all excitation wavelengths, emission was recorded at 535 nm. For [Na+]i measurements, the F340/F380 ratio was calculated after background subtraction and calibrated at the end of each experiment using divalent-free solutions with 0, 10, or 20 mM extracellular [Na+] in the presence of 10 μM gramicidin and 100 μM strophanthidin.20 Ca2+ transients are reported as the Fluo-4 F/F0 ratios. All measurements were at room temperature (21–25°C).
2.3. Ouabain dose–response curves
Myocytes were pre-treated with 1 μM thapsigargin for 10 min and voltage clamped in the whole-cell configuration using patch electrodes made from borosilicate glass capillaries, as previously described.7 When filled with pipette solution, the electrode resistance was 1.5–2.5 MΩ. Current signals were recorded using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, USA). Membrane capacitance and series resistance were calculated from a 5 mV voltage step from a holding potential of −70 mV. The pipette solution contained (in millimolar): 30 NaCl, 70 NaOH, 70 aspartic acid, 20 K+-aspartate, 20 TEA-Cl, 10 HEPES, 5 Mg-ATP, 0.7 MgCl2 (1 mM free Mg), 3 BAPTA, 1.15 CaCl2 (100 nM free Ca2+), pH = 7.2. At this internal [Na+], the pump operates near the maximum rate. Ipump was activated, in the whole-cell voltage clamp, by rapid switch from 0 to 4 mM external K at a holding potential of −20 mV. We have shown previously that the K-activated current is ouabain sensitive.7 The external solution contained (in millimolar): 136 NaCl, 5 NiCl2, 2 BaCl2, 1 MgCl2, 5 HEPES, 5 glucose, ±4 KCl, pH = 7.4.
After reaching the whole-cell configuration, myocytes were held at −70 mV in K+-free external solution for at least 8 min, to allow equilibration of intracellular and pipette Na+. Then, IPump was activated at −20 mV by switching to 4 mM K+ external solution. Under these conditions, ∼75% of the pumps are activated (assuming a K1/2 for external K+ of 1.5 mM). We then recorded the current while adding increasing concentrations of ouabain. For each ouabain concentration, IPump was considered at steady state if the holding current was constant for at least 10 s. We also repeated the analysis using exponential extrapolation to idealized steady state at longer times, and obtained virtually identical results (not shown). Control experiments (not shown) indicated that IPump does not run down significantly during the duration of the experiment. We calculated the percentage of IPump inhibition for each ouabain concentration as 100 (IPump0− IPumpOua)/IPump0, where IPump0 is IPump in the absence of ouabain (i.e. the outward current induced by adding 4 mM K+ to the external solution) and IPumpOua is IPump at the respective [ouabain]. The mean data were fit with a two-binding site equation: B1[Ouabain]/(K1 + [Ouabain]) + (100−B1)[Ouabain]/(K2 + [Ouabain]), to derive the K1/2 for ouabain binding to NKA-α1 and NKA-α2 and their relative contribution to IPump. The fit was done using Origin software (Microcal Software, Inc., Northampton, MA, USA) and the best fit was identified by a minimum χ2 test.
2.4. Immunofluorescence
Myocytes from WT and SWAP mice were fixed with 4% paraformaldehyde, permeabilized with saponin (0.5 µg/mL for 2 min), blocked with 2% goat serum and labelled with primary antibodies that bind specifically to NKA-α1 (clone C464.6 from Millipore) and NKA-α2 (AB9094 from Millipore). Alexa-Fluor546-linked secondary antibodies were used for detection. Images were acquired with a laser scanning confocal microscope.
2.5. Statistical analysis
The statistical differences between the groups were determined using Student's t-test. The data are presented as mean ± standard error. A statistical difference was achieved for a P-value < 0.05.
3. Results
3.1. Low ouabain concentration specifically inhibits NKA-α2 in WT and NKA-α1 in SWAP mice
In the mouse (and rat), NKA-α1 and NKA-α2 can be separated based on their different ouabain affinities. The dominant NKA-α1 isoform is ouabain resistant, whereas NKA-α2 is ouabain sensitive. SWAP mice carry two amino acid mutations in NKA-αisoforms so that NKA-α1 is ouabain sensitive and NKA-α2 is ouabain resistant.14 The mutations do not affect the haemodynamic parameters and the expression, tissue distribution, and enzymatic activity of NKA-α1 and NKA-α2.
We measured the dose-dependent IPump inhibition by ouabain in myocytes from WT and SWAP mice (Figure 1). For both WT and SWAP myocytes, data could be fit with a two-binding sites equation, which indicates the presence of two ouabain-binding sites (Figure 1). There was no significant difference in the ouabain K1/2 for either the high (K1/2= 0.22 ± 0.02 µM vs. 0.25 ± 0.02 µM) or the low (K1/2= 117 ± 5 µM vs. 105 ± 6 µM) affinity sites between WT and SWAP mice. In WT mice, the low affinity sites (i.e. NKA-α1) are dominant (∼72% of total NKA; Figure 1A). This is somewhat lower than we previously reported for mouse myocytes,9 maybe due to the different background mouse strain used (C57 BL/6 previously vs. Black Swiss here). NKA-α1 is still the major isoform in SWAP mice (∼80%), only here it is ouabain sensitive (Figure 1B). Thus, the relative proportion of NKA-α1 and NKA-α2 is comparable in WT and SWAP mice. Of note, we have shown previously21 that the apparent affinity for internal Na+ is similar for the α1 and α2 isoforms, both under basal conditions and during activation of β-adrenergic receptors with isoproterenol.
Figure 1.
Dose-dependent IPump inhibition by ouabain and NKA isoform localization in myocytes from WT and SWAP mice. (A and B) IPump inhibition by ouabain in myocytes from WT (A) and SWAP mice (B). K1/2 and the relative NKA-α1:NKA-α2 density were derived by fitting with a two-binding sites equation; n = 6 myocytes for both WT and SWAP mice. (C) Immunofluorescence images showing the localization of NKA-α1 and NKA-α2 in myocytes from WT and SWAP mice.
Immunofluorescence images of myocytes labelled with antibodies specific for NKA-α1 and NKA-α2 show that NKA-α1 is localized at the T-tubules and external sarcolemma, whereas NKA-α2 is present primarily at the T-tubules for both WT and SWAP mice (Figure 1C), consistent with prior analysis of IPump distribution in WT mice.7–9 Thus, swapping the ouabain sensitivity of NKA isoforms in the SWAP mice did not significantly affect NKA-α1 and NKA-α2 localization.
3.2. Selective NKA-α2 inhibition has greater consequences on cardiac myocyte [Ca2+]i than NKA-α1 blockade
In WT mice, 5 μM ouabain blocks NKA-α2 almost completely, whereas NKA-α1 is practically unaffected (Figure 1). In SWAP mice, 5 μM ouabain selectively blocks NKA-α1 isoform. Thus, we can use myocytes from WT and SWAP mice to determine the effect of selective NKA-α2 and NKA-α1 inhibition on cardiac myocyte Ca2+ cycling. However, because NKA-α1 represents ∼80% of the total NKA, 5 μM ouabain would inhibit a much larger fraction of NKA in SWAP mice than in WT. This would likely lead to different elevations in [Na+]i and SR Ca2+ content in WT and SWAP mice, making the SWAP mouse experiments a less appropriate control. Based on the dose–response curve in Figure 1B, we used 0.1 μM ouabain in SWAP mice to attain a similar level of total NKA inhibition (∼25%) as that produced by 5 μM ouabain in WT mice.
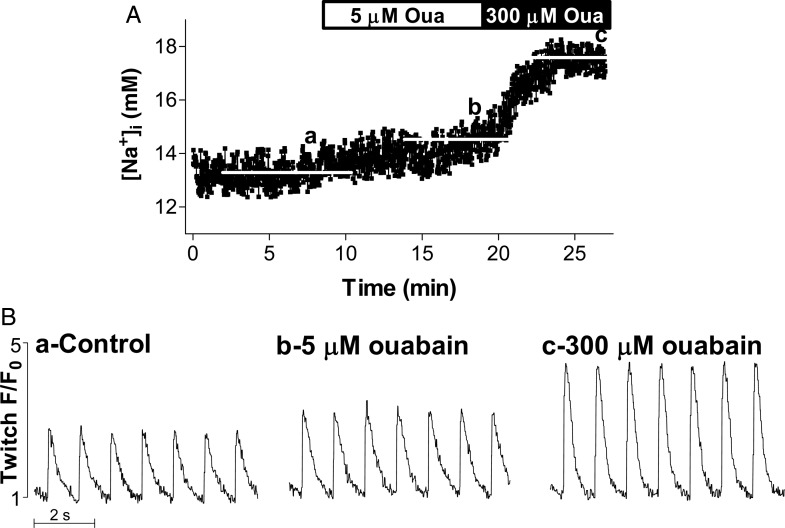
We measured simultaneously [Na+]i and Ca2+ transients in myocytes contracting at 1 Hz under control conditions (Figure 2, point a) and then in the presence of low ouabain concentration (5 μM for WT, 0.1 μM for SWAP mice) (Figure 2, point b). Higher ouabain levels, 300 μM for WT and 5 μM for SWAP mice, were added in some experiments (Figure 2, point c). SR Ca2+ content (with and without ouabain) was determined in select experiments from the amplitude of the Ca2+ transient triggered by 10 mM caffeine.
Figure 2.
Simultaneous [Na+]i and Ca2+ transient measurements; the effect of ouabain. (A) [Na+]i; (B) Ca2+ transients. Representative example in a cell from WT mice. Myocytes dually loaded with SBFI and Fluo-4 were paced at 1 Hz until [Na+]i and Ca2+ transients reached steady state (point a). Selective NKA-α2 inhibition (5 µM ouabain) slightly raised [Na+]i and significantly increased Ca2+ transients (point b). A higher dose of ouabain (300 μM) was then applied. At this concentration, all NKA-α2 and ∼70% of NKA-α1 are blocked. Both [Na+]i and Ca2+ transient amplitude were increased considerably (point c).
Under control conditions, [Na+]i (10.2 ± 1.1 vs. 9.3 ± 0.9 mM), Ca2+ transient amplitude (ΔF/F0= 1.5 ± 0.1 vs. 1.5 ± 0.1) and SR Ca2+ content (ΔF/F0= 5.9 ± 0.4 vs. 6.3 ± 0.5) were similar in myocytes from WT and SWAP mice (Figures 3A–B and 4). Moreover, there was no difference in Ca2+ transient decay during both twitch (τ = 437 ± 42 ms in WT and 446 ± 36 ms in SWAP mice) and caffeine application (τ = 2.9 ± 0.4 s in WT and 2.7 ± 0.4 s in SWAP mice). Thus, at baseline the net function of Na+ and Ca2+ cycling proteins is similar in WT and SWAP mice.
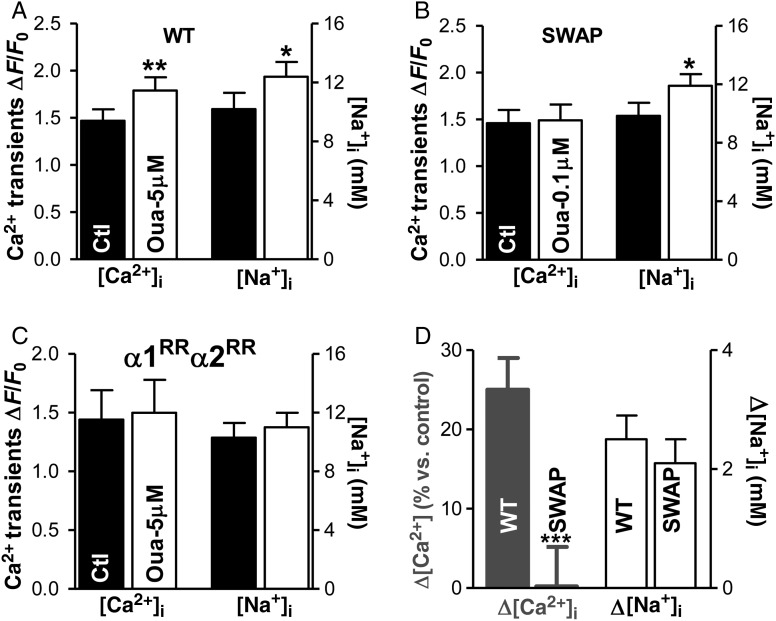
Figure 3.
At low concentration, ouabain increases Ca2+ transient amplitude in myocytes from WT but not SWAP mice. (A–C) The mean data showing the effect of the low concentration of ouabain on Ca2+ transient amplitude and [Na+]i in myocytes from (A) WT mice (NKA-α2 inhibition; n = 17 cells, 7 mice), (B) SWAP mice (NKA-α1 block; 15 cells, 7 mice), and (C) mice with both NKA-isoforms ouabain-resistant (no NKA inhibition; n = 10 cells, 5 mice). (D) Average change in Ca2+ transient amplitude and [Na+]i produced by selective NKA-α2 inhibition in WT mice and NKA-α1 inhibition in SWAP mice.
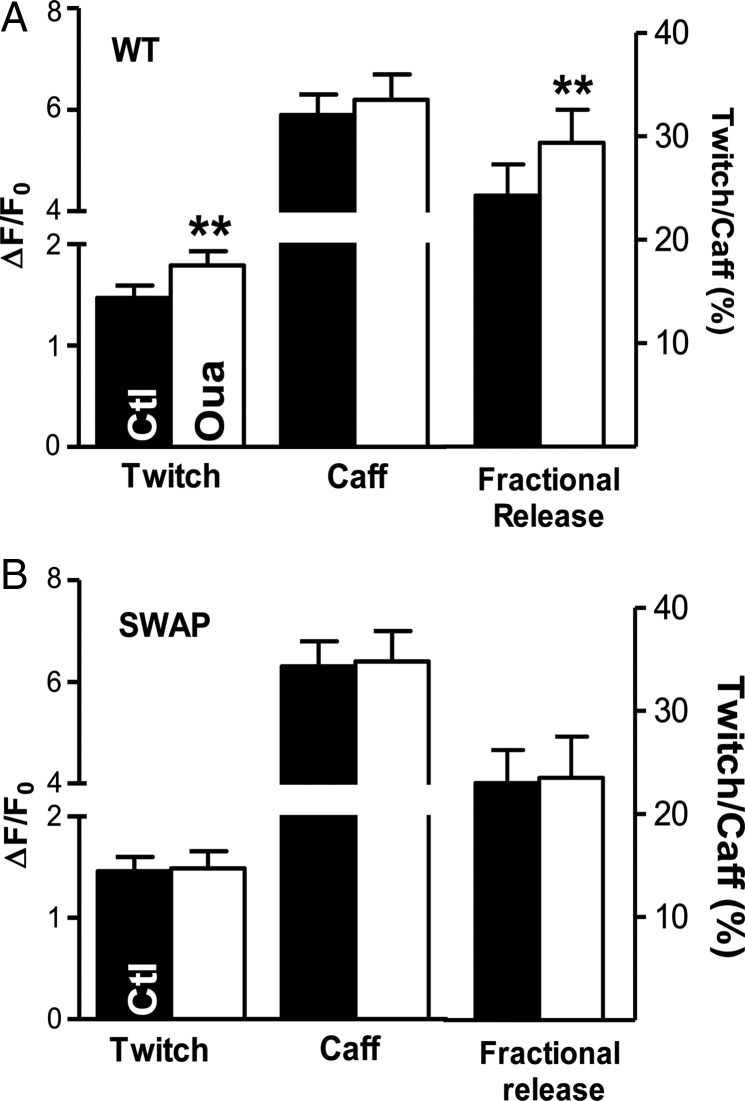
Figure 4.
The effect of ouabain at low concentration on Ca2+ transients, SR Ca2+ content, and fractional SR Ca2+ release in myocytes from WT [NKA-α2 inhibition (A)] and SWAP mice [NKA-α1 inhibition (B)].
Upon selective NKA-α1/NKA-α2 blockade, [Na+]i increased modestly and to the same extent in myocytes from both mice (by 2.5 ± 0.4 mM in WT and by 2.1 ± 0.4 mM in SWAP mice; Figure 3A, B, and D). This indicates a similar level of total NKA inhibition in myocytes from WT and SWAP mice. However, NKA-α2 inhibition in WT mice resulted in significantly larger amplitude of Ca2+ transients (by 25 ± 4%, Figure 3D), whereas comparable NKA-α1 inhibition in SWAP mice had no effect (Figure 3B and D).
Direct effects on ryanodine receptors22 and Ca2+ flux through Na+ channels23 have also been proposed for explaining the inotropic effect of cardiac glycosides. To elucidate whether such mechanisms contribute to the ouabain-induced Ca2+ transient augmentation in WT mice, we repeated the measurements in mice in which both NKA-α1 and NKA-α2 are ouabain resistant (α1RR α2RR mice; Figure 3C). As expected, ouabain had no effect on [Na+]i in these mice. Furthermore, ouabain did not alter Ca2+ transients either, which indicates that the enhancement observed in WT mice is caused by NKA-α2 inhibition.
SR Ca2+ content was not significantly altered by ouabain in either WT (ΔF/F0= 6.3 ± 0.5 with ouabain vs. 5.9 ± 0.4 without) or SWAP mice (6.4 ± 0.6 vs. 6.3 ± 0.5; Figure 4). However, low concentrations of ouabain significantly increased the fractional SR Ca2+ release (calculated as the ratio between the amplitude of twitch- and caffeine-induced Ca2+ transients) in WT and had no effect in SWAP mice (Figure 4).
4. Discussion
Whether NKA-α2 preferentially (vs. NKA-α1) regulates cardiac myocyte [Ca2+]i has not been determined unequivocally because the ouabain-resistant NKA-α1 cannot be selectively blocked to assess its effect. We overcame this by using mice in which NKA-α1 is ouabain sensitive and NKA-α2 is ouabain resistant (SWAP mice). At baseline, Na+ and Ca2+ regulation are similar in WT and SWAP mice. For equal levels of total NKA inhibition (∼25%), ouabain significantly increased Ca2+ transients and fractional SR Ca2+ release in WT (NKA-α2 block) but not in SWAP myocytes (NKA-α1 block), despite a similar [Na+]i rise. These findings support the hypothesis that, similar to smooth muscle and astrocytes, NKA-α2 has a more prominent role in modulating cardiac myocyte Ca2+ release.
4.1. Local [Ca2+]i control by NCX and NKA-α2
In cardiac myocytes contracting at steady state, NCX must remove ∼10 μmoL/L Ca2+ at each beat, the amount that enters via Ca2+ channels.1 Thus, at each beat NCX brings in ∼32 μmoL/L Na+.24 To maintain low [Na+]i and therefore facilitate NCX-mediated Ca2+ extrusion, Na+ must be pumped out of the myocytes by NKA. Thus, the interplay between NCX and NKA is essential for regulating [Na+]i and [Ca2+]i and profoundly influences cardiac contractility and arrhythmogenesis. Functional, co-immunoprecipitation and immunofluorescence studies support a mechanism where NCX and NKA interact functionally via local, microdomain [Na+]i rather than bulk [Na+]i. Rapid NKA blockade can slow the decline of caffeine-induced Ca2+ transients and NCX current25 and increase outward NCX current,26 under conditions such that bulk [Na+]i and SR Ca2+ load are not expected to change. NCX and NKA have been shown to co-immunoprecipitate14,15 and co-localize,15 which indicate a close physical association. The question is to what extent the microdomain controlled by NCX and NKA overlaps with the cleft at the sarcolemma–SR membrane junctions and if NKA isoform matters. During excitation, [Ca2+]i increases faster and higher in the junctional cleft vs. bulk cytosol. NCX also senses an early rise in local [Ca2+]i during SR Ca2+ release,27–29 suggesting that it has some access to Ca2+ in or near the junctional cleft. Indeed, Ca2+ entry via reverse-mode NCX can trigger the SR Ca2+ release, although with reduced efficiency compared with L-type Ca2+ channels.30–32 Data from NCX-KO mice indicate that by priming the cleft with Ca2+, NCX is essential for the normal triggering of Ca2+ release.33 Moreover, a recent immunofluorescence study found that NCX partially co-localizes with ryanodine receptors (27%).34 All these data are consistent with the idea that the junctional cleft and the microdomain controlled by NCX and NKA are functionally (and maybe also physically) overlapping.
Our data support this hypothesis and further point to α2 as the NKA isoform that is functionally important in controlling the cleft [Ca2+]i and SR Ca2+ release. The ouabain-induced local [Ca2+]i increase that sensitizes ryanodine receptors to release more Ca2+ during electrical stimulation may also partially inactivate L-type Ca2+ channels (as in the NCX-KO mice), thus partially masking the inotropic effect of NKA-α2 inhibition. Prior work provided indirect evidence for such a preferential role of NKA-α2 in cardiac myocytes.7–9,11,12 For example, selective NKA-α2 inhibition was found to increase rat myocyte contractility by 40%8 and to slow NCX-mediated Ca2+ extrusion12 although global [Na+]i was not affected. However, this is the first study to demonstrate that selective NKA-α1 inhibition has a significantly smaller effect on the cardiac myocyte SR Ca2+ release than the inhibition of a similar number of NKA-α2 pumps. We have previously shown that although NKA-α2 function is concentrated in the T-tubules, where Ca2+ release sites are predominantly located, the amount of NKA-α1 and NKA-α2 in the T-tubules is comparable.7 Both NKA-α1 and NKA-α2 were found to co-immunoprecipitate with NCX,14,15 thus both might create microdomains with NCX. It is possible, however, that the NCX–NKA-α2 microdomains are localized in the immediate vicinity of the sarcolemma–SR junctions, as in the smooth muscle and astrocytes. In contrast, NKA-α1 might predominate in the non-junctional area of the T-tubules. Alternatively, the NCX–NKA-α1 microdomains may also be localized at or near the junctions but the effect of NKA-α1 inhibition is diluted by the uniform distribution of NKA in the T-tubules and external sarcolemma. Both possibilities result in NKA-α1 being less important in regulating the local cleft [Na+]i (and implicitly local [Ca2+]i). Instead, NKA-α1, as the dominant isoform, is central to determining global [Na+]i.
4.2. NKA-α2 specific glycosides as inotropic agents in heart failure
Cardiac glycosides have been used for treating congestive heart failure (HF) for over 200 years. By raising [Na+]i and thus impeding NCX-mediated Ca2+ extrusion, they increase the cellular and SR Ca2+ content and cause inotropy. However, their use is currently limited by the induction of lethal cardiac arrhythmias caused by spontaneous diastolic SR Ca2+ release. Our data indicate that selectively blocking NKA-α2 may produce inotropy associated with a smaller rise in [Na+]i and SR Ca2+ load. Since diastolic SR Ca2+ release, the primary cause for triggered arrhythmias, depends steeply on the SR Ca2+ content, cardiac glycosides that specifically block NKA-α2 may have a lower risk of triggering arrhythmias and thus a wider therapeutic window than regular cardiac glycosides. Cardiac glycosides may also have other adverse effects35 and using a glycoside targeted to the NKA-α2 isoform may result in an inotropic effect at a drug concentration that is low enough to minimize these adverse effects. In the human heart, NKA-α2 represents 15–35% of the total NKA.4,5 All cardiac glycosides currently known have comparable affinity for the three human NKA isoforms. Thus, finding cardiac glycosides with selectivity for human NKA-α2 may lead to new treatment options for patients with HF, especially those with low output syndrome, left ventricular systolic dysfunction, and low systolic blood pressure that need inotropic agents to improve symptoms and end-organ function.36,37
In summary, we found that for a similar increase in [Na+]i, selective NKA-α2 inhibition generates a greater enhancement of cardiac myocyte Ca2+ transient and SR Ca2+ release than that produced by selective NKA-α1 blockade. Thus, NKA-α2 preferentially (vs. NKA-α1) regulates cardiac inotropy.
Funding
This work was partially supported by the American Heart Association (0735084N to S.D.) and the National Institutes of Health (HL109501 to S.D., HL81526 to D.M.B., HL28573 to J.L.).
Conflict of interest: none declared.
References
- 1.Bers DM. Excitation-contraction Coupling and Cardiac Contractile Force. 2nd ed. Kluwer Academic Press, Dordrecht, The Netherlands; 2001. [Google Scholar]
- 2.Hensley CB, Azuma KK, Tang MJ, McDonough AA. Thyroid hormone induction of rat myocardial Na+/K+-ATPase: α1-, α2-, and β1-mRNA and-protein levels at steady state. Am J Physiol. 1992;262:C484–C492. doi: 10.1152/ajpcell.1992.262.2.C484. [DOI] [PubMed] [Google Scholar]
- 3.Sweadner KJ, Herrera VLM, Amato S, Moellmann A, Gibbons DK, Repke KRH. Immunologic identification of Na+,K+-ATPase isoforms in myocardium. Circ Res. 1994;74:669–678. doi: 10.1161/01.res.74.4.669. [DOI] [PubMed] [Google Scholar]
- 4.Shamraj OI, Melvin D, Lingrel JB. Expression of Na+,K+-ATPase isoforms in human heart. Biochem Biophys Res Commun. 1991;179:1434–1440. doi: 10.1016/0006-291x(91)91733-s. [DOI] [PubMed] [Google Scholar]
- 5.Zahler R, Gilmore-Herbert M, Baldwin JC, Franco K, Benz EJ., Jr Biochim Biophys Acta. 1993;1149:189–194. doi: 10.1016/0005-2736(93)90200-j. [DOI] [PubMed] [Google Scholar]
- 6.Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci USA. 1997;94:1800–1805. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Despa S, Bers DM. Functional analysis of Na+/K+-ATPase isoform distribution in rat ventricular myocytes. Am J Physiol Cell Physiol. 2007;293:C321–C327. doi: 10.1152/ajpcell.00597.2006. [DOI] [PubMed] [Google Scholar]
- 8.Swift F, Tovsrud N, Enger UH, Sjaastad I, Sejersted OM. The Na+,K+-ATPase α2-isoform regulates cardiac contractility in rat cardiomyocytes. Cardiovasc Res. 2007;75:109–117. doi: 10.1016/j.cardiores.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse Na+/K+-ATPase α1 and α2-subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res. 2007;73:92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, et al. Identification of a specific role for the Na+,K+-ATPase α2 isoform as a regulator of calcium in the heart. Mol Cell. 1999;3:5555–5563. doi: 10.1016/s1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Su Z, Moseley AE, Kadono T, Zhang J, Cougnon M, et al. Relative abundance of α2 Na+ pump isoform influences Na+-Ca2+ exchanger currents and Ca2+ transients in mouse ventricular myocytes. J Mol Cell Cardiol. 2005;39:113–120. doi: 10.1016/j.yjmcc.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Swift F, Tovsrud N, Sjaastad I, Sejersted OM, Niggli E, Egger M. Functional coupling of α2-isoform Na+/K+-ATPase and Ca2+ extrusion through the Na+/Ca2+-exchanger in cardiomyocytes. Cell Calcium. 2010;48:54–60. doi: 10.1016/j.ceca.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Moseley AE, Huddleson JP, Bohanan CS, James PF, Lorenz JN, Aronow BJ, et al. Genetic profiling reveals global changes in multiple biological pathways in the hearts of Na+, K+-ATPase alpha 1 isoform haploinsufficient mice. Cell Physiol Biochem. 2005;15:145–158. doi: 10.1159/000083647. [DOI] [PubMed] [Google Scholar]
- 14.Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The α1 isoform of Na+,K+-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na+/Ca2+ exchanger in heart. J Biol Chem. 2004;279:54053–54061. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 15.Mohler PJ, Davis JQ, Bennet V. Ankyrin-B coordinates the Na+/K ATPase, Na+/Ca2+ exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, et al. The α2 isoform of Na+,K+-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem. 2003;278:53026–53034. doi: 10.1074/jbc.M308547200. [DOI] [PubMed] [Google Scholar]
- 17.Price EM, Lingrel JB. Structure-function relationships in the Na+,K+-ATPase α subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988;27:8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- 18.Price EM, Rice DA, Lingrel JB. Structure-function studies of Na+,K+-ATPase. Site-directed mutagenesis of the border residues from the H1-H2 extracellular domain of the α subunit. J Biol Chem. 1990;265:6638–6641. [PubMed] [Google Scholar]
- 19.DeSantiago J, Maier LS, Bers DM. Phospholamban is required for CaMKII-dependent recovery of Ca2+ transients and SR Ca2+ reuptake during acidosis in cardiac myocytes. J Mol Cell Cardiol. 2002;34:975–984. doi: 10.1016/j.yjmcc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na+/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 21.Bossuyt J, Despa S, Han F, Hou Z, Robia SL, Lingrel JB, et al. Isoform specificity of the Na+/K+-ATPase association and regulation by phospholemman. J Biol Chem. 2009;284:26749–26757. doi: 10.1074/jbc.M109.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagawa T, Sagawa K, Kelly JE, Tsushima RG, Wasserstrom JA. Activation of ryanodine receptors by cardiac glycosides. Am J Physiol. 2001;280:H1201–H1207. doi: 10.1152/ajpheart.2001.280.3.H1201. [DOI] [PubMed] [Google Scholar]
- 23.Santana LF, Gómez AM, Lederer WJ. Ca2+ flux through promiscuous cardiac Na+ channels: slip-mode conductance. Science. 1998;279:1027–1033. doi: 10.1126/science.279.5353.1027. [DOI] [PubMed] [Google Scholar]
- 24.Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res. 2003;57:897–912. doi: 10.1016/s0008-6363(02)00656-9. [DOI] [PubMed] [Google Scholar]
- 25.Terracciano CMN. Rapid inhibition of the Na+-K+ pump affects Na+-Ca2+ exchanger-mediated relaxation in rabbit ventricular myocytes. J Physiol. 2001;533:165–173. doi: 10.1111/j.1469-7793.2001.0165b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Z, Zou A, Nonaka A, Zubair I, Sanguinetti MC, Barry WH. Influence of prior Na+ pump activity on pump and Na+/Ca2+ exchange currents in mouse ventricular myocytes. Am J Physiol. 1998;275:H1808–H1817. doi: 10.1152/ajpheart.1998.275.5.H1808. [DOI] [PubMed] [Google Scholar]
- 27.Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J Physiol. 1995;488:577–586. doi: 10.1113/jphysiol.1995.sp020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber CR, Piacentino V, III, Ginsburg KS, Houser SR, Bers DM. Na+/Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circ Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- 29.Acsai K, Antoons G, Livshitz L, Rudy Y, Sipido KR. Microdomain [Ca2+] near ryanodine receptors as reported by L-type Ca2+ and Na+/Ca2+ exchange currents. J Physiol. 2011;589:2569–2583. doi: 10.1113/jphysiol.2010.202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasserstrom JA, Vites AM. The role of Na+-Ca2+ exchange in activation of excitation-contraction coupling in rat ventricular myocytes. J Physiol. 1996;493:529–542. doi: 10.1113/jphysiol.1996.sp021401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sipido KR, Maes M, Van de Werf F. Low efficiency of Ca2+ entry through the Na+-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. A comparison between L-type Ca2+ current and reverse-mode Na+-Ca2+ exchange. Circ Res. 1997;81:1034–1044. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- 32.Litwin SE, Li J, Bridge JH. Na+-Ca2+ exchange and the trigger for sarcoplasmic reticulum Ca2+ release: studies in adult rabbit ventricular myocytes. Biophys J. 1998;75:359–371. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neco P, Rose B, Huynh N, Zhang R, Bridge JH, Philipson KD, et al. Sodium-calcium exchange is essential for effective triggering of calcium release in mouse heart. Biophys J. 2010;99:755–764. doi: 10.1016/j.bpj.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayasinghe ID, Cannell MB, Soeller C. Organization of ryanodine receptors, transverse tubules, and sodium-calcium exchanger in rat myocytes. Biophys J. 2009;97:2664–2673. doi: 10.1016/j.bpj.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamimura D, Ohtani T, Sakata Y, Mano T, Takeda Y, Tamaki S, et al. Ca2+ entry mode of Na+/Ca2+ exchanger as a new therapeutic target for heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1408–1416. doi: 10.1093/eurheartj/ehr106. [DOI] [PubMed] [Google Scholar]
- 36.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 37.Teerlink JR, Metra M, Zacà V, Sabbah HN, Cotter G, Gheorghiade M, et al. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond . Heart Fail Rev. 2009;14:243–253. doi: 10.1007/s10741-009-9153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]