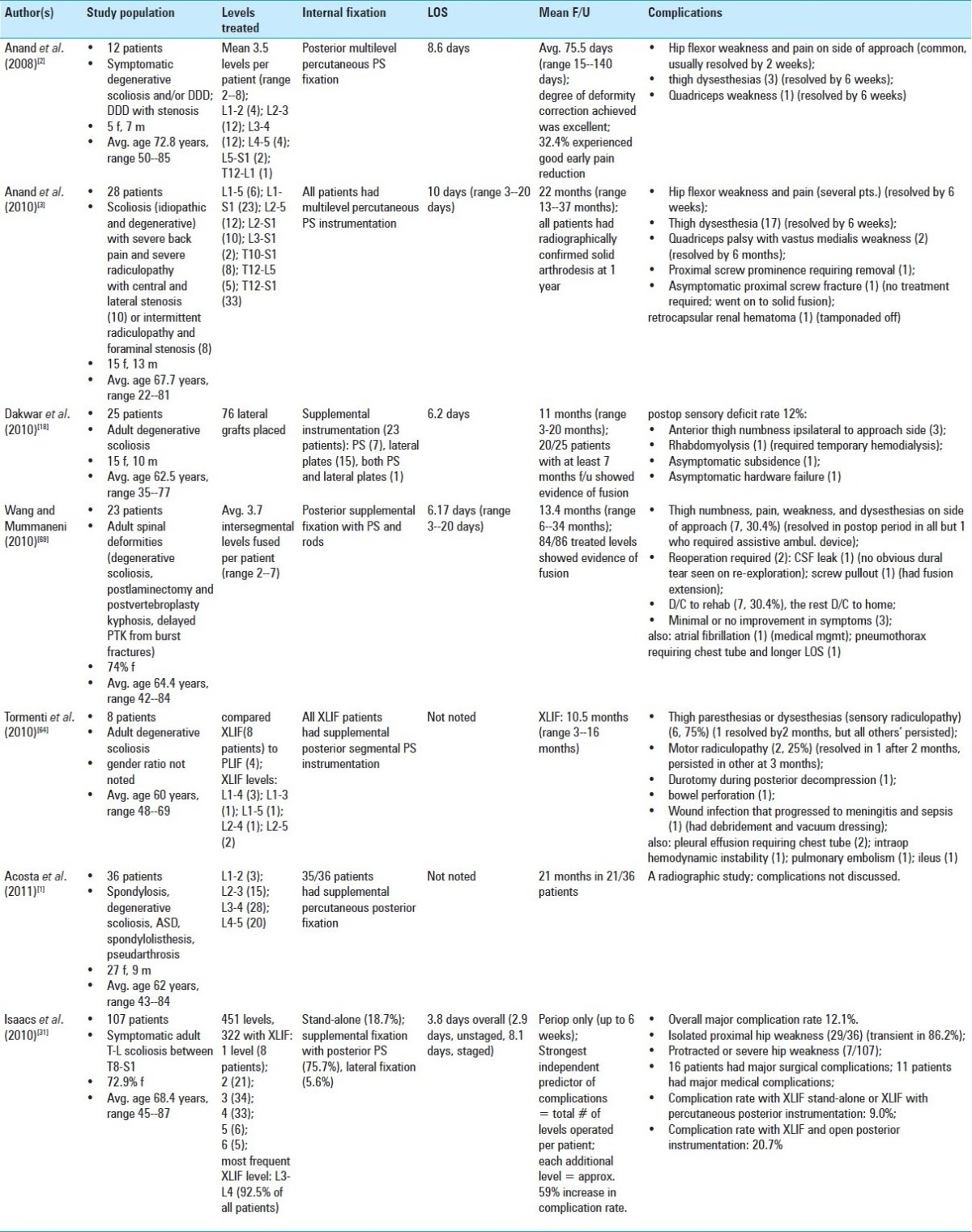
Abstract
Background:
In the last several years, the lateral transpsoas approach to the thoracic and lumbar spine, also known as extreme lateral interbody fusion (XLIF) or direct lateral interbody fusion (DLIF), has become an increasingly common method to achieve fusion. Several recent large series describe several advantages to this approach, including less tissue dissection, smaller incisions, decreased operative time, blood loss, shorter hospital stay, reduced postoperative pain, enhanced fusion rates, and the ability to place instrumentation through the same incision. Indications for this approach have expanded and now include degenerative disease, tumor, deformity, and infection.
Methods:
A lateral X-ray confirms that the patient is in a truly lateral position. Next, a series of tubes and dilators are used, along with fluoroscopy, to identify the mid-position of the disk to be incised. After continued dilation, the optimal site to enter the disk space is the midpoint of the disk, or a position slightly anterior to the midpoint of the disk. XLIF typically allows for a larger implant to be inserted compared to TLIF or PLIF, and, if necessary, instrumentation can be inserted percutaneously, which would allow for an overall minimally invasive procedure.
Results:
Fixation techniques appear to be equal between XLIF and more traditional approaches. Some caution should be exercised because common fusion levels of the lumbar spine, including L4-5 and L4-S1, are often inaccessible. In addition, XLIF has a unique set of complications, including neural injuries, psoas weakness, and thigh numbness.
Conclusion:
Additional studies are required to further evaluate and monitor the short and long-term safety, efficacy, outcomes, and complications of XLIF procedures.
Keywords: Lateral transpsoas approach, extreme lateral interbody fusion, direct lateral interbody fusion, lumbar spine, lumbosacral plexus, surgical technique
INTRODUCTION
The minimally invasive lateral transpsoas approach to the lumbar and thoracic spine, also known as extreme lateral interbody fusion (XLIF) or direct lateral interbody fusion (DLIF), was first described in 2001.[44,49] This technique has become an increasingly popular approach for achieving interbody fusion. The reported advantages include minimally invasive access to the spine, less blood loss compared to open procedures, decreased operative times, shorter hospital stays, and less postoperative pain.[20,43,44] The lateral transpsoas approach has been used in the management of adult degenerative disease as well as degenerative scoliosis.[1,2,6] Total disk replacement has also been achieved via this technique.[48] Biomechanical studies have shown equivalency between XLIF and anterior approaches to the lumbar spine.[27,38]
BACKGROUND
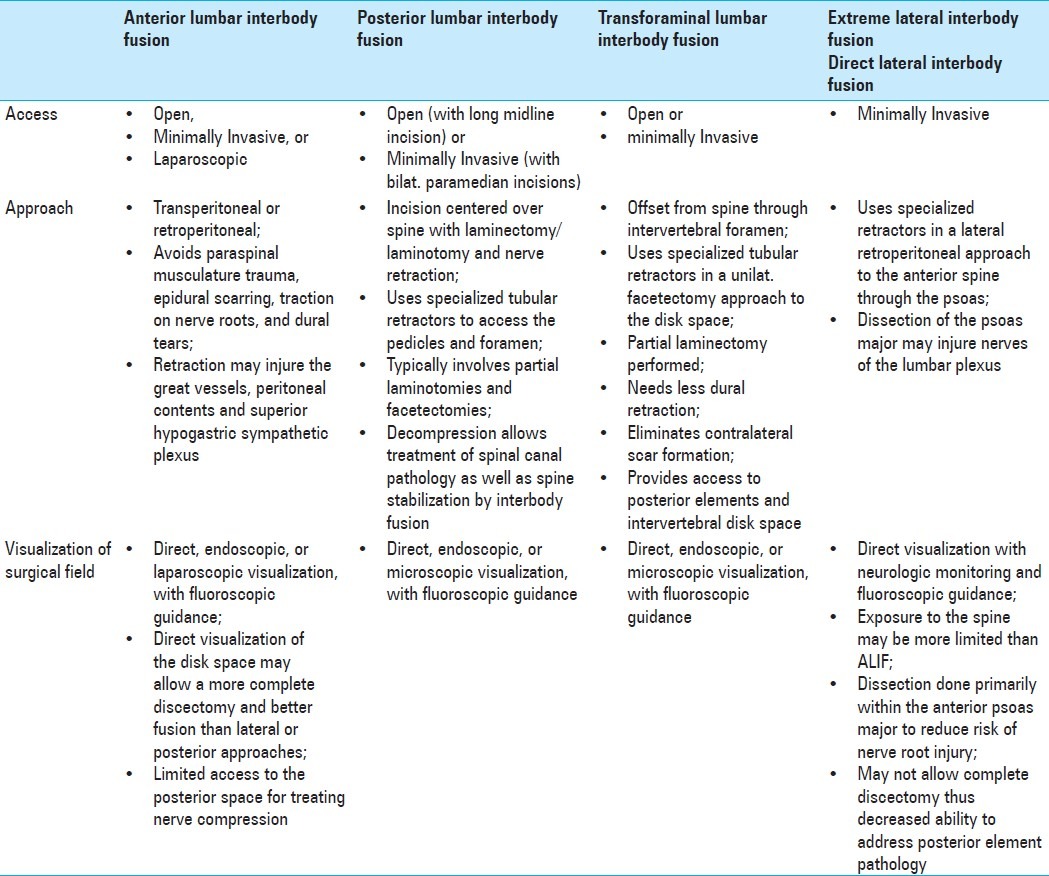
Differences between the lateral transpsoas approach, anterior lumbar interbody fusion, posterior lumbar interbody fusion, and transforaminal interbody fusion
The lateral transpsoas procedure differs from traditional anterior lumbar interbody fusion (ALIF), traditional posterior lumbar interbody fusion (PLIF), and transforaminal lumbar interbody fusion (TLIF) in several important ways[4,22,30,31,36,37,53,54,56,59,68,70,71] [Table 1]. In the lateral transpsoas procedure, the patient is placed in the lateral decubitus position rather than being prone. Neural monitoring, including electromyography (EMG), is mandatory with the XLIF, because it employs a muscle-splitting technique that exposes the lumbar plexus to potential injury.[5,37,63] In fact, injury to this plexus is one of the main risk factors of this procedure.[5,35,41]
Table 1.
Comparison of minimally invasive surgical approaches for lumbar interbody fusion
METHODS OF THE LATERAL TRANSPSOAS APPROACH TO THE SPINE
Monitoring and x-ray confirmation of proper positioning
After the patient is properly positioned and the appropriate surgical area is localized, electrodes are placed that correspond to the myotomes L2-L5. Stimulation is then performed to achieve adequate twitch strength, allowing for accurate and reproducible EMG recordings. A lateral X-ray confirms that the patient is in a truly lateral position.
Performing the lateral transpsoas approach utilizing multiple tubes/dilators
Several techniques utilize the XLIF approach to the disk space. A series of tubes and dilators are used, along with fluoroscopy, to identify the mid-position of the disk to be incised. The first dilator is introduced through a small incision, and from a second small posterior incision, the surgeon's index finger directs the dilator through the retroperitoneal space to the psoas muscle.
Positioning of the dilator and exposure for the lateral transpsoas approach
The surgeon's index finger, now in the retroperitoneal space, guides the dilator from the first incision to the psoas muscle, taking care not to injure the intra-abdominal organs. The fibers of the psoas muscles are separated with the initial dilator, and the neural monitoring system can evaluate how close the dilator is to the lumbar nerve roots, which is a critical step in guarding against neural injury. The closer the tip of the electrode is to a nerve, the greater the current adjacent to the nerve. However, direct vision of the surgical field may reveal nerve tissue that does not respond to customary EMG stimulation. This stimulation usually localizes the lumbosacral plexus to the inferior posterior quadrant of the dilator tube over the lateral disk space. Thus, with continued dilation, the optimal site to enter the disk space is the midpoint of the disk, or a position slightly anterior to the midpoint of the disk.
Application of the retractor for the lateral transpsoas approach
After the second and then third dilators are introduced over the initial dilator, a retractor is inserted over the last dilator and fixed in place to the operating room table. The retractor is then opened to the surgical field over the disk space and neural monitoring is again checked to assure the neural elements are not being stretched across the operative field.
Disk excision utilizing the lateral transpsoas approach
The disk can now be incised and removed. Fluoroscopy is useful to ascertain the depth to which the disk is resected; XLIF typically allows for a larger implant to be inserted compared to either TLIF or PLIF. If instrumentation is necessary, it can be inserted percutaneously, which will allow for an overall minimally invasive procedure. Ozgur et al. provides a comprehensive discussion of the details of XLIF.[44]
ANATOMY
Definition of “safe” working zones for the lateral transpsoas approach
Because nerve injury during the transpsoas approach is the most common and potentially the most devastating complication of the XLIF procedure,[28,37] several studies have looked at defining “safe” working zones. These studies have included cadaver,[5,41,47,65] electrical,[66] and radiographic[24,25,51] evaluations.
Cadaver studies for the lateral transpsoas approach
Several cadaver studies defined the anatomy of the lumbar plexus and proposed an appropriate working space where dilators could be placed at each level of the lumbar spine.[5,41,47,65] The position of the lumbar plexus and the location of where the genitofemoral nerve emerged into the abdominal space were identified [Figures 1 and 2]. Generally, these studies showed that when approaching the lumbar spine from L3, L2, or L1, the psoas muscle should be split into the ventral three-quarters of the vertebral body (VB) to avoid nerve injury.[24] There is risk to the genitofemoral nerve if the psoas major muscle is split at L3 or L4. The lumbosacral plexus is most dorsally positioned at the posterior endplate of L1-2, with a general trend of progressive ventral migration of the plexus on the disk space from L2-3 to L4-5. Placing the dilator or retractor in a posterior position may result in nerve injury, especially at L4-5.[5,32,47] Uribe et al. discussed the potential of injury to the ilioinguinal, iliohypogastric, and lateral femoral cutaneous nerves in the retroperitoneal space.[65] Hu et al. showed similar findings in a magnetic resonance imaging (MRI) study.[29] EMG monitoring during surgery is essential to preventing neural injury during the XLIF.[63]
Figure 1.
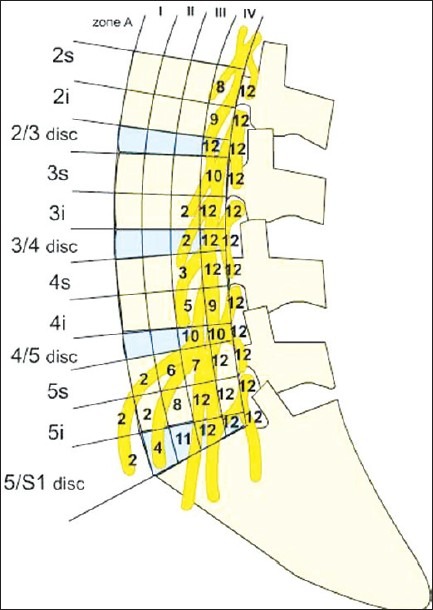
Diagram shows “safe zone” for placement of retractor
Figure 2.
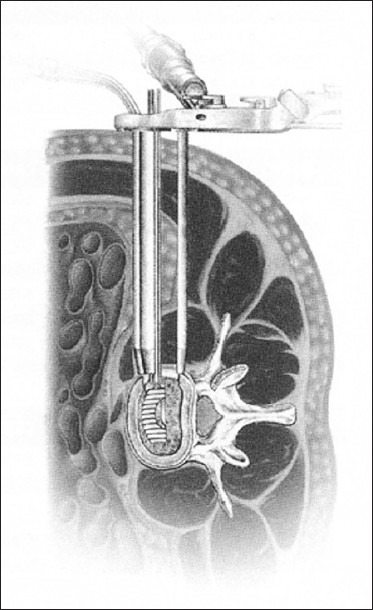
Schematic drawing of exposure before disk removal
RESULTS OF XLIF SURGERY
Levels and limitations of XLIF surgery
The most common XLIF procedure involves treatment of one disk level, although four- and five-level disease has been treated with this approach. The L5-S1 disk space is usually inaccessible due to the presence of the sacrum, and nearly half the time the L4-5 interspace is similarly obscured.[10,61] Smith et al. also found that approaching a lumbarized sacrum via this approach was a relative contraindication.[61]
Multiple indications for XLIF surgery
The majority of XLIF procedures are performed for degenerative conditions, including spondylolisthesis, herniated disk, degenerative disk disease, postlaminectomy kyphosis, adjacent segment disease, and degenerative scoliosis. Rarely has the procedure been used to treat osteomyelitis or tumor [Table 2].
Table 2.
Recent extreme lateral interbody fusion studies: Diagnoses in the study population

Most common indication for XLIF (degenerative lumbar disease) and outcomes
One of the most common indications for XLIF is degenerative disease of the lumbar and thoracolumbar spine. Ozgur et al., in advancing the technology from endoscopy to the XLIF, published the first feasibility study in 2006.[44] They reported no complications in their first 13 patients, although surgical indications were not discussed.
Fusion rates and outcomes after XLIF surgery
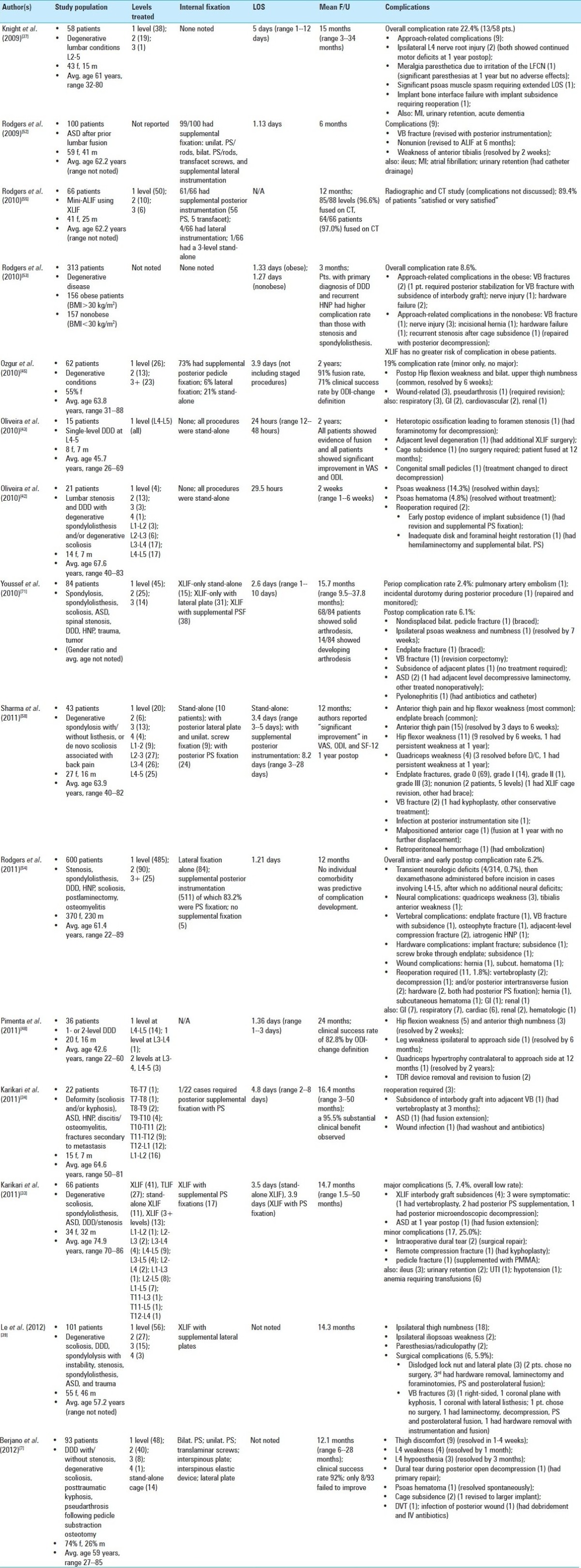
The bulk of the large series detailing outcomes and complications for XLIF were published in the past few years. Most of these studies were retrospective, and surgical procedures were typically performed at one or two levels accompanied by supplemental fixation (plates or pedicle screws)[37,42,43,45,52,53,55,71] [Table 3A]. Knight et al. published an early complication profile in 2009 in which 58 patients underwent mostly one- and two-level fusions for degenerative lumbar disease.[37] There was a 22.4% overall complication rate, and most complications were approach related. Significantly, two patients continued to have L4 motor deficits one year after surgery. Clinical outcomes were not discussed in more detail.
Table 3A.
Extreme lateral interbody fusion for degenerative conditions: recent large series reporting outcomes and complications (adjacent segment disease, degenerative disk disease, HNP, postlaminectomy kyphosis, spondylolisthesis, stenosis)
Complications after XLIF surgery
Rodgers et al. further assessed fusion rates and patient outcomes in 66 patients one year after surgery; 96.6% of levels were judged as fused on CT scan, with nearly 90% of patients “satisfied or very satisfied.”[55] Complications other than those described in their previous reports were not discussed in this series.[52,53]
Ozgur et al. reported a series of 62 patients who had two-year follow-up following XLIF.[45] They reported a 91% fusion rate and 75% frequency of “clinical success” (ODI-change definition). There was a 19% minor complication rate. The most frequent complication was hip flexion weakness that typically resolved within six weeks after surgery. Additionally, one patient with pseudarthrosis required revision surgery.
Complication rate for XLIF in obese patients not increased
Rodgers et al. reported on a series of 156 obese patients who underwent XLIF and found that they were no more likely to experience complications than the nonobese patients.[53] However, the obese patients had an approximate 7% complication rate, and four patients required secondary surgery. Nevertheless, there were fewer neural injuries in the obese vs. the nonobese population. When Rodgers et al. reported on another series of 100 patients in whom XLIF was used to treat adjacent segment disease, patients achieved excellent results with short hospital stays and minimal complications.[52]
XLIF surgery with bone morphogenetic protein rhBMP-2 [INFUSE® Bone Graft, (Medtronic Sofamor Danek Inc., Memphis TN, USA)]
Oliveira et al. reported on a series of 15 patients who underwent one-level stand-alone XLIFs supplemented with bone morphogenetic protein (rhBMP-2: INFUSE®).[43] Although all patients achieved solid fusion, two (13.3%) required repeat surgery. One secondary procedure addressed excessive (ectopic) bone formation that led to nerve root compression, which is a commonly described complication directly attributable to rhBMP-2/INFUSE® Bone Graft. The other secondary procedure addressed the failure of “indirect decompression” attributed to congenital small pedicles. Otherwise, all patients experienced significant improvement utilizing standard outcome measures. Furthermore, the average hospital length of stay was a remarkable 30 hours.
Dramatic increase in use of rhBMP-2 (INFUSE®) in the last decade
The use of rhBMP-2 in spinal fusion surgeries increased dramatically in the last decade. The results of preliminary human trials of rhBMP-2 in lumbar fusion were published in 2000 and 2002, and neither study reported any adverse events directly related to rhBMP-2.[8,9] From 2003 to 2009, several industry-sponsored or industry-associated studies again reported no adverse events directly related to rhBMP-2.[11–14,19,21,23,26]
Safety concerns regarding rhBMP-2 (INFUSE®) since 2002
As early as 2002, however, safety concerns regarding the use of rhBMP-2 in spine fusions were reported.[16,50] These safety issues included bony overgrowth or uncontrolled bone formation (heterotopic ossification), graft subsidence, loss of fixation, inflammation, infection, cancer risk, toxicity (local, systemic, and reproductive), neurological events/deterioration, retrograde ejaculation, radiculitis, and functional loss.[16,50] Despite those concerns, the nationwide usage of rhBMP-2 (INFUSE®) in spine fusions increased from 0.7% in 2002 to 24.3% in 2006.[15]
Intense scrutiny of rhBMP-2 (INFUSE®) since 2006 by United States Food and Drug Administration
In 2006, the first of a series of studies describing serious complications associated with the use of rhBMP-2 was published.[62,67] Soon rhBMP-2 and its manufacturer, Medtronic Inc. (Memphis TN, USA), came under intense scrutiny by the FDA, the U.S. Justice Department, and a U.S. Senate Committee.
In 2009, Cahill et al. conducted a retrospective cohort study of 328,468 patients who underwent spinal fusion procedures, including 17,623 patients in whom rhBMP-2 was used. The authors found that rhBMP-2 use in thoracic and lumbar fusions was not associated with any increased frequency of postoperative inpatient complications. (Notably, delayed outpatient complications were not analyzed.)[15] In 2011, Carragee et al. reported revised estimates of the risks of adverse events associated with the use of rhBMP-2 in various types of spinal fusions.[16] They calculated a 25-50% risk of rhBMP-2-associated adverse events occurring in PLIF, including osteolysis, graft migration, subsidence, cyst formation, and neuritis. They calculated a 10-15% risk of rhBMP-2-associated adverse events occurring in ALIF (for which it was FDA approved), including the above events as well as urinary retention and retrograde ejaculation.
XLIF indirect decompression of nerve roots in patients with degenerative disk disease and stenosis
Oliveira et al. also looked at the ability of XLIF procedures to indirectly decompress nerve roots in a small series of patients with degenerative disk disease and stenosis.[43] They noted substantial dimensional improvement on all radiographic parameters in 15 patients undergoing stand-alone XLIF. However, three patients had transient psoas weakness and two patients required another operation for decompression. The authors noted that XLIF provided adequate neural decompression for central or lateral stenosis but that this approach may not be appropriate for congenital stenosis. Furthermore, implant subsidence may also limit the utility of XLIF in patients with stenosis.
Youssef et al. reported minimal complications, good fusion rates, and good patient outcomes with XLIF in their series of 84 patients with an average follow-up of 16 months.[71]
A variety of complications of XLIF
In the last year, several additional series have reported a variety of complications attributed to the XLIF procedure.[7,33,34,39,48,54,58] Sharma et al. evaluated 43 patients treated with XLIF with a one-year follow-up, and found that 25% had transient postoperative anterior thigh pain and another 25% had postoperative hip flexor or quadriceps weakness; notably, two patients still had the latter deficit one year after surgery.[58] Additionally, there were five nonunions, one VB fracture (which required kyphoplasty), one infection, one malpositioned cage, and one retroperitoneal hemorrhage. Despite these issues, the authors reported “significant improvement” in outcome scores (Visual Analog Scale [VAS], Oswestry Disability Index [ODI], and SF-12) one year after surgery.
Complications of XLIF: transient neurological deficits and requirement for reoperations
Rodgers et al. reported on the largest series of XLIF procedures, and found a 6.2% complication rate in the early (six weeks) postoperative period in 600 procedures.[54] The authors noted shorter hospitalizations and fewer vascular, neurologic, or infectious complications compared with traditional open procedures; specifically, they observed four transient but no permanent neurologic injuries. The revision rate (reoperation rate) in their series of 1.8% was also comparable to that found in other series, and included five revisions for fractures, two for hardware, and two abdominal procedures. Similar results were reported in the other recent series [Table 3A].
Advantages of the XLIF approach with total disk replacement
Pimenta et al. concluded that the XLIF was safer and less invasive than the anterior approach (ALIF), demonstrated minimal morbidity (maintaining pain relief and functional improvement), avoided mobilization of the great vessels, preserved the anterior longitudinal ligament (ALL), resulted in biomechanical stability, and offered broader revision options.[48] When Pimenta et al. evaluated the clinical (pain and function) and radiographic ROM outcomes of a true lateral transpsoas (XLIF) approach for lumbar total disk replacement (TDR), they found that XLIF offered several advantages over the traditional anterior approach.[48] The authors prospectively evaluated 36 patients (mean age 42.6 years) with 1- or 2-level DDD who underwent TDR procedures and were followed for a minimum of 24 months, and observed that all patients were walking within 12 hours of surgery. Furthermore, at two years’ follow-up, the average VAS and ODI scores had improved 69.6% and 61.4%, respectively; ROM averaged 8.6°, which was well within normal limits.
Neurological complications of the XLIF approach with total disk replacement
Nevertheless, in the Pimenta et al. study, significant neurological complications were observed following XLIF for TDR. For instance, five patients had new psoas weakness and three had new anterior thigh numbness; fortunately, both conditions resolved within 2 postoperative weeks. However, one patient had leg weakness ipsilateral to the approach side which required 6 months to resolve, while another patient had quadriceps hypertrophy contralateral to the approach side which required 12 months to resolve. In two cases, removal of the TDR device and revision to fusion were required for pain that failed to resolve within 2 postoperative years.
Outcomes and complications of XLIF utilized to address scoliosis, tumors, prior fusions, thoracic disks, and discitis/osteomyelitis
Karikari et al. evaluated clinical, radiographic, operative, postoperative, and functional outcomes of 22 patients (mean age 64.6 years) treated with XLIF for various conditions including degenerative scoliosis, pathological fractures from tumors, adjacent level disease from prior fusions, thoracic disk herniations, and discitis/osteomyelitis.[34] In patients treated for degenerative scoliosis, the mean preoperative and postoperative coronal Cobb angles were 22° and 14°, respectively. The mean preoperative and postoperative sagittal angles were 39 and 44, respectively, and the average estimated blood loss and length of stay were 227.5 mL and 4.8 days, respectively. There were three complications that required reoperations: wound infection, subsidence, and adjacent level disease. There were no neural, vascular, or visceral injuries, or deaths. At a mean follow-up of 16.4 months (range 3-50 months), they observed a 95.5% substantial clinical benefit. All patients at 6-month follow-up (95.5%) demonstrated radiographic evidence of fusion. The authors concluded that the XLIF technique was a feasible and safe treatment option for thoracic spine diseases with minimal complications and favorable initial outcomes. Although traditional open approaches achieve a higher degree of deformity correction, the reduced invasiveness of XLIF may be more tolerable for the elderly and for patients with significant medical comorbidities.
Results of minimally invasive interbody fusion (XLIF, TLIF) in the elderly
In a companion study published the same year involving minimally invasive interbody fusions (41 cases of XLIF and 27 cases of TLIF), Karikari et al. evaluated the rate of perioperative and postoperative complications in the elderly.[33] Sixty-six consecutive patients, aged 70 years or older (mean age 74.9 years, range 70-86 years), underwent minimally invasive interbody lumbar fusion; the mean follow-up interval was 14.7 months (range 1.5-50 months). The authors found a low rate of major complications, including four cases of interbody graft subsidence and one case of adjacent level disease. There were no intraoperative medical complications nor any myocardial infarctions, pulmonary embolisms, hardware complications requiring removal, or wound infections, nor were there any major visceral, vascular, or neural injuries, or deaths. The authors concluded that although the effects of even minor complications can be more pronounced in elderly patients (age 70 and older), complex minimally invasive interbody fusion in patients 70 years or older is safe and well tolerated, without significant morbidities or mortality.
Complications of minimally invasive thoracolumbar XLIF instrumented fusions
Le et al. investigated hardware-associated complications in 101 patients who underwent minimally invasive lateral interbody thoracolumbar fusions using lateral plates for multilevel fusions or deformity correction.[39] The authors found a 5.9% complication rate which included three hardware failures, two coronal plane VB fractures, and one lateral VB fracture related to the lateral plate. All complications occurred in multilevel cases, and all cases presented with recurrent back pain except one which was identified incidentally. The authors concluded that minimally invasive lateral interbody fusion is a safe, practical, and direct technique that avoids the complications associated with other types of instrumentation.
Clinical outcomes and complications of XLIF
Berjano et al. reported on the clinical outcomes and complications in 97 consecutive XLIF cases with a minimum 6-month follow-up (mean 12 months).[7] Transient thigh discomfort/numbness was observed in 9%, and transient neurological symptoms presented in 7% of cases; all conditions resolved within one postoperative month. No instances of permanent neurological impairment, vascular or visceral injuries, or wound infections were observed. The authors acknowledged a 92% clinical success rate six months postoperatively. The authors concluded that XLIF is a safe and effective minimally invasive technique for treating lumbar and thoracolumbar spinal pathologies requiring anterior spinal fusion.
Degenerative scoliosis: another indication for XLIF
In the last few years, surgeons have expanded the indications for XLIF to include degenerative scoliosis. Due to the nature of this disease, deformity procedures tend to involve several levels of fixation. Anand et al. published a feasibility study in 2008, reporting on their first 12 scoliotic patients; surgical procedures involved an average of 3.64 segments and an average 13° correction per patient.[2] All patients underwent percutaneous pedicle fixation, and all patients requiring sacral fusion underwent AxiaLIF® (axial lumbar interbody fusion, TranS1, Inc., Wilmington, NC, USA); all procedures utilized rhBMP-2 to supplement the fusions. There were no permanent postoperative complications. Two years later, these same authors reported on their mid-term and long-term results for degenerative scoliosis; all 28 patients fused and maintained their immediate postoperative correction.[3] Complications were minimal and clinical outcomes were good, despite a mean length of stay/hospitalization (LOS) of ten days.
Similarly, Dakwar et al. reported on a series of 25 patients who underwent XLIF for thoracolumbar degenerative deformity.[18] Although sagittal balance was not corrected in one-third of the patients, clinical outcomes were acceptable and were accompanied by minimal long-term complications over an average 11-month follow-up interval. Wang and Mummaneni published a comparable series[69] and achieved an average 20° correction, which was a greater deformity correction than that reported by Dakwar et al.[18] Their fusion rates were excellent, despite a higher complication rate of 30%. Although symptoms resolved in all but one patient, two patients required revision surgery - one for cerebrospinal fluid (CSF) leak and one for hardware failure.
Comparison of outcomes/morbidity of XLIF and TLIF for scoliosis
In a small study, Tormenti et al. compared the surgical treatment of adult scoliosis utilizing the XLIF approach (eight patients) vs. standard posterior-only TLIF (four patients).[64] Patients in the XLIF group achieved greater deformity correction but had more extensive complications, including bowel injury requiring laparotomy (one patient), permanent motor radiculopathy (one patient), and persistent sensory symptoms (five of six patients).
Morbidity of XLIF for deformity/scoliosis
Neural decompression and fusion in patients with adult degenerative scoliosis presents a surgical challenge. Recent studies on surgical treatment of adult scoliotic deformity have found that the lateral transpsoas approach, when compared to traditional open approaches, results in less blood loss, shorter lengths of stay, and earlier mobilization, along with lower rates of infection and fewer transfusions.[2,3,18,31,52,64,69] Nevertheless, these studies also observed more early reoperations and more major complications.[2,3,18,31,52,64,69]
XLIF resulted in excellent deformity correction for scoliosis
Acosta et al. analyzed changes in coronal and sagittal plane alignment following XLIF for degenerative scoliosis and noted excellent results for deformity correction in both planes.[1] Clinical outcomes were also excellent, and included sufficient long-term follow-up results. The authors concluded that the direct lateral transpsoas approach, when combined with posterior fixation, resulted in statistically significant improvement in segmental, regional, and global coronal plane alignment in patients with degenerative lumbar conditions, including degenerative scoliosis. However, the authors also found that there were no statistically significant improvements in regional lumbar lordosis or global sagittal alignment.[1]
Perioperative complications for XLIF with degenerative scoliosis
Isaacs et al. reported on perioperative complications in a prospective series of 107 patients treated for an average 4.4 level degenerative scoliosis.[31] The mean hospital length of stay was three days, and there was a 12.1% major complication rate. A lower major complication rate of 9% was seen for patients undergoing stand-alone XLIF or XLIF with percutaneous instrumentation, while a higher major complication rate of 20.7% was seen in patients undergoing XLIF with posterior instrumentation. Although the presence of at least one comorbidity increased the incidence of major complications, the strongest independent predictor of complications was the total number of levels treated per patient. The authors concluded that their rates of adverse events compared favorably to those cited in other degenerative deformity series [Table 3B].
Table 3B.
Extreme lateral interbody fusion for degenerative scoliosis: recent large series reporting outcomes and complications
XLIF with total disk arthroplasty
Pimenta et al. extended the XLIF indications when they published a series of 36 patients who underwent this procedure for total disk replacement rather than for fusion.[48] The patients underwent either a one- or two-level lumbar arthroplasty, and the authors reported excellent results at two-year follow-up. There were no long-term complications, although two patients required revision to fusion due to persistent pain.
XLIF for osteomyelitis or tumor
In three earlier mentioned series,[34,54,71] patients underwent successful XLIF surgery for the treatment of osteomyelitis or tumor.
XLIF and asymptomatic pseudarthrosis
When Youssef et al. evaluated outcomes of 84 patients who underwent XLIF for various degenerative and deformity conditions, including one patient treated for tumor, the overall complication rate was 6.1%.[71] At an average of 15.7 months postoperatively, 68 patients demonstrated solid arthrodesis on both CT and dynamic radiographs, while the remaining 14 patients developed pseudarthrosis but without complications. Average pain and function scores (VAS and ODI) at one year were significantly improved over preoperative scores. Their results corroborated prior reports that XLIF is a safe and effective approach for lumbar fusion, and that it carries a low morbidity rate. Furthermore, patients maintain long-term improvement in pain and function as well as long-term improvement on radiographic measures.
Results of XLIF with supplemental posterior instrumentation
Rodgers et al. were the first to delineate complications in the early postoperative period (within the first six weeks) in 600 XLIF cases, 511 of whom underwent supplemental posterior instrumentation.[54] The XLIF procedure was utilized primarily for deformity and degenerative conditions, though one case of osteomyelitis was included as well. The authors noted an immediate 65% improvement in VAS pain scores. The overall early complication rate was 6.2%. When compared to traditional open posterior or anterior approaches, there were fewer total and fewer serious complications using the XLIF approach. The authors suggested that rare and transient postoperative neural deficits might be prevented in patients undergoing surgery at L4-L5 by the preoperative administration of dexamethasone before skin incision.
Perioperative morbidities for thoracic and thoracolumbar disease
Karikari et al. reported on perioperative morbidities and initial clinical, radiographic, operative, and functional outcomes in 22 patients who underwent XLIF for isolated thoracic and thoracolumbar diseases.[34] This series also included one patient treated for osteomyelitis and another two patients treated for pathologic fracture secondary to tumor invasion.[34] Only one patient in the series required supplemental posterior instrumentation. All patients who reached at least the 6-month follow-up evaluation demonstrated radiographic evidence of fusion; furthermore, 21 of 22 patients achieved substantial clinical benefit (SCB) for both VAS and ODI at that point. At an average follow-up of 16.4 postoperative months, only 3 of 22 patients had developed a complication. Although XLIF was originally developed for treating lumbar spine diseases, the authors concluded that XLIF is a feasible and safe option for treating thoracic spine disease. Nevertheless, to date, patients with osteomyelitis or tumor represent a small percentage of those undergoing XLIF.
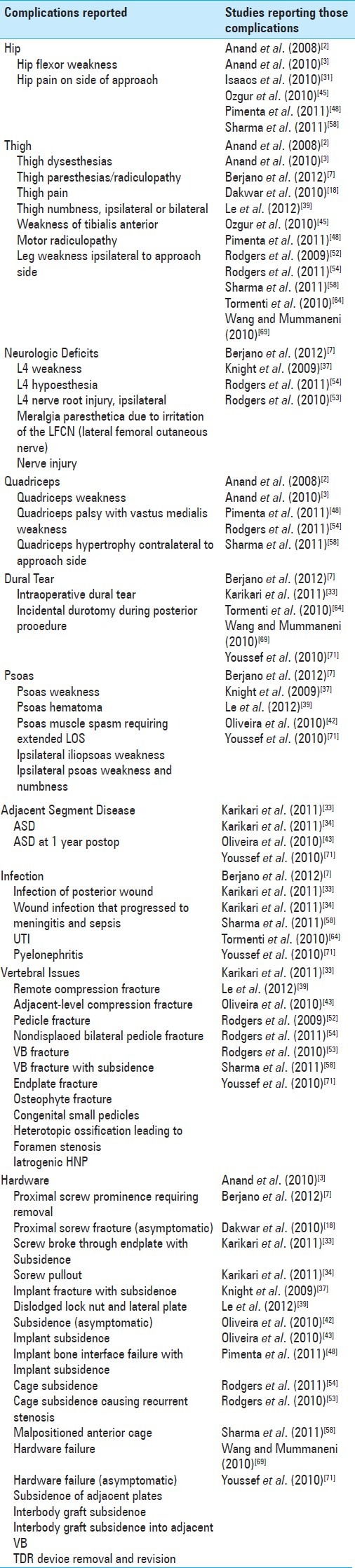
COMPLICATIONS OF XLIF
Although the most common complications following XLIF include thigh numbness, lower extremity radiculopathy with weakness, and pseudarthrosis, other unusual complications have been reported in smaller series or case reports[17,28,40,44,46,60,65,71] [Table 4]. Daffner and Wang reported a patient whose L3-L4 cage migrated one month after surgery.[17] Following cage revision utilizing a mini-open operation with lateral plate fixation, the patient fused and her leg pain resolved.
Table 4.
Extreme lateral interbody fusion for degenerative conditions: summary of reported complications
Contralateral femoral nerve compression following XLIF
Out of 14 patients who underwent XLIF, Papanastassiou et al. reported on two patients who developed the unusual complication of contralateral femoral nerve compression.[46] The first patient sustained a femoral nerve injury due to a displaced endplate fragment compressing the contralateral nerve, while the second patient developed a far lateral disk herniation. Although symptoms resolved in both patients following revision surgery, the authors cautioned against “overzealous” endplate removal in the opposite corner during surgery.
Ipsilateral nerve root injury during transpsoas approach for XLIF
Houten et al. described two patients who developed ipsilateral nerve root injuries during the transpsoas approach.[28] Neither deficit was detected on intraoperative EMG monitoring, leaving both patients with significant motor deficits that only partially recovered more than a year after surgery.
Failures and reoperations following XLIF with lateral fixation
XLIF has some significant technical shortcomings as indicated by the necessity for early reoperation to address chronic CSF leakage due to dural tears, infection, or displaced implants and/or instrumentation.[54] Of 101 patients who underwent XLIF and lateral fixation, Le et al. observed six complications - three VB fractures and three instances of hardware failure.[39] Additionally, one patient in the hardware failure group and two in the fracture group required reoperation or secondary surgery. In another report, a 55-year-old male presented in shock to a tertiary care center 48 hours following an L2-3 XLIF.[57] Following blood transfusions and fluid for resuscitation, CT demonstrated a large retroperitoneal hematoma. An angiogram revealed a traumatic pseudoaneurysm of the left L2 radicular artery adjacent to the superior left lateral L2 screw, and the pseudoaneurysm was embolized. Ultimately, the patient's condition stabilized and he was discharged two days later.
CONCLUSIONS
Popularity and high fusion rates of XLIF
The XLIF procedure has gained significant popularity in the last decade and is likely to become even more popular in the next several years. Indications for its use have increased, and some traumatic lesions may soon be treated with this approach as well. XLIF has a similar fusion rate and outcome profile when compared with more invasive procedures, and, as technology advances, the XLIF may even surpass them. In addition, XLIF appears to be as equally cost-effective as standard interbody fusion procedures.
Unique complications of XLIF
XLIF has its own set of unique complications, and surgeons who continue to utilize this technique must remain vigilant to observe, record, and avoid potential pitfalls. As is true of any new surgical procedure, successful XLIF is based on thorough knowledge of the anatomy, proper patient selection, attention to detail regarding surgical technique, and appropriate preoperative planning.
Footnotes
Disclaimer: The authors of this paper have received no outside funding and have nothing to disclose.
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/4/198/98583
Contributor Information
Paul M. Arnold, Email: parnold@kumc.edu.
Karen K. Anderson, Email: kanderson3@kumc.edu.
Robert A. McGuire, Jr., Email: rmcguire@umc.edu.
REFERENCES
- 1.Acosta FL, Liu J, Slimack N, Moller D, Fessler R, Koski T. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: A radiographic study. J Neurosurg Spine. 2011;15:92–6. doi: 10.3171/2011.3.SPINE10425. [DOI] [PubMed] [Google Scholar]
- 2.Anand N, Baron EM, Thaiyananthan G, Khalsa K, Goldstein TB. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: A technique and feasibility study. J Spinal Disord Tech. 2008;21:459–67. doi: 10.1097/BSD.0b013e318167b06b. [DOI] [PubMed] [Google Scholar]
- 3.Anand N, Rosemann R, Khalsa B, Baron EM. Mid-term to long-term clinical and functional outcomes of minimally invasive correction and fusion for adults with scoliosis. Neurosurg Focus. 2010;28:E6. doi: 10.3171/2010.1.FOCUS09272. [DOI] [PubMed] [Google Scholar]
- 4.Bagan B, Patel N, Deutsch H, Harrop J, Sharan A, Vaccaro AR, et al. Perioperative complications of minimally invasive surgery (MIS): Comparison of MIS and open interbody fusion techniques. Surg Technol Int. 2008;17:281–6. [PubMed] [Google Scholar]
- 5.Benglis DM, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139–44. doi: 10.3171/2008.10.SPI08479. [DOI] [PubMed] [Google Scholar]
- 6.Bergey DL, Villavicencio AT, Goldstein T, Regan JJ. Endoscopic lateral transpsoas approach to the lumbar spine. Spine. 2004;29:1681–8. doi: 10.1097/01.brs.0000133643.75795.ef. [DOI] [PubMed] [Google Scholar]
- 7.Berjano P, Balsano M, Buric J, Petruzzi M, Lamartina C. Direct lateral access lumbar and thoracolumbar fusion: preliminary results. Eur Spine J. 2012;21:37–42. doi: 10.1007/s00586-012-2217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: A prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–73. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages.Definitive evidence of osteoinduction in humans: A preliminary report. Spine. 2000;25:376–81. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 10.Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J. 2002;2:216–23. doi: 10.1016/s1529-9430(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 11.Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–49. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113–22. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Burkus JK, Sandhu HS, Gornet MF, Longley MC. Use of rhBMP-2 in combination with structural cortical allografts: Clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am. 2005;87:1205–12. doi: 10.2106/JBJS.D.02532. [DOI] [PubMed] [Google Scholar]
- 15.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 16.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Daffner SD, Wang JC. Migrated XLIF cage: Case report and discussion of surgical technique. Orthopedics. 2010;33:518. doi: 10.3928/01477447-20100526-21. [DOI] [PubMed] [Google Scholar]
- 18.Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28:E8. doi: 10.3171/2010.1.FOCUS09282. [DOI] [PubMed] [Google Scholar]
- 19.Dawson E, Bae HW, Burkus JK, Stambough JL, Glassman SD. Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation: A prospective randomized trial. J Bone Joint Surg Am. 2009;91:1604–13. doi: 10.2106/JBJS.G.01157. [DOI] [PubMed] [Google Scholar]
- 20.Deluzio KJ, Lucio JC, Rodgers WB. Value and cost in less invasive spinal fusion surgery: Lessons from a community hospital. SAS J. 2010;4:37–40. doi: 10.1016/j.esas.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31:2534–9. doi: 10.1097/01.brs.0000240715.78657.81. [DOI] [PubMed] [Google Scholar]
- 22.Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66:296–304. doi: 10.1227/01.NEU.0000363600.24074.D0. [DOI] [PubMed] [Google Scholar]
- 23.Glassman SD, Carreon L, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, et al. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007;7:44–9. doi: 10.1016/j.spinee.2006.06.381. [DOI] [PubMed] [Google Scholar]
- 24.Guérin P, Obeid I, Gille O, Bourghli A, Luc S, Pointillart V, et al. Safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: A morphometric study. Surg Radiol Anat. 2011;33:665–71. doi: 10.1007/s00276-011-0798-6. [DOI] [PubMed] [Google Scholar]
- 25.Guérin P, Obeid I, Bourghli A, Masquefa T, Luc S, Gille O, et al. The lumbosacral plexus: anatomic considerations for minimally invasive retroperitoneal transpsoas approach. Surg Radiol Anat. 2012;34:151–7. doi: 10.1007/s00276-011-0881-z. [DOI] [PubMed] [Google Scholar]
- 26.Haid RW, Jr, Branch CL, Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527–38. doi: 10.1016/j.spinee.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Heth JA, Hitchon PW, Goel VK, Rogge TN, Drake JS, Torner JC. A biomechanical comparison between anterior and transverse interbody fusion cages. Spine. 2001;26:E261–7. doi: 10.1097/00007632-200106150-00012. [DOI] [PubMed] [Google Scholar]
- 28.Houten JK, Alexandre LC, Nasser R, Wollowick AL. Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine. 2011;15:280–4. doi: 10.3171/2011.4.SPINE1127. [DOI] [PubMed] [Google Scholar]
- 29.Hu WK, He SS, Zhang SC, Liu YB, Li M, Hou TS, et al. An MRI study of psoas major and abdominal large vessels with respect to the X/DLIF approach. Eur Spine J. 2011;20:557–62. doi: 10.1007/s00586-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inamasu J, Guiot BH. Laparoscopic anterior lumbar interbody fusion: A review of outcome studies. Minim Invasive Neurosurg. 2005;48:340–7. doi: 10.1055/s-2005-915634. [DOI] [PubMed] [Google Scholar]
- 31.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine. 2010;356(Suppl):S322–30. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 32.Jahangiri FR, Sherman JH, Holmberg A, Louis R, Elias J, Vega-Bermudez F. Protecting the genitofemoral nerve during direct/extreme lateral interbody fusion (DLIF/XLIF) procedures. Am J Electroneurodiagnostic Technol. 2010;50:321–35. [PubMed] [Google Scholar]
- 33.Karikari IO, Grossi PM, Nimjee SM, Hardin C, Hodges TR, Hughes BD, Brown CR, et al. Minimally invasive lumbar interbody fusion in patients older than 70 years of age: Analysis of peri- and postoperative complications. Neurosurgery. 2011;68:897–902. doi: 10.1227/NEU.0b013e3182098bfa. [DOI] [PubMed] [Google Scholar]
- 34.Karikari IO, Nimjee SM, Hardin CA, Hughes BD, Hodges TR, Mehta AI, et al. Extreme lateral interbody fusion approach for isolated thoracic and thoracolumbar spine diseases: Initial clinical experience and early outcomes. J Spinal Disord Tech. 2011;24:368–75. doi: 10.1097/BSD.0b013e3181ffefd2. [DOI] [PubMed] [Google Scholar]
- 35.Kepler CK, Bogner EA, Herzog RJ, Huang RC. Anatomy of the psoas muscle and lumbar plexus with respect to the surgical approach for lateral transpsoas interbody fusion. Eur Spine J. 2011;20:550–6. doi: 10.1007/s00586-010-1593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JS, Choi WG, Lee SH. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis: Minimum 5-year follow-up. Spine J. 2010;10:404–9. doi: 10.1016/j.spinee.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Knight RQ, Schwaegler P, Hanscom D, Roh J. Direct lateral lumbar interbody fusion for degenerative conditions: Early complication profile. J Spinal Disord Tech. 2009;22:34–7. doi: 10.1097/BSD.0b013e3181679b8a. [DOI] [PubMed] [Google Scholar]
- 38.Laws CJ, Coughlin DG, Lotz JC, Serhan HA, Hu SS. Direct lateral approach to lumbar fusion is a biomechanically equivalent alternative to the anterior approach: An in vitro study. Spine. 2012;37:819–25. doi: 10.1097/BRS.0b013e31823551aa. [DOI] [PubMed] [Google Scholar]
- 39.Le TV, Smith DA, Greenberg MS, Dakwar E, Baaj AA, Uribe JS. Complications of lateral plating in the minimally invasive lateral transpsoas approach. J Neurosurg Spine. 2012;16:302–7. doi: 10.3171/2011.11.SPINE11653. [DOI] [PubMed] [Google Scholar]
- 40.Moller DJ, Slimack NP, Acosta FL, Jr, Koski TR, Fessler RG, Liu JC. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg Focus. 2011;31:E4. doi: 10.3171/2011.7.FOCUS11137. [DOI] [PubMed] [Google Scholar]
- 41.Moro T, Kikuchi S, Konno S, Yaginuma H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine. 2003;28:423–8. doi: 10.1097/01.BRS.0000049226.87064.3B. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine. 2010;356(Suppl):S331–7. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira L, Marchi L, Coutinho E, Abdala N, Pimenta L. The use of rh-BMP2 in standalone extreme lateral interbody fusion (XLIF®): Clinical and radiological results after 24 months follow-up. World Spinal Column J. 2010;1:19–25. [Google Scholar]
- 44.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–43. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Ozgur BM, Agarwal V, Nail E, Pimenta L. Two-year clinical and radiographic success of minimally invasive lateral transpsoas approach for the treatment of degenerative lumbar conditions. SAS J. 2010;4:41–6. doi: 10.1016/j.esas.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papanastassiou ID, Eleraky M, Vrionis FD. Contralateral femoral nerve compression: An unrecognized complication after extreme lateral interbody fusion (XLIF) J Clin Neurosci. 2011;18:149–51. doi: 10.1016/j.jocn.2010.07.109. [DOI] [PubMed] [Google Scholar]
- 47.Park DK, Lee MJ, Lin EL, Singh K, An HS, Phillips FM. The relationship of intrapsoas nerves during a transpsoas approach to the lumbar spine: Anatomic study. J Spinal Disord Tech. 2010;23:223–8. doi: 10.1097/BSD.0b013e3181a9d540. [DOI] [PubMed] [Google Scholar]
- 48.Pimenta L, Oliveira L, Schaffa T, Coutinho E, Marchi L. Lumbar total disc replacement from an extreme lateral approach: Clinical experience with a minimum of 2 years’ follow-up. J Neurosurg Spine. 2011;14:38–45. doi: 10.3171/2010.9.SPINE09865. [DOI] [PubMed] [Google Scholar]
- 49.Pimenta L. Paper presented at the VIII Brazilian Spine Society Meeting. Belo Horizonte, Minas Gerais, Brazil: 2001. May, Lateral endoscopic transpsoas retroperitoneal approach for lumbar spine surgery. [Google Scholar]
- 50.Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27(16 Suppl 1):S40–8. doi: 10.1097/00007632-200208151-00010. [DOI] [PubMed] [Google Scholar]
- 51.Regev GJ, Chen L, Dhawan M, Lee YP, Garfin SR, Kim CW. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine. 2009;34:1330–5. doi: 10.1097/BRS.0b013e3181a029e1. [DOI] [PubMed] [Google Scholar]
- 52.Rodgers WB, Cox CS, Gerber EJ. Minimally invasive treatment (XLIF) of adjacent segment disease after prior lumbar fusions. Int J Minimally Invasive Spinal Technol. 2009;3:1–7. [Google Scholar]
- 53.Rodgers WB, Cox CS, Gerber EJ. Early complications of extreme lateral interbody fusion in the obese. J Spinal Disord Tech. 2010;23:393–7. doi: 10.1097/BSD.0b013e3181b31729. [DOI] [PubMed] [Google Scholar]
- 54.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: An analysis of 600 cases. Spine. 2011;36:26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 55.Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: Analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4:63–6. doi: 10.1016/j.esas.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouben D, Casnellie M, Ferguson M. Long-term durability of minimal invasive posterior transforaminal lumbar interbody fusion: A clinical and radiographic follow-up. J Spinal Disord Tech. 2010 doi: 10.1097/BSD.0b013e3181f9a60a. [DOI] [PubMed] [Google Scholar]
- 57.Santillan A, Patsalides A, Gobin YP. Endovascular embolization of iatrogenic lumbar artery pseudoaneurysm following extreme lateral interbody fusion (XLIF) Vasc Endovascular Surg. 2010;44:601–3. doi: 10.1177/1538574410374655. [DOI] [PubMed] [Google Scholar]
- 58.Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: Clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24:242–50. doi: 10.1097/BSD.0b013e3181ecf995. [DOI] [PubMed] [Google Scholar]
- 59.Shunwu F, Xing Z, Fengdong Z, Xianggian F. Minimally invasive transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases. Spine (Phila Pa 1976) 2010;35:1615–20. doi: 10.1097/BRS.0b013e3181c70fe3. [DOI] [PubMed] [Google Scholar]
- 60.Simpson AK, Harrod C, White AP. Lateral Lumbar Trans-Psoas Interbody Fusion. Techn in Orthop. 2011;26:156–65. [Google Scholar]
- 61.Smith WD, Youssef JA, Christian G, Serrano S, Hyde JA. Lumbarized sacrum as a relative contraindication for lateral transpsoas interbody fusion at L5-6. J Spinal Disord Tech. 2011;26:156–65. doi: 10.1097/BSD.0b013e31821e262f. [DOI] [PubMed] [Google Scholar]
- 62.Smoljanovic T, Pecina M. Burkus JK, Sandhu HS, Gornet MF, editors. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 2006;31:775–81. doi: 10.1097/01.brs.0000206357.88287.5a. Spine (Phila Pa 1976) 2008;33:226. [DOI] [PubMed] [Google Scholar]
- 63.Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine. 2011;14:31–7. doi: 10.3171/2010.9.SPINE09871. [DOI] [PubMed] [Google Scholar]
- 64.Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7. doi: 10.3171/2010.1.FOCUS09263. [DOI] [PubMed] [Google Scholar]
- 65.Uribe JS, Arredondo N, Dakwar E, Vale FL. Defining the safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: an anatomical study. J Neurosurg Spine. 2010;13:260–6. doi: 10.3171/2010.3.SPINE09766. [DOI] [PubMed] [Google Scholar]
- 66.Uribe JS, Vale FL, Dakwar E. Electromyographic monitoring and its anatomical implications in minimally invasive spine surgery. Spine. 2010;35(26 Suppl):S368–74. doi: 10.1097/BRS.0b013e3182027976. [DOI] [PubMed] [Google Scholar]
- 67.Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89:342–5. doi: 10.1302/0301-620X.89B3.18270. [DOI] [PubMed] [Google Scholar]
- 68.Villavicencio AT, Burneikiene S, Roeca CM, Nelson EL, Mason A. Minimally invasive versus open transforaminal lumbar interbody fusion. Surg Neurol Int. 2010;1:12. doi: 10.4103/2152-7806.63905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: Initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28:E9. doi: 10.3171/2010.1.FOCUS09286. [DOI] [PubMed] [Google Scholar]
- 70.Wu RH, Fraser JF, Hartl R. Minimal access versus open transforaminal lumbar interbody fusion: Meta-analysis of fusion rates. Spine (Phila Pa 1976) 2010;35:2273–81. doi: 10.1097/BRS.0b013e3181cd42cc. [DOI] [PubMed] [Google Scholar]
- 71.Youssef JA, McAfee PC, Patty CA, Raley E, DeBauche S, Shucosky E, Chotikul L. Minimally invasive surgery: Lateral approach interbody fusion: results and review. Spine. 2010;35(26 Suppl):S302–11. doi: 10.1097/BRS.0b013e3182023438. [DOI] [PubMed] [Google Scholar]


