Abstract
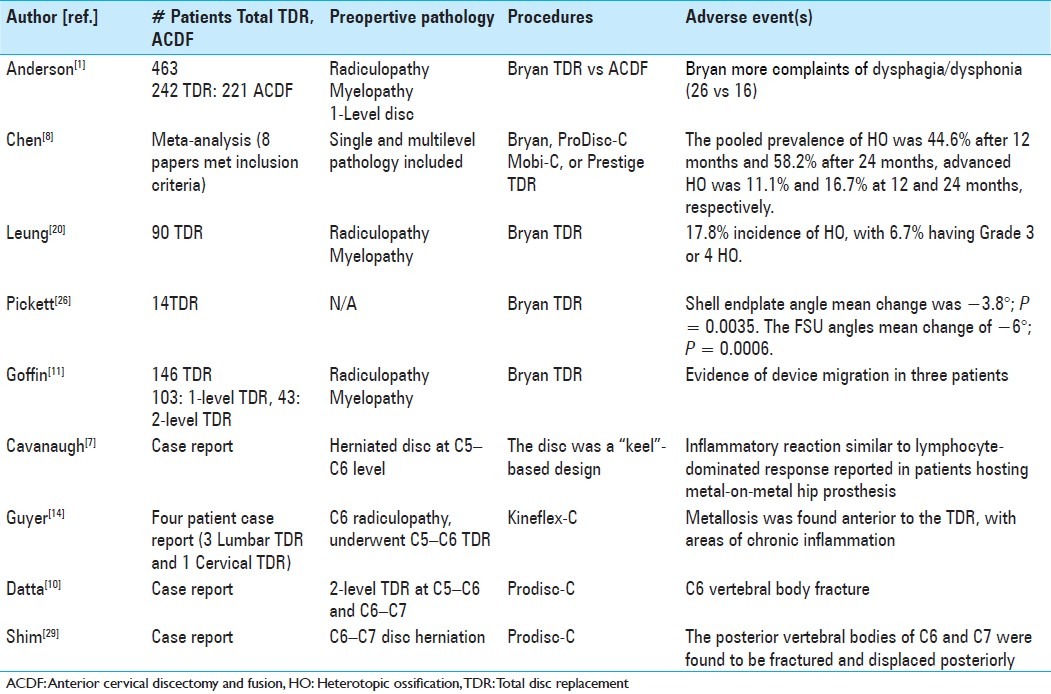
Background:
Cervical disc arthroplasty has emerged as a promising potential alternative to anterior cervical discectomy and fusion (ACDF) in appropriately selected patients. Despite a history of excellent outcomes after ACDF, the question as to whether a fusion leads to adjacent segment degeneration remains unanswered. Numerous US investigational device exemption trials comparing cervical arthroplasty to fusion have been conducted to answer this question.
Methods:
This study reviews the current research regarding cervical athroplasty, and emphasizes both the pros and cons of arthroplasty as compared with ACDF.
Results:
Early clinical outcomes show that cervical arthroplasty is as effective as the standard ACDF. However, this new technology is also associated with an expanding list of novel complications.
Conclusion:
Although there is no definitive evidence that cervical disc replacement reduces the incidence of adjacent segment degeneration, it does show other advantages; for example, faster return to work, and reduced need for postoperative bracing.
Keywords: Total disc replacement, anterior cervical discectomy and fusion, complications, adjacent segment degeneration, dysphagia, return to work, bracing
INTRODUCTION
Anterior cervical discectomy and fusion (ACDF) is the current gold standard for managing symptomatic anterior cervical degenerative disc disease.[2] Since its original description over 50 years ago, numerous studies have demonstrated the effectiveness of ACDF; patients generally experience rapid recoveries, and dramatic improvement in their quality of life.[4,5] However, when Hillibrand et al. in 1999 reported a 2.9% incidence of symptomatic adjacent segment disease attributed to ACDF fusions’ altered kinematics, cervical disc arthroplasty emerged as a new motion-sparing alternative to fusion.[17]
FDA-APPROVED TOTAL DISC REPLACEMENT DEVICES
To date, three cervical total disc replacement (TDR) devices have been approved by the Food and Drug Administration (FDA) for single-level anterior cervical disc procedures. They include the Bryan Disc (Medtronic Sofamor Danek, Memphis, TN, USA), the Prestige Disc (Medtronic Sofamor Danek, Memphis, TN, USA), and the ProDisc-C (Synthes Spine West Chester, PA, USA) [Table 1]. Several additional cervical TDRs are currently under investigation.
Table 1.
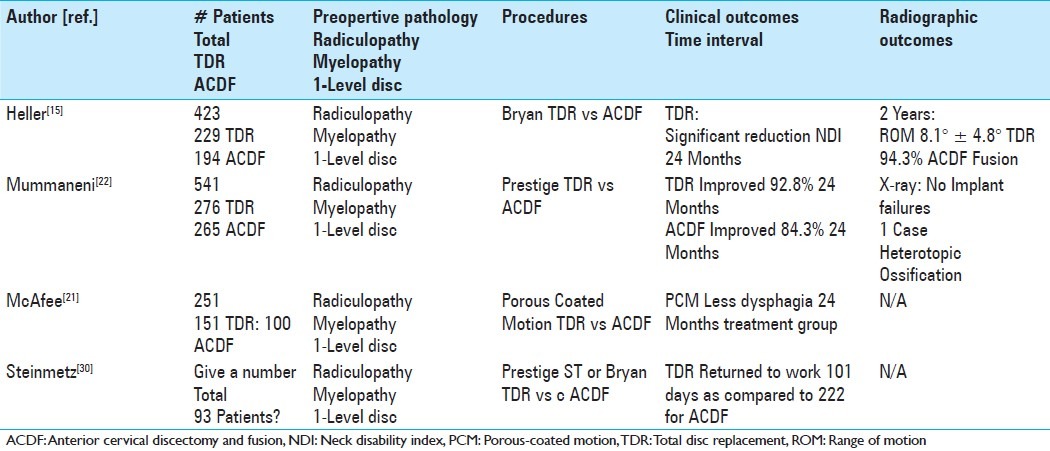
Indications for total disc replacement

There are several purposes of this review; to critically examine where cervical TDR stands today, to summarize what we have learned thus far, and to evaluate whether a new “gold standard” alternative is on the horizon.
THE ISSUE OF THE ADJACENT SEGMENT
The incidence of cervical disc degeneration after ACDF fusion has been studied extensively. Gore and Sepic examined 50 patients after anterior cervical fusion over a followup interval of 21 years.[13] Forty-eight of 50 patients had new degenerative findings on roentgenographic examinations, but only 8 required secondary surgery to address clinically significant radiculopathy, or myelopathy. Baba et al. followed 106 patients with cervical spondylotic myeloradiculopathy (42 with single-level fusion, 52 with two-level fusion, 12 with 3-level fusion) for an average of 8.5 years.[3] Dynamic instability resulted in spinal stenosis in 24% of patients, while another 15% developed anterior spondylolisthesis. Herkowitz et al. prospectively randomized 44 patients with cervical radiculopathy to either ACDF or posterior foraminotomy without fusion.[16] Forty-one percent of the patients in the ACDF group developed radiographic evidence of adjacent segment disease compared with 50% of patients following posterior foraminotomy alone.
THE PROGRESSION OF CERVICAL SPONDYLOSIS: ATTRIBUTED TO FUSION OR NATURAL HISTORY
Fusions, consisting of ACDF, did not seem to alter the natural history for the evolution of spondylotic changes. To assess clinically relevant adjacent segment degeneration after cervical fusion, Hilibrand et al. conducted a landmark Kaplan–Meier analysis.[17] In this work, following ACDF, symptomatic adjacent-segment disease was characterized by the onset of radiculopathy or myelopathy requiring surgical management. They reported a 2.9% incidence per year of increased adjacent segment degeneration, with 25.6% of patients developing new clinically significant disease within 10 years of the index fusion.
FUSION OF MORE ANTERIOR CERVICAL LEVELS REDUCED THE FREQUENCY OF ADJACENT SEGMENT DISEASE
If the need for additional surgery was caused by the fusion, then one would expect that the greater the number of levels fused, the higher the risk for developing new radiculopathy or myelopathy.[17] Importantly, the researchers found that the risk of new disease at an adjacent level was significantly lower following a multilevel fusion compared with a single-level arthrodesis. These results led to the suggestion that adjacent segment degeneration was a result of the natural progression of cervical spondylosis, and not the result of the fusion. Further corroboration of this hypothesis can be found in the work by Lehto et al. wherein they showed that disc degeneration, disc protrusions, narrowing of disc spaces, and/or dorsal osteophytes were seen in 62% of subjects older than 40 years of age.[19] By 65 years of age, nearly 95% of men and 75% of women had radiographic evidence of at least one degenerative level.[13]
WILL CERVICAL TDR MINIMIZE OR REDUCE THE RISK OF ADJACENT SEGMENT DISEASE?
The present data raise significant doubt as to whether cervical TDR will reduce or eliminate the risk of adjacent segment disease. Unfortunately, the followup intervals utilized by the US Investigational Device Exemption (IDE) studies have not yet provided enough long-term data to address this crucial issue. Recently Nunley et al. examined the 4- to 7-year outcomes of patients enrolled in 5 different cervical TDR trials; they reported a 2.3% incidence of symptomatic adjacent segment disease.[25] When Murrey et al. pooled the outcomes from 6 different cervical TDR IDEs from a single institution, they found no difference (after a mean followup interval of 41.5 months) in the rate of additional surgery for symptomatic adjacent segment disease in patients undergoing cervical fusion versus cervical TDR.[23]
CERVICAL TDR OUTCOMES: EQUIVALENT TO ACDF
In addition to the paucity of data justifying cervical TDR in reducing adjacent segment disease, there remain the crucial questions as to whether cervical TDR confers improved outcomes in other domains; for example, postoperative pain and neurologic function? The FDA IDEs conducted thus far provide the highest quality data available to address these controversies.[9,15,22,24] Results of the ProDisc-C trial, comparing cervical TDR versus fusion (ACDF), demonstrated no statistically significant differences at 24 postoperative months in visual analog scale (VAS) results for neck pain or arm pain.[24] Furthermore, there were no statistically significant differences in neurologic outcomes between ProDisc-C and patients fused (ACDF).[24]
CERVICAL TDR OUTCOMES: BETTER THAN OR SIMILAR TO ACDF?
Some series reported better neurologic outcomes with TDR versus ACDF. Mummaneni et al. reported statistically significant neurologic improvement/success in patients undergoing Prestige ST cervical TDR versus ACDF.[22] Heller et al. also reported statistically greater improvement in neck disability index (NDI), and overall success in patients undergoing Bryan cervical TDR versus ACDF.[15] Nevertheless, although those receiving Bryan TDR returned to work an average of 2 weeks earlier than ACDF subjects, there was no statistically significant difference in the rate of secondary surgical procedures.[15] In another series, although the overall success rate of patients undergoing Kineflex/C was significantly greater than those undergoing ACDF (2-year follow-up), patients enrolled in the Kineflex/C trial showed no statistically significant differences in NDI, VAS, neurologic outcomes, blood loss, operative time, length of stay, or adjacent-level reoperations compared with ACDF patients.[9] In another study, there were fewer secondary revision surgeries in patients undergoing cervical TDR versus ACDF, and they returned to work an average of 16 days earlier, but other outcome measures, such as NDI, SF-36, and neck pain, were not statistically different between the 2 patient groups.[22]
SUMMARY OF PROS AND CAVEATS FOR TOTAL DISC REPLACEMENT STUDIES
Taken together, the results of the US IDEs suggest that cervical TDR is a reasonable alternative to fusion, but with some notable caveats. First, patient selection bias was present; in each study, patients had to enter a trial to undergo TDR, but did not have to enter any trial to have an ACDF. Second, although patients were randomized and their treatment allotments were “blinded initially,” patients were “unblinded” on subsequent followup visits. These inherent biases may have negatively impacted the results of patients who were randomized to fusions. Third, there was also the potential for bias in favor of cervical TDR in the US IDEs as these were industry-sponsored trials; for example, many of the investigators disclosed financial relationships with industry sponsors, and data interpretation might have been inappropriately skewed in favor of TDR. Fourth, the noninferiority study design of these trials precluded conclusions regarding the superiority of cervical TDR over ACDF [Table 2].
Table 2.
Pros of total disc replacement
CONS OR CONTRAINDICATIONS FOR CERVICAL TOTAL DISC REPLACEMENT
Although cervical TDR may represent a viable alternative to fusion, opponents of cervical TDR have also raised issues regarding the number of contraindications to its use. Contraindications described by the FDA have included: isolated axial neck pain, ankylosing spondylitis or pregnancy, rheumatoid arthritis, autoimmune disease, diffuse idiopathic skeletal hyperostosis, severe spondylosis with bridging osteophytes or ossification of the posterior longitudinal ligament, disc height loss > 50%, spinal infection, metal allergy to components of the prosthesis, severe osteoporosis/osteopenia, active malignancy, metabolic bone disease, trauma, segmental instability, 3 or more levels requiring treatment, insulin dependent diabetes mellitus, human immunodeficiency virus, hepatitis B/C, morbid obesity, absence of motion < 2 degrees, and posterior facet arthrosis [Table 3]. Unless surgeons are prepared to spend the time and resources to screen patients clinically (including blood work, dual-energy X-ray absorptiometry scans, endocrine and rheumatologic consultations), cervical TDR outside of FDA trials may have greater risks of poor outcomes, device failures, and medicolegal suits [Table 4].
Table 3.
Contraindications for total disc replacement

Table 4.
Cons of total disc replacement
THE BEST CANDIDATES FOR MOTION SPARING DTR ARE YOUNGER PATIENTS, BUT LONGEVITY INCREASES WEAR AND TEAR
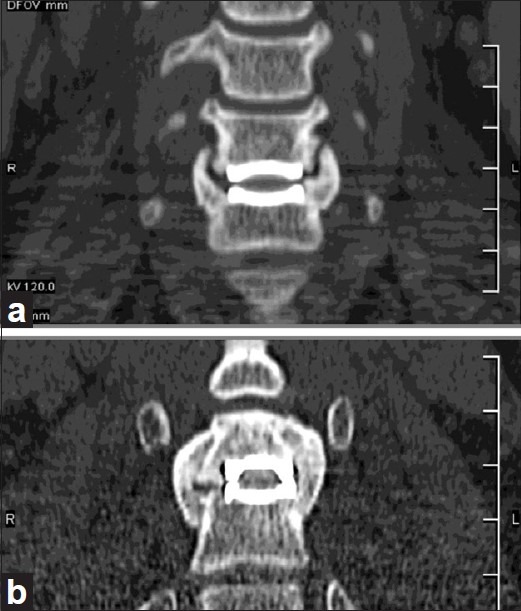
Since cervical TDR is motion sparing, and not motion restoring, the best candidates should be the younger patients with excellent baseline segmental motion [Figure 1]. However, further long-term studies are needed to examine this issue, as this younger population will also experience the greatest amount of prosthesis wear and tear due to both time and activity level. Furthermore, as cervical TDR implants undergo osteointegration over time, this can require substantial bone resection if they need to be removed; for example, one- or two-level anterior cervical corpectomy with complex fusion.
Figure 1.
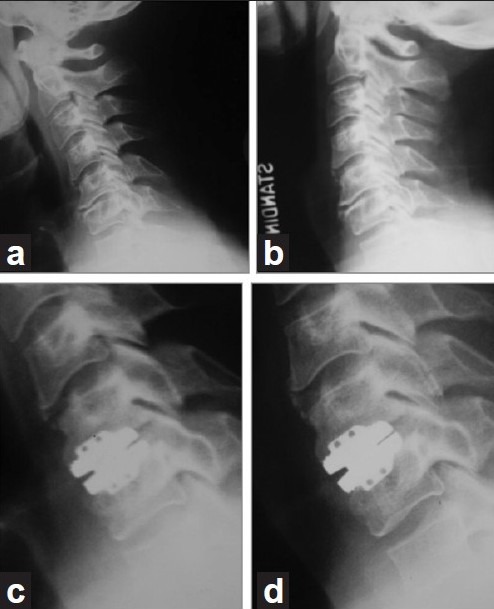
Flexion (a) and lateral (b) radiographs demonstrating virtually no motion at the C5–C6 level of a patient with cervical degenerative disc disease and cervical radiculopathy. Postoperative radiographs in flexion (c) and extension (d) following cervical total disc replacement show minimal motion. Cervical total disc replacement should not have been performed in this scenario
FURTHER ADVANTAGES OF CERVICAL TOTAL DISC REPLACEMENT
Dysphagia decreased by TDR
Dysphagia is a well-known complication following anterior cervical decompression and plating (ACDF) [Figure 2]. Indeed, proponents of cervical arthroplasty cite, among its many advantages, earlier mobilization, and earlier return to work, the reduced risk of dysphagia. Esophageal injury can occur during any phase of anterior cervical surgery; the surgical exposure itself, while resecting anterior cervical pathology, during retraction (most commonly resulting in an adynamic segment), as a result of primary plate placement or removal of a prior plate with placement with a new plate, and during the resection of scar related to prior surgery. Riley et al. examined 454 patients undergoing ACDF with plating, and reported a 21.5% incidence of dysphagia at 6 postoperative months, and a 21.3% incidence at 24 postoperative months.[27] Tortolani et al. found that intraesophageal pressures were significantly greater during single-level plating (ACDF) compared with disc replacement (TDR) at both the C3–C4 (P = 0.016) and C5–C6 levels (P = 0.016).[31] However, clinical data on the subject was inconclusive. In a randomized clinical trial, McAfee et al. reported a significantly lower incidence of dysphagia at 3 and 12 months following placement of the porous-coated motion (PCM) versus ACDF.[21] Long-term resolution of symptoms was also seen to occur at a higher 74% rate for TDR/PCM subjects compared with a lesser 41.4% for patients undergoing ACDF.
Figure 2.
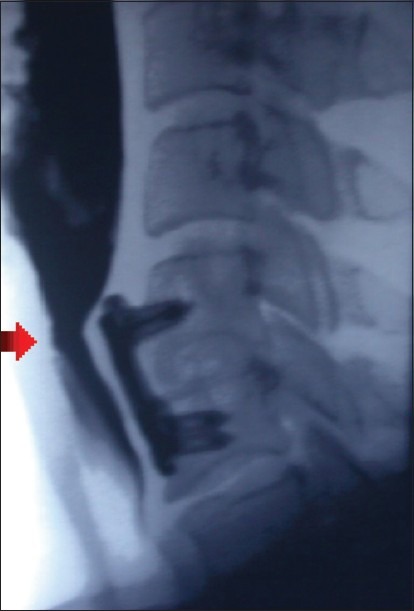
Lateral fluoroscopic image following a barium swallowing exam demonstrating severe esophageal narrowing from a cervical plate, which has migrated off of the anterior cervical spine. This patient presented with dysphagia
Disadvantages of TDR—dysphagia increased by TDR
In contrast to the studies presented, Anderson et al. found more rather than less frequent complaints of dysphagia in patients receiving the Bryan artificial disc (TDR) versus those undergoing ACDF.[1] In 242 patients who received the Bryan artificial disc, 26 reported dysphagia, while 16 of the 221 patients who underwent fusion had the same complaint. It was not assessed if these symptoms persisted over the long-term. The authors attributed this difference to longer operating times or the use of a specialized frame in the Bryan artificial disc cohort.[1]
Advantages of minimal to no bracing for TDR
Patients who undergo anterior cervical arthroplasty (TDR) may benefit from little to no bracing, along with earlier range of motion, fewer restrictions, and earlier return to work. Two studies reported that patients undergoing TDR returned to work in fewer days (13–16 days) versus those undergoing ACDF.[15,22] When Steinmetz et al. performed a subgroup analysis of the workers’ compensation population receiving the Prestige and Bryan TRD in randomized controlled trials versus ACDF, TDR patients returned to work a median of 101 days after arthroplasty versus 222 for ACDF.[30] This benefit was largely attributed to arthroplasty patients’ earlier resumption of normal activities because immobilization was not required. Another explanation was that some surgeons prevent their ACDF patients from returning to work earlier, to allow the fusion to heal completely. Additional studies have shown that the range of motion measured at the index level is maintained in the TDR/arthroplasty cohorts, but reduced in the fusion groups.[28,34] Even at long-term followup, Sasso et al. reported that at 24 and 48 months, the mean range of motion for the Bryan disc was 8.08° and 8.48°, respectively.
OTHER COMPLICATIONS OF CERVICAL TDR
Adverse events specifically associated with cervical TDR can be categorized by type: implant failure/wear, bone-implant failures, iatrogrenic deformity, segmental kyphosis, failed kinematics, neurologic injury, and infection. Another major risk factor that is often unanticipated is the host response to the arthroplasty, resulting in heterotopic ossification (HO) and osteolysis [Table 5].
Table 5.
Complications of total disc replacement
HETEROTOPIC OSSIFICATION: A COMPLICATION OF TDR
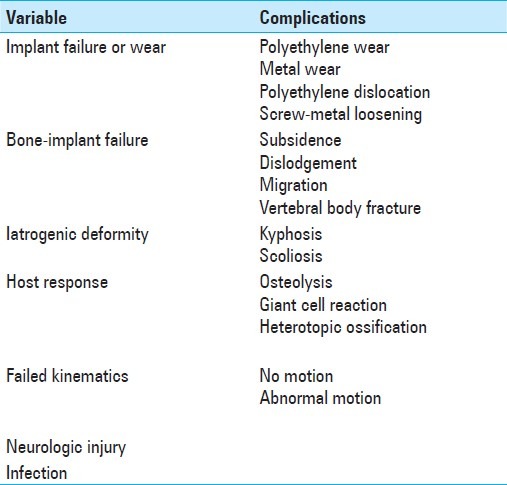
One of the most studied complications of TDR is HO [Figure 3]. A recent meta-analysis calculated a pooled prevalence of HO in 44.6% of patients 12 months after cervical arthroplasty (TDR); this rose to 58.2% at 24 months.[8] Advanced HO, defined as McAfee grade 3–4, was seen in 11.1% and 16.7% of patients at 12 and 24 months, respectively.[8] Leung et al. similarly reported an 18% incidence of HO with the Bryan cervical disc at one year followup.[20]
Figure 3.
Coronal computed tomography reformation demonstrating near complete (a) and complete (b) ankylosis in 2 separate patients with heterotopic ossification following cervical total disc replacement
ETIOLOGY OF HETEROTOPIC OSSIFICATION
The etiology of HO associated with cervical TDR is unknown. Some speculate that repeated trauma to the longus colli musclulature is a contributing factor, while others implicate the extensive vertebral endplate preparation or milling of the bone as a causative factor. Clearly, if HO reaches the point of autofusion in a large number of patients, the theoretic benefit of motion preservation is lost. An example of this was found in Leung et al. study, wherein 62% of patients had less than 2 degrees of motion at the affected surgical levels.[20] In order to reduce the incidence of HO, the protocol of the Bryan FDA IDE study required that patients receive a 2-week postoperative course of a nonsteroidal anti-inflammatory drug (surgeon's choice).[15]
KYPHOSIS FOLLOWING TDR
Multiple studies have reported postoperative kyphosis as an adverse outcome of cervical arthroplasty/TDR.[18,26] Kyphosis may be caused by both segmental misalignment of the functional spinal unit or, as in the case of the Bryan TDR, the prosthesis itself. Pickett et al. found that 100% of their 14 patients receiving the Bryan TDR had a mean change of –6° with respect to the functional spinal unit at final followup (24 months).[26] There was, however, no statistically significant adverse impact of kyphosis following TDR on clinical outcome.
SOME PATIENTS UNDERGOING TDR DEVELOP POSTOPERATIVE SEGMENTAL KYPHOSIS
Although the goal of cervical arthroplasty is to maintain the normal cervical range of motion and biomechanics, some patients develop postoperative segmental kyphosis. Troyanovich et al. argued that adjacent levels compensate for the kyphotic level, but that undue stress at these adjacent interspaces accelerates adjacent segment degeneration.[32] Two major factors contribute to segmental kyphosis include (1) over- or asymmetric milling of the endplates, and/or (2) the use of an undersized prosthesis in osteoporotic bone.[33] Appropriate modifications in surgical techniques addressing these two major issues have shown that adverse outcomes are indeed avoidable.[6] Again, however, it was observed that kyphotic deformity following TDR does not appear to adversely impact clinical outcome.
OTHER COMPLICATIONS OF TDR: IMPLANT MIGRATION OR SUBSIDENCE
Several authors have reported implant migration or subsidence leading to surgical failures following cervical TDR [Figure 4]. Goffin et al. described the migration of the Bryan device in 3 of 146 patients; this was attributed to a deficiency in endplate preparation.[11] Goffin et al. subsequently reported on a single case of implant subsidence, and recommended several strategies to prevent this complication.[12] First, the footprint of the implant should be as large as possible to maintain axial load while maximally preserving the underlying structural integrity of the endplate. Second, cervical arthroplasty should be avoided in patients with osteopenia or metabolic bone disease resulting in decreased bone quality (osteoporosis, osteopenia).[12]
Figure 4.
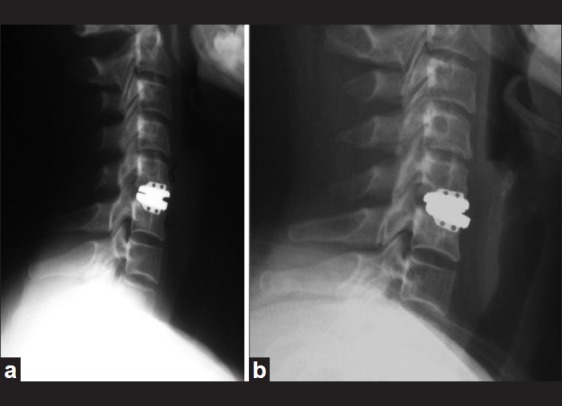
Immediate postoperative lateral radiograph (a) and 6-month followup lateral radiograph (b) demonstrating anterior migration of the inferior end-plate of a cervical total disc replacement
OTHER COMPLICATIONS OF TDR: WEAR DEBRIS
Other complications of cervical arthroplasty include the generation of wear debris, and the resultant immune reactions. Goffin et al. noted that with motion, the Bryan disc prosthesis generates wear debris that can incite an inflammatory reaction and a cascade of events leading to pain, osteolysis, and/or loosening [Figure 5].[11] Cavanaugh et al. reported one case involving a delayed hypersensitivity reaction to metal ions after cervical arthroplasty (TDR); surgical exploration of the disc space revealed chronic inflammatory debris, and abnormal cartilaginous tissue.[7] Guyer et al. described 4 patients (3 lumbar disc replacements, and 1 cervical TDR) who had undergone metal-on-metal disc replacements and sustained delayed reactions attributed to wear debris; although the subjects initially had good surgical outcomes, within a few months they developed axial pain and/or radicular symptoms.[14]
Figure 5.
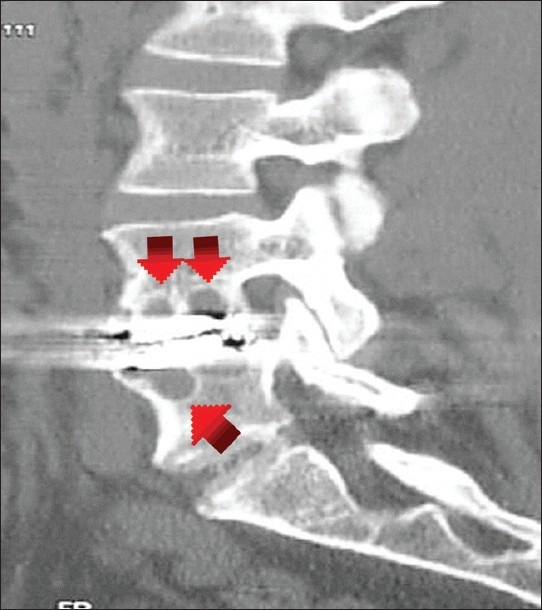
Sagittal computed tomography reformation demonstrating osteolysis in the vertebral bodies adjacent to a lumbar metal-on-polyethylene total disc replacement
METALLOSIS (MASS LESIONS ATTRIBUTE TO LYMPHOCYTIC REACTION) FOLLOWING TDR
Further studies are needed to investigate the prevalence of metal ion debris, and to evaluate its long-term clinical consequences after TDR. In Guyer et al. imaging studies after TDR revealed a mass lesion attributed to metal ion debris in all cases.[14] The patient with cervical spine involvement had gray-tinged tissue observed during a second operative procedure (revision surgery) that suggested metallosis; pathology confirmed a lymphocytic reaction attributed to the implant.[14] Nevertheless, it remains unclear as to whether these devices fail from preexisting metal hypersensitivity, or whether the patient becomes hypersensitive to the implanted metal.
VERTEBRAL BODY FRACTURES ATTRIBUTED TO INSERTING TDR
The insertion of certain TDR devices carries the potential risk of iatrogenic vertebral body fractures. In 1 case, Datta et al. observed an intraoperative sagittal split fracture produced within the C6 vertebral body during insertion of adjacent “keeled” prostheses at contiguous C5–C6 and C6–C7 levels.[10] The cause of the C6 fracture was attributed to the two centralized keel cuts involving the cephalad and caudad C6 vertebral body and end plates. Shim et al. similarly reported on an avulsion fracture that developed utilizing a keeled prosthesis.[29] In the latter case, a box cutting chisel was used to remove excess bone to allow the disc replacement to be positioned more posteriorly. This resulted in a fracture of the central aspect of the caudal C6 and cranial C7 vertebral bodies, and allowed the TDR to become posteriorly displaced. Methods to avoid vertebral body fractures when inserting TDR include the following: (1) use of sharp keel osteotomes only, (2) keeping the keel and box osteotomes parallel to the disc space, and (3) being cautious when making 2 keel cuts in the same vertebral body (placing TDR at adjacent levels).[10]
CONCLUSIONS
No clear evidence of superiority of TDR versus ACDF
Over the past 10 years, cervical TDR has emerged as an alternative to ACDF for carefully selected patients. To date, definitive evidence to support a reduction in the incidence of adjacent segment disease following index-level TDR surgery is still lacking. This point is worth emphasizing given the fact that the US IDEs were powered to answer this very question.
Pros of TDR
Distinct advantages of cervical TDR seem to be a more rapid return to work, the avoidance of harvesting iliac crest bone graft (using instead) allograft bone, and the lack of necessity for postoperative bracing.
TDR: Not a new gold standard for treating cervical spondylotic radiculopathy or myelopathy
Because of the multiplicity of contraindications and the previously reported/emerging complication profiles, which may require life-long surveillance, cervical TDR does not, at this time, appear to represent a new gold standard for the treatment of cervical spondylotic radiculopathy or myelopathy. Continued followup on patients enrolled in the US IDE studies will undoubtedly shed new light on the relative merits (pros) and demerits (cons) of cervical TDR.
Footnotes
Disclaimer: The authors of this paper have received no outside funding and have nothing to disclose.
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/4/216/98582
Contributor Information
Bradley Moatz, Email: Bwm142@gmail.com.
P. Justin Tortolani, Email: justintortolani@gmail.com.
REFERENCES
- 1.Anderson PA, Sasso RC, Riew KD. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. Spine. 2008;33:1305–12. doi: 10.1097/BRS.0b013e31817329a1. [DOI] [PubMed] [Google Scholar]
- 2.Agrillo U, Faccioli F, Fachinetti P, Gambardella G, Guizzardi G, Profeta G. Guidelines for the diagnosis and management of the degenerative diseases of the cervical spine. J Neurol Sci. 1999;43:11–14. [PubMed] [Google Scholar]
- 3.Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167–73. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy.Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298–307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Brodke DS, Zdeblick TA. Modified Smith-Robinson procedure for anterior cervical discectomy and fusion. Spine. 1992;17:S427–30. doi: 10.1097/00007632-199210001-00014. [DOI] [PubMed] [Google Scholar]
- 6.Cao JM, Zhang YZ, Shen Y, Xu JX, Ding WY, Yang DL, et al. Clinical and radiological outcomes of modified techniques in Bryan cervical disc arthroplasty. J Clin Neurocsci. 2011;18:1308–12. doi: 10.1016/j.jocn.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh DA, Nunley PD, Kerr EJ, 3rd, Werner DJ, Jawahar A. A delayed hypersensitivity to metal ions after cervical disc arthroplasty. Spine. 2009;34:262–5. doi: 10.1097/BRS.0b013e318195dd60. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Wang X, Bai W, Shen X, Yuan W. Prevalence of heterotopic ossification after cervical total disc arthroplasty: a meta-analysis. Eur Spine J. 2012;21:674–80. doi: 10.1007/s00586-011-2094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coric D, Nunley PD, Guyer RD. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine. 2011;15:348–58. doi: 10.3171/2011.5.SPINE10769. [DOI] [PubMed] [Google Scholar]
- 10.Datta JC, Janssen ME, Beckham R, Ponce C. Sagittal split fractures in multilevel cervical arthroplasty using a keeled prosthesis. J Spinal Disord Tech. 2007;20:89–92. doi: 10.1097/01.bsd.0000211258.90378.10. [DOI] [PubMed] [Google Scholar]
- 11.Goffin J, Van Calenbergh F, van Loon J, Casey A, Kehr P, Liebig K, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan cervical disc prosthesis: single-level and bi-level. Spine. 2003;28:2673–8. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 12.Goffin J. Complications of cervical disc arthroplasty. Semin Spine Surg. 2006;18:87–97. [Google Scholar]
- 13.Gore DR, Sepic SB, Gardner GM. Neck pain: a long-term follow-up of 205 patients. Spine. 1987;12:1–5. doi: 10.1097/00007632-198701000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Guyer RD, Shellock J, MacLennan B, Hanscom D, Knight RQ, McCombe P, et al. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine. 2011;36:E492–7. doi: 10.1097/BRS.0b013e31820ea9a2. [DOI] [PubMed] [Google Scholar]
- 15.Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fessler RG, Hacker RJ, et al. Comparison of the BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: Clinical and radiographic results of a randomized, controlled, clinical trial. Spine. 2009;34:101–7. doi: 10.1097/BRS.0b013e31818ee263. [DOI] [PubMed] [Google Scholar]
- 16.Herkowitz H, Kurz L, Overholt D. Surgical management of cervical soft disc herniation: a comparison between the anterior and posterior approach. Spine. 1990;15:1026–30. doi: 10.1097/00007632-199015100-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–28. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JP, Lauryssen C, Cambron HO, Pashman R, Regan JJ, Anand N, et al. Sagittal alignment and the Bryan cervical artificial disc. Neurosurg Focus. 2004;17:E14. doi: 10.3171/foc.2004.17.6.14. [DOI] [PubMed] [Google Scholar]
- 19.Lehto IJ, Tertti MO, Komu ME, Paajanen HE, Tuominen J, Kormano MJ. Age-related MRI changes at 0.1 T in cervical discs in cervical discs in aymptomatic subjects. Neuroradiology. 1994;36:49–53. doi: 10.1007/BF00599196. [DOI] [PubMed] [Google Scholar]
- 20.Leung C, Casey A, Goffin J, Kehr P, Liebig K, Lind B, et al. Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trial. Neurosurgery. 2005;57:759–63. doi: 10.1093/neurosurgery/57.4.759. [DOI] [PubMed] [Google Scholar]
- 21.McAfee PC, Cappuccino A, Cunningham BW, Devine JG, Phillips FM, Regan JJ, et al. Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial. J Spinal Disord Tech. 2010;23:1–8. doi: 10.1097/BSD.0b013e31819e2ab8. [DOI] [PubMed] [Google Scholar]
- 22.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: A randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 23.Murrey DB, Zigler JE, Goldstein JA, Darden B, Delamarter R. Five-Year Results of the ProDisc-C Multi-Center Randomized Clinical Trial. Cervical Spine Res Soc. 2011 [Google Scholar]
- 24.Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275–86. doi: 10.1016/j.spinee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Nunley PD, Coric D, Jawahar A, Kerr EJ, Gordon C, Utter PA, et al. Total Disc Replacement in Cervical Spine: 4-7 Years Follow-up for Primary Outcomes and Symptomatic Adjacent Segment Disease. Cervical Spine Res Soc. 2011 [Google Scholar]
- 26.Pickett GE, Mitsis DK, Sekhon LH, Sears WR, Duggal N. Effects of cervical disc prosthesis on segmental and cervical spine alignment. Neurosurg Focus. 2004;17:E5. doi: 10.3171/foc.2004.17.3.5. [DOI] [PubMed] [Google Scholar]
- 27.Riley LH, 3rd, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine. 2005;30:2564–9. doi: 10.1097/01.brs.0000186317.86379.02. [DOI] [PubMed] [Google Scholar]
- 28.Sasso RC, Anderson PA, Riew KD, Heller JG. Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am. 2011;93:1684–92. doi: 10.2106/JBJS.J.00476. [DOI] [PubMed] [Google Scholar]
- 29.Shim C, Shin HD, Lee SH. Posterior avulsion fracture at adjacent vertebral body during cervical disc replacement with ProDisc-C A Case Report. J Spinal Disord Tech. 2007;20:468–72. doi: 10.1097/bsd.0b013e31803b95db. [DOI] [PubMed] [Google Scholar]
- 30.Steinmetz MP, Patel R, Traynelis V, Resnick DK, Anderson PA. Cervical disc arthroplasty compared with fusion in a workers’ compensation population. Neurosurgery. 2008;63:741–7. doi: 10.1227/01.NEU.0000325495.79104.DB. [DOI] [PubMed] [Google Scholar]
- 31.Tortolani PJ, Cunningham BW, Vigna F, Hu N, Zorn CM, McAfee PC. A comparison of retraction pressure during anterior cervical plate surgery and cervical disc replacement: a cadaveric study. J Spinal Disord Tech. 2006;19:312–7. doi: 10.1097/01.bsd.0000210117.01897.ca. [DOI] [PubMed] [Google Scholar]
- 32.Troyanovich SJ, Stroink AR, Kattner KA, Dornan WA, Gubina I. Does anterior plating maintain cervical lordosis versus conventional fusion techniques? A retrospective analysis of patients receiving single-level fusion. J Spinal Disord Tech. 2002;15:69–74. doi: 10.1097/00024720-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Xu JX, Zhang YZ, Shen Y, Ding WY. Effect of modified techniques in Bryan cervical disc arthroplasty. Spine. 2009;34:1012–7. doi: 10.1097/BRS.0b013e31819c4a5b. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Zhang X, Chen C, Zhang Y, Wang Z, Wang B, et al. Randomized, controlled, multicenter, clinical trial comparing Bryan cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine. 2012;37:433–8. doi: 10.1097/BRS.0b013e31822699fa. [DOI] [PubMed] [Google Scholar]


