Abstract
Background:
Intraoperative neurophysiologic monitoring (IONM) is a technique that is helpful for assessing the nervous system during spine surgery.
Methods:
This is a review of the field describing the basic mechanisms behind the techniques of IONM. These include the most often utilized trancranial motor evoked potentials (Tc-MEPs), somatosensory evoked potentials (SSEPs), and stimulated and spontaneous EMG activity. It also describes some of the issues regarding practices and qualifications of practitioners.
Results:
Although the anatomic pathways responsible for the Tc-MEP and SSEP are well known and these clinical techniques have a high sensitivity and specificity, there is little published data showing that monitoring actually leads to improved patient outcomes. It is evident that IONM has high utility when the risk of injury is high, but may be only marginally helpful when the risk of injury is very low. The monitoring team must be well trained, be able to provide the surgeon feedback in real time, and coordinate activities with those of the surgical and anesthesia teams.
Conclusions:
Although IONM is a valuable technique that provides sensitive and specific indications of neurologic injury, it does have limitations that must be understood. Maintaining a high quality of practice with appropriately trained personnel is critical.
Keywords: Intraoperative neurophysiologic monitoring, motor evoked potentials, somatosensory evoked potentials, spine
INTRODUCTION
Intraoperative neurophysiologic monitoring (IONM) is a valuable technique for assessing the nervous system. It replaces the neurologic examination when the patient is under general anesthesia and cannot cooperate with a face-to-face examination. It allows for assessment of many neural structures including the neuromuscular junction, peripheral nerve, spinal cord, brainstem, and cortex during surgery. One goal of this review is to summarize the techniques used for IONM of the spine. The most commonly employed techniques during spinal procedures are: (1) transcranial motor evoked potentials (Tc-MEPs), (2) upper and lower somatosensory sensory evoked potentials (upper and lower SSEP), (3) pedicle screw simulation, and (4) spontaneous electromyography (EMG). A number of other techniques have been used over the years that include direct spinal cord stimulation and reflex monitoring.
This review is broken up into three sections: one discusses the basic techniques used, another one discusses the application of these techniques in different common surgical procedures of the spine, and the third one discusses the typical qualifications for personnel involved in IONM.
TECHNIQUES
Trancranial motor evoked potentials
Tc-MEPs have been used to perform intraoperative monitoring for more than 20 years.[8,27] They have been available on a routine basis since the approval of the first device designed to producing them by the FDA in 2002. The Tc-MEP involves applying a train of high-voltage stimuli to electrodes on the surface of the head to activate motor pathways and produce either a motor contraction (muscle MEP) or a nerve action potential (D-wave) that can be recorded.
Basic physiology of Tc-MEPs in the awake patient
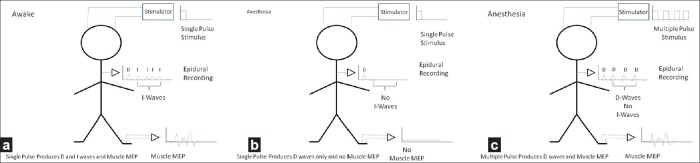
In a normal awake patient, electrical stimulation of the cortex/subcortical white matter with a single electrical pulse produces a number of responses that can be recorded by an epidural electrode placed over the upper thoracic spinal cord [Figure 1]. The first of these waves is called the direct or D-wave and the succeeding waves are termed indirect or I-waves. The D-wave is the orthodromic nerve action potential that results from stimulating white matter directly.[1] It involves no synaptic activity. The I-waves represent the volleys produced by the cortical neurons that were excited by the same stimulus. These require synaptic activity and are hence strongly suppressed with general anesthesia. This is important because of the characteristics of the anterior horn cell which is the final common pathway for all motor responses.[55] These cells respond optimally not to single stimuli but to multiple sequential time locked stimuli. Thus, an anterior horn cell will fire easily in response to a train of stimuli, but will not fire readily with just a single stimulus.
Figure 1.
Illustration of the responses to a single-pulse transcranial electrical stimulation in the (a) awake and (b) anesthetized patients. (c) Shows the effect of multipulse stimulation in the anesthetized patient
Trancranial motor evoked potentials and anesthesia
The clinical importance of the physiology described above is that a single stimulus applied to the scalp of an awake person may produce a muscle contraction because of the train of D- and I-waves reaching the anterior horn cell. However, under general anesthesia, a single stimulus may not be effective since the I-waves are diminished and the anterior horn cell sees only the single D-wave. In addition, during general anesthesia, there is a reduction in spontaneous activity in the interneurons of the spinal cord, reducing the overall level of excitation reaching the anterior horn cell. During clinical IONM studies, these problems are overcome by using trains of stimuli rather than single stimuli [Figure 1]. As the depression of intrinsic spinal cord activity is greatest with the halogenated anesthetic agents and nitrous oxide, these agents should generally not be used for the recording of muscle MEP.[57] Despite the recommendation against certain anesthetics, muscle MEP responses can be recorded in the presence of low-dose halogenated agents such as isoflurane, sevoflurane, or desflurane and/or low concentrations of nitrous oxide. The clinical question relating to the use of these halogenated agents during monitoring is “When there is a change in the muscle MEP during surgery, can the monitoring team be certain that it was not due to the effects of anesthesia?” This is a complex question because the effects of the inhalational anesthetic agents on evoked potentials are not simply related to the end-tidal concentrations of the agents but also on other factors such as the time over which the anesthetic has been administered and the presence of prior nervous system injury. It is much better not to use precious time answering this question when there is an intraoperative change, and so the use of the halogenated anesthetic and nitrous oxide should be avoided. The use of total intravenous anesthesia (TIVA) with propofol is preferred when monitoring muscle MEP because it suppresses this response to a lesser degree than the inhalational agents. Other intravenous agents such as ketamine or etomidate may be helpful in obtaining the muscle MEP. Narcotics do not cause problems with the muscle MEP although the benzodiazepines may. Since the D-wave is purely a nerve action potential and does not involve synaptic activity, it is relatively insensitive to the effects of anesthesia.
Stimulus parameters and trancranial motor evoked potentials
The train stimuli used during TC-MEP range in amplitude from about 75 to 900 V, with maximal currents up to 0.9 A. The stimulation voltage and current required is markedly dependent on the type of electrode used. The highest current levels are required if EEG cup electrodes are used. Lower thresholds are seen with subdermal needle electrodes and corkscrew electrodes. The duration of each pulse is between 50 and 500 msec.[17] The longer duration pulses are associated with a lower threshold. The number of pulses ranges between 3 and 12, with the frequency of the pulses at 150–500 Hz.
Safety and complications of trancranial motor evoked potentials
Safety and the prevention of complications are important issues when discussing motor evoked potentials.[53] Safety is an issue for two major reasons: first because of the high voltage and high current delivered during stimulation, there is risk of tissue injury or shock to OR personnel who inadvertently touch stimulating electrodes during stimulation. The second risk is that the spread of current can cause direct stimulation of the trigeminal nerve, causing jaw contractions. The most common complication of Tc-MEP is tongue bite. It is prevented by placing soft spacers between the teeth. It is not optimal to use a hard plastic “bite block” because of the risk of damage to teeth. One effective approach is to make two large cotton wads from 4 × 4's and place them bilaterally between the molars on each side. Another problem is that the patient may move during the elicitation of the Tc-MEP. It is important to make sure that the surgeon is aware of this possibility prior to performing each test so that that patient does not move during a critical surgical maneuver. In patients at risk for seizures, it is common to perform the Tc-MEP study provided that the stimulus is not given very frequently. There is a tiny risk for seizures, and the monitorist should be vigilant and the surgeons aware of this possibility. If a patient undergoing Tc-MEP has an implanted defibrillator, it is prudent not to perform the study unless there is a very high risk of motor injury. In that case, consultation in advance with a cardiologist is suggested. For patients with a pacemaker without defibrillator, although the risk of damage or aberrant firing of the pacemaker is low, the issue should be discussed with a cardiologist in advance.
Electrode locations for performing trancranial motor evoked potentials
Stimulating electrodes are typically placed over the C1 and C2 locations (located midway between the traditional C3 and C4 electrode positions and Cz in the 10–20 system) which are near the motor cortex. Other locations including more lateral placements may optimize stimulation in some patients. Midline stimulation may be helpful at times for eliciting responses from the lower extremities. However, even with these placements, the point of maximal stimulation is not the cortex, but the deep white matter likely in the corona radiate.[26] This means that although the technique may not be sensitive to cortical injury, especially if the stimulation level is far above the threshold, it would still be sensitive to injury to motor pathways in the brainstem or spinal cord. When there is risk to the cortex, direct stimulation of the motor cortex using subdural electrodes may provide additional information.[63]
Muscle MEP
The muscle MEP is the most commonly used Tc-MEP. Recordings are of high amplitude and can be obtained with a single trial. Thus, they can provide the surgeon with nearly instantaneous information, unlike the SSEP which requires prolonged averaging. The problem with the muscle MEP is that the waveform is complex. Thus, many schemes have been devised to try to determine when there is a significant change.
Interpretative criteria for the muscle MEP
One criterion is the threshold criterion proposed by Calancie.[9] This criterion was based upon the fact that the stimulus threshold for obtaining a muscle MEP increases when there is damage to the corticospinal tract. Typically, increases of more than 100 V in the threshold for obtaining a muscle MEP are considered an early sign of injury. The difficulty with this criterion is that thresholds generally increase gradually during surgery and are significantly influenced by even small changes in anesthesia.[33] Another criterion that is often used is complete disappearance of the muscle MEP. Clearly, this indicates a significant change, but it does not always indicate a permanent injury. In a study[50] of monitoring during surgery for intramedullary spinal cord tumors, loss of the muscle MEP without more than a 50% change in the D-wave was associated only with transient neurologic deficits. The problem is that in most spinal surgeries, other than spinal cord tumors, D-wave recording is difficult and limited.[68] Other investigators[32] have proposed that a reduction in amplitude of 50% or more should be considered as significant. The problem with this is that there is quite a bit of natural variability in the muscle MEP, which may increase the false-positive and false-negative rate. Although some investigators have proposed other criteria,[47] they are not yet standardized.
The effect of pre-operative damage to the motor pathways and the trancranial motor evoked potentials
It should also be noted that the state of the motor pathways prior to surgery is very critical to the generation of the muscle MEP. If there is injury pre-operatively, even if the patient has good strength pre-operatively, the MEPs may be difficult to obtain. This is because activation of the anterior horn cell requires a highly synchronized volley of inputs that can easily be desynchronized by a minor disruption of conduction.
D-wave
Because the D-wave has a simple morphology and is insensitive to anesthesia, criteria for interpretation are much simpler than for the muscle MEP. It is generally considered[16] that 50% decline in amplitude is an appropriate alert criterion. Disappearance of the D-wave is generally associated with a significant neurologic deficit. Although this technique is very powerful and responses can be obtained even in the presence of neuromuscular blockade with a single stimulus, it does have some limitations. First, it must be recorded with an epidural electrode. Second, it is difficult to record below the mid-thoracic region. Finally, the changes in the configuration of the spinal canal during deformity surgery can lead to changes in the D-wave, and thereby limit its use in these cases. Despite these limitations, D-wave recording remains particularly important during spinal cord tumor surgery.[31]
Upper and lower extremity somatosensory evoked potentials
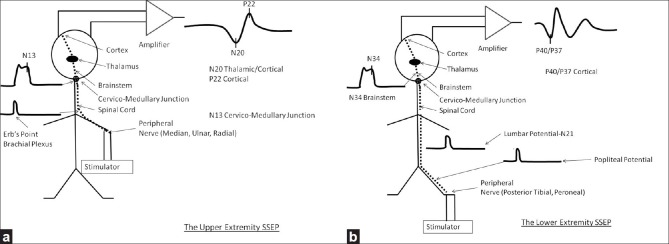
The SSEP [Figure 2] was the first effective means for monitoring the function of the spinal cord during surgery.[42] In the upper extremity,[65] this involves stimulating a peripheral nerve which is usually the median or ulnar nerve near the wrist. Alternate sites of stimulation may be used if needed. These include the radial nerve between the thumb and first finger on the dorsal surface of the hand, and the median nerve or the ulnar nerve at the elbow. In the lower extremity, the typical site of stimulation is most typically the posterior tibial nerve at the foot. Alternate stimulation sites include the peroneal nerve at the fibular head and the tibial nerve in the popliteal fossa.
Figure 2.
Illustrations of the anatomy underlying the upper (a) and lower (b) somatosensory evoked potentials
Anatomy of the somatosensory evoked potential
Impulses from the upper and lower extremity travel back to the spinal cord utilizing different pathways. The impulses from the upper extremity are conducted to the spinal cord through the peripheral nerve and brachial plexus where the ERB’ s point potential is generated. These fibers synapse in the dorsal column nuclei, where in response to upper extremity stimulation, the N13 potential is seen. The fibers then pass through the medical lemniscus in the brainstem and reach the thalamus where the upper extremity SSEPs generate part of the N20 potential. Arriving at the primary sensory cortex, the upper extremity SSEPs generate a cortical N20 and P22.
From the lower extremity, somatosensory potentials travel past the popliteal fossa where the popliteal potential is generated before they reach the lumbosacral plexus. As the impulses from the lower extremity enter the cauda equina, a lumbar potential (N21) is generated. Both the popliteal and lumbar potentials can be difficult to record, especially in patients who are overweight. The orthodromic action potentials then travel along the dorsal root and enter the spinal cord posteriorly. Most of the fibers monitored with this technique travel in the dorsal columns although there is evidence that some do travel in the dorsal spinocerebellar pathways.[51] The lower extremity SSEPs, when arriving at the cortex, produce the P37/P40 potential.
Of note, the slower conducting fibers in the spinothalamic pathways are not monitored by this technique.
Criteria for change in somatosensory evoked potentials
In comparison with the Tc-MEPs, SSEP responses are very low in amplitude and require prolonged averaging. Therefore, depending on the ambient level of noise, the time required to determine if a significant change has occurred may be 3–5 minutes or more. SSEP responses have a simple waveform, and so are simpler to quantify than the muscle MEPs. Injury to the large fiber dorsal column pathways is typically expected when there is a >50% decrease in amplitude or 10% increase in latency of any of the above potentials. Interpretation of what represents a significant change remains controversial because of intraobserver variability and because the optimal criteria for determining when there is a significant change are very dependent on the type of procedure.[60,61] However, since some of the potentials recorded clinically are 2–3 synapses away from the stimulus, they can be sensitive to anesthesia and this will influence the criteria for significant change as well. The SSEPs generally are thought to have a high specificity but low sensitivity to injury. One of the primary times when the SSEP is most critical during spinal operations is when sublaminar wires[35] are passed. The reason for this is the possibility of direct damage to the dorsal columns during this maneuver, which may not be detected utilizing motor evoked potentials, as the latter primarily monitor the lateral columns of the spinal cord.
Spontaneous electromyography
The recording of spontaneous EMG activity from a muscle provides information on the state of the peripheral nerves that innervate that muscle. Compression or stretch of a nerve as well as hypothermia and ischemia produce depolarization of the axons resulting in the appearance of spontaneous action potentials. These action potentials subsequently produce contractions of muscle fibers that can be recorded by electrodes placed in the muscle.
Theoretical limitations of spontaneous EMG recording
There are a number of important clinical issues regarding these responses. The first is that the spontaneous activity in the different axons during injury is not synchronized so that there is generally no large-scale muscle movement; rather, there may be only contractions of a few fibers at a time. Thus, the placement and type of the recording electrodes is critical since spontaneous activity may be noted in one location and not another within the same muscle.[6,29] In addition, it is important to distinguish any intraoperatively recorded EMG activity from the fibrillations caused by chronic denervation of muscle fibers or fasiculations produced by chronic injury to axons and anterior horn cells. The main factor that will help distinguish these types of activity from the effects of acute nerve injury is that fibrillations and fasiculations take weeks to develop, and so will be present from the beginning of the recordings. It is also important to note that if a patient begins to awaken from general anesthesia, there can also be intermittent muscle activity that might appear similar on the recording to the spontaneous activity. However, if a patient is waking up, there are increasing amounts of muscle activity over time and eventually these findings become continuous.
Patterns of spontaneous electromyography activity associated with nerve damage
One of the critical issues is which patterns of EMG activity are most highly associated with damage to the nerve.[7] The traditional answer is that high frequency discharges are more likely associated with true injury. Romstock[48] has classified this spontaneous activity into A, B, and C trains, and has indicated that the A trains which are characterized by high frequency activity are the most likely to be associated with significant injury. However, since the appearance of spontaneous EMG activity is due to depolarization of the nerve, sudden sharp section of the nerve may create no spontaneous activity.
Pros and cons of using spontaneous EMG activity
The most useful characteristic of spontaneous EMG activity is that it is instantaneous. Alternatively, the most disadvantageous factors attributed to spontaneous EMGs include its extreme sensitivity to neuromuscular blockade, complex criteria for significant abnormality, and dependence on anesthesia.[59]
Triggered electromyography
For instrumented spinal fusions, electrical stimulation facilitates proper screw placement. The most common use is to determine whether a screw that has already been placed is properly located. The basic principle[10] is that if the screw is electrically close to one of the nerve roots, then electrically stimulating the screw will activate the nearby nerve root at a lower current level. The term “electrically close” means that a low-resistance pathway exists between the screw and the nerve. This could occur because the screw is physically close to the nerve, or because there is a low impedance pathway between the screw and the nerve. This can occur if there is, for example, a breach of the medial wall, or in the setting of very severe osteoporosis. Toleikis has demonstrated[64] in a study of 662[67] patients that lumbar pedicle screws with thresholds less than 10 mA (with a stimulus duration of 0.2 msec) should be inspected. Screws with a stimulation threshold less than or equal to 5 mA were most often misplaced, while screws with stimulation thresholds greater than 10 mA were generally well placed.
Factors that may confound the interpretation of triggered electromyography responses
Although the technique for interpreting triggered EMG responses appears simple, there are a number of potential issues that may confound the evaluation of results. The first is that if the nerve root has been previously injured, the threshold will rise, and it is possible that a high threshold may be recorded even when the screw is electrically near the nerve. If the monitorist is not certain that the nerve root under study is uninjured, it is sometimes possible to stimulate the nerve root directly. For spinal nerve roots, the threshold for stimulation is typically at a current of approximately 2 mA. Significantly higher thresholds might indicate nerve damage that could falsely elevate the threshold for stimulation of the pedicle screw.[23] As a double check, it is often helpful for the monitoring team to review with the surgeon any screws with a threshold markedly higher or lower than the other screws in a given patient. Another problem with the pedicle screw stimulation technique is that the thresholds may be elevated by even moderate amounts of neuromuscular blockade.[39] Another important issue is that some pedicle screws are galvanized or coated with a non-conducting surface layer. These screws[2] may be difficult to stimulate and may have aberrant electrical properties. The monitorist must be aware of these potential issues as well as the issue that current shunting can markedly change the results of stimulation. This can occur if the rod is placed before the screw testing occurs or if there is an extremely high level of saline near the stimulated screw to shunt the current away.
Threshold levels vary with spinal location
It is also important to know that the[14,40,45] threshold level that optimally classifies abnormal screws will vary depending on the location of the screw because the anatomy of the thoracic pedicles is different from that of the lumbar pedicles.
Other monitoring techniques to facilitate screw placement
There are other techniques that may assist the surgeon with placement of pedicle screws. These involve continuous stimulation of the tools producing the screw holes and testing the screw holes before the screw is placed.[64] Although these techniques may be helpful, norms are less clearly defined than with the stimulation of screws once they are in place.
H-reflexes and F-waves
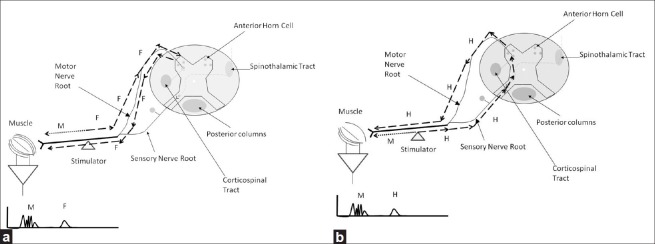
There has been significant interest in using H-reflexes and F-waves as a means to monitor the function of the spinal cord and proximal nerve roots during spine surgery. As in Figure 3, the F-wave appears when a peripheral nerve is stimulated. With stimulation, the action potentials travel both toward and away from the periphery. The impulses that travel toward the periphery activate the muscle and produce the M response or compound motor action potential (CMAP). Action potentials traveling toward the spinal cord reach the anterior horn cell which then sometimes fire an impulse back toward the periphery. This is the F-wave. In addition, action potentials traveling proximally along sensory fibers enter the dorsal horn of the spinal cord and can activate the monosynaptic reflex arc and produce an H-reflex. The F- and H-waves are distinguished by a number of factors. The F-waves have clear variability in latency that is not seen with the H-reflex. The F-wave is best seen with supramaximal stimulation, but the H-wave is best seen with stimuli of intermediate intensity. F-waves and H-reflexes offer the opportunity to monitor part of the proximal nervous system in a different way than the SSEP or muscle MEP. The problem is that like the muscle MEP, they are strongly dependent on anesthesia. Some authors have demonstrated the clinical utility of these methods which are still not considered part of the standard clinical practice.[3,34,56]
Figure 3.
Illustration of the physiology of the (a) F-wave and (b) H-reflex
Direct spinal cord stimulation
Spinal cord stimulation techniques, originally championed by Owen,[13,30] involved stimulating the spinal cord either directly or through a long intraosseous electrode. Recordings were made from peripheral nerves. This technique was originally proposed as a means of monitoring motor function that could be achieved even with total paralysis since only the peripheral nerve action potential was recorded, and not the muscle response. However, collision studies[66] have demonstrated that the responses are mainly the result of conduction along large fiber somatosensory pathways similar to those used by the SSEP, and hence this technique does not provide additional information to that already conveyed by the SSEP and MEP.
Table 1 compares the different techniques discussed above as an indicator of spinal cord injury and Table 2 compares these techniques when used as an indicator of peripheral nerve injury.
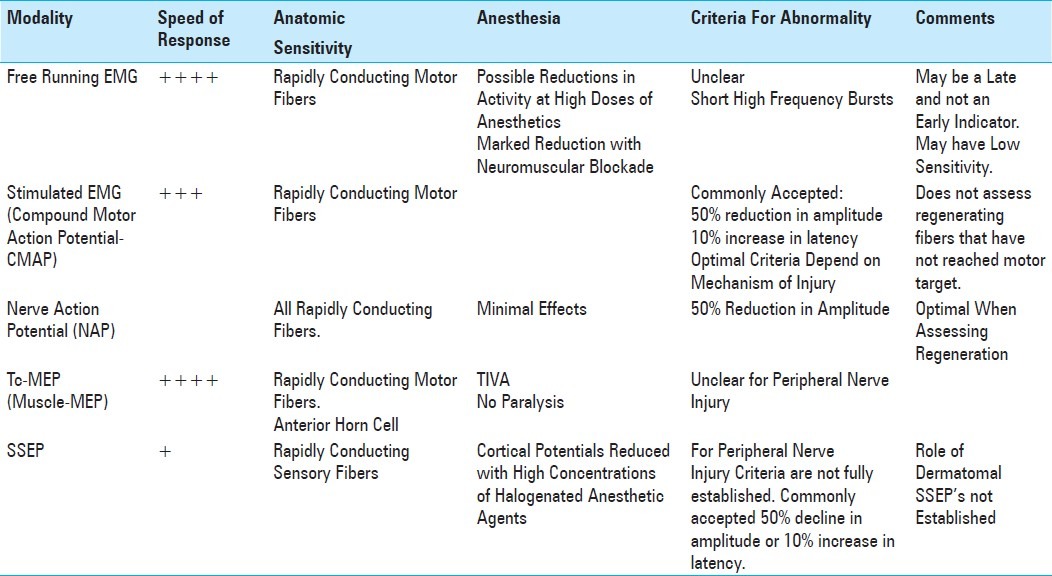
Table 1.
Comparison of the characteristics of various spinal cord monitoring techniques
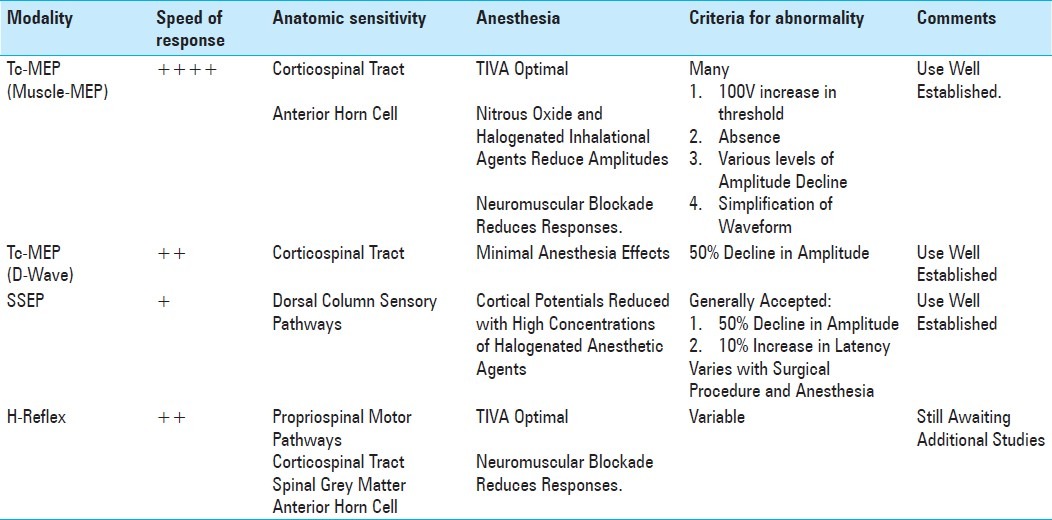
Table 2.
Comparison of the characteristics of various peripheral nerve techniques
SPECIFIC APPLICATIONS
Detecting intraoperative spinal cord injury
Although it does have some limitations, neurophysiologic monitoring is a relatively new technique that can be very helpful in preventing injury to the nervous system. The first limitation relates to the overall frequency of neurologic injury during an operation. If the frequency of injury during a particular surgery is extremely low, the type of monitoring utilized must be highly sensitive and specific in order to provide useful information. When the frequency of injury is relatively high, monitoring need not have the same sensitivity and specificity in order to be helpful. This is illustrated in Figure 4 where Bayes theorem is used to determine the probability of a warning (W) being associated with a true injury (I). In this Figure the conditional probability P(I/W) (the probability that there is a real injury when a warning is issued) is plotted as a function of the a priori probability that there will be injury, P(I), in a given surgery. Multiple plots are generated for different values of P(W/NI) the conditional probability that a warning (W) is issued when there is no injury (NI) (false-positive rate). The probability that a warning is given for every injury is taken as 100% (P(W/I) = 1.0) in creating the plots. It is clear that when the probability of injury is small, the false-positive rate must be very low in order to produce warnings that have a high expectation of being correlated with a true injury. The anterior cervical discectomy with fusion (ACDF) illustrates this point. In one study of monitoring during ACDF surgery, the incidence of cord injury in patients who did not have pre-operative myelopathy was less than 0.1%.[58] In this same cohort, it was found that there was no difference in outcome for patients who were monitored versus those who were not monitored.[58]
Figure 4.
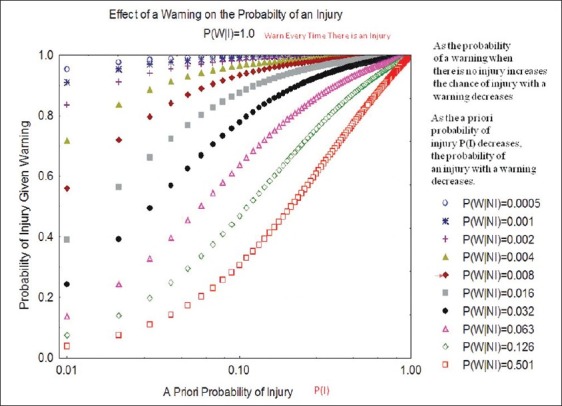
Illustration of the effect that the a priori probability P(I) has on the probability that a warning is associated with true injury when a warning is made with every injury (100% sensitive) and varying levels of false-positive warnings P(W/NI). W = warning, I = injury, NI = no injury, P(W/NI) is the conditional probability of a warning when there is no injury
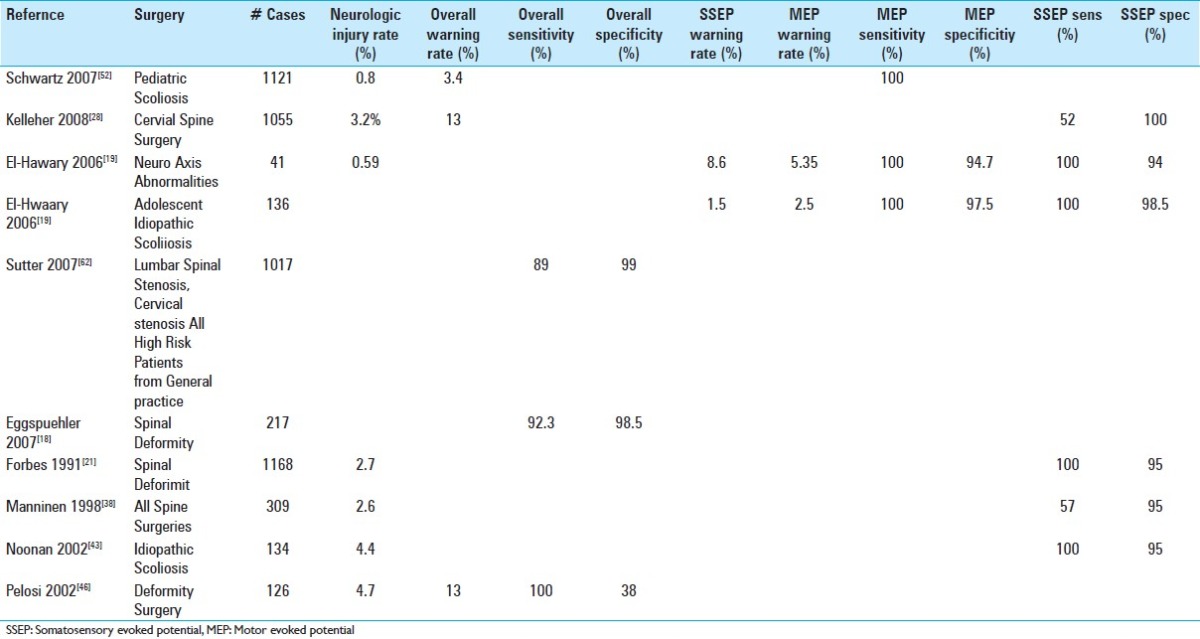
In scoliosis surgery, the risk of spinal cord injury is much higher than with the ACDF, roughly 0.3–0.8%. Thus, the likelihood that monitoring will be helpful is increased.[4,15,36,52] In one large scoliosis series involving the correction of deformity, the overall rate of postoperative new neurologic deficits was 0.8%.[52] The muscle MEP was 100% sensitive in detecting postoperative paraplegia, while the SSEP was only 43% sensitive. Alerts were issued in 3.4% of procedures. Since the actual number of postoperative deficits was only 0.8%, the probability of an alert being associated with a postoperative neurologic deficit was only 24%. It is important to understand that not all warnings that are not associated with a postoperative neurologic deficit are “false positives.” It is possible and desirable that once a warning is issued, the surgeon and anesthesiologist are able to make changes that prevent a permanent injury. Some warnings may be attributed to transient hypotension that is rectified after the warning. In other cases, a warning may lead to alteration of the surgical procedure that may have prevented neurological injury. These results described above are typical of those found in other studies,[11,18,19,41,43,46,49] some of which are shown in Table 3. In particular, Malhotra[37] suggested the importance of multimodality monitoring in order to increase sensitivity and reduce false positives. In this review of the literature, monitoring provided false-negative results in 0–0.79% of cases, while false-positive warnings were provided in 0.6–1.38% of cases. Taking the rate of postoperative neurologic injury as 0.8%, then false-negative results would occur at a rate of 0–100% of the neurologic injury rate. Warnings were issued at a rate between the neurologic injury rate and twice that rate. Much of the data regarding specificity and sensitivity is difficult to interpret because of different patient populations, different levels of surgical skill, and different criteria for the interpretation of monitoring. In addition, studies also differ significantly on whether patients with baseline abnormalities are excluded or included. They also differ by the percentage of patients in which reliable recordings can be achieved, which in some studies can be on the order of only 90%. When Fehlings[20] performed a meta-analysis of studies on monitoring during spine surgery, he found that IONM is very sensitive and specific for detecting intraoperative neurologic injury; nevertheless, the level of evidence supporting the claim that response to an alert improves outcomes could not be clearly documented. This is expected, since the users of IONM feel strongly about its clinical value, and hence a prospective randomized trial would be impossible. In many studies, the advantage of using both the SSEP and Tc-MEP (multimodality monitoring) over using either modality alone has been suggested. One large survey,[44] however, did demonstrate improved outcomes in patients who had intraoperative monitoring.
Table 3.
Results of Selected Studies of intraoperative neurophysiologic monitoring in spinal surgeries. No attempt was made to be exhaustive. It should be noted that due to differences in criteria used for interpretation and different presentation of results, the numbers obtained from different studies are not always comparable. There are also significant differences between the differing surgical populations and different definitions of a neurologic deficit, anesthesia used and monitoring technique
The Neurophysiology Research and Education Consortium (https://www.nrec.info) is beginning in the next 6 months a national database regarding intraoperative monitoring that, once completed, will allow information from any monitoring team to be entered in an Health Insurance Portability and Accountability Act (HIPAA) compliant manner. This will help provide additional insight into the value of IONM.
Intraoperative monitoring for peripheral nerve injury
There are a number of types of peripheral nerve injury that may occur during spine surgery. Injury may occur during positioning to the ulnar nerve[12] or brachial plexus.[25,54,69] SSEP and muscle MEP have been shown to have some value in potentially detecting these injuries.
When should monitoring be used?
One important question in the current climate of cost-effective medicine is determining which spine surgeries should be monitored. One answer to this question is that every case should be monitored because even if there is a tiny chance that a patient could benefit, then some patients will have improved outcomes. Beyond this, there are a number of cost-effectiveness arguments that can be applied. One of the arguments relates to the cost of responding to a warning. Each time a warning is issued by the monitoring team, a number of events occur. This includes the surgical and anesthesia teams double checking what they are doing. Very commonly attempts will be made to increase the blood pressure, administer medications, or change the level of anesthesia. Thus, there is a cost in terms of OR time and medications involved with a response to a warning. The cost of this is Co(W), the operative cost of responding to a warning. The total cost would be Co(W) × P(W), where P(W) is the probability that a warning is issued. In addition, there is a cost to providing the monitoring for each case Cm, which is not dependent on the rate of issuing a warning. The cost saved by responding to a warning is the cost of an injured patient C(I) multiplied by the chance that monitoring will prevent an injury. This is the probability that a warning is issued P(W) times the conditional probability that the warning is associated with a true injury P(I/W) (probability of injury given a warning) times the probability that a true warning can be acted on to prevent injury PC. Thus, the total cost of monitoring is Cm + Co(W) × P(W) – C(I) × P(I/W)P(W) × PC. If this number is positive, then monitoring is not cost effective. If it is negative, then monitoring is cost effective. Consider some very rough estimates. For a scoliosis procedure, the cost of providing a warning may be $1000 [both Co(W) and Cm] and the cost of an injury being permanent paraplegia might be $10,000,000. If PC is say 0.5 (the surgeon can prevent injury in half of cases where there is a true injury), then since P(I/W) is on the order of 0.24 (above), then the above equation for total cost will read as 1000 – P(W) × 1,250,000. Thus, if P(W) is greater than about 0.1%, monitoring is cost effective. Using the numbers quoted above for scoliosis, monitoring would be very cost effective, but at least for the investigators cited above regarding ACDF, the advantage would be marginal. In surgical procedures where the cost of an injury is less, monitoring becomes cost effective by this calculation only when the risk of injury is high.
This is not the complete calculation for the cost effectiveness of monitoring because it did not include the advantages that might be gained if the costs of monitoring were used for another service that might benefit the patient. In fact, the cost estimated above needs to be compared not to zero but to the cost that might be saved by using the amount Cm for another patient care expense that could also help prevent injury. Consider the case of a surgeon who performs three spine surgeries a week. The total cost of monitoring for these cases would be conservatively estimated at $150,000/year. This might be enough for the surgeon to hire an additional physician's assistant and a nurse just to take care of these three patients every week. It is hard to estimate the advantage that hiring these additional medical personnel would have, but it is clear that the advantage will be more in the medically ill elderly patient than in the medically healthy young person. This is especially true since there is a known 2–10% rate of making errors in writing medication orders,[5,22] which might be prevented by additional staff. Thus, the advantage of monitoring remains clear for the younger patients undergoing scoliosis surgery, but for the elderly patient with multiple comorbidities undergoing ACDF, there is more rationale to consider the option of not providing monitoring.
PERSONNEL
IONM is performed at two levels. The first is a technical level that involves placement of electrodes, setting up monitoring equipment, and performing the testing as described above. The other level is the interpretative level that involves deciding which testing is appropriate for a given surgical case and elucidating the clinical meaning of any change in the waveforms during the procedure.
The practice of monitoring
Practitioners at these two levels, however, cannot practice solely within the narrow boundaries described above. The practitioners who perform the technical level of monitoring must understand the interpretative process or else they will not be able to function efficiently. By the same token, interpreting providers must understand all aspects of the equipment being used and the technical problems that might arise which could interfere with signal acquisition.
Working with a team
Because responses to changes in the monitored waveforms need to be acted on quickly in order to prevent injury, it is critical that this team be highly trained, and work well with both the surgical and anesthesia teams. This, in particular, means that it is not optimal for a new monitoring team to arrive in the OR and just start working with a particular surgeon and anesthesiologist. It is important that the surgeon and monitoring team share expectations and protocols in advance. There must also be ongoing discussions between the monitoring team, and surgical and anesthesia teams beyond the contact that occurs during provision of services to individual patients in order to enhance quality assessment (QA) and quality improvement (QI) activities. This will also be an important venue in which to bring forward new monitoring techniques and new clinical problems, for joint discussion.
Credentials for intraoperative monitoring at the technical level
What are the credentials and training considered to be important for the technical level of monitoring, as defined by national societies? The appropriate credential is the Certification in Neurophysiologic Intraoperative Monitoring (CNIM) through the American Board of Registration of Electroencephalograpic and Evoked Potential Technologists (ABRET) (www.abret.org). Although there are a number of different pathways, the candidate must have performed at least 150 surgical monitoring cases, passed a written examination, and hold either a bachelor's degree or another credential in neurodiagnostics such as the R.EEG.T or R.EP.T (registered EEG or EP technologist). ASET (www.aset.org), the society that represents neurodiagnostic technologists, goes further and has created a number of job descriptions for technologists in which there is a graded level of supervision for technologists depending on their level of education, experience, and the credentials they hold. Technologists with limited experience will require personal supervision by a provider who has a higher level of skill, while very experienced technologists may perform well with purely remote supervision of the interpreting provider. The level of supervision is indeed critical. First, ASET and American Society of Neurophysiologic Monitoring (ASNM) (www.asnm.org) oppose unattended monitoring which is defined by intraoperative monitoring delivered by an electronic device when a provider who understands and can control the functions of the device and its connections is not present. Second, it is clearly preferable that the provider who interprets the recordings be present in the operating room. This is often not feasible, and so a remote monitoring system is used. In this system, a provider of the technical level of monitoring is physically present in the operating room and the interpreting provider is at a distance reviewing the acquired waveforms in real time. Often the interpreter is reviewing many simultaneous studies. This requires that the remote computer link is reliable and secure. There should be plans in place for how interpretative services will be delivered in case of a networking problem. The remote interpreter should be fully involved in the case and should be aware of the patient's medical history. The patient should also have been informed that this model is being used and given the names of the interpreters who might be involved prior to surgery. Finally, the remote interpreter must be able to respond quickly and not review so many simultaneous cases that individual attention to each is impossible.
Interpretation of intraoperative neurophysiologic monitoring and the practice of medicine
It is useful to begin by recalling a statement of the AMA House of Delegates Resolution 201 June 2008: “…it is the policy of the American Medical Association that supervision and interpretation of intraoperative neurophysiologic monitoring constitutes the practice of medicine, which can be delegated to nonphysician personnel who are under the direct or online real time supervision of the operating surgeon or another physician trained in, or who has demonstrated competence, in neurophysiologic techniques and is available to interpret the studies and advise the surgeon during the surgical procedures.” It is important to dissect this statement. Every state has laws that define what constitutes the practice of medicine. In the state of New York, according to the 2010 New York Code Title 8 Article 131-6521: “The practice of the profession of medicine is defined as diagnosing, treating, operating or prescribing for any human disease, pain, injury, deformity or physical condition.” Similar statements are issued by other states. The AMA policy is consistent with this definition of the practice of medicine. However, a physician is not the only person who can make a diagnosis or treat a patient. For example, a physical therapist may treat a patient with back pain. The general principle under which providers whose activities are not explicitly prescribed by the state medical code function is by delegation from a physician. It has always been the ability of the physician to delegate medical activities to non-licensed providers, provided that they have appropriate training and supervision and that there are no regulations that forbid the delegation of the particular service to a specified provider. It is thus important for an operating surgeon who delegates the interpretation of intraoperative monitoring to a non-physician check relevant law in the state and make sure that the person to whom this service is delegated has the appropriate credentials, education, and training. It is also important to recognize that the physician delegating that service remains responsible for it.
The state of Ohio presents an interesting application of the above principles in regard to diagnostic electromyography (EMG). The state allows the physician to delegate nerve conduction studies to non-physicians because definite criteria for performance and interpretation exist but not EMG because it cannot “safely be performed according to exact, unchanging directions” (State Medical Board of Ohio-Your Report-Summer 1999).
There are outstanding non-physician practitioners of IONM whose interpretative skills can provide great benefit to the patient, but it is very important to be sure, based upon state laws and regulations, that the process of delegation is consistent with good medical practice and consistent with state law.
Credentials for interpreting IONM
What are the credentials and training considered to be important for the interpretative level by national societies? There are a number of board certifications that directly apply to the field of IONM. The first is the American Board of Neurophysiologic Monitoring (ABNM) (www.abnm.info) which offers a written and oral examination leading to the DABNM or diplomate of the ABNM. This certification requires that the provider have a doctoral degree, have taken graduate level courses in neuroanatomy and neurophysiology, submit proof of training, and have taken the primary responsibility for interpreting at least 300 cases. The other credential is through the American Board of Clinical Neurophysiology (ABCN) which consists of two written examinations open to licensed physicians who are board certified in neurology, neurosurgery, or psychiatry, and who have done at least 1 year of fellowship training in clinical neurophysiology. There are other credentials that convey a measure of achievement and knowledge in clinical neurophysiology. They include other examinations provided by the ABCN as well as the added qualification in clinical neurophysiology by the American Board of Psychiatry and Neurology (ABPN) as well as the examination provided by the American Board of Electrodiagnostic Medicine (ABEM).
Policies for practicing intraoperative monitoring
Regarding policies for the practice of intraoperative monitoring, the ASNM (www.asnm.org) and the American Clinical Neurophysiology Society (ACNS) have issued a number of evidence-based guidelines relating to the science underlying IONM. The American Academy of Neurology (AAN) (www.aan.com), ABRET-LAB (www.abret.org), and American Board for Neurophysiologic Monitoring Programs (ABNMP) (www.abnmp.org) have each issued policies regarding the practice of IONM. The ASNM has published a white paper[24] regarding credentialing and IONM.
SUMMARY
Ttrancranial motor evoked potential
Tc-MEP using muscle responses (muscle MEP) provides an effective means of monitoring motor pathways in the spinal cord. Although optimal warning criteria have not been elucidated, simple criteria such as disappearance of the response are very useful guides.
Care must be taken to prevent injury and to assure that anesthesia does not affect the muscle MEP responses.
Significant changes in the muscle MEP during scoliosis surgery bear a strong correlation with cord injury.
Although D-wave monitoring is limited to cervical and upper thoracic cord and requires an epidural recording electrode, it is helpful in addition to muscle MEP monitoring because it is not anesthesia dependent, is not dependent on neuromuscular blockade, and has simple criteria for interpretation.
Somatosensory evoked potentials
Upper and lower SSEP can be used to monitor the dorsal column (and possible dorsal spinocerbellar) sensory pathways during spine surgery.
Changes in the SSEP have a high specificity but low sensitivity for detecting spinal cord injury.
Continuous and triggered EMG activity
Continuous EMG is one technique that detects peripheral nerve injury quickly and easily. Its use is limited by the fact that not all injuries produce spontaneous EMG activity. It is also limited by the fact that the criteria for determining which types of activity are associated with significant injury.
Triggered EMG is an excellent technique for determining whether lumbar pedicle screws are properly placed. Use of the technique for screws in other locations may be helpful although normative data are less clear.
Multimodality monitoring (SSEP + Tc-MEP + EMG) provides the surgeon with optimal information about the state of the nervous system. This helps to increase sensitivity and provide the surgeon with additional information on the specificity of any warnings issued.
Use of monitoring
IONM has a high sensitivity and specificity for detecting injury.
In procedures where there is a high risk of severe injury, monitoring is clearly critical to improving outcomes.
The advantage of monitoring during procedures with a low risk of injury or where the expected injuries are minor is not well defined.
Personnel
It is critical for IONM to be helpful that it be performed by practitioners skilled at both the technical and interpretative aspects of monitoring.
The activities of the monitoring team must integrate well with those of the surgical team and the anesthesia team, and should involve joint QA and QI activities.
The monitoring team must be able to respond quickly to changes in the recorded signals and provide the surgeon with appropriate interpretations in real time.
The surgeon and/or hospital responsible for the monitoring services must be sure that each provider has adequate training and supervision and all delegation of interpretations is consistent with each state's practice of medicine regulations.
Footnotes
Disclaimer: The author of this paper has received no outside funding and has nothing to disclose.
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/4/174/98579
REFERENCES
- 1.Amassian V. Animal and human motor system neuorphysiology related to intraoperative monitoring. In: Deletis V, Shils J, editors. Neurophysiology in Neurosurgery. San Diego California: Academic Press; 2002. pp. 3–23. [Google Scholar]
- 2.Anderson DG, Wierzbowski LR, Schwartz DM, Hilibrand AS, Vaccaro AR, Albert TJ. Pedicle screws with high electrical resistance: A potential source of error with stimulus-evoked EMG. Spine (Phila Pa 1976) 2002;27:1577–81. doi: 10.1097/00007632-200207150-00018. [DOI] [PubMed] [Google Scholar]
- 3.Baars JH, Kalisch D, Herold KF, Hadzidiakos DA, Rehberg B. Concentration-dependent suppression of F-waves by sevoflurane does not predict immobility to painful stimuli in humans. Br J Anaesth. 2005;95:789–97. doi: 10.1093/bja/aei252. [DOI] [PubMed] [Google Scholar]
- 4.Bar-On Z, Zeilig G, Blumen N, Ohry A, Azaria M. Paraplegia following surgical correction of scoliosis with Cotrel-Dubousset instrumentation. Bull Hosp Jt Dis. 1995;54:32–4. [PubMed] [Google Scholar]
- 5.Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med. 2006;166:2525–31. doi: 10.1001/archinte.166.22.2525. [DOI] [PubMed] [Google Scholar]
- 6.Bigelow DC, Patterson T, Weber R, Stecker MM, Judy K. Comparison of endotracheal tube and hookwire electrodes for monitoring the vagus nerve. J Clin Monit Comput. 2002;17:217–20. doi: 10.1023/a:1020729832385. [DOI] [PubMed] [Google Scholar]
- 7.Bose B, Wierzbowski LR, Sestokas AK. Neurophysiologic monitoring of spinal nerve root function during instrumented posterior lumbar spine surgery. Spine (Phila Pa 1976) 2002;27:1444–50. doi: 10.1097/00007632-200207010-00014. [DOI] [PubMed] [Google Scholar]
- 8.Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Assessment of corticospinal and somatosensory conduction simultaneously during scoliosis surgery. Electroencephalogr Clin Neurophysiol. 1992;85:388–96. doi: 10.1016/0168-5597(92)90052-d. [DOI] [PubMed] [Google Scholar]
- 9.Calancie B, Harris W, Brindle GF, Green BA, Landy HJ. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001;95:161–8. doi: 10.3171/spi.2001.95.2.0161. [DOI] [PubMed] [Google Scholar]
- 10.Calancie B, Madsen P, Lebwohl N. Stimulus-evoked EMG monitoring during transpedicular lumbosacral spine instrumentation.Initial clinical results. Spine (Phila Pa 1976) 1994;19:2780–6. doi: 10.1097/00007632-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZY, Wong HK, Chan YH. Variability of somatosensory evoked potential monitoring during scoliosis surgery. J Spinal Disord Tech. 2004;17:470–6. doi: 10.1097/01.bsd.0000133465.89618.c8. [DOI] [PubMed] [Google Scholar]
- 12.Chung I, Glow JA, Dimopoulos V, Walid MS, Smisson HF, Johnston KW, et al. Upper-limb somatosensory evoked potential monitoring in lumbosacral spine surgery: A prognostic marker for position-related ulnar nerve injury. Spine J. 2009;9:287–95. doi: 10.1016/j.spinee.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Darden BV, 2nd, Hatley MK, Owen JH. Neurogenic motor evoked-potential monitoring in anterior cervical surgery. J Spinal Disord. 1996;9:485–93. [PubMed] [Google Scholar]
- 14.de Blas G, Barrios C, Regidor I, Montes E, Burgos J, Piza-Vallespir G, et al. Safe pedicle screw placement in thoracic scoliotic curves using t-EMG: Stimulation threshold variability at concavity and convexity in apex segments. Spine (Phila Pa 1976) 2012;37:E387–95. doi: 10.1097/BRS.0b013e31823b077b. [DOI] [PubMed] [Google Scholar]
- 15.Delank KS, Delank HW, Konig DP, Popken F, Furderer S, Eysel P. Iatrogenic paraplegia in spinal surgery. Arch Orthop Trauma Surg. 2005;125:33–41. doi: 10.1007/s00402-004-0763-5. [DOI] [PubMed] [Google Scholar]
- 16.Deletis V. Intraoperative neuorphysiology and methodologies used to monitor the functional integrity of the motor system. In: Deletis V, Shils J, editors. Neurophysiology in Neurosurgery. San Diego, California: Academic Press; 2002. pp. 25–51. [Google Scholar]
- 17.Deletis V, Isgum V, Amassian VE. Neurophysiological mechanisms underlying motor evoked potentials in anesthetized humans. Part 1. Recovery time of corticospinal tract direct waves elicited by pairs of transcranial electrical stimuli. Clin Neurophysiol. 2001;112:438–44. doi: 10.1016/s1388-2457(01)00461-8. [DOI] [PubMed] [Google Scholar]
- 18.Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Porchet F, Dvorak J. Multimodal intraoperative monitoring (MIOM) during cervical spine surgical procedures in 246 patients. Eur Spine J. 2007;16(Suppl 2):S209–15. doi: 10.1007/s00586-007-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Hawary R, Sucato DJ, Sparagana S, McClung A, Van Allen E, Rampy P. Spinal cord monitoring in patients with spinal deformity and neural axis abnormalities: A comparison with adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 2006;31:E698–706. doi: 10.1097/01.brs.0000232707.98076.37. [DOI] [PubMed] [Google Scholar]
- 20.Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery: Does it make a difference? Spine (Phila Pa 1976) 2010;35:S37–46. doi: 10.1097/BRS.0b013e3181d8338e. [DOI] [PubMed] [Google Scholar]
- 21.Forbes HJ, Allen PW, Waller CS, Jones SJ, Edgar MA, Webb PJ, et al. Spinal cord monitoring in scoliosis surgery.Experience with 1168 cases. J Bone Joint Surg Br. 1991;73:487–91. doi: 10.1302/0301-620X.73B3.1670455. [DOI] [PubMed] [Google Scholar]
- 22.Hendey GW, Barth BE, Soliz T. Overnight and postcall errors in medication orders. Acad Emerg Med. 2005;12:629–34. doi: 10.1197/j.aem.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Holland NR, Lukaczyk TA, Riley LH, 3rd, Kostuik JP. Higher electrical stimulus intensities are required to activate chronically compressed nerve roots.Implications for intraoperative electromyographic pedicle screw testing. Spine (Phila Pa 1976) 1998;23:224–7. doi: 10.1097/00007632-199801150-00014. [DOI] [PubMed] [Google Scholar]
- 24.Isley MR, Pearlman RC. Credentialing and competency policy statement for intraoperative neuromonitoring. [Last Accessed on 2012 Apr 17]. Available online at: http://www.asnm.org/ASNM-Credentialing.htm .
- 25.Jahangiri FR, Holmberg A, Vega-Bermudez F, Arlet V. Preventing position-related brachial plexus injury with intraoperative somatosensory evoked potentials and transcranial electrical motor evoked potentials during anterior cervical spine surgery. Am J Electroneurodiagnostic Technol. 2011;51:198–205. [PubMed] [Google Scholar]
- 26.Journee H. Motor EP physiology, risks and specific anesthetic effects. In: Nuwer MR, editor. Handbook of Clinical Neurophysiology. Vol. 8. Amsterdam, NL: Elsevier; 2008. pp. 218–34. [Google Scholar]
- 27.Kalkman CJ, Drummond JC, Kennelly NA, Patel PM, Partridge BL. Intraoperative monitoring of tibialis anterior muscle motor evoked responses to transcranial electrical stimulation during partial neuromuscular blockade. Anesth Analg. 1992;75:584–9. doi: 10.1213/00000539-199210000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Kelleher MO, Tan G, Sarjeant R, Fehlings MG. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: A prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8:215–21. doi: 10.3171/SPI/2008/8/3/215. [DOI] [PubMed] [Google Scholar]
- 29.Khan A, Pearlman RC, Bianchi DA, Hauck KW. Experience with two types of electromyography monitoring electrodes during thyroid surgery. Am J Otolaryngol. 1997;18:99–102. doi: 10.1016/s0196-0709(97)90095-8. [DOI] [PubMed] [Google Scholar]
- 30.Komanetsky RM, Padberg AM, Lenke LG, Bridwell KH, Russo MH, Chapman MP, et al. Neurogenic motor evoked potentials: A prospective comparison of stimulation methods in spinal deformity surgery. J Spinal Disord. 1998;11:21–8. [PubMed] [Google Scholar]
- 31.Kothbaurer K. Motor evoked potential monitoring for intramedullary spinal cord surgery. In: Deletis V, Shils J, editors. Neurophysiology in Neurosurgery. San Diego, California: Academic Press; 2002. pp. 73–92. [Google Scholar]
- 32.Krammer MJ, Wolf S, Schul DB, Gerstner W, Lumenta CB. Significance of intraoperative motor function monitoring using transcranial electrical motor evoked potentials (MEP) in patients with spinal and cranial lesions near the motor pathways. Br J Neurosurg. 2009;23:48–55. doi: 10.1080/02688690802563349. [DOI] [PubMed] [Google Scholar]
- 33.Langeloo DD, Journee HL, de Kleuver M, Grotenhuis JA. Criteria for transcranial electrical motor evoked potential monitoring during spinal deformity surgery A review and discussion of the literature. Neurophysiol Clin. 2007;37:431–9. doi: 10.1016/j.neucli.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Leppanen RE, Abnm D. American Society of Neurophysiological M. Intraoperative monitoring of segmental spinal nerve root function with free-run and electrically-triggered electromyography and spinal cord function with reflexes and F-responses. A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2005;19:437–61. doi: 10.1007/s10877-005-0086-2. [DOI] [PubMed] [Google Scholar]
- 35.Loder RT, Thomson GJ, LaMont RL. Spinal cord monitoring in patients with nonidiopathic spinal deformities using somatosensory evoked potentials. Spine (Phila Pa 1976) 1991;16:1359–64. doi: 10.1097/00007632-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 36.MacEwen GD, Bunnell WP, Sriram K. Acute neurological complications in the treatment of scoliosis.A report of the Scoliosis Research Society. J Bone Joint Surg Am. 1975;57:404–8. [PubMed] [Google Scholar]
- 37.Malhotra NR, Shaffrey CI. Intraoperative electrophysiological monitoring in spine surgery. Spine (Phila Pa 1976) 2010;35:2167–79. doi: 10.1097/BRS.0b013e3181f6f0d0. [DOI] [PubMed] [Google Scholar]
- 38.Manninen PH. Monitoring evoked potentials during spinal surgery in one institution. Can J Anaesth. 1998;45:460–5. doi: 10.1007/BF03012582. [DOI] [PubMed] [Google Scholar]
- 39.Minahan RE, Riley LH, 3rd, Lukaczyk T, Cohen DB, Kostuik JP. The effect of neuromuscular blockade on pedicle screw stimulation thresholds. Spine (Phila Pa 1976) 2000;25:2526–30. doi: 10.1097/00007632-200010010-00016. [DOI] [PubMed] [Google Scholar]
- 40.Montes E, De Blas G, Regidor I, Barrios C, Burgos J, Hevia E, et al. Electromyographic thresholds after thoracic screw stimulation depend on the distance of the screw from the spinal cord and not on pedicle cortex integrity. Spine J. 2012;12:127–32. doi: 10.1016/j.spinee.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 41.More RC, Nuwer MR, Dawson EG. Cortical evoked potential monitoring during spinal surgery: Sensitivity, specificity, reliability, and criteria for alarm. J Spinal Disord. 1988;1:75–80. [PubMed] [Google Scholar]
- 42.Nash CL, Jr, Lorig RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop Relat Res. 1977;126:100–5. [PubMed] [Google Scholar]
- 43.Noonan KJ, Walker T, Feinberg JR, Nagel M, Didelot W, Lindseth R. Factors related to false- versus true-positive neuromonitoring changes in adolescent idiopathic scoliosis surgery. Spine (Phila Pa 1976) 2002;27:825–30. doi: 10.1097/00007632-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 44.Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: Results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. doi: 10.1016/0013-4694(94)00235-d. [DOI] [PubMed] [Google Scholar]
- 45.Parker SL, Amin AG, Farber SH, McGirt MJ, Sciubba DM, Wolinsky JP, et al. Ability of electromyographic monitoring to determine the presence of malpositioned pedicle screws in the lumbosacral spine: Analysis of 2450 consecutively placed screws. J Neurosurg Spine. 2011;15:130–5. doi: 10.3171/2011.3.SPINE101. [DOI] [PubMed] [Google Scholar]
- 46.Pelosi L, Lamb J, Grevitt M, Mehdian SM, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113:1082–91. doi: 10.1016/s1388-2457(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 47.Quinones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56:982–93. discussion 982-93. [PubMed] [Google Scholar]
- 48.Romstock J, Strauss C, Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000;93:586–93. doi: 10.3171/jns.2000.93.4.0586. [DOI] [PubMed] [Google Scholar]
- 49.Roy EP, 3rd, Gutmann L, Riggs JE, Jones ET, Byrd JA, Ringel RA. Intraoperative somatosensory evoked potential monitoring in scoliosis. Clin Orthop Relat Res. 1988;229:94–8. [PubMed] [Google Scholar]
- 50.Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: A historical control study. Neurosurgery. 2006;58:1129–43. doi: 10.1227/01.NEU.0000215948.97195.58. discussion 1129-43. [DOI] [PubMed] [Google Scholar]
- 51.Schieppati M, Ducati A. Effects of stimulus intensity, cervical cord tractotomies and cerebellectomy on somatosensory evoked potentials from skin and muscle afferents of cat hind limb. Electroencephalogr Clin Neurophysiol. 1981;51:363–72. doi: 10.1016/0013-4694(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz DM, Auerbach JD, Dormans JP, Flynn J, Drummond DS, Bowe JA, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440–9. doi: 10.2106/JBJS.F.01476. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz DM, Sestokas AK, Dormans JP, Vaccaro AR, Hilibrand AS, Flynn JM, et al. Transcranial electric motor evoked potential monitoring during spine surgery: Is it safe? Spine (Phila Pa 1976) 2011;36:1046–9. doi: 10.1097/BRS.0b013e3181ecbe77. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz DM, Sestokas AK, Hilibrand AS, Vaccaro AR, Bose B, Li M, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20:437–44. doi: 10.1007/s10877-006-9032-1. [DOI] [PubMed] [Google Scholar]
- 55.Sherrington C. The integrative action of the nervous system. New Haven, CT: Yale University Press; 1906. [Google Scholar]
- 56.Slimp JC. Electrophysiologic intraoperative monitoring for spine procedures. Phys Med Rehabil Clin N Am. 2004;15:85–105. doi: 10.1016/s1047-9651(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 57.Sloan T. Anesthesia and motor evoked potential monitoring. In: Deletis V, Shils J, editors. Neurophysiology in Neurosurgery. San Diego, California: Academic Press; 2002. pp. 452–74. [Google Scholar]
- 58.Smith PN, Balzer JR, Khan MH, Davis RA, Crammond D, Welch WC, et al. Intraoperative somatosensory evoked potential monitoring during anterior cervical discectomy and fusion in nonmyelopathic patients--A review of 1039 cases. Spine J. 2007;7:83–7. doi: 10.1016/j.spinee.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Stecker MM, Baylor K, Wolfe J, Stevenson M. Acute nerve stretch and the compound motor action potential. J Brachial Plex Peripher Nerve Inj. 2011;6:4. doi: 10.1186/1749-7221-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stecker MM, Cheung AT, Patterson T, Savino JS, Weiss SJ, Richards RM, et al. Detection of stroke during cardiac operations with somatosensory evoked responses. J Thorac Cardiovasc Surg. 1996;112:962–72. doi: 10.1016/S0022-5223(96)70096-X. [DOI] [PubMed] [Google Scholar]
- 61.Stecker MM, Robertshaw J. Factors affecting reliability of interpretations of intra-operative evoked potentials. J Clin Monit Comput. 2006;20:47–55. doi: 10.1007/s10877-005-9006-8. [DOI] [PubMed] [Google Scholar]
- 62.Sutter MA, Eggspuehler A, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during 409 lumbosacral surgical procedures in 409 patients. Eur Spine J. 2007;16(Suppl 2):S221–8. doi: 10.1007/s00586-007-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szelenyi A, Langer D, Beck J, Raabe A, Flamm ES, Seifert V, et al. Transcranial and direct cortical stimulation for motor evoked potential monitoring in intracerebral aneurysm surgery. Neurophysiol Clin. 2007;37:391–8. doi: 10.1016/j.neucli.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Toleikis RJ. Neurophysiological monitoring during pedicle screw placement. In: Deletis V, Shils J, editors. Neurophysiology in Neurosurgery. San Diego, California: Academic Press; 2002. pp. 231–64. [Google Scholar]
- 65.Toleikis JR. American Society of Neurophysiological Monitoring. Intraoperative monitoring using somatosensory evoked potentials. A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2005;19:241–58. doi: 10.1007/s10877-005-4397-0. [DOI] [PubMed] [Google Scholar]
- 66.Toleikis JR, Skelly JP, Carlvin AO, Burkus JK. Spinally elicited peripheral nerve responses are sensory rather than motor. Clin Neurophysiol. 2000;111:736–42. doi: 10.1016/s1388-2457(99)00317-x. [DOI] [PubMed] [Google Scholar]
- 67.Toleikis JR, Skelly JP, Carlvin AO, Toleikis SC, Bernard TN, Burkus JK, et al. The usefulness of electrical stimulation for assessing pedicle screw placements. J Spinal Disord. 2000;13:283–9. doi: 10.1097/00002517-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Ulkatan S, Neuwirth M, Bitan F, Minardi C, Kokoszka A, Deletis V. Monitoring of scoliosis surgery with epidurally recorded motor evoked potentials (D wave) revealed false results. Clin Neurophysiol. 2006;117:2093–101. doi: 10.1016/j.clinph.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Uribe JS, Kolla J, Omar H, Dakwar E, Abel N, Mangar D, et al. Brachial plexus injury following spinal surgery. J Neurosurg Spine. 2010;13:552–8. doi: 10.3171/2010.4.SPINE09682. [DOI] [PubMed] [Google Scholar]