Abstract
A surprising result of the groundbreaking CAPRISA-004 trial, which demonstrated the efficacy of vaginal tenofovir 1% gel in reducing the risk of human immunodeficiency virus (HIV)-1 infection by 39% in heterosexual women, was the added benefit of this microbicide in reducing acquisition of herpes simplex virus type 2 (HSV-2) by 51%. HSV-2 is the most common cause of genital ulcer disease worldwide, and is responsible for considerable morbidity among women and neonates. The virus is further implicated in increasing the risk of both HIV acquisition and transmission, and may have additional adverse consequences in HIV-coinfected persons, making HSV-2 prevention an important clinical and public health objective. While tenofovir had not previously been widely considered to be an anti-herpes drug, in vitro activity against HSV is well documented, raising interest in potential future applications of tenofovir and its prodrugs in HSV-2 control. This article reviews the currently available data for tenofovir as an anti-herpes agent, as well as unanswered questions about delivery systems, drug formulation, rectal administration, drug resistance, and clinical applications.
Keywords: tenofovir, herpes simplex virus type 2, microbicide, CAPRISA-004
Introduction
Herpes simplex virus type 2 (HSV-2) is the most common cause of genital ulcer disease worldwide, with an estimated seroprevalence of 20%–80% in different settings around the world and a greater burden of infection in women than in men due to increased susceptibility.1,2 Upon contact with nonintact skin or mucosal surfaces, the virus classically causes recurrent vesicular or pustular ulcerative lesions that may be accompanied by painful, itching, and burning sensations, as well as tender lymphadenopathy.3 HSV-2 is associated with morbidity, social stigma, and increased transmission of human immunodeficiency virus type 1 (herein referred to as HIV) infection, rendering the potential prevention of HSV-2 in both women and men an important clinical and public health objective.
When the groundbreaking results of the CAPRISA-004 trial (conducted by the Centre for the AIDS Programme of Research in South Africa, CAPRISA) were first presented at the XVIII International AIDS Conference in July 2010, showing that vaginal application of tenofovir 1% gel was associated with a 39% decrease in HIV acquisition risk, the additional finding of a 51% reduction in HSV-2 acquisition among susceptible participants was a welcome surprise.4,5 Although this finding regarding HSV-2 has thus far neither been formally published in the scientific literature nor replicated in another clinical trial, it has spawned considerable interest in the potential of this antiretroviral agent to prevent this ubiquitous infection. This paper reviews the characteristics of tenofovir and the results of the CAPRISA-004 trial, provides an explanation for the drug’s anti-herpetic activity, discusses the potential benefits of HSV-2 prevention in women independent of HIV infection status, and raises some unresolved questions about the potential for using this drug for this purpose.
Tenofovir
Tenofovir has been widely used for the treatment of established HIV and hepatitis B infections for several years, and is available as an oral tablet containing 300 mg of the prodrug tenofovir disoproxil fumarate (TDF). Its active metabolite is tenofovir diphosphate, an adenosine analog that acts as an obligate chain terminator of the HIV reverse transcriptase. TDF is available both independently and in fixed-dose coformulated tablets containing another antiretroviral drug, emtricitabine (FTC), and is commonly recommended as part of first-line combination regimens in HIV treatment guidelines worldwide.6–10 Important clinical features of this agent are its potent antiviral activity against both HIV and hepatitis B virus, long intracellular half-life that allows for once-daily dosing, and relatively favorable toxicity profile.
Formulation of tenofovir as a 1% gel represents the first development of an existing antiretroviral drug as a microbicide against HIV. Microbicides are products designed for topical application to the vagina or rectum for the purpose of reducing the risk of acquiring sexually transmitted infections such as HIV and HSV-2. Given the relentless spread of HIV infection worldwide, as well as the biological and social factors which predispose many women to a heightened risk of acquiring sexually transmitted pathogens, there has been considerable interest in developing a female-controlled microbicide against HIV for many years. In particular, difficulties in negotiating condom use by male partners represent a particularly important challenge for many women rooted in gender-based power dynamics, and microbicides could be used without requiring the explicit knowledge or consent of sexual partners. Unfortunately, several earlier HIV microbicide candidates, including the spermicide nonoxynol-9, polyanion products such as Carraguard® and PRO 2000®, the surfactant C31G or SAVVY, and the acid-buffering agent BufferGel® have proven to be ineffective in preventing HIV, and in some cases even enhanced HIV susceptibility, most likely by increasing mucosal ulceration and inflammation.11–15
Tenofovir 1% gel contains 40 mg of 9-[(R)-2-phosphonomethoxy] propyl]adenine monohydrate (PMPA) in a purified water solution with edetate disodium, citric acid, glycerin, methylparaben, propylparaben, and hydroxyethylcellulose. This agent was deemed to be a good candidate for an HIV microbicide given its long half-life and favorable safety profile. Early studies using the gel showed the product to be safe and well tolerated when used twice daily for 2 weeks in a trial of 48 sexually abstinent women in the US,16 and when used in either a once-daily or coitally-dependent fashion in a 6-month trial of 200 sexually active women in India and the US.17 Acceptability was also high, with 94% and 90%–92% of participants in each trial, respectively, agreeing that they would be willing to use the product if it was demonstrated to have anti-HIV efficacy.16,17 Further, data from animal models18,19 and ex vivo culture systems20 provided proof of concept for the efficacy of this microbicide in preventing HIV. However, until the CAPRISA-004 trial, robust human efficacy data were not available.
CAPRISA-004 trial
CAPRISA-004 was a randomized, double-blind, placebocontrolled clinical trial of tenofovir 1% vaginal gel as a strategy for preventing incident HIV infections among women aged 18–40 years not infected with HIV.5 Participants were instructed to apply one dose of the gel within 12 hours Before coitus, followed by a second dose as soon as possible within 12 hours Afterwards, to a maximum of Two doses per 24-hour period (ie. the so-called “BAT24” dosing strategy). All participants also received comprehensive HIV prevention services, including pretest and post-test counseling, sexually transmitted infection monitoring and treatment, and condoms, as well as an adherence support program involving motivational interviewing. In the primary efficacy analysis, the incidence of HIV (95% confidence interval [CI]) was 5.6 (4.0–7.7) per 100 woman-years in the active arm versus 9.1 (6.9–11.7) in the placebo arm, giving an incidence rate ratio of 0.61 (95% CI 0.40–0.94). Acceptability of the gel was high at 97.4%.
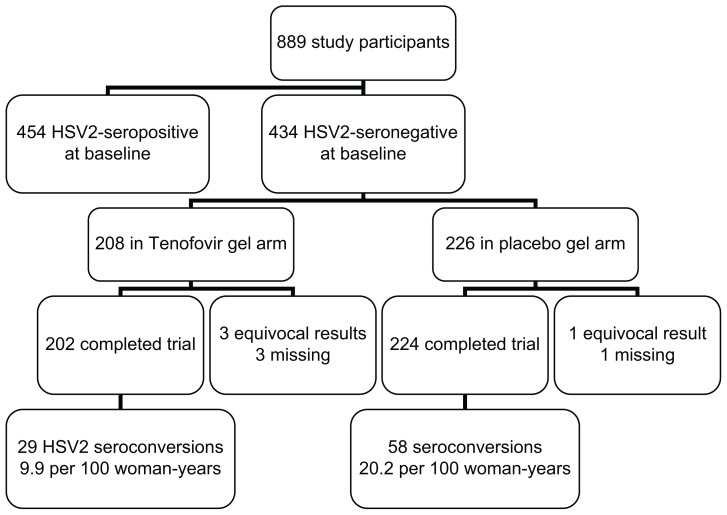
Unexpectedly, the trial also found tenofovir 1% gel to be associated with a decreased incidence of serologically confirmed HSV-2 infection, with an incidence (95% CI) of 9.9 (6.6–14.2) per 100 woman-years in the active arm versus 20.2 (15.3–26.1) in the placebo arm, giving an incidence rate ratio of 0.49 (0.30–0.78) or 51% (22%–70%) efficacy ( Figure 1).4 Sensitivity analyses that variously assigned equivocal HSV-2 serology results as HSV-2 seronegative or seropositive did not significantly change the conclusions, and adjustment for potential confounders including study site, age, condom use, sexually transmitted infections, contraceptive use,
Figure 1.
Herpes simplex virus type 2 prevention with tenofovir 1% vaginal gel in the CAPRISA-004 trial. number of sexual partners, and parity gave a similar efficacy estimate of 47% (33%–83%).
Tenofovir: an antiherpes drug?
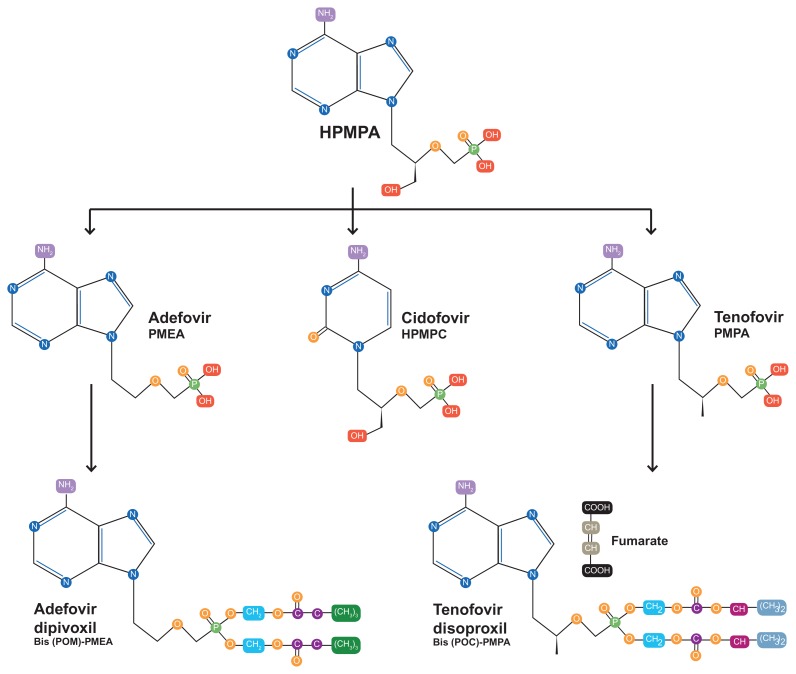
While previous microbicides such as PRO-2000, Carraguard, Buffergel, and others had in part been conceived of as HSV-2 prevention tools based on data from in vitro systems,21 animal models,22–24 and early human data,25 the CAPRISA result was surprising because tenofovir had not previously been widely conceived of as an anti-herpetic agent. Indeed, early studies during the development of tenofovir showed the compound to have little if any anti-HSV activity.26 However, more detailed examination of the molecular chemistry of tenofovir reveals close similarity with the anti-cytomegalovirus agent, cidofovir (Figure 2). Both compounds, along with adefovir, are classified as acyclic nucleoside phosphonates, and are derivatives of (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl) adenine (HPMPA), itself originally developed as a broad-spectrum anti-DNA virus agent.27
Figure 2.
Chemical structure of tenofovir showing similarities to other anti-DNA virus compounds.
More recently, in an effort to explain the CAPRISA-004 findings, the direct activity of tenofovir against both laboratory and clinical strains of HSV-2 and HSV-1 has been confirmed in vitro using cell cultures of human embryonic lung fibroblasts and primary human keratinocytes, where the EC50 for HSV-2 was found to range from 85,000 to 193,000 ng/mL, as well as ex vivo human tonsillar and cervicovaginal tissues, and a mouse model.28 Although the concentrations required were 1–2 orders of magnitude greater than for traditional anti-herpetic drugs like acyclovir and cidofovir, replication of clinical HSV isolates was inhibited in a dose-dependent manner. The cell culture findings are similar to original reports showing an EC50 of 244 μM or 70,000 ng/mL for (R)-PMPA against HSV-2.26
However, importantly, standard doses of oral TDF do not appear to exhibit similarly meaningful anti-HSV activity in the genital tract. In a study of 40 HIV-1 seropositive persons coinfected with HSV-1 and/or HSV-2 who were taking daily oral TDF as part of suppressive combination antiretroviral therapy, for instance, we observed no decreases in HSV shedding from the oral, genital, or anal mucosa during a 28-day sampling period using polymerase chain reaction for detection of HSV.29 Further, a subanalysis of the iPrEx (Preexposure Prophylaxis Initiative) trial, the primary analysis of which demonstrated 44% efficacy of oral TDF compared with placebo in preventing HIV among men who have sex with men,30 found no difference in the rate of HSV-2 acquisition between the study arms in the 1416 participants who were HSV-2 seronegative at baseline.31
The most likely explanation for these discrepant results is that, in contrast with topical application, standard dose oral TDF produces inadequate genital tract concentrations to block HSV-2 replication and acquisition (Table 1).32–36 When used as part of a suppressive antiretroviral treatment regimen, TDF achieves excellent penetration of both the female and male genital tract, and the demonstrated efficacy of daily oral TDF in preventing sexually transmitted HIV infection in several recent clinical trials shows the adequacy of achievable genital tract concentrations (roughly 70–80 ng/mL in cervicovaginal fluid) for inhibiting HIV.30 However, these concentrations are low in comparison with the in vitro EC50 of tenofovir for HSV-2, cited above at 70,000–193,000 ng/mL in various studies.26,28 In contrast, the median cervicovaginal fluid tenofovir concentration achieved among tenofovir gel users who remained HIV-negative throughout the CAPRISA trial was 520 (range 0–1.3 million) ng/mL,32 and among healthy volunteers using a single dose of the gel was roughly 100,000 ng/mL.36 Still higher cervicovaginal fluid concentrations are achievable in healthy volunteers using multiple doses of the vaginal gel.36 Further supporting this pharmacokinetic hypothesis, a relationship was observed between cervicovaginal fluid tenofovir concentrations in the CAPRISA trial and HSV-2 acquisition risk, with 24% versus 6% of women acquiring HSV-2 if concentrations were < or > 100,000 ng/mL. Although higher doses of oral TDF might theoretically be able to overcome these pharmacokinetic challenges, the risk of treatment-limiting drug toxicities at such doses may preclude their use.
Table 1.
Tenofovir and tenofovir diphosphate C24h concentrations achieved in pharmacokinetic studies of oral TDF and tenofovir 1% vaginal gel
| Analyte | Tissue | Oral TDF 300 | Ref | Tenofovir 1% vaginal gela | Reference |
|---|---|---|---|---|---|
| TFV | Blood plasma | 41 (34–47) ng/mL | 32 | 0.3 (0.3,0.5) ng/mL | 35 |
| CVF | 69 (57–586) ng/mL | 32 | 100000 ng/mL | 35 | |
| 68.4 (28.2–112.6) ng/mLb | 33 | ||||
| 84.03 (95% CI 24.47–288.56) ng/mLb | 34 | ||||
| Vaginal tissue | 6.8 ng/g | 32 | 7000 ng/g | 35 | |
| Cervical tissue | 50 ng/g | 32 | |||
| Rectal tissue | 1877 ng/g | 32 | |||
| TFV-DP | Vaginal tissue | 1645 fmol/g | 32 | ||
| Cervical tissue | BLQ | 32 | |||
| Endocervical cells | 80,000 ng/mL | 35 | |||
| Rectal tissue | 206950 fmol/g | 32 |
Notes: All data are median (interquartile range) unless otherwise indicated.
Data shown are for single dose gel applications;
these results were obtained in HIV-infected women on antiretroviral therapy; all other data are from healthy volunteers.
Abbreviations: BLQ, below the limit of quantification; CVF, cervicovaginal fluid; HIV, human immunodeficiency virus; TDF, tenofovir disoproxil fumarate; TFV-DP, tenofovir diphosphate.
Further clinical trial data in different populations and study settings are needed to confirm that tenofovir 1% gel is an effective microbicide not only against HIV but HSV-2 as well. An unfortunate setback in this regard was the announcement in November 2011 that the VOICE (Vaginal and Oral Interventions to Control the Epidemic) trial was discontinuing the tenofovir and placebo gel arms of the trial early, upon recommendation from the trial’s independent data safety and monitoring board, due to lack of efficacy in preventing HIV. VOICE (also known as the Microbicide Trials Network or MTN-003 trial) was designed as a five-arm randomized trial comparing oral TDF, oral TDF-FTC, oral placebo, tenofovir 1% vaginal gel, and placebo gel as alternative strategies for HIV prevention among sexually active women in Uganda, Zimbabwe, and South Africa. The oral TDF arm of this trial was similarly discontinued prematurely for lack of efficacy in September 2011; the TDF-FTC and oral placebo arms of the trial continue. Data regarding HSV-2 acquisition in VOICE are not yet available, but follow-up of all trial participants is expected by August 2012, with final results by 2013. Importantly, unlike the CAPRISA-004 trial, the VOICE trial used a once-daily tenofovir gel dosing strategy, and detailed information on adherence and pericoital genital tract drug concentrations are not yet available.
Further data are also expected from MTN-021, a placebocontrolled trial of daily tenofovir 1% gel in sexually experienced adolescent females not infected with HIV, and from FACTS-001, a randomized trial of tenofovir 1% gel using the BAT24 dosing strategy being conducted among 18–30-year-old women at seven sites in South Africa. Data on pregnancy and lactation are also needed and will be obtained in the ongoing MTN-008 trial of 7-day courses of daily tenofovir 1% gel in 18–40-year-old American women. Although no differences in pregnancy outcomes or congenital anomalies were noted between the two CAPRISA-004 trial arms, participants in that trial were instructed to discontinue gel use if they became pregnant. Data on HIV-exposed uninfected infants of women who took oral TDF as part of antiretroviral therapy in pregnancy suggest no excess of small for gestational age or low birth weight, but slightly lower length and head circumference for age at one year.37 However, the overall clinical relevance of these findings and their applicability to topical tenofovir use are unknown.
Benefits of HSV-2 prevention in women not infected with HIV
If confirmed to be effective in these and other studies, important benefits could derive from the use of tenofovir-based HSV-2 prophylaxis in HIV-uninfected women, including the prevention of HSV-2 related morbidity and indirect benefits in preventing HIV acquisition.
In addition to causing morbidity in the form of recurrent genital ulceration, systemic symptoms such as fever, malaise, headache, and myalgia also occur during primary HSV-2 infection in 70% of women, and other manifestations may include dysuria, cervicitis, and central nervous system complications, including urinary retention and aseptic meningitis. HSV can also be transported via the lymphatics to cause disseminated infection, and HSV viremia can be detected using polymerase chain reaction in 24% of uncomplicated primary genital infections, although the clinical consequences of this viremia are unknown.38 These manifestations are further associated with considerable psychological distress and utilization of health care resources. Of still greater concern is the potential in pregnancy for neonatal HSV infection, a devastating and potentially fatal vertically transmitted condition, with skin, mucosal, neurological, and/or disseminated visceral manifestations. The risk of neonatal HSV is greatest if primary HSV infection (ie, infection in the absence of either HSV-1 or 2 antibodies) occurs during pregnancy,39 making the prevention of HSV-2 in pregnancy an important priority.
Preventing HSV-2 would have further indirect benefits to personal and public health because HSV-2 significantly increases the risk of HIV acquisition by a factor of approximately 2.1 (95% CI 1.4–3.2).40 This is particularly true because incident HSV-2 infection has a greater impact on increasing HIV acquisition risk than prevalent HSV-2.41 Further, in meta-analyses that considered the relative risk of HIV associated with HSV-2 infection separately by gender, the summary adjusted relative risk was higher in women at 3.1 (95% CI 1.7–5.6), than in men (relative risk 2.7, 95% CI 1.9–3.9) and in men who have sex with men (relative risk 1.7, 95% CI 1.2–2.4).42
Several biological mechanisms may underlie this negative synergy. Microscopic or macroscopic physical disruptions of the epithelial barrier of genital mucosal surfaces by HSV-2 may provide portals of entry and exit for HIV during sexual contact. Studies involving intensive daily sampling of HSV-2 infected adults have shown that roughly 40%–50% of shedding episodes in immunocompetent adults are asymptomatic and brief, at only 12 hours or less,43,44 and mathematical modeling further suggests that neuronal release of small numbers of HSV virions into the genital tract is nearly constant.45 This frequent, low-level viral shedding is difficult to suppress even with high doses of anti-HSV medication.46 In addition, the host immune response to HSV-2 reactivation involves an influx of CD4-positive T cells, thereby increasing the pool of locally susceptible target cells in persons sexually exposed to HIV.47–49
Of note, detailed analyses from the CAPRISA-004 trial show that tenofovir gel reduced the risk of HIV acquisition to a similar degree in participants with and without HSV-2 infection; further, after adjustment for prevalent HSV-2 infection, the effect of tenofovir on HIV prevention was not significantly changed. In contrast, mathematical modeling suggests a partial role of HSV-2 prevention in preventing HIV.50 Taken together, these findings suggest that tenofovir-related HSV-2 prevention at best contributed modestly to its HIV prevention effect in this trial, although long-term benefits of HSV-2 prevention in reducing HIV risk likely exist.
Benefits of HSV-2 prevention in HIV-infected women
Among HIV-infected populations, the estimated HSV-2 seroprevalence is 60%–95%.51 Preventing HSV-2 in the remaining 5%–40% of HIV-infected women is also important for several reasons. First, the usual manifestations of HSV-2 are exacerbated in the context of HIV. Mucocutaneous shedding of HSV is greater in frequency and quantity in the context of HIV. A study among 176 women showed the prevalence of asymptomatic genital HSV-2 culture positivity to be roughly four times higher in HIV-infected than in uninfected participants (13.2% versus 3.6%, P = 0.04; odds ratio 4.1, 95% CI 1.0–27.4).52 Symptomatic reactivations of HSV-2 are also more frequent and severe with increasing HIV-related immunosuppression,53 and can cause extensive lesions featuring deep ulceration and necrosis, and chronic mucocutaneous HSV infection is considered an acquired immune deficiency syndrome (AIDS)-defining illness.54,55 As in other immunocompromised populations, HSV-2 can also produce atypical hypertrophic, verrucous, and nodular lesions in HIV-infected hosts regardless of CD4 count that can be challenging to treat.56,57 Further complicating the management of HIV and HSV-2 coinfection is the risk of acyclovir resistance which may necessitate second-line therapies; resistant strains of HSV-2 are largely confined to patients with advanced AIDS and chronic herpetic ulcerations.58 These exacerbations and perturbations of the usual clinical manifestations of HSV-2 in the setting of HIV are felt to be attributable to HIV-related impairment of T cell immunity against the virus. Both the breadth and the magnitude of T cell responses to HSV-2 are downregulated in HIV, and HSV-2 coinfected individuals compared with HSV-2 monoinfected individuals.59,60
Second, because HSV-2 reactivations are associated with increases in plasma HIV viral load,61 preventing HSV-2 in HIV-infected women could theoretically avoid an adverse impact of HSV-2 on HIV disease progression. Importantly, however, definitive evidence from longitudinal studies that HSV-2 accelerates HIV disease progression is lacking.62 Although two clinical trials from Sub-Saharan Africa demonstrated modest attenuation in HIV disease progression among HSV-2 coinfected adults randomized to receive acyclovir 400 mg twice daily,63,64 it is likely that these benefits derive at least in part from the direct anti-HIV effects of acyclovir rather than from the reversal of HSV-2-related effects.65,66
Finally, HSV-2 prevention among HIV-infected persons could have downstream benefits in decreasing onward HIV transmission, given that coinfection is thought to increase HIV transmissibility.67 A study in Uganda showed that genital ulcer disease, the bulk of which was caused by HSV-2, increased the risk of transmitting HIV to sexual partners by roughly four-fold.68
Unanswered questions
Although the CAPRISA-004 trial provided proof-of-concept that topical tenofovir can prevent HIV and HSV-2 infections in women, several important questions remain regarding delivery systems, drug formulation, rectal administration, drug resistance, and usage in the management of established HSV infection, among other issues.
Delivery systems
The optimal delivery system for achieving relevant genital tract concentrations using topical tenofovir remains to be determined. Limitations of the vaginal gel formulation are that it is relatively short-acting (hence requiring repeated applications), requires use of an applicator, and can be associated with leakage. Extended-release systems that require reapplication only every 1–3 months, such as intravaginal rings, may thus be an attractive alternative, offering advantages including coital independence, improved adherence, and greater acceptability.69 Such systems further offer the potential for coadministration with more than one active ingredient, such as a contraceptive or additional microbicide, although not without their own challenges. Given the water solubility of tenofovir, for instance, coformulation with hydrophobic drugs such as levonorgestrel and the investigational antiretroviral, dapivirine, may require a segmented design,70 and technical challenges may preclude inclusion of more than two agents.71 Also, there remain unanswered questions about whether hormonal contraception potentiates HIV acquisition.72
At present, a tenofovir-loaded polyurethane ring capable of releasing 10 mg tenofovir daily for 90 days is under evaluation, as is a combination ring containing levonorgestrel. The combination of tenofovir with acyclovir has also been studied in animal models as a strategy for improving efficacy in preventing HSV-2, and shown to produce constant drug release rates.73,74 Other novel delivery systems under investigation for delivering tenofovir to the vagina include a pH-responsive nanoparticle system,75,76 and could include bioadhesive films and vaginal tablets, although none of these methods would offer extended-release possibilities. Combination of tenofovir with novel HIV non-nucleoside reverse transcriptase inhibitors has also been explored, although retaining the gel formulation.77,78
Formulation
Whether tenofovir or a prodrug offers a better formulation for HSV-2 preventive applications is another important question. Although TDF cannot be formulated into a gel because of its hydrolytic instability,79 TDF is suitable for formulation in an intravaginal ring, and confers the advantage over tenofovir of increased hydrophobicity, potentially resulting in improved uptake into cells.80,81 GS-7340 is another prodrug of tenofovir, which, when taken orally, has a longer half-life in the bloodstream than TDF, and more intracellular rather than bloodstream metabolism compared with tenofovir.82 These properties of GS-7340 are being exploited in clinical trials for the treatment of established HIV infection, where it is hoped that greater antiviral efficacy and lower systemic toxicity can be achieved with a lower drug dose. Whether the formulation will achieve clinically relevant concentrations in the vaginal tract for preventing HSV-2 remains to be determined.
Rectal application
Because receptive anal intercourse is another important potential route for transmission of both HIV and HSV-2, tenofovir 1% gel has been studied for its safety and tolerability as a rectal microbicide. In a Phase I trial of 18 participants, including four women, the original vaginal formulation was associated with a suboptimal gastrointestinal side effect profile when applied rectally.83 A reduced glycerin formulation was thus developed specifically for rectal application, the decreased osmolality of which (836 mOsmol/kg compared with 3111 mOsmol/kg for the vaginal gel) was associated with acceptable systemic and mucosal safety, acceptability, and adherence in a trial of 65 participants (including 20 women);84 further testing in MTN-017, an 8-week Phase II trial, is planned.
Drug resistance
An important consideration with prophylactic tenofovir is the potential for the development of antiviral drug resistance, particularly in HIV, but whether clinically relevant tenofovir resistance mutations exist in HSV-2 is unknown. One scenario in which such resistance could theoretically arise is in women not infected with HIV who develop breakthrough infection but continue to use topical tenofovir before the diagnosis is made, even though systemic absorption of the gel is known to be low. Another scenario in which such resistance could arise is if topical tenofovir is used for HSV-2 prevention by women already known to have HIV infection. Such use could be restricted to patients already receiving highly active antiretroviral therapy to minimize the risk of systemic drug resistance, although resistance in the genital compartment could theoretically emerge, with public health implications.
Importantly, of the 35 CAPRISA-004 participants who acquired HIV in the tenofovir arm and whose plasma samples could be tested for resistance, no tenofovir-related resistance mutations (K65R or K70E) were observed on bulk sequencing,5 nor were there clinically relevant mutation frequencies by deep pyrosequencing.85 Among the 21 women whose cervicovaginal swab specimens could be tested for resistance, only one had detectable K65R at a frequency of 0.8% from proviral DNA only; this level is in the range observed for drug-naïve HIV patients.86 These encouraging results must be interpreted in light of the limited exposure of the virus to the drug in these women; resistance assays in plasma were conducted an estimated 3–4 weeks after HIV acquisition in these women, and only three of the 21 cervicovaginal specimens contained detectable drug at all.5,86
Role in clinical management of HSV infection
If tenofovir is an anti-herpes medication, it is reasonable to envisage potential roles for the drug in the management of established HSV infection and reduction of onward HSV transmission, in addition to primary prevention of HSV. Such roles could involve chronic suppressive therapy for individuals with frequent outbreaks or for prevention purposes, as well as short-course treatment of herpes flares, akin to current therapeutic indications for acyclovir and related compounds. Topical formulations would be best suited to this role, give that oral TDF appears unlikely to have a clinically meaningful impact on HSV shedding or symptoms,29 and could offer the additional advantage of decreased systemic absorption and related toxicities. A Phase IV trial of oral and topical tenofovir for reducing HSV shedding in HSV-2 seropositive, HIV-uninfected persons (NCT01448616) is currently underway. The documented activity of tenofovir against HSV-1 in addition to HSV-2 is also relevant in this regard,26,28 given the increasingly important role of HSV-1 in genital herpes infection in many settings and its potential relevance to HIV infection.87
Conclusion
Although not previously considered to be an anti-herpes medication, tenofovir has recently been demonstrated to have in vitro activity against HSV-2 and in vivo protective efficacy against HSV-2 acquisition as a vaginal microbicide. Further study is needed to confirm these clinical findings, assess its potential use in other populations, and optimize formulation and delivery systems. These efforts may eventually identify HSV-2 prevention as a new use for this existing drug, with unique advantages over existing strategies, including potential for use without requiring the consent of sexual partners. Nevertheless, the available data suggest an incomplete protective effect of tenofovir 1% gel against HSV-2 acquisition at only 51%, cautioning that this strategy must be complemented by ongoing efforts to develop other novel anti-herpetic drugs and a protective HSV-2 vaccine.
Acknowledgment
The author receives postdoctoral fellowship funding from the Ontario HIV Treatment Network.
Footnotes
Disclosure
The author reports no relevant conflicts of interest in this work.
References
- 1.Paz-Bailey G, Ramaswamy M, Hawkes SJ, Geretti AM. Herpes simplex virus type 2: epidemiology and management options in developing countries. Sex Transm Infect. 2007;83:16–22. doi: 10.1136/sti.2006.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 3.Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 4.Karim SS, Karim Q. Effectiveness and safety of vaginal microbicide 1% tenofovir gel for prevention of HIV infection in women. Abstract TUSS0204 presented at the XVIII International AIDS Conference; July 18–23, 2010; Vienna, Austria. [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed April 10, 2012]. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 7.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 8.European AIDS Clinical Society. Guidelines: clinical management and treatment of HIV infected adults in Europe. [Accessed June 2, 2012]. Available at: http://www.europeanaidsclinicalsociety.org/index.php?option=com_content&view=article&id=59&Itemid=41.
- 9.Gazzard BG, Anderson J, Babiker A, et al. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organisation. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. [Accessed July 2, 2012]. Available at: http://www.who.int/hiv/pub/arv/advice/en/index.html.
- 11.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 12.Peterson L, Nanda K, Opoku BK, et al. SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS One. 2007;2:e1312. doi: 10.1371/journal.pone.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 14.McCormack S, Ramjee G, Kamali A, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 17.Hillier SL, Joshi S, Cyrus-Cameron E, et al. Safety and acceptability of coitally dependent use of 1% tenofovir gel over six months of use. Abstract 655 presented at the Microbicides Conference; February 24–27, 2008; New Delhi, India. [Google Scholar]
- 18.Cranage M, Sharpe S, Herrera C, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008;5:e157. doi: 10.1371/journal.pmed.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheshenko N, Keller MJ, MasCasullo V, et al. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob Agents Chemother. 2004;48:2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacharopoulos VR, Phillips DM. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diagn Lab Immunol. 1997;4:465–468. doi: 10.1128/cdli.4.4.465-468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maguire RA, Bergman N, Phillips DM. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex Transm Dis. 2001;28:259–265. doi: 10.1097/00007435-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Zeitlin L, Hoen TE, Achilles SL, et al. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis. 2001;28:417–423. doi: 10.1097/00007435-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Keller MJ, Zerhouni-Layachi B, Cheshenko N, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- 26.Balzarini J, Holy A, Jindrich J, et al. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Clercq E. The acyclic nucleoside phosphonates from inception to clinical use: historical perspective. Antiviral Res. 2007;75:1–13. doi: 10.1016/j.antiviral.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Andrei G, Lisco A, Vanpouille C, et al. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe. 2011;10:379–389. doi: 10.1016/j.chom.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan DH, Kaul R, Raboud JM, Walmsley SL. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS. 2011;25:207–210. doi: 10.1097/QAD.0b013e328341ddf7. [DOI] [PubMed] [Google Scholar]
- 30.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lama J, Mayer K, Schechter M, et al. Oral TDF and its impact in HSV-2 acquisition and clinical expression. Abstract 1003 presented at the 18th Conference on Retroviruses and Opportunistic Infections; February 27–March 2, 2011; Boston, MA. [Google Scholar]
- 32.Kashuba AD, Abdool Karim SS, Kraft E, et al. Do systemic and genital tract tenofovir concentrations predict HIV seroconversion in the CAPRISA 004 tenofovir gel trial. Abstract TUSS0503 presented at the XVIII International AIDS Conference; July 18–23, 2010; Vienna, Austria. [Google Scholar]
- 33.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112–114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwara A, Delong A, Rezk N, et al. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis. 2008;46:719–725. doi: 10.1086/527387. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz JL, Rountree W, Kashuba AD, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One. 2011;6:e25974. doi: 10.1371/journal.pone.0025974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siberry GK, Williams PL, Mendez H, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–1159. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston C, Magaret A, Selke S, Remington M, Corey L, Wald A. Herpes simplex virus viremia during primary genital infection. J Infect Dis. 2008;198:31–34. doi: 10.1086/588676. [DOI] [PubMed] [Google Scholar]
- 39.Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509–515. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 40.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds SJ, Risbud AR, Shepherd ME, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis. 2003;187:1513–1521. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 42.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 43.Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of oral and anogenital herpes simplex virus shedding in HIV-infected adults. J Acquir Immune Defic Syndr. 2010;54:482–488. doi: 10.1097/QAI.0b013e3181d91322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffer JT, Abu-Raddad L, Mark KE, et al. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med. 2009;1:7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston C, Saracino M, Kuntz S, et al. Standard-dose and highdose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet. 2012;379:641–647. doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest. 1985;75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 49.Koelle DM, Corey L, Burke RL, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boily MC, Dimitrov D, Mâsse B. How much of the overall microbicide effectiveness against HIV is due to the protection of TFV gel against HSV-2 The CAPRISA-004 trial. Abstract 999 presented at the 18th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2011; Boston, MA. [Google Scholar]
- 51.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2008;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 52.Augenbraun M, Feldman J, Chirgwin K, et al. Increased genital shedding of herpes simplex virus type 2 in HIV-seropositive women. Ann Intern Med. 1995;123:845–847. doi: 10.7326/0003-4819-123-11-199512010-00006. [DOI] [PubMed] [Google Scholar]
- 53.Bagdades EK, Pillay D, Squire SB, O’Neil C, Johnson MA, Griffiths PD. Relationship between herpes simplex virus ulceration and CD4+ cell counts in patients with HIV infection. AIDS. 1992;6:1317–1320. doi: 10.1097/00002030-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Safrin S, Ashley R, Houlihan C, Cusick PS, Mills J. Clinical and serologic features of herpes simplex virus infection in patients with AIDS. AIDS. 1991;5:1107–1110. doi: 10.1097/00002030-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Siegal FP, Lopez C, Hammer GS, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305:1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 56.Yudin MH, Kaul R. Progressive hypertrophic genital herpes in an HIVinfected woman despite immune recovery on antiretroviral therapy. Infect Dis Obstet Gynecol. 2008;2008:592532. doi: 10.1155/2008/592532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmes A, McMenamin M, Mulcahy F, Bergin C. Thalidomide therapy for the treatment of hypertrophic herpes simplex virus-related genitalis in HIV-infected individuals. Clin Infect Dis. 2007;44:e96–e99. doi: 10.1086/517513. [DOI] [PubMed] [Google Scholar]
- 58.Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheth PM, Sunderji S, Shin LY, et al. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J Infect Dis. 2008;197:1394–1401. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- 60.Kaul R, Pettengell C, Sheth PM, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Gray RH, Li X, Wawer MJ, et al. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J Infect Dis. 2004;18:1209–1215. doi: 10.1086/382750. [DOI] [PubMed] [Google Scholar]
- 62.Tan DHS, Murphy K, Shah PS, Walmsley SL. Systematic review of the impact of herpes simplex virus type 2 on HIV disease progression. Abstract 305 presented at the Ontario HIV Treatment Network Research Conference; November 14–15, 2011; Toronto, Canada. [Google Scholar]
- 63.Lingappa JR, Baeten JM, Wald A, et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12:441–448. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–31293. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–270. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 68.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 69.Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. Advances in microbicide vaginal rings. Antiviral Res. 2010;88(Suppl 1):S30–S39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39:203–212. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Friend DR. Drug delivery in multiple indication (multipurpose) prevention technologies: systems to prevent HIV-1 transmission and unintended pregnancies or HSV-2 transmission. Expert Opin Drug Deliv. 2012;9:417–427. doi: 10.1517/17425247.2012.668183. [DOI] [PubMed] [Google Scholar]
- 72.Blish CA, Baeten JM. Hormonal contraception and HIV-1 transmission. Am J Reprod Immunol. 2011;65:302–307. doi: 10.1111/j.1600-0897.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly L, Carr D, Clark MR, et al. In vivo release in sheep of a dual loaded microbicide polyurethane vaginal ring. Paper presented at the American Association of Pharmaceutical Scientists Annual Meeting and Exposition; October 23–27, 2011; Washington, DC. [Google Scholar]
- 74.Moss JA, Malone AM, Smith TJ, et al. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother. 2012;56:875–882. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang T, Sturgis TF, Youan BB. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur J Pharm Biopharm. 2011;79:526–536. doi: 10.1016/j.ejpb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alukda D, Sturgis T, Youan BB. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. J Pharm Sci. 2011;100:3345–3356. doi: 10.1002/jps.22529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ham AS, Ugaonkar SR, Shi L, et al. Development of a combination microbicide gel formulation containing IQP-0528 and tenofovir for the prevention of HIV infection. J Pharm Sci. 2012;101:1423–1435. doi: 10.1002/jps.23026. [DOI] [PubMed] [Google Scholar]
- 78.Kiser PF, Mahalingam A, Fabian J, et al. Design of tenofovir-UC781 combination microbicide vaginal gels. J Pharm Sci. 2012;101:1852–1864. doi: 10.1002/jps.23089. [DOI] [PubMed] [Google Scholar]
- 79.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66:240–250. doi: 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moss JA, Baum MM, Malone AM, et al. Tenofovir and tenofovir disoproxil fumarate pharmacokinetics from intravaginal rings. AIDS. 2012;26:707–710. doi: 10.1097/QAD.0b013e3283509abb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mesquita PM, Rastogi R, Segarra TJ, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012;67(7):1730–1738. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruane P, DeJesus E, Berger D, et al. GS-7340 25 mg and 40 mg demonstrate superior efficacy to tenofovir disoproxil fumarate 300 mg in a 10-day monotherapy study of HIV-1+ patients. Abstract 103 presented at the 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA. [Google Scholar]
- 83.Anton P, Cranston R, Carballo-Dieguez A, et al. RMP-02/MTN-006: A phase 1 placebo-controlled trial of rectally applied 1% vaginal TFV gel with comparison to oral TDF. Abstract 34 LB presented at the 18th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2011; Boston, MA. [Google Scholar]
- 84.McGowan I, Hoesley C, Andrew P, et al. MTN-007: a Phase 1 randomized, double-blind, placebo-controlled rectal safety and acceptability study of tenofovir 1% gel. Abstract 34LB presented at the19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA. [Google Scholar]
- 85.Fischer W, Hunt G, Sibeko S, et al. Tenofovir resistance mutation frequencies assessed by deep pyrosequencing of plasma virus from breakthrough HIV infections: CAPRISA 004 microbicide trial. Abstract 1063 presented at the 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA. [Google Scholar]
- 86.Wei X, Morris L, Naranbhai V, et al. Sensitive tenofovir resistance screening of HIV-1 from the genital tract of women with breakthrough infections: CAPRISA 004 tenofovir gel trial. Abstract 33 presented at the 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA. [Google Scholar]
- 87.Tan DH, Kaul R, Walsmley S. Left out but not forgotten: should closer attention be paid to coinfection with herpes simplex virus type 1 and HIV? Can J Infect Dis Med Microbiol. 2009;20:e1–e7. doi: 10.1155/2009/965263. [DOI] [PMC free article] [PubMed] [Google Scholar]