Platelets may be small, but their behavior in vivo is governed by molecular mechanisms every bit as complex as those in larger cells and new molecules that provide unexpected insights turn up with surprising regularity. One recent example is the connexin family member, Cx37 or GJA4. The role of Cx37 in platelets is examined in this issue by Vaiyapuri et al. 1, but a related study by Angelillo-Scherer, et al. 2 appeared here a year ago and both will be considered together.
Cx37 is a member of a family of gap junction proteins that can assemble a comparatively non-selective channel when cells come into close contact with each other. These channels are large enough to allow free passage of soluble molecules such as cAMP, Ca++ and IP3 between cells. They are formed by the interaction of two Cx37 hemichannels on the surface of opposing cells. Hemichannels themselves can also pass small molecules, but with different specificities than the complete channel (see Kar, et al. 3 for a recent review).
The identification of a previously-unsuspected molecule in platelets inevitably leads to questions about its role in either platelets or megakaryocytes. Platelets have limited synthetic capability. According to current models, they are formed by carefully orchestrated events in which proteins synthesized by megakaryocytes are transshipped to the tip of a developing proplatelet projection 4. Presumably anything found in mature platelets is there for a reason. What, then, is the role of Cx37? Based on studies in other cells it is possible to make some reasoned guesses. However, as will be discussed below, published evidence leaves the answer in platelets somewhat uncertain.
First, consider that a major design goal of platelets is to be able to form a stable hemostatic plug in response to vascular injury while, at the same time, avoiding either unnecessary platelet activation or excessive platelet accumulation. There are both positive and negative regulators of platelet activation that have a substantial impact. The initial capture of rapidly-moving platelets is mediated by the binding of glycoprotein (GP) Ibα on the platelet surface to the von Willebrand factor (VWF) that decorates collagen fibrils in the damaged vessel wall. Activation often begins with the collagen receptor, GP VI, and the thrombin receptors, PAR1 and PAR4 (Figure 1). It is reinforced by local accumulation of ADP (secreted by platelets and released by damaged cells) and thromboxane A2 (released by activated platelets). As additional platelets join the growing hemostatic mass, integrin áIIbâ3 on the platelet surface binds to fibrinogen, fibrin and von Willebrand factor, forming an array of adhesive connections between platelets. áIIbâ3 is present at very high copy number on resting platelets and increases to even higher levels of surface expression when platelets are activated. Events within the platelet trigger the conformational shift that allows áIIbâ3 to bind its ligands and hold platelet aggregates together.
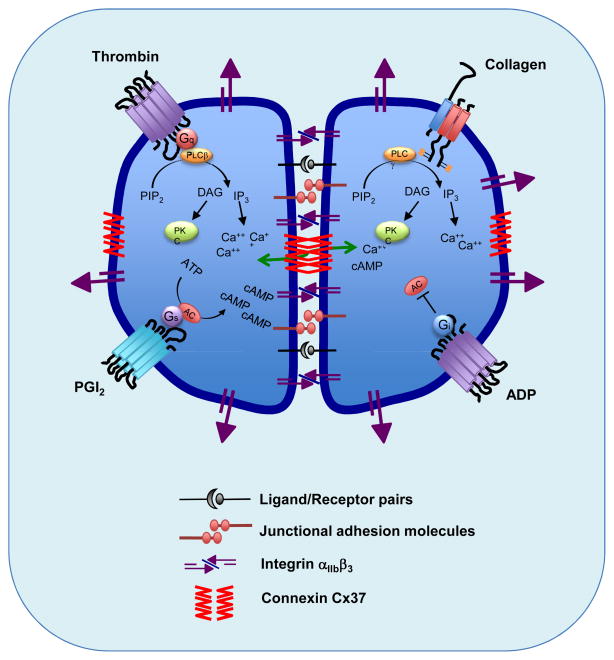
Figure 1.
The figure illustrates the accumulation (established or potential) of proteins at the interface between adjacent platelets within a growing hemostatic plug. It also shows some of the signaling events downstream of thrombin and collagen receptors in platelets, and the stimulation and inhibition of cAMP formation in response to PGI2 and ADP. Finally, the figure illustrates one of the hypotheses discussed in the text, which is that channels formed by Cx37 are able to pass small molecules between platelets once stable contacts have formed.
The major pathways that support platelet activation have been mapped, mutated and targeted by the pharmaceutical and biotech industries, producing drugs that have found widespread clinical use. However, platelet biology does not begin or end with a rapid response to vascular injury. In addition to identifying roles for platelets outside of hemostasis, past studies have shown that the quiescent state of platelets is actively maintained prior to injury and that events occurring after platelet aggregation help to stabilize the hemostatic plug. Cx37, the focus of this brief commentary, appears to play a role in regulating thrombus growth and stability.
Formation of platelet-platelet “junctions”
As activated platelets come into áIIbâ3-mediated contacts with each other, other proteins on the surface of adjacent platelets come close enough for long enough to interact in trans (Figure 1). In a recent review we cited a number of examples of this phenomenon, including the ephrin and semaphorin family members, ephrinB1 and sema4D. Like Cx37, ephrinB1 and sema4D entered the platelet literature as surface proteins whose role in hemostasis was unknown 5. It is now known that by binding to their cognate receptors on adjacent platelets, ephrinB1 and sema4D are able to initiate signaling events that reinforce platelet activation 6, 7. Loss of either impairs subsequent platelet activation. Examples of adhesive proteins include PECAM-1, CEACAM-1, and four members of the CTX family (JAM-A, JAM-C, ESAM and CD226), many of which have been shown to accumulate at the junctions between cells via either homophilic or heterophilic trans-interactions. Note, however, that although these are classified as “adhesive” proteins, it is not clear that they actually contribute to the forces holding activated platelets in contact with each other. Additional candidates for junctional roles in platelets include SLAM 8, CD84 8, G6b-B 9, 10, CD148 11 and TLT-1 12. Interestingly, platelet function has been found to be increased, rather than decreased in mice that lack PECAM-1 13, CEACAM-1 14, JAM-A 15 or ESAM 16, suggesting that this group of molecules normally serves to restrain, rather than promote, thrombus growth and stability.
Despite this lengthy list of junction-associated molecules and the growing evidence for contact-dependent signaling in platelets, it remains unclear whether adjacent platelets form any of the classic junctional structures found in other cell types, such as tight, adherens or gap junctions. The narrowing distances between activated platelets in a growing hemostatic plug or thrombus brings adjacent platelet membranes close enough to each other that protein:protein interactions are possible. The gaps that remain provide a relatively protected environments in which soluble molecules can accumulate and the entry (or exit) of plasma proteins can be restrained. However, the evidence that platelets form actual junctional structures is limited, even though a number of reports have documented the presence of junction forming molecules in platelets (see 17 among others). Close contacts between platelets have been documented by a number of investigators using electron microscopy, including, most recently, Vaiyapuri et al. 1. However, it is not yet clear from those studies that there are actual junctional structures.
Connexin 37
In this context, the identification of Cx37 on platelets is especially intriguing. The evidence that it is expressed by platelets is compelling. The evidence for its role is somewhat contradictory. Both of the groups that have looked at Cx37 function in platelets were able to show that small molecules can pass between adjacent platelets in an aggregate: neurobiotin in one case 2 and calcein in the other 1. Blockade or deletion of the Cx37 channels prevented passage of the tracers.
After that, however, their observations diverge. Angelillo-Scherer and her colleagues 2, found that deleting Cx37 in mice reduced the tail bleeding time, shortened the time to occlusive thrombosis, accelerated mortality in a disseminated thrombosis model, and enhanced platelet aggregation in response to suboptimal concentrations of ADP, thrombin and collagen. A Cx37-blocking peptide mimicked the effects of the knockout by promoting platelet aggregation. The authors proposed that Cx37 forms a channel when platelets are in stable contact, permitting passage of a molecule such as cAMP from one platelet to another. Since rising cAMP levels suppress platelet reactivity, transferred cAMP would act as an inhibitor and preventing transfer would conceivably cause the observed gain of function in the Cx37 knockout. Note, however, that while the transfer of cAMP between cells other than platelets has been demonstrated, cAMP transfer between platelets remains an interesting speculation.
In contrast, Vaiyapuri, et al. 1 in the current issue of Circulation found a loss of function when they studied platelets from what appears to be the same Cx37 knockout mouse line that was studied by Angelillo-Scherer et al. Deleting Cx37 reduced platelet aggregation. It also inhibited fibrinogen binding and á-granule secretion under conditions when platelet:platelet contacts were excluded. Cx37 blockers had comparable inhibitory effects, which were seen when single platelets were observed by flow cytometry as well as under conditions when aggregation occurred. Receptor-proximal signaling events seemed to occur normally, but the rise in cytosolic Ca++ concentration that usually accompanies platelet activation was blunted. These results suggest that the contribution of Cx37 is not limited to settings in which platelets come into stable contact with each other, but also occurs when only hemichannels are present.
Thus, although there is agreement that platelets express Cx37, there is no consensus on its role, one group concluding that Cx37 helps to repress platelet activation, while another concludes that it promotes platelet activation. The reasons for these differences are not clear. The inhibitors used by both groups vary in their mechanisms of action and are vulnerable to off-target effects. The Cx37 knockout was not limited to platelets. Effects of the knockout on cells other than platelets may have contributed to the effects observed in vivo, but are less obviously relevant in studies performed with isolated platelets. Each report made use of multiple methods and is internally consistent, so the conundrum remains. Therefore, however tempting it may be to assign Cx37 a role in communication between platelets after stable platelet:platelet contacts have formed, it is still premature to do so. The tale of gaps and junctions between platelets is far from complete.
Footnotes
Conflict of Interest Disclosures: None (both authors)
References
- 1.Vaiyapuri S, Jones CI, Sasikumar P, Morae LA, Munger SJ, Wright JR, Marfoua SA, Sage T, Kaiser WJ, Tucker KL, Stain CJ, Bye AP, Jones S, Oviedo-Orta E, Simon AM, Mahout-Smith MP, Gibbins JM. Gap junctions and connexin hemichannels underpin haemostasis and thrombosis. Circulation. 2012;125:XXX–XXX. doi: 10.1161/CIRCULATIONAHA.112.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelillo-Scherrer A, Fontana P, Burnier L, Roth I, Sugamele R, Brisset A, Morel S, Nolli S, Sutter E, Chassot A, Capron C, Borgel D, Saller F, Chanson M, Kwak BR. Connexin 37 limits thrombus propensity by downregulating platelet reactivity. Circulation. 2011;124:930–939. doi: 10.1161/CIRCULATIONAHA.110.015479. [DOI] [PubMed] [Google Scholar]
- 3.Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Archives of biochemistry and biophysics. 2012 Mar 17; doi: 10.1016/j.abb.2012.03.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thon JN, Italiano JE. Platelet formation. Semin Hematol. 2010;47:220–226. doi: 10.1053/j.seminhematol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brass LF, Wannemacher KM, Ma P, Stalker TJ. Regulating thrombus growth and stability to achieve an optimal response to injury. J Thromb Haemost. 2011;9 (Suppl 1):66–75. doi: 10.1111/j.1538-7836.2011.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevost N, Woulfe D, Tanaka T, Brass LF. Interactions between eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci USA. 2002;99:9219–9224. doi: 10.1073/pnas.142053899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4d in platelet responses to vascular injury. Proc Natl Acad Sci U S A. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanda N, Andre P, Bao M, Clauser K, Deguzman F, Howie D, Conley PB, Terhorst C, Phillips DR. Platelet aggregation induces platelet aggregate stability via slam family receptor signaling. Blood. 2005;106:3028–3034. doi: 10.1182/blood-2005-01-0333. [DOI] [PubMed] [Google Scholar]
- 9.Newland SA, Macaulay IC, Floto AR, de Vet EC, Ouwehand WH, Watkins NA, Lyons PA, Campbell DR. The novel inhibitory receptor G6b is expressed on the surface of platelets and attenuates platelet function in vitro. Blood. 2007;109:4806–4809. doi: 10.1182/blood-2006-09-047449. [DOI] [PubMed] [Google Scholar]
- 10.Senis YA, Tomlinson MG, Garcia A, Dumon S, Heath VL, Herbert J, Cobbold SP, Spalton JC, Ayman S, Antrobus R, Zitzmann N, Bicknell R, Frampton J, Authi K, Martin A, Wakelam MJ, Watson SP. A comprehensive proteomics and genomics analysis reveals novel transmembrane proteins in human platelets and mouse megakaryocytes including G6B-b, a novel ITIM protein. Mol Cell Proteomics. 2006;6:548–564. doi: 10.1074/mcp.D600007-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senis YA, Tomlinson MG, Ellison S, Mazharian A, Lim J, Zhao Y, Kornerup KN, Auger JM, Thomas SG, Dhanjal T, Kalia N, Zhu JW, Weiss A, Watson SP. The tyrosine phosphatase CD148 is an essential positive regulator of platelet activation and thrombosis. Blood. 2009;113:4942–4954. doi: 10.1182/blood-2008-08-174318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, Feltz R, De La Mota A, Schubert RL, Gomez-Rodriguez J, Cheng J, Dutra A, Pak E, Chertov O, Rivera L, Morales J, Lubkowski J, Hunter R, Schwartzberg PL, McVicar DW. Trem-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119:1489–1501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patil S, Newman DK, Newman PJ. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood. 2001;97:1727–1732. doi: 10.1182/blood.v97.6.1727. [DOI] [PubMed] [Google Scholar]
- 14.Wong C, Liu Y, Yip J, Chand R, Wee JL, Oates L, Nieswandt B, Reheman A, Ni H, Beauchemin N, Jackson DE. Ceacam1 negatively regulates platelet-collagen interactions and thrombus growth in vitro and in vivo. Blood. 2009;113:1818–1828. doi: 10.1182/blood-2008-06-165043. [DOI] [PubMed] [Google Scholar]
- 15.Naik MU, Stalker TJ, Brass LF, Naik UP. Jam-a protects from thrombosis by suppressing integrin alphaiibbeta3-dependent outside-in signaling in platelets. Blood. 2012;119:3352–60. doi: 10.1182/blood-2011-12-397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stalker TJ, Wu J, Morgans A, Traxler EA, Wang L, Chatterjee MS, Lee D, Quertermous T, Hall RA, Hammer DA, Diamond SL, Brass LF. Endothelial cell specific adhesion molecule (esam) localizes to platelet-platelet contacts and regulates thrombus formation in vivo. J Thromb Haemost. 2009;7:1886–1896. doi: 10.1111/j.1538-7836.2009.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elrod JW, Park JH, Oshima T, Sharp CD, Minagar A, Alexander JS. Expression of junctional proteins in human platelets. Platelets. 2003;14:247–251. doi: 10.1080/0953710031000118894. [DOI] [PubMed] [Google Scholar]