Abstract
Introduction
Ebolaviruses cause severe viral hemorrhagic fever in humans and non-human primates, with case fatality rates of up to 90%. Currently, neither a specific treatment nor a vaccine licensed for use in humans is available. However, a number of vaccine candidates have been developed in the last decade that are highly protective in non-human primates, the gold standard animal model for Ebola hemorrhagic fever.
Areas covered
This review analyzes a number of scenarios for the use of ebolavirus vaccines, discusses the requirements for ebolavirus vaccines in these scenarios, and describes current ebolavirus vaccines. Among these vaccines are recombinant Adenoviruses, recombinant Vesicular Stomatitis viruses, recombinant Human Parainfluenza viruses and virus-like particles. Interestingly, one of these vaccine platforms, based on recombinant Vesicular Stomatitis viruses, has also demonstrated post-exposure protection in non-human primates.
Expert opinion
The most pressing remaining challenge is now to move these vaccine candidates forward into human trials and towards licensure. In order to achieve this, it will be necessary to establish the mechanisms and correlates of protection for these vaccines, and to continue to demonstrate their safety, particularly in potentially immunocompromised populations. However, already now there is sufficient evidence that, from a scientific perspective, a vaccine protective against ebolaviruses is possible.
Keywords: filoviruses, vaccine, intervention, ebolavirus, hemorrhagic fever
1. Introduction
Since their discovery in 1976, ebolaviruses have caused numerous outbreaks of severe hemorrhagic fevers in Africa. The severity of disease, the perceived threat of bioterrorism associated with these viruses, and the potential for imported infections into the western world, as demonstrated by recent importation of two cases of the closely related Marburgvirus into the Netherlands and the USA, have triggered tremendous interest in these viruses. Nevertheless, despite intense research there is still no effective treatment against Ebola hemorrhagic fever (EHF). However, in recent years there has been significant progress towards vaccines against ebolaviruses, and particularly the last years have seen a surge in vaccine research, although the first attempts at vaccination were reported as early as 1980. However, despite this progress and the availability of vaccines that are highly protective in non-human primates (NHPs), which are the gold standard animal model for EHF, there are still a number of steps that have to be taken before an ebolavirus vaccine can be used in the field. This review tries to provide an overview about current ebolavirus vaccines, and possible scenarios for their use and the resulting requirements for an ideal ebolavirus vaccine.
1.1 Taxonomy and biology of ebolaviruses
Ebolaviruses, together with the closely related marburgviruses, form the family Filoviridae in the order Mononegavirales. There are four accepted and one proposed species of ebolaviruses, with four species (Zaire ebolavirus (ZEBOV); Sudan ebolavirus (SEBOV); Cote d'Ivoire ebolavirus (CIEBOV); Bundibugyo ebolavirus (BEBOV)) occuring in Africa, whereas the fith species (Reston ebolavirus (REBOV)) originates from the Philippines 1, 2. Interestingly, only the African ebolavirus species cause disease in humans, whereas REBOV has not yet been reported to cause any disease in humans, despite evidence of human infections with this virus 3. However, it remains pathogenic in non-human primates, and has been responsible for several outbreaks among animals imported into the USA and Italy. The genetic differences underlying the differences in pathogenic potential are not understood and, therefore, it is difficult to predict the potential for this virus to become pathogenic in humans. This is of particular concern since REBOV has recently been identified in pigs on commercial pig farms, so that there is a potential for REBOV being introduced into the food chain 4.
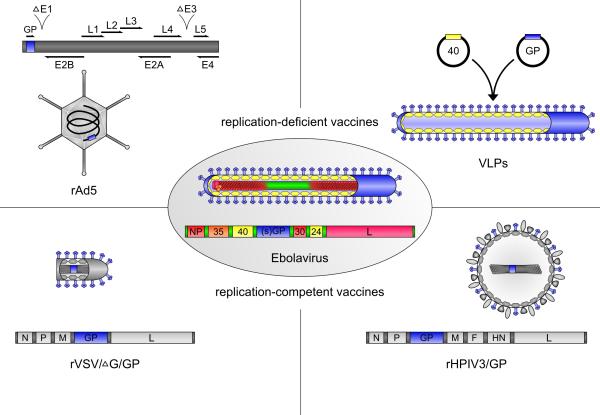
Ebolavirus particles have a characteristic threadlike appearance and consist of seven structural proteins 1 (Figure 1). The viral RNA genome is encapsidated by the nucleoprotein NP and together with the viral polymerase L, the polymerase cofactor VP35 and the transcriptional activator VP30, as well as VP24, forms a central nucleocapsid in the virus particle 5. In the space surrounding the nucleocapsid the matrix protein VP40 is found, which plays a role in particle morphogenesis and budding 1. Virus particles are surrounded by a host cell-derived membrane in which the surface glycoprotein GP is embedded.
Figure 1. Structure of ebolavirus and of ebolavirus vaccines protective in NHPs.
Shown are schematic representations of ebolavirus (center) and VLP, rAd5, rVSV and rHPIV3 vaccine particles, as well as of the recombinant virus genomes for rAd5, rVSV and rHPIV3. Ebolavirus genes and proteins are shown in color (blue: glycoprotein, yellow: matrix proteins, red: nucleocapsid proteins), whereas genes and proteins of the vaccine vectors are shown in gray. For the rAd5 genome deletions in the E1 and E3 regions are indicated.
1.2 Clinical features and treatment
The first symptoms of EHF are relatively unspecific and include a high fever with sudden onset, headaches, muscle and joint pain and general malaise, as well as gastrointestinal symptoms such as diarrhea, nausea and vomiting 6, 7. Hemorrhagic symptoms appear later in the course of disease (5 to 7 days after onset of symptoms) and most frequently occur in the gastrointestinal tract leading to hematemesis, hematochezia and melena. In contrast, overt bleeding, for example from venipuncture sites, is not as common, nor is intradermal bleeding. Death usually occurs between day 6 and 10 after onset of symptoms, and is caused by multi-organ failure and a syndrome resembling septic shock 6, 7. Our current knowledge regarding the immune response to EBOV infection in patients is limited; however, survivors show an IgM response as early as 2 days post onset of symptoms, and an IgG response that develops within 5 to 8 days post symptom onset 8, 9. In contrast, in fatal cases an IgG response seems to be lacking, and IgM antibodies are only detected in about 30% of patients. Also, there seems to be a decrease in T-cell numbers in fatal cases prior to death 8, 10. The case fatality rate of EHF varies depending on the ebolavirus species involved, with ZEBOV having a case fatality rate of 60-90%, whereas SEBOV and BEBOV show lower average case fatality rates of 40-60% and 25%, respectively 6. CIEBOV has caused only a single severe infection in humans; therefore, the lethality rate is unknown.
Ebolaviruses are usually spread through close contact with infected individuals, and particularly with their body fluids. Nosocomial transmission has also played a major role in some outbreaks due to poor infection control practices. Barrier nursing techniques have been shown to be sufficient protection for medical personal, who belong to the high-risk group for EHF infection.
There is no approved specific treatment for EHF currently available. However, experiments in NHPs, which are the gold standard gold model for vaccine and drug testing, have shown that recombinant nematode anticoagulant protein c2 (rNAPc2), administered as late as 24 hours post-infection (p.i.), produced a 33% survival rate in an otherwise uniformly lethal model of infection 11. Activated protein C, which is used for treating patients with severe sepsis, resulted in an 18% survival rate 12. The most promising results so far were obtained in a recent study using siRNAs, in which a 100% survival rate in NHPs was achieved, when animals were treated daily for 7 days, with the first treatment starting 30 minutes after challenge 13. Passive antibody treatment starting as late as 48 hours p.i. with concentrated IgG from vaccinated NHPs which survived ebolavirus challenge has recently been shown to protect non-vaccinated NHPs from otherwise lethal challenge14. Also a postexposure vaccination with a recombinant Vesicular Stomatitis virus (VSV) expressing ebolavirus GP has shown promise as a possible treatment option (see section 3.2.1). In human patients treatment is currently restricted to supportive measures including management of pain and fever (avoiding nonsteroidal anti-inflammatory drugs), fluid replacement, treatment of renal failure and control of secondary infectons 15, 16.
1.3 Ebolavirus ecology
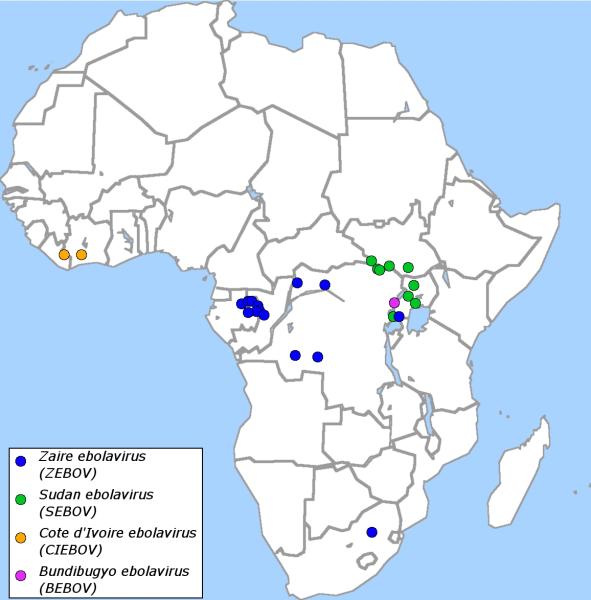
Fruit bats have been identified as a possible natural reservoir for ebolaviruses 17 and it is believed that wildlife, in particular great apes, contract infections with ebolaviruses from bats 18. Indeed there have been reports of major die-offs among great apes that are thought to be due to infection with ebolaviruses 19, 20. The first EHF outbreaks among humans were reported in 1978, and since 1994 there has been on average one outbreak per year, all of which occurred or originated in central Africa (Figure 2). Some outbreaks are believed to have occured after contact of humans with infected NHPs, with hunting or butchering of these animals being risk factors for acquiring EHF. However, direct transmission from bats to humans has also been suggested as an additional possible route of transmission 21.
Figure 2. Ebolavirus outbreaks among humans.
Shown are all ebolavirus outbreaks since the initial discovery in 1976 (blue: ZEBOV, green: SEBOV, orange: CIEBOV, purple BEBOV).
In the case of REBOV, the natural reservoir has not been established; however, bats have been shown to be seropositive for REBOV, and are considered the most likely candidate 22. In addition to the transmission routes established for African ebolaviruses, transmission from pigs to humans is considered likely, and it has been shown that pigs shed virus via the nasopharynx, and under some circumstances also via the fecal route 23.
1.4 Requirements for vaccines against ebolavirus hemorrhagic fever
Outbreaks of EHF are sporadic in nature, and compared to other infectious diseases, have only a limited impact on public health in terms of case numbers and fatalities. However, outbreaks of EHF often result in closure of affected health facilities and the death of health care providing staff, thus causing a much higher impact on public health than is apparent based on case numbers alone. Also, ebolaviruses are perceived by the public as a considerable threat, and are classified as potential bioterrorism agents. These properties of ebolaviruses create particular requirements for an EHF vaccine. In addition, different scenarios under which an EHF vaccine might be needed result in different requirements for an optimal vaccine, so that more than one vaccine might be neccessary to meet all these requirements (Table 1).
Table 1.
Desired characteristics of an ebolavirus vaccine for different vaccination scenarios
| characteristic | outbreak response vaccination | preventative vaccination of risk groups | wild-life vaccination |
|---|---|---|---|
| rapid protection | essential | not essential | not essential |
| long-lasting immunity | desirable, but not essential | essential | essential |
| cross-protection against multiple species | desirable, but not essential, since species causing outbreak can be rapidly determined | essential | essential |
| number of vaccinations | single vaccination essential | multiple vaccination tolerable | single vaccination essential |
There are several different scenarios for an EHF vaccination. The most obvious scenario would be the vaccination of the complete population living in areas where EHF outbreaks occur. However, the fact that outbreaks are sporadic and affect comparatively few people, together with the size of the affected areas (Figure 2) and logistical challenges make a vaccination of large numbers of people problematic. A much more likely scenario for vaccination is the control of an ongoing outbreak, during which people in the directly affected area are vaccinated in order to limit spread of the disease (commonly referred to as ‘ring vaccination’). This scenario would be very similar to a bioterrorist attack, where vaccination would also be initiated only after the attack has been identified. Under these circumstances the timespan between first vaccination and development of immunity is of paramount importance, and should be as brief as possible. Therefore, a vaccine should ideally require only a single vaccination, and provide rapid protection. If viral vectors are used for the vaccine, preexisting immunity against vector components is a considerable problem under these conditions. Also, due to logistical challenges a vaccine that remains effective in the absence of a continous cold chain from production to administration is desirable.
A second scenario for vaccination is of individuals who are at an increased professional risk to encounter ebolaviruses, which includes health care providers (either in areas where EHF outbreaks can occur or as part of outbreak response teams) and researchers working with these viruses. In this case vaccination would happen most likely a long time before any potential exposure, so that multiple vaccinations or a longer timespan between vaccination and the development of immunity are tolerable. Also, preexisting immunity might be of less concern, since current data suggest that a prime-boost approach is able to induce a sufficient level of protection even in the presence of pre-existing immunity 24. Similarily, vaccine stability in the absence of a reliable cold chain is less important in this instance. However, a broad immunity against as many ebolavirus species as possible as well as long-lasting immunity is extremely important in this case.
The third scenario is the vaccination of great apes, which are at considerable risk for EHF, and protection of which is not only desirable in terms of conservation efforts, but would also decrease the risk for human outbreaks considerably, since at least some outbreaks could be traced back to index cases who acquired the infection from these wildlife species. In this scenario vaccination would most likely be performed preemptively, since most outbreaks in NHPs are only recognized in retrospect. Therefore, the timespan between vaccination and development of immunity is of low importance; however, due to the challenges of vaccinating wild animals it would be crucial that a single vaccination suffices to induce protective immunity against as many ebolavirus species as possible. The two principal means of administering a wild-life vaccine are darting and baiting, with only the latter seeming to be practical. Therefore, a vaccine that is tolerant against prolonged incubation at ambient temperatures would be desirable. Alternatively, a diseminating vaccine based on a recombinant virus expressing ebolavirus antigens that, once released, circulates among the wild-life, could be used. In any case it has to be ensured that the vaccine is not only safe in the target population, i.e. NHPs, but also does not cause any adverse side effects in other animals that may come in contact with the vaccine.
2. Animal models for vaccine evaluation
2.1 Mice
While wild-type filoviruses do not result in lethal disease in adult immunocompetent mice, there are several models now available based either on immunodeficient mouse strains or mouse-adapted virus strains. Serial passage in progressively older animals has been used to produce a mouse-adapted ZEBOV strain 25. This virus is uniformly lethal in BALB/c mice when given intraperitoneally even at extremely low doses (<1 pfu) 25, 26. Alternatively, either severe combined immunideficient (SCID), signal transducer and activator of transcription 1 knock-out (STAT1-/-) or interferon α/β receptor knock-out (IFNAR-/-) mouse strains can also be used to provide a lethal model after challenge with wild-type ZEBOV strains 27, 28. While ZEBOV infection in mice results in infection of the same primary target cells and tissues as human and NHP infection 25, 27, 28, the major limitation of mouse models of EHF is that there appears to be little involvement of coagulation abnormalities in the disease process 29, which is an important hallmark of EHF in NHPs and humans. Further, while mouse models continue to serve as a first-line choice for the evaluation of new vaccine approaches, their predictive value for subsequent success in the NHP model is limited, a feature which has been suggested to be linked to their strong innate antiviral responses 27. Nevertheless, it is unlikely that approaches that do not show complete protection in the mouse model will be successful in other animal models. Thus, regardless of their limitations, they will no doubt continue to be used for screening purposes.
2.2 Guinea pigs
Infection of guinea pigs with wild-type ebolaviruses results in no or very limited clinical signs, and the establishment of a model has again been based on the generation of guinea pig-adapted ZEBOV strains 30, 31. As with the mouse model, infection of target cells and organs mirrors what is seen in NHPs and humans, and in addition there is some evidence of coagulation abnormalities, although changes in fibrinogen levels are not observed 29, 30. However, for reasons that remain unclear the guinea pig provides a more stringent evaluation of vaccine efficacy and is somewhat more predictive of success in the NHP model than is the mouse model. Unfortunately, significantly fewer tools are available for immunological research using this animal model.
2.3 Syrian hamsters
Recently, a Syrian golden hamster model has also been developed for studying ebolavirus infection 32. This model is based on infection of adult hamsters with mouse-adapted ZEBOV and shows a uniformly lethal outcome. In contrast to other rodent models, the hamster accurately models the severe coagulation abnormailties associated with EHF, including alterations in fibrinogen and Protein C levels 32. In addition, this model shows the dysregulated cytokine production and suppression of Type I interferon responses believed to contribute to the pathophysiology of filovirus infection 32. However, as with the guinea pig model, immunological reagents are limited for the hamster model.
2.4 Non-human primates
Ebolavirus infections have been extensively studied in several species of NHPs. While most research focuses on the use of either cynomolgus (Macaca fascicularis) or rhesus macaques (Macaca mulatta), in some cases African green monkeys (Chlorocebus aethiops) or baboons (Papio hamadryas) have also been used. The NHP model is the gold standard animal model for vaccine and drug testing, since it exhibits both the characteristic pathological and clinical features seen in human infection 33, 34. Indeed, with almost 100% lethality the NHP model can be considered to be an even more stringent test of efficacy than would use in the human population, where infection with the most virulent ebolavirus strains results in an average case fatality of 78%. Despite being a necessary final step in the demonstration of vaccine efficacy and a prerequisite for consideration for human use, given the technical difficulty, expense and especially the ethical considerations associated with NHP research, the decision to test an approach in NHPs must usually be based on strong supporting evidence for efficacy in one or more small animal model.
2.5 Immune mechanisms, correlates of protection and the animal rule
Vaccine licensing using the “animal rule”, introduced in 2002 by the US Food and Drug Administration (FDA), is intended to facilitate licensure for vaccines targeting those agents for which conventional clinical efficacy trials are not possible. This includes pathogens such as ebolaviruses, where outbreaks occur only sporadically and high-risk populations in which to conduct efficacy trials cannot be identified. In these cases the ”animal rule” permits efficacy data from studies using animal models that accurately recapitulate human disease (i.e. the NHP model in the case of EHF) and safety and immunogenicity data in humans to be used to pursue licensure for use in the human population (reviewed in 35). However, for this purpose the correlate(s) of protection predictive of survival in the relevant animal model have to be defined, so that these data can be used to predict human efficacy.
Unfortunately, until now there has been only limited success in defining these correlates of immunity, although it could be shown that there is some correlation between antibody titres (but not necessarily neutralizing antibody titres) and survival 35. Further, while the mechanism of protection for ebolavirus vaccines is not well understood, it has recently been shown that passive antibody transfer from animals vaccinated with a recombinant Adenovirus expressing ebolavirus GP is not sufficient to protect against EHF, and that protection with this vaccine might rather be dependent on CD8+ T cells, even though other factor still seem to play a role 36. On the other hand, in a recent study it was possible to protect NHPs against otherwise lethal EBOV infection by passive immunization using serum from NHPs surviving ebolavirus challenge, showing that at least in principal antibodies alone can be sufficient to protect against EHF 14. Therefore, the definition of immune mechanisms and correlates of protection will be an extremely important avenue of vaccine research in the foreseeable future.
3. Ebolavirus vaccines
Ebolavirus vaccines can be broadly divided into non-replicating and replicating vaccines (Figure 1)(Table 2), with the former being further divided into inactivated vaccines, subunit vaccines and vector-based vaccines. In the case of subunit vaccines the immunogen (in most cases the glycoprotein GP) is delivered in the form of virus-like particles or recombinantly expressed purified proteins, whereas in vector-based vaccines it is expressed in the vaccinee from DNA or viral vectors. Non-replicating viral vectors carry deletions of genes essential for the life cycle of the vector virus. Therefore, these vectors have to be produced while providing the proteins encoded by the deleted genes in trans, and their life span is restricted to a single infectious cycle, eliminating many concerns with regard to vaccine safety. However, in order for these vaccines to be efficacious, often higher doses and/or multiple vaccinations are required.
Table 2.
Overview of EHF vaccines
| System | % protection, number of vaccinations, time required for vaccination schemea) | Remarks | Key references | ||
|---|---|---|---|---|---|
| NHPs | guinea pigs | mice | |||
| Non-replicating vaccines | |||||
| inactivated ebolavirus | 10%b), 3, 11w | 64%, 3, 21w | 100%, 2, 4w | 38, 39 | |
| replicons | 0%, 3, 15w | 100%, 3, 22w | 100%, 2, 8w | 39, 40 | |
| DNA | n/a | 100%, 3, 19w | 100%, 2, 8w | safe and inmmunogenic in human phase 1 clinical trials | 43-45 |
| DNA + Ad5 | 100%, 4, 32w | n/a | n/a | 44 | |
| Ad5 | 100%, 1, 4w | 100%, 1, 4w | 100%, 1, 3w | safe and inmmunogenic in human phase 1 clinical trials; problems with preexisting immunity, but this can be overcome by multiple vaccinations | 46, 50, 53, 55 |
| baculovirus-derived GP | n/a | 50%, 3, 11w | n/a | 58 | |
| recombinant GP-Fc fusion protein | n/a | n/a | 83%, 4, 11w | 59 | |
| GP-immunocomplexes | n/a | n/a | 80%, 4, 12w | 60 | |
| VLPs | 100%, 3, 15w | n/a | 100%, 2, 7w | 63, 64 | |
| rEBOVΔVP30 | n/a | 100%, 2, 9w | 100%, 2, 11w | 68 | |
| inactivated rRABV (BSNP333-GP) | n/a | n/a | 100%, 1, 11w | only inactivated vaccine shown to protect after a single vaccination; extensive experiences with this vaccine platform for wildlife vaccination | 89 |
| Replicating vaccines | |||||
| rVSV/ΔG/GP | 100%, 1, 4wc) | 100%, 1, 3wc) | 100%, 1, 1dc) | capable of post-exposure protection | 69-71, 91 |
| rHPIV3/GP | 100%, 2, 10w | 100%, 1, 4w | n/a | potential problems with preexisting immunity | 85, 86 |
| rHPIV3/ΔHN-F/GP | n/a | 100%, 1, 4w | n/a | no problems with preexisting immunity | 87 |
| rRABV (BNSPΔG-GP) | n/a | n/a | 100%, 1, 11w | 89 | |
| rCMV | n/a | n/a | 100%, 2, 10w | disseminating vaccine | 90 |
abbreviations: w = weeks, d = days, n/a = no data from challenge experiments available
no protection was observed in 8 cynomolgus macaques; however, 1 out of 2 rhesus macaques was protected
postexposure vaccination protects 50% of NHPs and 83% of guinea pigs if given 0.5 to 1 hour post infection, and 100% of mice as late as 24 hours post infection
In contrast, for replication-competent ebolavirus vaccines, recombinant viruses (based on viruses other than filoviruses) are generated with the gene encoding the immunogen integrated into their genome. In contrast to other viruses live-attenuated ebolavirus-based vaccines, which would also fall into the category of replication-competent vaccines, do not exist. Vaccines based on recombinant viruses are often very efficacious, but since they are replicating, it has to be ensured that they cannot cause disease in vaccinees. This is particularly important when considering the high HIV-prevalence in Africa, and the potential for underlying (infectious) diseases in vaccinees.
3.1 Non-replicating ebolavirus vaccines
3.1.1 Inactivated vaccines
The first attempts at developing an ebolavirus vaccine used inactivated viruses, and were performed only a few years after the discovery of ZEBOV and SEBOV. Formalin or heat-inactivated virus preparations were used for immunization of guinea pigs, which were subsequently protected from challenge with wild-type ZEBOV 37. However, since wild-type rather than a guinea-pig adapted ZEBOV variant was used for challenge, only 29% of non-vaccinated control animals succumbed to infection, raising some doubt about the efficacy of the vaccine. Later attempts at using inactivated virus for vaccination utilized irradiated ZEBOV, which was 100% protective in a nearly 100% lethal mouse model of infection after two vaccinations if formulated together with liposomes and given intravenously 38. However, this vaccine failed to protect NHPs, despite the fact that the animals developed an antibody response against the vaccine, including neutralizing responses 38, 39.
3.1.2 Replicons
Another early attempt at vaccine development involved alphavirus replicons. To this end the structural genes of Venezuelan equine encephalitis virus (VEEV) were replaced by ZEBOV GP, and the resulting replicon was expressed from an RNA expression vector and packaged into virus-like particles by providing the structural VEEV proteins in trans. These replicon-containing VLPs were then used for vaccination, and in vaccinated animals the non-structural VEEV proteins encoded by the replicon led to its replication and high-level expression of ZEBOV GP. However, since in vaccinated animals no structural VEEV proteins are expressed, no progeny VEEV particles can be produced, limiting the life span of the VEEV replicon-containing VLPs to a single infectious cycle. These vaccines were highly protective in mice after two vaccinations and in guinea pigs after three vaccinations 40, but they failed to protect NHPs 39.
Recently, a similar approach was taken using Kunjin virus replicons and protected between 25% and 86% of ZEBOV challenged guinea pigs, depending on the vaccine dose and the immunogen used 41. However, this vaccine has not been evaluated in NHPs, and based on the experiences with the VEEV replicon, which showed 100% protection in guinea pigs, it is doubtful whether the Kunjin virus replicon in its current form will be protective in these animals.
3.1.3 DNA vaccines
DNA vaccines for ebolaviruses have been shown to be protective in mice and guinea pig models. While initially 4 to 5 vaccinations were required to reach 100% protection in mice 42, increasing the dose led to 100% survival in mice after two vaccinations and 100% survival in guinea pigs after 3 vaccinations 43, 44. In NHPs DNA vaccines have been tested in combination with a boost using a replication-deficient recombinant Adenovirus expressing ZEBOV GP 44, and this was the first approach to succesfully protect 100% of NHPs against an otherwise lethal challenge. However, given the fact that the recombinant Adenovirus by itself is able to induce 100% protection, it is not clear to what extent the DNA component of this approach contributed to this success. Nevertheles, in an initial phase 1 clinical trial a DNA vaccine has been shown to be safe and produce antibody as well as T-cell responses in humans after three vaccinations 45.
3.1.4 Recombinant Adenoviruses
Recombinant Adenoviruses were first used as a boost in combination with DNA vaccines. While this vaccination strategy protected 100% of challenged NHPs, more than 6 months were required to complete the immunizations. However, when using the recombinant Adenovirus alone, 100% protection was achieved after only 4 weeks, even though antibody titers were lower than when using the combined DNA/Adenovirus strategy 46. Vaccination dose has been shown to be extremely important for vaccine efficacy, with at least 1×1010 virus particles being required to achieve 100% protection in NHPs 47. Attempts to lower this dose by using optimized expression casettes for the immunogen have been successful in mice 48, but have not yet been evaluated in NHP models.
Adenovirus-based vaccines have also been used to achieve protection against multiple strains of ebolaviruses. A recombinant Adenovirus expressing both the glycoprotein of ZEBOV and of SEBOV protected NHPs against challenge with either virus after a single vaccination 24. Also, a blended vaccine containing Adenoviruses expressing either ZEBOV GP or SEBOV GP and given as part of a combined DNA/Adenovirus vaccination scheme induced crossprotection against BEBOV, an ebolavirus strain that was not included in the vaccination 49. This demonstrates that it should be possible to generate a broad immune protection against ebolaviruses that covers newly emerging species.
Similar to DNA vaccination, the Adenovirus vaccine has been evaluated in a phase 1 clinical trial, and was found to be safe in all vaccinees 50. GP-specific T-cell responses, which have been shown to be the major correlate of protection for the Adenovirus vaccine in NHPs 36, were detected in 25 to 45% of vaccinees, depending on the vaccination dose and the species from which the GP gene was derived.
One considerable problem with the Adenovirus vaccine platform is that of preexisting immunity. The original recombinant Adenovirus vaccine is based on Adenovirus serotype 5 (Ad5), to which between 60 and 90% of adults are seropositive, depending of their country of origin 51. In rodent 52-55 as well as NHP models 56 preexisting immunity against Ad5 has severly impaired the efficacy of the Ad5 vaccine, and in the clinical trial of the Ad5 vaccine individuals who were seropositive for Ad5 before vaccination had a significantly lower antibody response rate and antibody reponse magnitude after vaccination 50. Several strategies have been developed to overcome this problem, including the use of different Adenovirus serotypes as vectors 55-57, changes in vaccine delivery route 52-54, and changes in the number of vaccinations 24. In the NHP model increasing the number of Ad5 vaccinations to 2 has been shown to overcome preexisting immunity against Ad5 24. Also, vaccines based on Ad26 and Ad35, to which seroprevalence is much lower, have been shown to provide 100% protection against challenge with ZEBOV, but only after 2 vaccinations 56.
3.1.5 Subunit vaccines
Subunit vaccines for ebolaviruses can be divided in classical subunit vaccines which use purified recombinantly expressed viral proteins, and virus-like particles (VLPs). There are few studies that have attempted the use of classical subunit vaccines for ebolaviruses. Baculovirus-derived GP given subcutaneously into guinea pigs resulted in 50% protection after 3 immunizations 58. Recently, a subunit vaccine in which the ectodomain of ZEBOV GP is fused to a human Fc fragment for the purpose of purification was shown to protect 83% of challenged mice after 4 vaccinations 59. Further, one study has evaluated the use of immunocomplexes as vaccines. In this approach GP1 fused to an anti-GP antibody was produced in plants, and the resulting immunocomplexes were shown to protect 80% of mice against challenge after 4 vaccinations 60. However, compared to other vaccination strategies these classical subunit vaccines currently lack in protective efficacy, and further improvements will be necessary before tests in NHPs can be justified.
In contrast the more complex VLP-based vaccines are highly promising. Expression of the matrix protein VP40 in mammalian cells leads to the budding of VLPs. These particles incorporate other viral proteins if they are coexpressed, and this has been exploited for vaccine purposes by generating VLPs containing VP40 and GP, and in some cases also NP. These VLPs have been shown to be protective in rodents 61, 62, and also in an NHP model where they protected against challenge after 3 vaccinations 63. At least in mice vaccination efficacy has been shown to be dose dependant 64, and it might be difficult to produce the amounts of VLPs needed for vaccination of humans using 293T cells, which are commonly used for VLP production. In order to address this problem, recent studies have shown that VLPs can also be produced in insect cells using the baculovirus expression system, which is more amenable to large-scale production under good manufacturing production (GMP) conditions, and that they protect mice, although they have not yet been evaluated in NHPs 65.
3.1.6 Replication-deficient Ebolaviruses
Reverse genetics have made it possible to generate genetically engineered ebolaviruses 66, and this has been exploited to generate an ebolavirus in which the gene encoding for the transcriptional activator VP30 has been deleted (rEBOVΔVP30) 67. In order to grow this virus, VP30 has to be provied in trans (e.g. by a cell line stably expressing VP30, which was developed for this purpose), and while the produced rEBOVΔVP30 can infect target cells, it does not produce infectious progeny in the absence of VP30, thus limiting the life span of this virus to one infectious cycle. Consequently, this virus has been shown to be non-pathogenic in STAT1-KO mice. However, rEBOVΔVP30 was still able to protect 100% of both mice and guinea pigs against an otherwise lethal challenge after 2 vaccinations 68.
Because of the recombinant nature of rEBOVΔVP30 and the fact that its genome still contains more than 95% of the original ZEBOV genome, some concerns exist with respect to vaccine safety, and particularly the generation of viruses which have reintegrated VP30 into their genome. However, sequential passaging in VP30-expressing Vero cells has shown that there are no recombination events, which could lead to the reintegration of VP30 into the genome, over at least 7 passages. Also, according to our current understanding of filovirus biology there is no possibility for such recombinantion events.
3.2 Replicating ebolavirus vaccines
3.2.1 Recombinant VSV
The first replicating ebolavirus vaccine shown to be protective in NHPs was based on a recombinant VSV (rVSV). In this virus the VSV glycoprotein was replaced with ZEBOV GP, and the resulting virus (rVSV/ΔG/GP) was 100% protective in NHPs after a single vaccination 69. No ZEBOV replication could be detected by virus isolation or RT-PCR in vaccinated NHPs after challenge, and vaccinated animals showed no signs of disease. Since rVSV/ΔG/GP is replication competent, a relatively small dose of recombinant virus (compared to the replication-deficient Adenovirus platform) was required for successful vaccination (1×107 PFU of rVSV/ΔG/GP vs. 1×1010 particles of Ad5/GP), and in experiments in mice the dose for 100% successful vaccination could be be lowered to as little as 2 PFU 70. A transient rVSV viremia may occur in vaccinated animals, but no adverse effects were observed despite the rVSV/ΔG/GP or closely related vaccines using the same platform being administered to more than 100 NHPs 69, 71-82. Neurovirulence, which can be a potential problem after infection with wild-type VSV, does not occur in NHPs intrathalamically infected with rVSV/ΔG/GP 82. Also, rVSV/ΔG/GP was tested in NHPs infected with simian-human immunodeficiency virus (SHIV), and despite their immunocompromised status the vaccine did not cause any clinical illness, fever, or local reaction at the vaccination site 74. rVSV viremia in immunocompromised animals was low and did not last longer than in immunocompetent animals. Despite their underlying SHIV-infection the vaccine was able to protect 4 out of 6 NHPs, with the two animals that succumbed to infection showing the lowest CD4+ cell counts. This study not only underlines the safety of rVSV/ΔG/GP, but is of particular importance due to the high prevalence of HIV in areas with ebolavirus outbreaks. The rVSV vaccine has been shown to be 100% protective in NHPs after mucosal immunization through the oral or intranasal route 76, and to protect against a ZEBOV aerosol challenge, which is considered the most likely scenario for a bioterrorist attack 73. Recent studies have focused on trying to generate vaccines that protect against more than one ebolavirus species, and a blended formulation of three rVSV/ΔG/GP viruses encoding GP from ZEBOV, SEBOV and Marburg virus was able not only to protect against challenge with any of those viruses, but also against challenge with CIEBOV 75.
Surprisingly, the recombinant VSV platform has also shown success in post-exposure vaccination, and 100% of challenged mice as well as hamsters can be protected by vaccination with rVSV/ΔG/GP as late as 24 hours post challenge 71, 83. In guinea pigs and NHPs the survival rates are somewhat lower, but still reach 83% and 50%, respectively, if the vaccine is administered within 30 to 60 minutes post exposure 71, which is a realistic timeframe for response to a laboratory accident. Given the fact that EHF seems to be somewhat less pathogenic in humans than in NHPs, this timeframe might even be longer for humans. In fact, after an accidental exposure to ZEBOV in a biosafety level 4 laboratory the researcher was treated with rVSV/ΔG/GP 48 hours after the incident 84. Transient low level rVSV viremia was observed on day 1 and 2 post vaccination, and on day 1 after vaccination the patient showed fever and myalgia. Other then that, no adverse effects were observed, and RT-PCRs against ZEBOV L remained negative. While it is not possible to confirm whether the patient was actually infected with ZEBOV, this case is a first indication of the safety of the rVSV vaccine platform in humans. Nevertheless, a perceived safety risk remains a problem for this platform, although there are numerous studies clearly showing its safety in NHPs, together with the fact that the correlates of protection, as well as the mechanism of action for the postexposure vaccination, are not known.
3.2.2 Recombinant Human Parainfluenzavirus type 3
Another negative-sense RNA virus that has been successfully used as a vaccine platform against ebolaviruses is human parainfluenza virus type 3 (HPIV3). HPIV3 is a common human respiratory pathogen, and was chosen in order to attempt vaccination via the respiratory route. A transcription casette encoding the GP gene was inserted between the P and M genes of HPIV3, giving rise to a recombinant HPIV3 virus (rHPIV3/GP) which in addition to its own surface proteins also carried ZEBOV GP on its surface 85. This virus was 100% protective in guinea pigs after a single vaccination 85, and protected 100% of NHPs after 2 vaccinations, with no signs of EHF disease or viremia in these animals 86.
Similar to Adenoviruses, there is a high seroprevalence for HPIV3 among humans, which could cause problems with respect to preexisting immunity. In order to overcome these problems, a second generation rHPIV3 vaccine has been developed in which the genes for the HPIV3 surface proteins HN and F were deleted, and the ZEBOV GP takes over their function (rHPIV/ΔHN-F/GP) 87. This virus was highly attenuated in vivo and did not disseminate beyond the respiratory tract in guinea pigs, yet it protected 100% of challenged guinea pigs after a single vaccination. In NHPs this vaccine was equally immunogenic in both HPIV3 seropositive and HPIV3 seronegative animals, but no data from challenge experiments are available at this point 88.
3.2.3 Rabies
Rabies virus, which is closely related to VSV, has also been explored as a vaccine platform against ebolaviruses. This platform is particularly interesting since the live-attenuated Rabies strain that was used as the basis for the vaccine is already used for rabies wildlife vaccination in Europe. For the purposes of the ebolavirus vaccine, this strain was further attenuated by introducing a point mutation in the Rabies glycoprotein G gene, in order to further reduce neurovirulence. ZEBOV GP was introduced as an additional transcriptional unit between the N and P gene, and variants both retaining rabies G (BNSP333-GP) or with a deletion of G (BNSPΔG-GP) were generated. None of the recombinant viruses caused any disease after intramuscular, intranasal or intraperitoneal infection of mice 89. Further, even intracerebral inoculation of suckling mice, which is associated with neurovirulence in the case of the Rabies vaccine strains currently in use for wildlife vaccination, did not result in any clinical signs or lethality in the case of the BNSPΔG-GP virus, even though the BSNP333-GP remained neurovirulent in this very stringent model. A single vaccination with the BSNP333-GP virus protected 100% of mice from ZEBOV lethal challenge, and interestingly inactivated BNSPΔG-GP was also able to protect 100% of mice after a single vaccination if a modified form of the ZEBOV GP, which consisted of the ZEBOV GP ectodomain and transmembrane domains but the rabies G cytoplamic domain, was used 89. This is of particular interest since inactiviation protocols for human Rabies virus vaccines are well established, and since this vaccine is the only inactivated EHF vaccine that completely protects against challenge after a single vaccination in the rodent model.
3.2.4 Cytomegalovirus
Recently, a new approach aimed at an ebolavirus wildlife vaccine has been proposed using Cytomegalovirus (CMV) as a disseminating vaccine platform. This herpesvirus, which establishes a life-long persistent, but benign infection within its host is highly immunogenic, and in a proof-of-concept study a recombinant CMV expressing a single T-cell epitope from ZEBOV NP was used to fully protect mice against challenge after two vaccinations 90. While the idea of introducing a recombinant virus as a disseminating vaccine into the wild raises significant concerns, several arguments have been brought forward to meet these concerns. First it has been argued that CMV is already ubiquitous in all primate species studied, and second it has been argued that CMV should be highly host specific. Nevertheless, significant further study both with respect to efficay and safety will be required before this vaccine can be considered for use.
4. Conclusion
While no ebolavirus vaccine is currently licensed, a number of vaccine candidates are 100% protective in NHPs, which represent the most sensitive model for EHF. Most of the successful vaccines are based on recombinant viruses, in whose genome the ebolavirus GP gene was introduced. In addition, virus-like particles bearing GP on their surface have been shown to protect NHPs. While non-replicating vaccines are associated with fewer perceived safety concerns than replicating vaccines, the latter generally produce a better protection even at lower doses and with fewer vaccinations. Also, despite extensive testing in the case of some of the replicating vaccines, until now no adverse effects have been observed in NHPs for the most succesful candidates. Open questions that remain are the duration of immunity, since there is currently only very limited data on long-term protection, and the mechanisms and correlates of protection. Also, cross-protection using a single vector has yet to be achieved, although approaches using blended vaccines are already able to provide cross-protection.
Expert opinion
The last years have seen significant progress in terms of ebolavirus vaccine development, and we now have a number of candidates that perform extremely well in the NHP model. The challenge that remains is to proceed with these vaccine candidates into preclinical and clinical trials and eventually seek licensure under the animal rule. The vaccine candidate that has progressed the furthest in this respect is the Ad5-based vaccine, which has been been produced under GMP conditions and has been clinically tested with results showing that it was well tolerated in 23 healthy adults. However, if T-cell responses really are the major mechanism of protection, a single vaccination might not be sufficient for this vaccine platform, since only 25% to 45% of vaccinees developed T-cell responses, depending on the vaccination protocol. Nevertheless, this vaccine is the most promising candidate for a preventive vaccination of individuals that are at an increased risk for exposure to ebolaviruses, since for this group a multiple vaccination approach would be acceptable. A feasible alternative would be the VLP-based vaccine, although more vaccinations will be required for this vaccine to be protective, and large scale production of this vaccine remains an issue.
However, for an outbreak or bioterrorist attack scenario the Ad5 vaccine might be less adequate, since in this case a rapid protection after only a single injection is paramount. Most promising in this respect is the rVSV vaccine, which seems to be at least partially protective even post-expoure. This vaccine was safe in more than 100 NHPs and the one human who has received it to date. Unfortunately, until now it has not been produced under GMP conditions, and also neither the correlates nor the mechanisms of protection have been established, and these two points will have to be addressed before this vaccine can be tested in humans. However, the case of an potential laboratory acquired infection in Germany has shown that already now it might be prudent to use experimental vaccines for laboratory acquired infections, due to the low risks associated with these vaccines as compared to the often fatal outcome of a laboratory acquired ebolavirus infection. Biosafety level 4 laboratories working with ebolaviruses should explore possibilities to establish protocols for the use of the rVSV vaccine in case of such an accident, and the possibility to store it on-site to ensure a minimal time between accidental exposure and post-exposure vaccination.
While for a wild-life vaccination the rVSV platform would most likely also be very efficient, the fact that VSV is an animal pathogen might prohibit release of rVSV-GP in the wild, although this virus is considerably attenuated as compared to wild-type VSV. For this scenario the Rabies platform is extremely promising, particularly since there is considerable experience using this platform for wild-life vaccination against Rabies. However, this vaccine has not yet been tested in NHPs, and this is the obvious next step in moving this vaccine platform forward.
Article highlights.
a number of vaccine candidates against Ebola hemorrhagic fever that are highly protective in non-human primates already exist
future efforts should focus on advancing these candidates into a vaccine licensed for use in humans and/or wildlife (i.e. NHP) populations
in order to do so future studies need to establish the correlates and mechanisms of protection for these vaccines, and address remaining safety concerns
recombinant Adenoviruses expressing ebolavirus GP have been shown to be highly protective in non-human primates as well as safe and immunogenic in phase 1 clinical trials in humans, and are presently the most promising candidate for a preventative vaccine
recombinant Vesicular Stomatitis Viruses expressing ebolavirus GP have been shown to be highly protective and safe in non-human primates, and are the only platform that offers post-exposure protection; these viruses are the most promising candidate for a vaccine to be used in an outbreak situation or in response to a bioterrorist attack
recombinant Rabies viruses expressing ebolavirus GP are a promising candidate for wildlife vaccinations due to the extensive practical experience with this platform, if they can be shown to be protective in wildlife species such as the great apes
Acknowledgements
Research on filoviruses by the authors was supported in part by the Intramural Research Program of the NIH, NIAID.
References
- 1.Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola Viruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1410–48. [Google Scholar]
- 2.Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008 Nov;4(11):e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO WHO experts consultation on Ebola Reston pathogenicity in humans. 2009.
- 4.Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009 Jul 10;325(5937):204–6. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- 5.Beniac DR, Melito PL, Devarennes SL, Hiebert SL, Rabb MJ, Lamboo LL, et al. The organisation of ebola virus reveals a capacity for extensive, modular polyploidy. PloS one. 2012;7(1):e29608. doi: 10.1371/journal.pone.0029608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011 Mar 5;377(9768):849–62. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoenen T, Gottschalk R, Becker S. Hofmann F, editor. [Ebolavirus hemmorrhagic fever]. [Manual of infectious diseases] 2007. pp. VIII–6.11.1-15. Landsberg/Lech: ecomed Medizin.
- 8.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nature medicine. 1999 Apr;5(4):423–6. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 9.Ksiazek TG, Rollin PE, Williams AJ, Bressler DS, Martin ML, Swanepoel R, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999 Feb;179(Suppl 1):S177–87. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez A, Lukwiya M, Bausch D, Mahanty S, Sanchez AJ, Wagoner KD, et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004 Oct;78(19):10370–7. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, Paragas J, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003 Dec 13;362(9400):1953–8. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 12.Hensley LE, Stevens EL, Yan SB, Geisbert JB, Macias WL, Larsen T, et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J Infect Dis. 2007 Nov 15;196(Suppl 2):S390–9. doi: 10.1086/520598. [DOI] [PubMed] [Google Scholar]
- 13.Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010 May 29;375(9729):1896–905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dye JM, Herbert AS, Kuehne AI, Barth JF, Muhammad MA, Zak SE, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A. 2012 Mar 27;109(13):5034–9. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffs B. A clinical guide to viral haemorrhagic fevers: Ebola, Marburg and Lassa. Tropical doctor. 2006 Jan;36(1):1–4. doi: 10.1258/004947506775598914. [DOI] [PubMed] [Google Scholar]
- 16.Nkoghe D, Formenty P, Nnegue S, Mve MT, Hypolite I, Leonard P, et al. [Practical guidelines for the management of Ebola infected patients in the field]. Medecine tropicale : revue du Corps de sante colonial. 2004;64(2):199–204. [PubMed] [Google Scholar]
- 17.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005 Dec 1;438(7068):575–6. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 18.Groseth A, Feldmann H, Strong JE. The ecology of Ebola virus. Trends Microbiol. 2007 Sep;15(9):408–16. doi: 10.1016/j.tim.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Bermejo M, Rodriguez-Teijeiro JD, Illera G, Barroso A, Vila C, Walsh PD. Ebola outbreak killed 5000 gorillas. Science. 2006 Dec 8;314(5805):1564. doi: 10.1126/science.1133105. [DOI] [PubMed] [Google Scholar]
- 20.Leroy EM, Rouquet P, Formenty P, Souquiere S, Kilbourne A, Froment JM, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004 Jan 16;303(5656):387–90. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 21.Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez JP, Muyembe-Tamfum JJ, et al. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector borne and zoonotic diseases. 2009 Dec;9(6):723–8. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi S, Watanabe S, Masangkay JS, Omatsu T, Ikegami T, Alviola P, et al. Reston Ebolavirus antibodies in bats, the Philippines. Emerging infectious diseases. 2011 Aug;17(8):1559–60. doi: 10.3201/eid1708.101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsh GA, Haining J, Robinson R, Foord A, Yamada M, Barr JA, et al. Ebola Reston virus infection of pigs: clinical significance and transmission potential. J Infect Dis. 2011 Nov;204(Suppl 3):S804–9. doi: 10.1093/infdis/jir300. [DOI] [PubMed] [Google Scholar]
- 24.Pratt WD, Wang D, Nichols DK, Luo M, Woraratanadharm J, Dye JM, et al. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clinical and vaccine immunology : CVI. 2010 Apr;17(4):572–81. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis. 1999 Feb;179(Suppl 1):S248–58. doi: 10.1086/514292. [DOI] [PubMed] [Google Scholar]
- 26.Ebihara H, Takada A, Kobasa D, Jones S, Neumann G, Theriault S, et al. Molecular determinants of Ebola virus virulence in mice. PLoS Pathog. 2006 Jul;2(7):e73. doi: 10.1371/journal.ppat.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bray M. The role of the Type I interferon response in the resistance of mice to filovirus infection. J Gen Virol. 2001 Jun;82(Pt 6):1365–73. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- 28.Raymond J, Bradfute S, Bray M. Filovirus infection of STAT-1 knockout mice. J Infect Dis. 2011 Nov;204(Suppl 3):S986–90. doi: 10.1093/infdis/jir335. [DOI] [PubMed] [Google Scholar]
- 29.Bray M, Hatfill S, Hensley L, Huggins JW. Haematological, biochemical and coagulation changes in mice, guinea-pigs and monkeys infected with a mouse-adapted variant of Ebola Zaire virus. Journal of comparative pathology. 2001 Nov;125(4):243–53. doi: 10.1053/jcpa.2001.0503. [DOI] [PubMed] [Google Scholar]
- 30.Ryabchikova E, Kolesnikova L, Smolina M, Tkachev V, Pereboeva L, Baranova S, et al. Ebola virus infection in guinea pigs: presumable role of granulomatous inflammation in pathogenesis. Arch Virol. 1996;141(5):909–21. doi: 10.1007/BF01718165. [DOI] [PubMed] [Google Scholar]
- 31.Connolly BM, Steele KE, Davis KJ, Geisbert TW, Kell WM, Jaax NK, et al. Pathogenesis of experimental Ebola virus infection in guinea pigs. J Infect Dis. 1999 Feb;179(Suppl 1):S203–17. doi: 10.1086/514305. [DOI] [PubMed] [Google Scholar]
- 32.Ebihara H, Zivcec M, Gardner D, Falzarano D, LaCasse R, Rosenke R, et al. A Syrian Golden Hamster Model Recapitulating Ebola Hemorrhagic Fever. J Infect Dis. 2012 doi: 10.1093/infdis/jis626. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baskerville A, Bowen ET, Platt GS, McArdell LB, Simpson DI. The pathology of experimental Ebola virus infection in monkeys. The Journal of pathology. 1978 Jul;125(3):131–8. doi: 10.1002/path.1711250303. [DOI] [PubMed] [Google Scholar]
- 34.Bowen ET, Platt GS, Simpson DI, McArdell LB, Raymond RT. Ebola haemorrhagic fever: experimental infection of monkeys. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978;72(2):188–91. doi: 10.1016/0035-9203(78)90058-5. [DOI] [PubMed] [Google Scholar]
- 35*.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nature reviews Microbiology. 2009 May;7(5):393–400. doi: 10.1038/nrmicro2129. [reviews the requirements for approval of vaccines under the animal rule.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nature medicine. 2011 Sep;17(9):1128–31. doi: 10.1038/nm.2447. [defines the correlates of protection for the Ad5-based EHF vaccine.] [DOI] [PubMed] [Google Scholar]
- 37.Lupton HW, Lambert RD, Bumgardner DL, Moe JB, Eddy GA. Inactivated vaccine for Ebola virus efficacious in guineapig model. Lancet. 1980 Dec 13;2(8207):1294–5. doi: 10.1016/s0140-6736(80)92352-1. [DOI] [PubMed] [Google Scholar]
- 38.Rao M, Bray M, Alving CR, Jahrling P, Matyas GR. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: protection in mice requires CD4(+) T cells. J Virol. 2002 Sep;76(18):9176–85. doi: 10.1128/JVI.76.18.9176-9185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerging infectious diseases. 2002 May;8(5):503–7. doi: 10.3201/eid0805.010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, Sanchez A, et al. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine. 2000 Aug 15;19(1):142–53. doi: 10.1016/s0264-410x(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 41.Reynard O, Mokhonov V, Mokhonova E, Leung J, Page A, Mateo M, et al. Kunjin virus replicon-based vaccines expressing Ebola virus glycoprotein GP protect the guinea pig against lethal Ebola virus infection. J Infect Dis. 2011 Nov;204(Suppl 3):S1060–5. doi: 10.1093/infdis/jir347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanderzanden L, Bray M, Fuller D, Roberts T, Custer D, Spik K, et al. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology. 1998 Jun 20;246(1):134–44. doi: 10.1006/viro.1998.9176. [DOI] [PubMed] [Google Scholar]
- 43.Riemenschneider J, Garrison A, Geisbert J, Jahrling P, Hevey M, Negley D, et al. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003 Sep 8;21(25-26):4071–80. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000 Nov 30;408(6812):605–9. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 45.Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clinical and vaccine immunology : CVI. 2006 Nov;13(11):1267–77. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003 Aug 7;424(6949):681–4. doi: 10.1038/nature01876. [first report of protection of NHPs by the Ad5-based vaccine alone.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan NJ, Geisbert TW, Geisbert JB, Shedlock DJ, Xu L, Lamoreaux L, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS medicine. 2006 Jun;3(6):e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson JS, Yao MK, Tran KN, Croyle MA, Strong JE, Feldmann H, et al. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PloS one. 2009;4(4):e5308. doi: 10.1371/journal.pone.0005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hensley LE, Mulangu S, Asiedu C, Johnson J, Honko AN, Stanley D, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus Species. PLoS Pathog. 2010 May;6(5):e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010 Dec 16;29(2):304–13. doi: 10.1016/j.vaccine.2010.10.037. [first evidence of safety of the Ad5-based EHF vaccine in humans.] [DOI] [PubMed] [Google Scholar]
- 51.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010 Jan 22;28(4):950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 52.Choi JH, Schafer SC, Zhang L, Kobinger GP, Juelich T, Freiberg AN, et al. A Single Sublingual Dose of an Adenovirus-Based Vaccine Protects against Lethal Ebola Challenge in Mice and Guinea Pigs. Molecular pharmaceutics. 2012 Jan 1;9(1):156–67. doi: 10.1021/mp200392g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson JS, Abou MC, Tran KN, Kumar A, Sahai BM, Kobinger GP. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. J Infect Dis. 2011 Nov;204(Suppl 3):S1032–42. doi: 10.1093/infdis/jir332. [DOI] [PubMed] [Google Scholar]
- 54.Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PloS one. 2008;3(10):e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, et al. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006 Mar 15;346(2):394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 56.Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011 May;85(9):4222–33. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy S, Zhi Y, Kobinger GP, Figueredo J, Calcedo R, Miller JR, et al. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J Gen Virol. 2006 Sep;87(Pt 9):2477–85. doi: 10.1099/vir.0.81989-0. [DOI] [PubMed] [Google Scholar]
- 58.Mellquist-Riemenschneider JL, Garrison AR, Geisbert JB, Saikh KU, Heidebrink KD, Jahrling PB, et al. Comparison of the protective efficacy of DNA and baculovirus-derived protein vaccines for EBOLA virus in guinea pigs. Virus Res. 2003 Apr;92(2):187–93. doi: 10.1016/s0168-1702(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 59.Konduru K, Bradfute SB, Jacques J, Manangeeswaran M, Nakamura S, Morshed S, et al. Ebola virus glycoprotein Fc fusion protein confers protection against lethal challenge in vaccinated mice. Vaccine. 2011 Apr 5;29(16):2968–77. doi: 10.1016/j.vaccine.2011.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phoolcharoen W, Dye JM, Kilbourne J, Piensook K, Pratt WD, Arntzen CJ, et al. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20695–700. doi: 10.1073/pnas.1117715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005 Apr 27;23(23):3033–42. doi: 10.1016/j.vaccine.2004.11.070. [shows that vaccination with VLPs can fully protect NHPs against EHF.] [DOI] [PubMed] [Google Scholar]
- 62.Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15889–94. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007 Nov 15;196(Suppl 2):S430–7. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 64.Warfield KL, Posten NA, Swenson DL, Olinger GG, Esposito D, Gillette WK, et al. Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J Infect Dis. 2007 Nov 15;196(Suppl 2):S421–9. doi: 10.1086/520612. [DOI] [PubMed] [Google Scholar]
- 65.Sun Y, Carrion R, Jr., Ye L, Wen Z, Ro YT, Brasky K, et al. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology. 2009 Jan 5;383(1):12–21. doi: 10.1016/j.virol.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoenen T, Groseth A, de Kok-Mercado F, Kuhn JH, Wahl-Jensen V. Minigenomes, transcription and replication competent virus-like particles and beyond: reverse genetics systems for filoviruses and other negative stranded hemorrhagic fever viruses. Antiviral Res. 2011 Aug;91(2):195–208. doi: 10.1016/j.antiviral.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halfmann P, Kim JH, Ebihara H, Noda T, Neumann G, Feldmann H, et al. Generation of biologically contained Ebola viruses. Proc Natl Acad Sci U S A. 2008 Jan 29;105(4):1129–33. doi: 10.1073/pnas.0708057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halfmann P, Ebihara H, Marzi A, Hatta Y, Watanabe S, Suresh M, et al. Replication-deficient ebolavirus as a vaccine candidate. J Virol. 2009 Apr;83(8):3810–5. doi: 10.1128/JVI.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nature medicine. 2005 Jul;11(7):786–90. doi: 10.1038/nm1258. [first report of protection of NHPs by the rVSV-based EHF vaccine.] [DOI] [PubMed] [Google Scholar]
- 70.Jones SM, Stroher U, Fernando L, Qiu X, Alimonti J, Melito P, et al. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007 Nov 15;196(Suppl 2):S404–12. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 71**.Feldmann H, Jones SM, Daddario-DiCaprio KM, Geisbert JB, Stroher U, Grolla A, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007 Jan;3(1):e2. doi: 10.1371/journal.ppat.0030002. [describes successful post-exposure vaccination of NHPs against EHF.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, Stroher U, Hensley LE, Grolla A, et al. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J Virol. 2006 Oct;80(19):9659–66. doi: 10.1128/JVI.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008 Dec 9;26(52):6894–900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008 Nov;4(11):e1000225. doi: 10.1371/journal.ppat.1000225. [shows safety and efficacy of the rVSV-based EHF vaccine in severely immunocompromised NHPs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol. 2009 Jul;83(14):7296–304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PloS one. 2009;4(5):e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, Geisbert JB, Leung A, Feldmann F, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol. 2008 Jun;82(11):5664–8. doi: 10.1128/JVI.00456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geisbert TW, Hensley LE, Geisbert JB, Leung A, Johnson JC, Grolla A, et al. Postexposure treatment of Marburg virus infection. Emerging infectious diseases. 2010 Jul;16(7):1119–22. doi: 10.3201/eid1607.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daddario-DiCaprio KM, Geisbert TW, Stroher U, Geisbert JB, Grolla A, Fritz EA, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006 Apr 29;367(9520):1399–404. doi: 10.1016/S0140-6736(06)68546-2. [DOI] [PubMed] [Google Scholar]
- 80.Geisbert TW, Jones S, Fritz EA, Shurtleff AC, Geisbert JB, Liebscher R, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS medicine. 2005 Jun;2(6):e183. doi: 10.1371/journal.pmed.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Falzarano D, Feldmann F, Grolla A, Leung A, Ebihara H, Strong JE, et al. Single immunization with a monovalent vesicular stomatitis virus-based vaccine protects nonhuman primates against heterologous challenge with Bundibugyo ebolavirus. J Infect Dis. 2011 Nov;204(Suppl 3):S1082–9. doi: 10.1093/infdis/jir350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mire CE, Miller AD, Carville A, Westmoreland SV, Geisbert JB, Mansfield KG, et al. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS neglected tropical diseases. 2012 Mar;6(3):e1567. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuda Y, Safronetz D, Brown K, LaCasse R, Marzi A, Ebihara H, et al. Protective efficacy of a bivalent recombinant vesicular stomatitis virus vaccine in the Syrian hamster model of lethal Ebola virus infection. J Infect Dis. 2011 Nov;204(Suppl 3):S1090–7. doi: 10.1093/infdis/jir379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Gunther S, Feldmann H, Geisbert TW, Hensley LE, Rollin PE, Nichol ST, et al. Management of accidental exposure to Ebola virus in the biosafety level 4 laboratory, Hamburg, Germany. J Infect Dis. 2011 Nov;204(Suppl 3):S785–90. doi: 10.1093/infdis/jir298. [describes the first use of the rVSV-based EHF vaccine as post-exposure vaccination in humans.] [DOI] [PubMed] [Google Scholar]
- 85.Bukreyev A, Yang L, Zaki SR, Shieh WJ, Rollin PE, Murphy BR, et al. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J Virol. 2006 Mar;80(5):2267–79. doi: 10.1128/JVI.80.5.2267-2279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, et al. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol. 2007 Jun;81(12):6379–88. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bukreyev A, Marzi A, Feldmann F, Zhang L, Yang L, Ward JM, et al. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology. 2009 Jan 20;383(2):348–61. doi: 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bukreyev AA, Dinapoli JM, Yang L, Murphy BR, Collins PL. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology. 2010 Apr 10;399(2):290–8. doi: 10.1016/j.virol.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89*.Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, et al. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J Virol. 2011 Oct;85(20):10605–16. doi: 10.1128/JVI.00558-11. [describes a recombinant Rabies virus which is a promising candidate for a wildlife vaccination platform.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, et al. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS neglected tropical diseases. 2011 Aug;5(8):e1275. doi: 10.1371/journal.pntd.0001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marzi A, Ebihara H, Callison J, Groseth A, Williams KJ, Geisbert TW, et al. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J Infect Dis. 2011 Nov;204(Suppl 3):S1066–74. doi: 10.1093/infdis/jir348. [DOI] [PMC free article] [PubMed] [Google Scholar]