Abstract
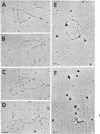
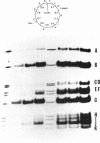
SV40 replicating chromosomes were extracted from infected cells using a detergent free extraction method. This procedure also extracts 2 forms of the non-replicating chromosome, one of which corresponds to the well characterized 50-55S SV40 minichromosome. The other is a more compact structure which has a sedimentation coefficient of 80-85S. The replicating chromosomes sediment between the 2 conformations of the mature chromosome. Electron microscopy of the replicating chromosomes suggests an overall conformation that resembles the 50-55S form of the mature chromosome rather than that of the 80-85S structure. Nucleosomes are present on both sides of the replication forks. When the replicating chromosomes were incubated in an in vitro DNA synthesis assay all regions of the SV40 genome were synthesized and a significant fraction of the replicating chromosomes completed replication. The progeny chromosomes co-sedimented with the 50-55S chromosomes which were present prior to the incubation. The sedimentation coefficients and relative amounts of the two forms of the mature chromosome were unaffected by the incubation.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen M. C., Birkenmeier E., Salzman N. P. Simian virus 40 DNA replication: characterization of gaps in the termination region. J Virol. 1976 Feb;17(2):614–621. doi: 10.1128/jvi.17.2.614-621.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. C., Chang K. S., Salzman N. P. Studies of polyoma virus DNA: cleavage map of the polyoma virus genome. J Virol. 1975 Jan;15(1):191–198. doi: 10.1128/jvi.15.1.191-198.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremisi C., Pignatti P. F., Croissant O., Yaniv M. Chromatin-like structures in polyoma virus and simian virus 10 lytic cycle. J Virol. 1975 Jan;17(1):204–211. doi: 10.1128/jvi.17.1.204-211.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Waqar M. A., Huberman J. A. Subnuclear systems for synthesis of simian virus 40 DNA in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4392–4396. doi: 10.1073/pnas.73.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Khoury G., Salzman N. P. Self-annealing of 4 S strands from replicating simian virus 40 DNA. J Mol Biol. 1973 Jul 5;77(3):457–462. doi: 10.1016/0022-2836(73)90451-8. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Sebring E. D., Salzman N. P. Cleavage of replicative intermediates of simian virus 40 deoxyribonucleic acid by the restriction endonuclease of Escherichia coli B. J Biol Chem. 1972 Sep 25;247(18):5872–5879. [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hall M. R., Meinke W., Goldstein D. A. Nucleoprotein complexes containing replicating Simian virus 40 DNA: comparison with polyoma nucleoprotein complexes. J Virol. 1973 Oct;12(4):901–908. doi: 10.1128/jvi.12.4.901-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Specific origin in SV40 DNA replication. Nat New Biol. 1972 Apr 19;236(68):200–202. doi: 10.1038/newbio236200a0. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. In vitro replication of simian virus 40 DNA in a nucleoprotein complex. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3466–3470. doi: 10.1073/pnas.73.10.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Nedospasov S. A., Schmatchenko V. V., Bakayev V. V., Chumackov P. M., Georgiev G. P. Compact form of SV40 viral minichromosome is resistant to nuclease: possible implications for chromatin structure. Nucleic Acids Res. 1977 Oct;4(10):3303–3325. doi: 10.1093/nar/4.10.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Benyajati C. Higher order coiling of DNA in chromatin. Cell. 1977 Sep;12(1):83–100. doi: 10.1016/0092-8674(77)90187-8. [DOI] [PubMed] [Google Scholar]