Abstract
Pdx1 has been shown to convert hepatocytes into both exocrine and endocrine pancreatic cells in mice, but it fails to selectively convert hepatocytes into pure insulin-producing cells (IPCs). The molecular mechanisms underlying the transdifferentiation remain unclear. In this study, we generated a stably transfected rat hepatic cell line named WB-1 that expresses an active form of Pdx1 along with a reporter gene, RIP-eGFP. Our results demonstrate that Pdx1 induces the expression of multiple genes related to endocrine pancreas development and islet function in these liver cells. We do not however find any expression of the late-stage genes (Pax4, Pax6, Isl-1, and MafA) related to β-cell development, and the cells do not secrete insulin upon the glucose challenge. Yet when WB-1 cells are transplanted into diabetic NOD-scid mice, these genes become activated and hyperglycemia is completely reversed. Detailed comparison of gene expression profiles between pre- and posttransplanted WB-1 cells demonstrates that the WB-1 cells have similar properties as that seen in pancreatic β-cells. In addition, in vitro culture in high-glucose medium is sufficient to induce complete maturation of WB-1 cells into functional IPCs. In summary, we find that Pdx1-VP16 is able to selectively convert hepatic cells into pancreatic endocrine precursor cells. However, complete transdifferentiation into functional IPCs requires additional external factors, including high glucose or hyperglycemia. Thus, transdifferentiation of hepatocytes into functional IPCs may serve as a viable therapeutic option for patients with type 1 diabetes.
The liver and pancreas have an intimate relationship during embryogenesis. Indeed, it has been proposed that these two organs are derived from a common progenitor cell (1), and transdifferentiation between the liver and pancreas has been demonstrated under certain conditions (2). We previously demonstrated that hepatic stem cells could be induced in vitro to transdifferentiate into insulin-producing pancreatic endocrine-like cells (3). Recent studies have shown that ectopic and transient expression of the transcription factor Pdx1 in the mouse liver induces transdifferentiation into pancreatic cells, including both exocrine and endocrine cells, and reduces hyperglycemia in chemically induced diabetic mice (4 –7). However, the conversion from liver to endocrine pancreatic cells mediated by Pdx1 alone is incomplete and nonselective, resulting in severe hepatitis due to the production of by-products such as the exocrine enzymes amylase and trypsin (7).
To establish an in vitro system to study the molecular mechanism of selective liver to endocrine pancreas transdifferentiation, we generated a stably transfected rat hepatic cell line (WB-1) that overexpresses an activated form of Pdx1 (Pdx1-VP16) along with a reporter gene, RIP-eGFP, to monitor insulin gene expression. Pdx1-VP16 is a fusion of mouse Pdx1 with the VP16 activation domain from the Herpes simplex virus that creates a super active version of Pdx1 (5). In this study, we investigated the profile of gene expression induced by Pdx1-VP16 –mediated transdifferentiation of WB-1 cells in vitro and during in vivo cell transplantation. We find that expression of Pdx1-VP16 can transdifferentiate hepatic WB cells into glucose-insensitive endocrine precursor cells expressing multiple genes related to β-cell development and function but with no evidence of pancreatic exocrine gene expression. However, these precursor cells require either in vivo hyperglycemia or in vitro long-term high-glucose culture to become fully functional pancreatic endocrine cells. Thus, the conversion of hepatocytes into endocrine pancreatic cells may be a viable option for cell replacement therapy in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Plasmids and plasmid construction
Pdx1-VP16 was constructed by fusing the activation domain of VP16 (80 amino acids) to the mouse COOH-terminus of Pdx1 as follows. Full-length Pdx1 was isolated from IPF1-pcDNA3 (a gift from H.-P. Huang) using the T7 primer and a 3′ primer that included a ClaI site, 5′-TCG CAG TGG ATC GAT GCT GGA G-3′. The product was cut with HindIII and ClaI and subcloned into VP16-N in pCS2+ (a gift from Dr. Kessler) (8). Pdx1-VP16 was then subcloned into the HindIII and XbaI sites of pcDNA3. A vector containing a chimeric gene of the zeocin resistance gene (zeor) and a green fluorescence protein gene under the control of rat insulin-1 promoter–enhanced green fluorescence protein (RIP-eGFP) was constructed in our laboratory. To construct pRIP-eGFP, a 645-bp fragment of the 5′ untranscribed (promoter) region of the rat insulin I gene was amplified from DNA of rat pancreas. The PCR product was first cloned into pGEM-T vector (Promega, Madison, WI). The insulin promoter fragment was then cut out by BamHI and EcoRI and then cloned into pHR-eGFP (Stratagene, La Jolla, CA).
Cell lines and cell cultures
Rat liver epithelial cells (WB cells)
WB cells are derived from normal liver cells isolated from an adult male Fisher 344 rat and represent the cultured counterpart of liver stem-like cells (9,10). They are capable of differentiating into both mature hepatocytes and biliary epithelial cells (10,11). The WB cells (a gift from Dr. Grisham) (9) were maintained in Dulbecco’s modified Eagle’s medium/F12 medium supplemented with 10% FCS, 11.1 mmol/l D-glucose, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Rat insulinoma INS-1 cell line (clone 832/13)
The rat INS-1 cell line (clone 832/13) (a gift from Christopher B. Newgard) was derived from stable transfection of a plasmid containing the human proinsulin gene driven by a cytomegalovirus (CMV) promoter (12). The cells express and process both rat and human insulin and are capable of insulin release in response to glucose stimulation. The cells were continuously maintained in our laboratory for 1.5 years in RPMI 1640 medium as described (12) and used as a positive control for studies of insulin content and insulin release.
WB-1 cell lines
We have generated five cell lines containing a fusion gene of Pdx1-VP16 and a reporter gene RIP-eGFP from WB cells. In short, WB cells were cotransfected with plasmids of CMV-Pdx1-VP16 and RIP-eGFP by lipofectin reagent (Invitrogen, Carlsbad, CA). Double-positive cells were selected with antibiotics G418 (400 μg/ml) and Zeocin (400 μg/ml) for 3 weeks. Five single cell– derived green fluorescence protein–positive clones were randomly isolated using a cloning cylinder (Fisher) and were expanded into five cell lines designated WB-1 to -5. All of the five cell lines expressed similar levels of Pdx1 protein detected by Western blotting with anti-Pdx1 antibody and the insulin gene visualized under a fluorescence microscope. According to these criteria, we assumed that the five lines were similar and we therefore focused on a single clone, WB-1, for the rest of the experiments, as presented in this article. The WB-1 cells were maintained in RPMI 1640 medium supplemented with 10% FCS and 11.1 mmol/l D-glucose.
RT-PCR
Total RNA was prepared from cells or tissues using TRI-reagent. Transcriptional gene expression was determined by RT-PCR according to our previously published protocol (3). The forward and reverse primers of each PCR set were designed to be located in different exon(s) based on sequences obtained from GenBank to distinguish the PCR product from DNA contamination. The name and sequences of the primers, the sizes of PCR products, cycles, and annealing temperature for each pair are listed in Table 1.
TABLE 1.
List of primer information for RT-PCR
| Forward primer | Reverse primer | Size of PCR product (bp) | GenBank accession no. | Annealing temperature (°C) | No. of cycles | |
|---|---|---|---|---|---|---|
| Actin | cgt aaa gac ctc tat gcc aa | agc cat gcc aaa tgt ctc at | 351 | V01217 | 56 | 35 |
| Albumin | gcc cta ccc aca aag cct cag | pp gtg gct ttc tgt tgc tgt tca | 540 | NM_172320 | 55 | 35 |
| TGF-α | aca gct cgc tct gct agc gct | gga tct tca gac cac tgt ctc | 469 | NM_012671 | 57 | 35 |
| Gata-4 | cat gct tgc agt tgt gct ag | att ctc tgc tac ggc cag ta | 174 | NM_144730 | 56 | 35 |
| TTR | ctc tgc ctc gct gga ctg ata | agt ggt gct gta gga gta cgg | 393 | AF479660 | 55 | 35 |
| HNF-4 | aca tgg ctg act aca gtg ctg | ctg ctg tcc tcg tag ctt gac | 392 | NM_022180 | 58 | 35 |
| P48 | att aac ttc ctc agc gag ctg | tca aaa ggt ggt tcg ttc tct | 338 | NM_053964 | 58 | 35 |
| Amylase | agc agg aag acc ag ctg ta | atg act ggg tct gtg aac at | 201 | J00703 | 55 | 35 |
| Elastase | ggt cta ctc tct cca caa cat | tgg tac cat gat cct ccg ga | 180 | NM_012552 | 56 | 35 |
| Carboxypeptidase A1 | gcatcc att cta ggg agt ggg | gaa aga gta ctt gat gcc ctg | 602 | NM_016998 | 55 | 35 |
| HNF-1 | ttc taa gct gag cca gct gca gac g | gct gag gtt ctc cgg ctc ttt cag a | 275 | X54423 | 56 | 35 |
| mPdx-1 | tac aag ctc gct ggg atc act | gca gta cgg gtc ctc ttg tt | 309 | X_74342 | 56 | 35 |
| rPdx-1 | cgg cca cac agc tct aca agg | gag gtt acg gca caa tcc tgc | 667 | NM_022852 | 56 | 35 |
| Ngn3 | ctg cgc ata gcg gac cac agc ttc | ctt cac aag aag tct gag aac acc ag | 324 | NM_021700 | 58 | 35 |
| NeuroD | ctt ggc caa gaa cta cat ctg g | gga gta ggg atg cac cgg gaa | 225 | NM_019218 | 57 | 35 |
| Nkx2.2 | gtacacgcgctggctggccag | gtacacgcgctggctggccag | 304 | NM_010919 | 58 | 35 |
| Pax4 | cag cag cat gga cca gct tgg | ctc ctg taa tgc ccg cag gac | 214 | XM_133023 | 55 | 35 |
| Nkx6.1 | atg gga aga gaa aac aca cca gac | taa tcg tcg tcg tcc tcc tcg ttc | 280 | AF357883 | 58 | 35 |
| Pax6 | gag aca gat tac tct ccg agg | acc aca cct gta tcc ttg ctt ag g | 465 | NM_013001 | 55 | 35 |
| Isl1 | cgg gag gat ggg ctt ttc tg | agc tgc ttt tgg ttg agc aca g | 191 | NM_017339 | 56 | 35 |
| MafA | gac atc tcc cca tac gaa gtg | ccg cta cta cgt ttc tta tct | 462 | NM_008814 | 55 | 35 |
| Glut-2 | tcc agt aca ttg cgg act tcc | ggt gta gtc cta cac tca tg | 304 | J03145 | 58 | 35 |
| GK | aag gga aca aca tcg tag ga | cta tgg cgg tct tca tag ta | 126 | X53589 | 56 | 35 |
| Insulin I | tac aat cat aga cca tca gca | cag ttg gta gag gga gca gat | 355 | Gi:204956 | 56 | 35 |
| Insulin II | agc cct aag tga cca gct aca | tgc caa ggt ctg aag gtc ac | 343 | V01243 | 56 | 35 |
| PP | gtc gca tac tac tgc ctc tcc | aga cag aag gga ggc tac aaa tcc | 336 | NM_012626 | 57 | 35 |
| Glucagon | gac cgt tta cat cgt ggc gg | cgg ttc ctc ttg gtg ttc atc aac | 249 | NM_012707 | 58 | 35 |
| Somatostatin | atg ctg tcc tgc cgt ctc c | tcg agt tgg cag acc tct g | 277 | NM_012659 | 56 | 35 |
| GLP-1R | tct ctt ctg caa ccg aac ct | ctg gtg cag tgc aag tgt ct | 351 | S75952 | 58 | 35 |
| SUR-1 | Aag atc atg cac ttg tct act | aga cag cag gaa cag cgg tgt | 593 | AF039595 | 56 | 35 |
| SNAP-25 | Agt agt ggc cag cca gcc tg | atc tgg cga ttc tgg gtg tca | 200 | NM_030991 | 57 | 35 |
| Hexokinase | Tga acc acg aga aga acc aga | Aca atg tta gca tca tag tcc | 322 | NM_012734 | 58 | 35 |
| Kir6.2 | Acc acg ctg gtg gac ctc aag | Gca cca cct gca tat gaa tgg | 481 | RNU44897 | 60 | 35 |
| IAPP | Ggc tgt agt tcc tga agc tt | aag gtt gtt gct gga gcg aa | 260 | NM_012586 | 56 | 35 |
| Chromogranin A | Act aag gtg atg aag tgt gt | tct cta cag tgt cct tgg ag | 353 | NM_021655 | 56 | 35 |
| PC-1 | Ttt gtc agt atg cgt gct aac | Ctg tga cga tgc tgt aat gat | 554 | AB071596 | 58 | 35 |
| PC-2 | Agg tgg tga ggg att acc aa | Aga act gtg gac caa gga ga | 177 | NM_012746 | 58 | 35 |
Enzyme-linked immunosorbent assay
WB-1 cells were maintained in the medium containing 11.1 mmol/l glucose after they were cultured under a high (25 mmol/l)-glucose medium for 1 month. The static insulin production in the culture medium was measured regularly (twice a week). For insulin secretion experiments, the WB-1 cells were switched to low (5.5 mmol/l)-glucose medium for 1 week. After overnight starvation with a serum-free medium containing 0.5% BSA and 3.0 mmol/l glucose, the cells were stimulated with 20.0 mmol/l glucose for 2 h. The culture media were collected and frozen at −70°C until assay for insulin release. Serum-free culture medium containing 0.5% BSA was used as a medium control. For insulin content measurement, the cellular proteins were extracted with radioimmunoprecipitation assay buffer, and cellular proteins were quantitated. Insulin release and insulin content were detected by using an ultrasensitive mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (ALPCO, Windham, NH) following the manufacturer’s protocols. Three separate experiments were performed. INS-1 cells or parental WB cells were used as positive or negative controls and treated the same way as the WB-1 cells, as described above in the insulin secretion experiments.
SDS-PAGE and Western blot
Detection of Pdx1 and insulin proteins was accomplished according to our previously published procedure (13) with minor modification. In brief, cell lysates (50 μg/lane) were separated by SDS-PAGE using 12% gels for Pdx1 and 16.5% for insulin detection (Bio-Rad). The proteins were transferred to PVDF membranes, blotted with rabbit anti-Pdx1 serum (1:5,000) (a gift from C. Wright), followed by horseradish peroxidase– conjugated secondary anti-rabbit polyclonal antibody (1:20,000) and visualized by enhance chemiluminescence.
Cell transplantation and nephrectomy
The 8- to 10-week-old male NOD-scid mice (Pathology Animal Core Facility, University of Florida) were made hyperglycemic by intraperitoneal injections of streptozotocin (STZ) at 50 μg/g body wt daily for 5 days, as previously described (14). When blood glucose reached levels >350 mg/dl, 1 × 106 WB-1 cells/mouse were transplanted into the left renal subcapsular space. Control mice receiving 1 × 106 WB cells/mouse or 1 × 106 INS-1 cells/mouse in 50 μl volume of PBS served as negative and positive controls, respectively. The blood glucose levels were monitored regularly at 1600 in the nonfasting condition. Three of the six WB-1 cell–implanted mice underwent nephrectomy at day 40 posttransplantation to assess metabolic activity of the transplanted cells. The hyperglycemic mice were terminated at day 30. The remaining mice with WB-1 and INS-1 cell transplantation were maintained and terminated at 4 months posttransplantation. The explanted tissues containing implanted WB-1 cells and INS-1 cells were collected for analyses of gene expression and morphologic evaluation.
Histology, immunohistochemistry, and immunocytochemistry
The explanted tissues were fixed and embedded in paraffin, and sections were stained with hematoxylin and eosin (H&E). Sections were incubated with antibodies against insulin, glucagon and albumin (Dako), and amylase (Santa Cruz Biotechnology) according to our previously published procedure (3) with minor modifications. Cytospin slides were made from cultured WB-1 and WB cells and fixed with methanol for 10 min. For Pdx1 staining, cytospin slides were incubated sequentially with rabbit anti-Pdx1 (1:5,000) antibody for 2 h and then biotinylated secondary anti-rabbit polyclonal antibody for 30 min. The antigen-antibody complex was visualized with DAB reagent (Vector Laboratories).
Electron microscopy with immunogold labeling
For immunogold localization of insulin, the cultured WB-1 cells and rat pancreas were embedded as previously described (14). For immunogold labeling, ultra-thin sections were blocked with 5% BSA and 5% normal goat serum in PBS and then incubated overnight at 4°C with rabbit anti-insulin antibody (Santa Cruz Biotechnology) diluted 1:50 in PBS containing 0.2% BSA and 10 mmol/l NaN3. After washing, the samples were incubated for 1.5 h at room temperature with the secondary goat anti-rabbit IgG antibody conjugated to 0.8 nm colloidal gold particles (Aurion EM Grade Ultra Small, EM Sciences). The gold particles were silver-enhanced for 45 min at room temperature (Aurion R-Gent SE EM, EM Sciences). The samples were counterstained and then viewed using a Zeiss EM-10A transmission electron microscope.
Statistical analysis
Statistical analyses were performed using an independent sample t test. A value of P < 0.05 was considered significant.
RESULTS
Generation and characterization of the WB-1 cell line
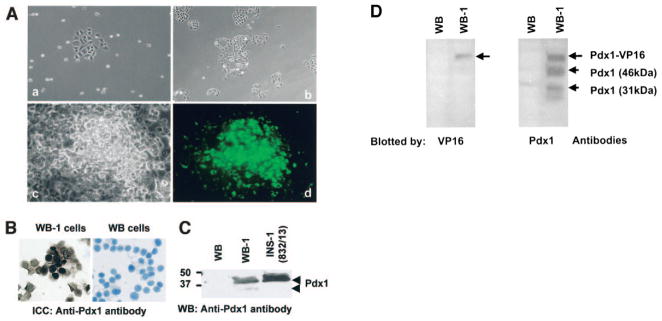
To establish an in vitro system to study the molecular mechanisms of the transdifferentiation of hepatic cells into insulin-producing cells (IPCs), rat WB cells were cotransfected with CMV-Pdx1-VP16/neor and RIP-eGFP/zeor plasmids. Five single cell– derived green fluorescence protein–positive clones (Fig. 1A, part a) were isolated and named WB-1 to -5. These clones were further expanded (Fig. 1A, b and c), and the cells were found to exhibit an intense cytoplasmic green fluorescence, indicating activation of the insulin promoter by Pdx1-VP16 (Fig. 1A, d). To confirm the nuclear localization of the Pdx1 protein, WB-1 cells were subjected to immunocytochemistry with anti-Pdx1 antibodies. As expected, Pdx1 protein was mainly distributed in the nuclei, as shown by dark brown nuclear staining in WB-1 cells (Fig. 1B, left), and there was no staining in WB cells (Fig. 1B, right). Cells stained with control antibody showed no detectable staining (data not shown). Western blot analysis of whole-cell lysates from WB, WB-1, and INS-1 cells demonstrated abundant expression of activated 46-kDa Pdx1 phosphorylated protein in WB-1 and INS-1 cells, but not in parental WB cells (Fig. 1C, WB-1). Similar expression of the Pdx1 protein was also observed in other WB-derived clones 2–5 (data not shown). Because all five Pdx1-VP16 – expressing WB clones expressed similar amounts of Pdx1-VP16 protein and RIP-eGFP, the remaining studies were performed with the WB-1 clone.
FIG. 1.
Generation and characterization of Pdx1-VP16–expressing WB cell lines. A: Generation of WB-1 cells. WB cells were transfected with Pdx1-VP16 and RIP-eGFP and double-positive single cell–derived GFP-positive clones were selected (a) and expanded (b) as described in RESEARCH DESIGN AND METHODS. Representative cloned cells (c) expressed insulin gene reflected by eGFP as green cells (d). B: Detection of Pdx1 protein by immunocytochemistry. Cytospin slides were made from WB-1 and WB cells and subjected to immunostaining with anti-Pdx1 antibody. Brown nuclear staining represents the Pdx1 protein located in WB-1 cells but not present in WB cells. C: Confirmation of Pdx1 protein by Western blot. Cell lysates extracted from WB, WB-1, and INS-1 cells were separated by SDS-PAGE, and the Pdx1 protein was detected by blotting with Pdx1 antibody. Two forms of Pdx1 proteins are indicated by arrows: activated 46-kDa form (upper band) and inactivated 31-kDa form (lower band). D: Identification of Pdx1-VP16 fusion protein in WB-1 cells. The cellular proteins from WB and WB-1 cells were blotted with anti-VP16 antibody (1:200; BD Biosciences) (left) and anti-Pdx1 antibody (right). Top arrows indicate the position of the exogenous Pdx1-VP16 fusion protein.
To confirm that the WB-1 cells indeed expressed the Pdx1-VP16 fusion protein, we performed a Western blot using anti-VP16 antibody. A single band at ~58 kDa of the Pdx1-VP16 fusion protein was detected in WB-1 cells, but not in the parental WB cells (Fig. 1D, left). When a Pdx1 antibody was used to examine the Pdx1 expression, we were able to not only detect the Pdx1-VP16 fusion protein, but also a phosphorylated/active (46-kDa) and an unphosphorylated/inactive (31-kDa) endogenous Pdx1 protein that did not normally express in WB cells (Fig. 1D, right). These results indicate that Pdx1-VP16 activates the expression of the endogenous Pdx1 during the transdifferentiation to pancreatic endocrine cells. In conclusion, we have successfully established an in vitro system consisting of a single hepatic cell– derived clone expressing Pdx1-VP16 with a built-in reporter RIP-eGFP gene to reflect the activity of the insulin promoter.
To characterize the gene expression profile of the newly generated WB-1 cells, we examined the expression of various genes related to pancreatic development and β-cell function by RT-PCR and compared the results with parental WB cells, rat insulinoma cells (INS-1), and normal pancreas (Fig. 2). Expression of Pdx1-VP16 in WB cells resulted in the transcription of multiple genes related to endocrine pancreas development and β-cell function. These include HNF1, endogenous Pdx1, NeuroD/Beta2, Ngn3, NKx2.2, NKx6.1, Glut-2, GK, insulin I and II, and glucagon. This gene expression profile is similar to that seen in the INS-1 cells and rat pancreas. However, there was no detectable expression of the genes Pax4, Pax6, Isl-1, or MafA, which are involved in the late stages of differentiation of pancreatic endocrine cells (15). To determine whether the newly generated WB-1 cells are capable of glucose-responsive insulin release, they were challenged with 20 mmol/l glucose for 2 h, and insulin secretion was determined by ELISA. We found that although the WB-1 cells express multiple pancreatic endocrine genes, including insulin, they do not respond to glucose stimulation by releasing insulin (data not shown). Moreover, there was no detectable mature insulin by Western blot with an anti-insulin antibody (data not shown). Taken together, these results indicate that Pdx1-VP16 – expressing WB-1 cells are precursors of pancreatic endocrine cells that do not exhibit mature β-cell function in the absence of further differentiation.
FIG. 2.
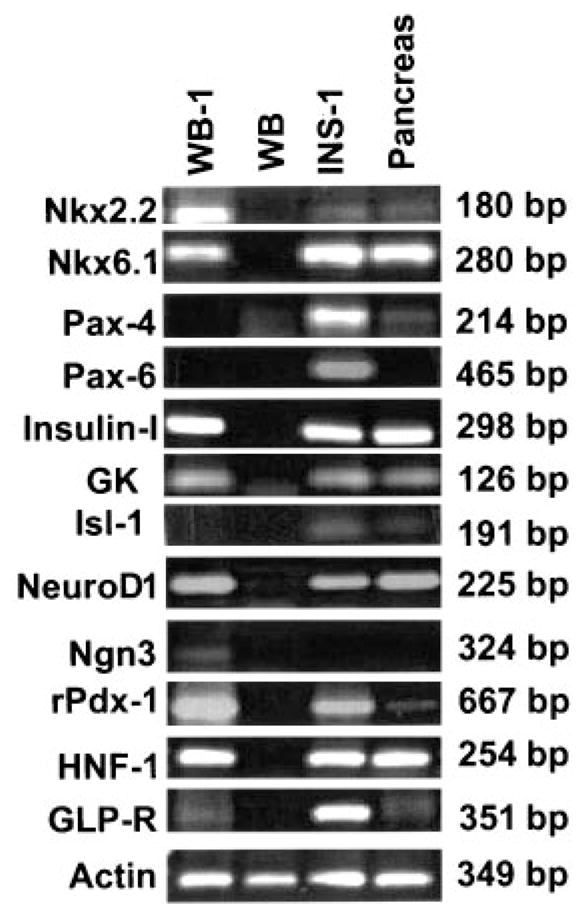
Comparison of gene expression among WB-1, WB, INS-1, and rat pancreas. Total RNA was extracted from the above cells, and RT-PCR was performed. All primers were designed across intron(s). Details regarding primers are presented in Table 1. WB-1 cells are newly generated cells.
Reversal of diabetes in WB-1 cell–transplanted diabetic mice
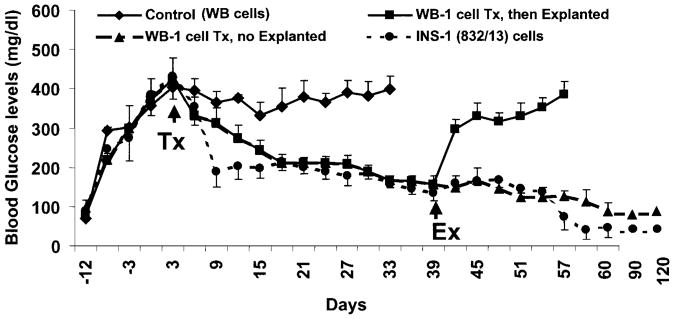
To determine whether the WB-1 cells possess the ability to further differentiate into mature functional pancreatic endocrine β-like cells, they were transplanted into the left renal subcapsular space of STZ-induced diabetic NOD-scid mice. As demonstrated in Fig. 3, WB-1 cells are capable of reducing blood glucose levels from ~400 to ~200 mg/dl in the diabetic mice within 2–3 weeks after cell transplantation, and by day 60, blood glucose levels were normalized (70 –100 mg/dl). In contrast, mice implanted with WB cells did not show any reduction in blood glucose levels and remained hyperglycemic during the entire observation period. As expected, mice receiving INS-1 cell transplantation showed a sharp reduction in blood glucose levels within 7–9 days and maintained blood glucose levels near 150 –200 mg/dl for a long time, but eventually became hypoglycemic (~30 – 40 mg/dl). Furthermore, removal of implanted WB-1 cells by left nephrectomy induced a rebound persistent hyperglycemia, confirming that the implanted WB-1 cells are indeed responsible for the reduction of blood glucose levels. To evaluate the long-term effects of the implanted WB-1 and INS-1 cells, the remaining mice from the two groups were continuously observed for 4 months. WB-1–transplanted mice displayed a consistent euglycemia (70 –100 mg/dl), whereas INS-1–transplanted mice had persistent hypoglycemia (30 – 40 mg/dl). All mice maintained normal body weight comparable to the aged-matched normal mice without diabetes (data not shown). These results demonstrate that although WB-1 cells do not appear to be mature endocrine cells in vitro, they are able to further differentiate and mature in vivo, as well as function like β-cells and rescue diabetic mice.
FIG. 3.
Cell transplantation. 1 × 106/mouse of WB (control, n = 3), WB-1 (n = 6), or INS-1 (n = 4) cells were implanted under the left renal subcapsule after the blood glucose levels reached 400 mg/dl (arrow, transplantation [Tx]) in repeatedly low-dose STZ-induced diabetic NOD-scid mice. The blood glucose levels were monitored regularly under nonfasting conditions through tapping the tail vein. The left kidney of three mice from the WB-1 group was removed at 40 days post-implantation (arrow, explanted [Ex]). The remaining WB-1– and INS-1–transplanted mice were terminated by the end of the observation (4 months).
Gene expression profiles of pre- and posttransplanted WB-1 cells
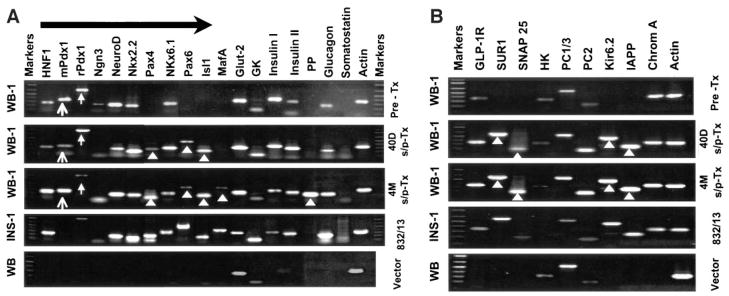
To explore the molecular mechanism responsible for the functional shift of WB-1 cells from being glucose insensitive in vitro to being functional in vivo, we compared the gene expression profiles of posttransplanted WB-1 cells at 40 days and 4 months to that of the functional rat insulinoma INS-1 cells and to pretransplanted WB-1 cells as well as to their parental WB cells. We observed several noticeable changes in the expression of some genes related to β-cell development and function (Fig. 4A). First, after 40 days in vivo, the WB-1 cells now express the genes Pax4, Pax6, and Isl-1, which were not expressed before transplantation. Second, Ngn3, a key transcription factor that is transiently expressed in the pancreatic endocrine precursors but not in mature pancreatic endocrine cells (15–17), was now undetectable. Third, we found increased expression of NKx2.2, GK, and insulin 2 genes in the day 40 posttransplanted WB-1 cells. Fourth, MafA and PP genes became activated in WB-1 cells 4 months after transplantation. Last, we confirmed that the exogenous Pdx1-VP16 fusion gene was persistently expressed in the explanted WB-1 cells throughout the entire observation period. (see supplemental figure [available at http://diabetes.diabetesjournals.org]). The profile of gene expression at 4 months posttransplantation is similar to that of INS-1 cells. Taken together, the changes in gene expression profiles of WB-1 cells suggest a correlation of the sequence of gene activation, β-like cell differentiation and maturation, and the ability of glucose-regulating function in the WB-1 cells.
FIG. 4.
Comparison of gene expression profiles among various stages of WB-1 cells (A and B). Total RNA was extracted from the cells, and RT-PCR was performed. All primers were designed cross intron(s). Details regarding primers are presented in Table 1. Gene expression profiles in pre-and posttransplanted (Tx) (40 days and 4 months) WB-1 cells were compared. INS-1 and WB cells transfected with an empty plasmid vector served as positive and negative controls, respectively. Arrows indicate exogenous (mPdx1) and endogenous (rPdx1) gene expression of Pdx1. Arrowheads indicate newly activated genes (Pax4, Pax6, Isl-1, MafA, and PP in A) as well as β-cell function–related genes (SUR1, Kir6.2, SNAP25, and IAPP in B) in 40 days and 4 months posttransplanted WB-1 cells.
To characterize the molecular components of the glucose sensing, insulin secretion– coupling machinery, and β-cell function, we investigated the expression profiles of genes known to be involved in β-cell function: SUR1, Kir6.2, Snare 25, PC1/3, PC2, IAPP, and chromagranin A (Chrom A). SUR1 and Kir6.2 are ATP-sensitive K+ channel proteins, Snare 25 is involved in coupling and fusing vesicles to the cell membrane, IAPP is colocalized with insulin in secretory granules, and Chrom A, an abundant protein, is present in all islet cells. We also examined the gene expression of hexokinase (HK) to compare the levels to glucokinase (GK) during various stages of WB-1 cell maturation. Several interesting findings are demonstrated in Fig. 4B. 1) The gene expression of GLP-1R and PC2 is weak in WB-1 cells (pretransplantation) but becomes strong in mature (posttransplantation) WB-1 cells at 40 days and 40 months posttransplantation. 2) Several genes including SUR1, Kir6.2, Snare 25, and IAPP are not expressed in immature WB-1 cells but become highly expressed in mature WB-1 cells, indicating that these proteins are related to mature β-cell functions. 3) HK gene expression appears to gradually decrease as the cells become mature. In contrast, GK gene expression increases as the WB-1 cells become mature during their in vivo differentiation. These results suggest that the estimated GK/HK ratio increases as the WB-1 cells undergo maturation. 4) Chrom A, a widely distributed protein in all islet cells, is expressed at all stages of WB-1 cell maturation, but is not detected in the parental liver epithelial WB cells. As expected, the rat insulinoma cell line INS-1 (823/13) expresses all genes with the exception of HK, whereas the parental WB cells express GK, HK, PC1/3, and PC2. These results demonstrate that upon maturation, the WB-1 cells indeed express many of the molecular components involved in regulated insulin secretion in mature β-cells.
Histology and pancreatic hormone production in the explanted tissues
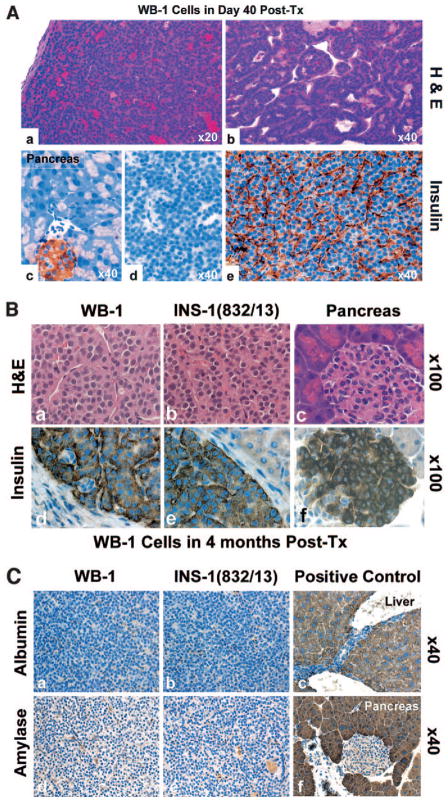
To further characterize the identity of the transplanted WB-1 cells, we examined their histological appearance and insulin protein expression after in vivo differentiation at 40 and 120 days posttransplantation. The explanted WB-1 cells formed glandular or islet-like clusters with a rich network of microvasculature (Fig. 5A and B, H&E). The cytology of the posttransplanted WB-1 cells at 40 days revealed large nuclei and relatively scant cytoplasm, which is morphologically consistent with less mature cells (Fig. 5A). In contrast, at 120 days, the cells showed a decreased nucleus/cytoplasm ratio with “salt and pepper” chromatin and abundant cytoplasm, which is characteristic of mature pancreatic endocrine cells (Fig. 5B, H&E). Immunostaining for insulin protein showed that >95% of the implanted cells expressed insulin (Fig. 5A, e and 5B, d) with no detectable glucagon-positive cells present (data not shown). Neither amylase nor albumin was detectable in WB-1 and INS-1 cells in comparison to positive controls (Fig. 5C). These results demonstrate that in vivo, Pdx1-VP16 – expressing WB-1 cells selectively differentiate into mature functional IPCs with no detectable pancreatic exocrine or liver proteins.
FIG. 5.
Histology and immunostaining of the explanted tissues. Paraffin sections of explanted tissues from 40 days (A) and 4 months (B) posttransplantation were stained with H&E (upper panels of A and B). The sections were immunostained with antibodies to insulin (1:500), amylase (1:200), and albumin (1:200) and incubated with matched secondary antibodies. The labeled proteins were visualized with DAB reagent. Lower panels of A represent insulin staining (c, pancreatic islet; d, negative control; e, insulin in the explanted tissue) in 40-day explanted WB-1 cells. Lower panels of B represent insulin staining on the explanted tissues containing WB-1 (left) cells, INS-1 (middle), and mouse pancreas tissue (right) as positive controls, as indicated in the figures. Each figure in B contains an internal negative control. C shows immunostaining of amylase and albumin in WB-1, INS-1, and positive controls (liver and pancreas), as indicated in the figure. Tx, transplanted.
Effects of high-glucose culture on WB-1 cell maturation
Based on the results of cell transplantation, as well as our previous studies on hepatic oval cells (3) and bone marrow– derived stem cells (14), we hypothesize that high-glucose condition is a critical factor to promote further differentiation of precursor WB-1 cells. To determine whether WB-1 cells could be induced to mature in vitro into functional IPCs, we cultured them in high-glucose media for 4 weeks. Table 2 summarizes the insulin content and release in WB, WB-1, and INS-1 cells upon stimulation with 20 mmol/l glucose. We found a 1.8-fold increase in insulin release in WB-1 cells in response to glucose stimulation when compared with unstimulated WB-1 cells. A similar ratio is seen with INS-1 cells under our experimental conditions, demonstrating that high glucose can indeed promote the maturation of nonfunctional WB-1 cells into functional IPCs. These results support our hypothesis that Pdx1-VP16 – expressing hepatic cells are endocrine precursor cells that selectively differentiate into mature functional IPCs only when placed in the proper microenvironment, such as in high-glucose culture or in a hyperglycemic diabetic mouse.
TABLE 2.
Insulin content and insulin release after 2 h of glucose stimulation
| Cell type | Insulin content (ng/ml) | Insulin release (ng/ml)
|
Fold of insulin release (stimulated/control) | |
|---|---|---|---|---|
| 20 mmol/l glucose (−) (control) | 20 mmol/l glucose (+) (stimulated) | |||
| Media | <0.025 | <0.025 | <0.025 | — |
| WB | <0.025 | <0.025 | <0.025 | — |
| WB-1 | 15.22 ± 1.549 | 10.38 ± 1.893 | 18.74 ± 0.051* | 1.851 ± 0.373 |
| INS-1 | 1225 ± 344.1 | 91.80 ± 6.184 | 165.7 ± 10.19* | 1.666 ± 0.348 |
P < 0.001. Note that 0.025 ng/ml is the lowest detectable level.
To examine whether the functional WB-1 cells can maintain their differentiated state when glucose levels are switched back to basic levels, we continuously followed the WB-1 cells in the basic medium (11.1 mmol/l glucose) and measured static insulin levels in the culture medium. We observed that the static insulin secretion in the medium was maintained at a high level (>1.7 ng/ml culture medium) for five passages, decreased to 0.8 ng/ml for the next two passages, and then remained at 0.3 ng/ml for six additional passages before it became undetectable. This phenomenon of changing sensitivity to glucose is commonly observed and has been well documented, even in true β-cell lines or genetically engineered β-cell lines (18,19) when they were cultured in vitro for a long time. These results indicate that functional WB-1 cells can indeed maintain their differentiated state during in vitro culture conditions.
Selective transdifferentiation of WB-1 into IPCs without evidence of exocrine differentiation
Previously, we showed that amylase protein is not detected in the explanted WB-1 cells. To confirm that transdifferentiation is selective toward an endocrine cell fate, we examined the expression of both early (p48) and late exocrine (amylase, elastase, and carboxypeptidase) genes in various stages of WB-1 cells by RT-PCR. In agreement with our hypothesis that WB-1 cells are pancreatic endocrine precursors, we found no detectable expression of exocrine markers (Fig. 6A). In contrast, we do find expression of hepatic mRNA, though we do not detect protein expression (Fig. 6B). These results indicate that expression of Pdx1-VP16 selectively transdifferentiates hepatic cells into pancreatic endocrine IPCs cells and the gene expression profile that we examined in WB-1 cells become identical to that of mature β-cell line INS-1 cells after transplantation into diabetic mice.
FIG. 6.
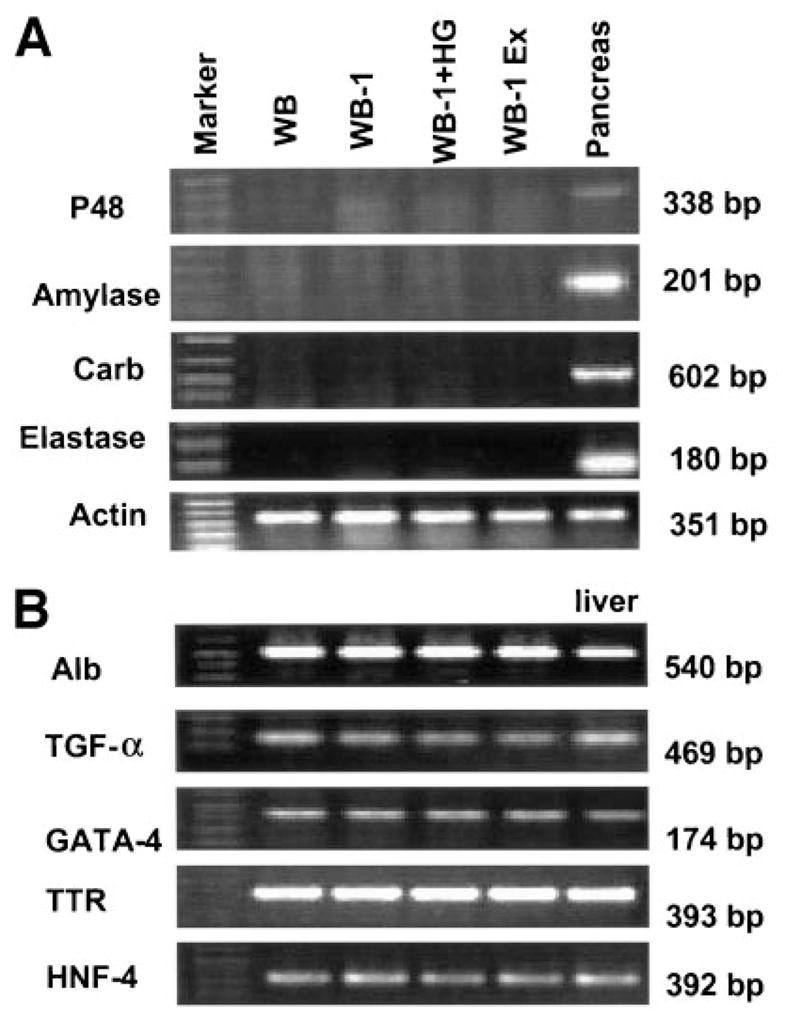
Gene expression of pancreatic exocrine (A) and hepatic markers (B) by RT-PCR. Gene expression studies were performed on WB cells, WB-1 (newly generated), high-glucose–cultured WB-1 cells (WB-1 + HG), and 40-day explanted WB-1 cells (WB-1 Ex), as well as rat liver and pancreas as positive controls. Pancreatic exocrine genes include p48, amylase, carboxypeptidase, and elastase. The liver genes include three transcription factors (TGF-α, GATA-4, and HNF-4) and two genes of liver functional proteins (albumin [Alb] and transthyretin [TTR]).
Analysis of insulin protein and insulin secretory granules
To determine if the in vitro– differentiated WB-1 cells process proinsulin to insulin and if there are any insulin secretory granules present in these cells, we performed Western blotting to detect mature insulin and electron microscopy studies combined with immunogold labeling with anti-insulin antibody to detect insulin secretory granules. WB-1 cells were continuously cultured in high-glucose medium for further differentiation and maturation. The presence of mature insulin in high-glucose–cultured WB-1 cells was evaluated after the cells became glucose responsive to release insulin, as detected by ELISA. As indicated in Fig. 7A, WB-1 cells in high glucose culture (lane 2) produce mature insulin (arrow), compared with the positive control INS-1 (823/13) cells (lane 4). No insulin was detected in immature WB-1 (lane 3) or WB (lane 1) cells. At the ultrastructural level, these cultured mature WB-1 cells show scattered cytoplasmic globular structures containing insulin molecules, which were confirmed by immunogold-labeled anti-insulin antibody. As shown in Fig. 7B, insulin-containing electron dense granules were present in mature WB-1 cells (left) and were similar to that seen in β-cells (right). These results indicate that cultured mature WB-1 cells can indeed process insulin and form insulin secretory granules.
FIG. 7.
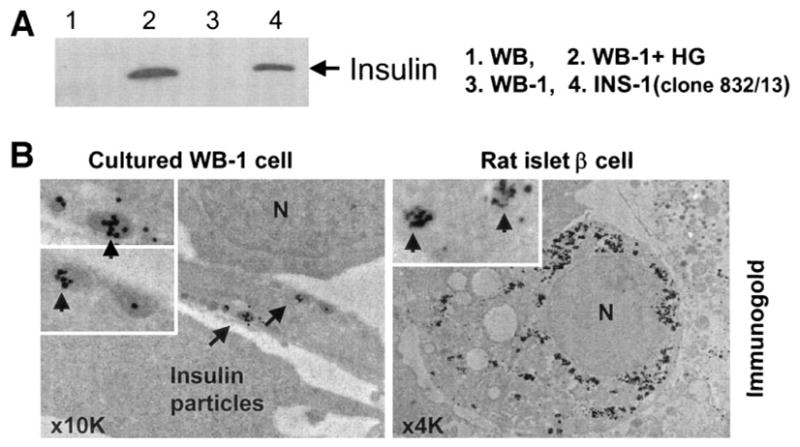
Detection of insulin and insulin secretory granules. A: Mature insulin was detected in the differentiated WB-1 cells (lane 2) by Western blot with anti-insulin antibody (1:500; Santa Cruz). All lanes were loaded with 50 μg protein except for INS-1 (loading only one-tenth protein [5 μg] to the well). B: Insulin secretory granules were detected by electron microscopy combined with immunogold labeling using anti-insulin antibody in an in vitro–differentiated WB-1 cell (B, left). Rat islet β-cells serve as the positive control (right). N represents the nucleus, and arrows indicate immunogold-labeled insulin particles.
DISCUSSION
We have successfully generated stably transfected WB rat liver cell lines expressing an activated form of Pdx1 and containing the reporter construct RIP-eGFP. The parental WB cells are derived from a normal rat adult liver and are believed to represent rat liver epithelial stem-like cells, having the capacity to differentiate into both hepatocytes and bile ductal cells (10,11). The WB-1 cell line has a gene expression profile resembling endocrine pancreatic precursor cells and expresses several pancreatic transcription factors related to pancreatic endocrine development (HNF1, endogenous Pdx1, Ngn3, NeuroD/Beta2, NKx2.2, and NKx6.1) as well as numerous genes related to pancreatic endocrine function (insulin I and II, glucagon, Glut-2, GK, GLP-R, PC1/3, PC2, HK, and Chrom A. However, Pdx1-VP16 is unable to activate the late-stage pancreatic transcription factors Pax-4, Pax-6, Isl-1, and MafA as well as β-cell function–related genes SUR1, Kir6.2, SNAP25, and IAPP. Moreover, although the WB-1 cells express insulin mRNA, they do not process and secrete insulin upon stimulation with glucose. It is possible that the lack of expression of Pax-4, Pax-6, Isl-1, SUR1, Kir6.2, SNAP25, and IAPP in early WB-1 cells may explain the results of the glucose insensitivity, since these genes are essential for late stages of β-cell differentiation and maintenance of mature β-cell function. In searching for external factors that can promote further differentiation of the WB-1 cells, we found that high-glucose environments (both in vitro and in vivo) are sufficient to induce the WB-1 cells to become fully functional IPCs.
Cell transplantation into diabetic NOD-scid mice
Although the WB-1 cells do not behave like mature β-cells, we found that they were able to differentiate further when transplanted into diabetic NOD-scid mice. Interestingly, the WB-1 cells were indeed capable of normalizing blood glucose levels and restoring weight loss in these mice, indicating that, in vivo, the implanted cells are fully functional. This pattern of reduction of blood glucose levels with WB-1 cells is similar to that seen with INS-1 cells, except that the INS-1 cells show a more rapid effect on glucose reduction. Because the INS-1 cells are fully mature β-cells, the difference between the effects of WB-1 and INS-1 cells is most likely due to the time needed for the WB-1 cells to differentiate and mature. Furthermore, upon removal of the implanted WB-1 cells, a persistent hyperglycemia returned, confirming that the implanted WB-1 cells were indeed responsible for the reduction of blood glucose levels. In contrast to the results seen with WB-1 and INS-1 cells, WB cells had no effect on blood glucose levels.
From day 57 posttransplantation and lasting to the end of the 4-month observation period, WB-1 cell mice maintained perfect euglycemia (70 –100 mg/dl), whereas INS-1 cell mice became hypoglycemic (30 – 40 mg/dl). This difference may be due to uncontrolled proliferation or unregulated insulin release from INS-1 cells, since these cells constitutively express insulin. In contrast, WB-1 cells are able to respond to blood glucose levels in a regulated manner similar to normal β-cells. Indeed, examination of the explanted tissue from both WB-1– and INS-1–implanted mice did not reveal any differences in the appearance of these cells, but more tissue mass was seen in the INS-1 cells.
These data indicate that in vivo hyperglycemia is a powerful factor in promoting pancreatic precursor cells to differentiate into mature β-like cells; indeed, liver-derived IPCs are able to function like pancreatic β-like cells and maintain long-term euglycemia without exocrine differentiation. The in vivo maturation of the implanted cells may be explained by at least two possible mechanisms: 1) a diabetic microenvironment such as hyperglycemia promotes immature cell differentiation and maturation, and 2) subrenal capsular implantation creates cell-cell contact in three-dimensional environments to allow cell– extracellular matrix interaction, promoting cell maturation and insulin production.
Novel points from gene expression studies
Little is known about the molecular mechanism of Pdx1-induced transdifferentiation from liver to pancreatic endocrine cells. By examining the expression of numerous pancreatic transcription factors, we found that there are some important differences in the sequence of gene activation between normal pancreas development and Pdx1-induced transdifferentiation of liver cells. For example, a recent review by Wilson et al. (15) describes that Nkx6.1 is positioned as an immediate downstream target gene of Pax4 during embryogenesis. We find, however, that Nkx6.1 can be activated in the absence of Pax4, suggesting that Nkx6.1 may act upstream of Pax4. Interestingly, Pax4, along with the other transcription factors Pax6, Isl-1, and MafA and β-cell function–related genes SUR1, Kir6.2, SNAP25, and IAPP, did become activated in WB-1 cells after transplantation into diabetic mice. Other genes such as Nkx2.2, insulin II, GLP-1R, GK, and PC2 also showed increased expression in WB-1 cells after transplantation. In contrast, Ngn3 became undetectable after transplantation, consistent with the notion that Ngn3 is transiently expressed in early pancreatic precursor cells. Another interesting observation is GK and HK gene expression during the WB-1 cell in vivo maturation. In pancreatic β-cells, glucose is phosphorylated to glucose-6-phosphate by GK, whereas in most other cells (e.g., liver cells), the process is mediated by HK (19,20). The comparison of gene expression between GK and HK in various stages of the WB-1 cells suggests that as WB-1 cells become more differentiated, GK expression increases. Conversely, HK gene expression appears to decrease as the WB-1 cells differentiate and mature. The ratio of GK/HK increases at the transcriptional level, as the WB-1 cells become mature. These results indicate that the in vivo diabetic environment plays a key role in the differentiation and maturation of WB-1 cells into fully functional pancreatic endocrine cells.
Selective pancreatic endocrine differentiation
Several groups have overexpressed Pdx1 protein in the liver of STZ-induced diabetic mice but have not succeeded in producing selective pancreatic endocrine differentiation (4,6,7,21). Instead, severe hepatitis, presumably caused by the production of exocrine enzymes such as amylase, elastase, and chymotrypsin (7), and dysmorphogenesis, such as abnormal lobe structures and multiple cystic lesions (21), were recently reported. The conclusion drawn from these in vivo animal studies is that ectopic expression of Pdx1 alone in the liver initiates both endocrine and exocrine pancreas differentiation and is insufficient to induce selective endocrine pancreas differentiation. Recent work by Zalzman et al. (22) has shown that introduction of Pdx1 into human fetal liver progenitor cells by lentivirus resulted in the expression of Ngn3, Beta2, and Nkx6.1 genes, but not Nkx2.2, Isl-1, Pax4, and Pax6 genes and was able to rescue diabetic mice, although a complete analysis was not performed. This observation is consistent with our unpublished data (manuscript in preparation) obtained with WB cells transduced with Pdx1 alone by the lentiviral vector. In contrast to the results with Pdx1 alone, we found that Pdx1-VP16 does not induce exocrine pancreas differentiation in WB-1 cells, as demonstrated by no detectable expression of early and late (p48, amylase, elastase, and carboxypeptidase) genes or amylase protein. These differences suggest that Pdx1 and Pdx1-VP16 have different activities when overexpressed in mature liver cells. It is possible that Pdx1 and Pdx1-VP16 interact with different protein partners in hepatic cells leading to the activation of different target genes (23–25).
Another interesting observation is that we consistently detected glucagon gene expression by RT-PCR, but we failed to detect glucagon protein expression. The same was true in the well-characterized pure rat insulinoma INS-1 cells (823/13). The glucagon gene was strongly expressed in INS-1 cells, but no glucagon-positive cells were detected by immunohistochemistry. The most likely explanation is that some WB-1 cells are still in the precursor stage. It is well documented that during pancreatic endocrine β-cell development, the β-cell precursors express both glucagon and insulin (26). Although in vivo transplantation promotes precursor WB-1 cells to further differentiate into mature cells, it is quite safe to assume that not all of the implanted WB-1 cells simultaneously mature to IPCs. Some WB-1 cells apparently maintain their precursor state. This assumption is supported by our observation of persistent euglycemia in the WB-1 cell–transplanted diabetic NOD-scid mice. The euglycemic stage in these mice lasted until the end of the observation (4 months). The data from animal experiments suggest that some transplanted WB-1 cells function like precursor cells. These cells respond to changes in glucose levels and allow a certain number of stem-like cells to undergo differentiation. At the same time, they duplicate themselves to maintain the number of pancreatic stem-like cells. This would explain why we detect not only many late-stage genes related to β-cell function, but also genes representing precursor cell features in a pooled sample. The reason we could not detect glucagon-expressing stem-like WB-1 cells is most likely due to both the low level of glucagon protein as well as low sensitivity of immunohistochemistry.
The same explanation can also apply to the phenomenon that albumin gene expression was detected, but no albumin protein was detected. As expected, when a WB cell undergoes a complete transdifferentiation into a pancreatic β-cell, the liver genes should be either downregulated or shut off. However, when a stem-like WB-1 cell gives rise to its progenies, it may undergo an asymmetric division, like all stem cells do, to duplicate itself and to give a daughter cell, allowing complete differentiation to take place. Therefore, the liver gene expression we detected by RT-PCR in the explanted WB-1 cells is probably due to residual precursor WB-1 cells that still maintain low levels of liver cell activity.
Effects of high glucose or hyperglycemia on cell differentiation
We have previously demonstrated that culture of hepatic stem cells and bone marrow– derived stem cells in high-glucose medium is critical to their transdifferentiation into insulin-producing cells (3,14). Our current study with WB-1 cells showed no detectable insulin protein or glucose-responsive insulin release even though the insulin genes were activated. Our results demonstrated that in vivo hyperglycemia or in vitro high-glucose culture is critical for promoting WB-1 cells to undergo further differentiation into functional β-like IPCs. Although the molecular mechanism of high-glucose–induced differentiation of stem/precursor cells into insulin-producing cells is unclear, it is well known that glucose is a growth factor for β-cells (26). It promotes β-cell replication in vitro and in vivo at the 20- to 30-mmol/l concentration (27). Those observations have been supported by two recent studies. Zalzman et al. (22) demonstrated that high-glucose (25 mmol/l) culture of immortalized Pdx1-expressing human fetal hepatocytes promoted the production, storage, and release of insulin in a regulated manner. Recently, Kojima et al. (28) showed that hyperglycemia produced by intraperitoneal injection of glucose into non-diabetic mice led to the appearance of proinsulin and somatostatin co-positive cells in the liver within 3 days, and mature insulin-positive cells were observed by day 15.
The results of our study demonstrate that hyperglycemic conditions in vivo turn on Pax4, Pax6, Isl-1, and MafA (late-stage genes in the developing endocrine pancreas) as well as β-cell function–related genes SUR1, Kir6.2, SNAP25, and IAPP, shut off Ngn3 expression, and upregulate multiple genes (Nkx2.2, GK, insulin II, PC2, and GLP-1R) that are related to glucose regulation in WB-1 pancreatic precursor cells. Based on these observations, we conclude that high glucose is a key factor in promoting the differentiation of WB-1 cells into functional IPCs. A detailed study of the molecular mechanism of the effect of a high-glucose condition on cell differentiation requires further investigation.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (M.E.H.) and by grants DK063270, DK068031, and K08-DK064054 (L.-J.Y.) from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health.
We thank Christopher Wright for the Pdx1 antibody, Christopher B. Newgard for the rat INS-1 cell line (clone 832/13), the University of Florida electron microscopy lab for technical support, and Kirsten Madsen for critical reading of the manuscript. We particularly thank Dr. Mark A. Atkinson for his unfailing support and encouragement.
Glossary
- ELISA
enzyme-linked immunosorbent assay
- GK
glucokinase
- H&E
hematoxylin and eosin
- HK
hexokinase
- IPC
insulin-producing cell
- RIP-eGFP
rat insulin-1 promoter– enhanced green fluorescence protein
- STZ
streptozotocin
Footnotes
Additional information for this article can be found in an online appendix at http://diabetes.diabetesjournals.org.
References
- 1.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871– 881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 2.Rao MS, Reddy JK. Hepatic transdifferentiation in the pancreas. Semin Cell Biol. 1995;6:151–156. doi: 10.1006/scel.1995.0021. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 5.Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 6.Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278:31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 7.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596– 603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 8.Kessler DS. Siamois is required for formation of Spemann’s organizer. Proc Natl Acad Sci U S A. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grisham JW, Coleman WB, Smith GJ. Isolation, culture, and transplantation of rat hepatocytic precursor (stem-like) cells. Proc Soc Exp Biol Med. 1993;204:270–279. doi: 10.3181/00379727-204-43663. [DOI] [PubMed] [Google Scholar]
- 10.Couchie D, Holic N, Chobert MN, Corlu A, Laperche Y. In vitro differentiation of WB-F344 rat liver epithelial cells into the biliary lineage. Differentiation. 2002;69:209–215. doi: 10.1046/j.1432-0436.2002.690414.x. [DOI] [PubMed] [Google Scholar]
- 11.Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, Grisham JW. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo: differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- 12.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel– dependent and –independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424– 430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 13.Yang LJ, Rhee SG, Williamson JR. Epidermal growth factor-induced activation and translocation of phospholipase C-gamma 1 to the cytoskeleton in rat hepatocytes. J Biol Chem. 1994;269:7156–7162. [PubMed] [Google Scholar]
- 14.Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech Dev. 2003;120:65– 80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 16.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 17.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 18.Knaack D, Fiore DM, Surana M, Leiser M, Laurance M, Fusco-DeMane D, Hegre OD, Fleischer N, Efrat S. Clonal insulinoma cell line that stably maintains correct glucose responsiveness. Diabetes. 1994;43:1413–1417. doi: 10.2337/diab.43.12.1413. [DOI] [PubMed] [Google Scholar]
- 19.Efrat S. Regulation of insulin secretion: insights from engineered beta-cell lines. Ann N Y Acad Sci. 2004;1014:88–96. doi: 10.1196/annals.1294.009. [DOI] [PubMed] [Google Scholar]
- 20.Matschinsky F, Liang Y, Kesavan P, Wang L, Froguel P, Velho G, Cohen D, Permutt MA, Tanizawa Y, Jetton TL. Glucokinase as pancreatic beta cell glucose sensor and diabetes gene. J Clin Invest. 1993;92:2092–2098. doi: 10.1172/JCI116809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, Umayahara Y, Watada H, Yamasaki Y, Magnuson MA, Miyazaki J, Hori M. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun. 2003;310:1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- 22.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann RS, Affolter M. Hox proteins meet more partners. Curr Opin Genet Dev. 1998;8:423– 429. doi: 10.1016/s0959-437x(98)80113-5. [DOI] [PubMed] [Google Scholar]
- 24.Dutta S, Gannon M, Peers B, Wright C, Bonner-Weir S, Montminy M. PDX:PBX complexes are required for normal proliferation of pancreatic cells during development. Proc Natl Acad Sci U S A. 2001;98:1065–1070. doi: 10.1073/pnas.031561298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goudet G, Delhalle S, Biemar F, Martial JA, Peers B. Functional and cooperative interactions between the homeodomain PDX1, Pbx, and Prep1 factors on the somatostatin promoter. J Biol Chem. 1999;274:4067– 4073. doi: 10.1074/jbc.274.7.4067. [DOI] [PubMed] [Google Scholar]
- 26.Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–219. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 27.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci U S A. 2004;101:2458–2463. doi: 10.1073/pnas.0308690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.