Abstract
Resistance to insecticides is one interesting example of a rapid current evolutionary change. DNA variability in the voltage-gated sodium channel gene (trans-membrane segments 5 and 6 in domain II) was investigated in order to estimate resistance evolution to pyrethroid in codling moth populations at the World level. DNA variation among 38 sequences revealed a unique kdr mutation (L1014F) involved in pyrethroid resistance in this gene region, which likely resulted from several convergent substitutions. The analysis of codling moth samples from 52 apple orchards in 19 countries using a simple PCR-RFLP confirmed that this kdr mutation is almost worldwide distributed. The proportions of kdr mutation were negatively correlated with the annual temperatures in the sampled regions. Homozygous kdr genotypes in the French apple orchards showed lower P450 cytochrome oxidase activities than other genotypes. The most plausible interpretation of the geographic distribution of kdr in codling moth populations is that it has both multiple independent origins and a spreading limited by low temperature and negative interaction with the presence of alternative resistance mechanisms to pyrethroid in the populations.
Introduction
The codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae), is one of the major insect pests in the orchards (mainly apple, pear and walnut orchards), worldwide distributed in the temperate regions [1]. Chemical insecticides remain the major means used to maintain populations of this pest at a low level. As a consequence of these treatments, C. pomonella developed resistance to numerous insecticides in Australia [2], Americas [3], [4], [5], and Eurasia [6], [7] including resistance to synthetic pyrethroids [8], [9].
Resistance to pyrethroids is mainly conferred by modification of their primary target site: the voltage-gated sodium channel [10]. Computer-generated 3D models characterized a small number of mutations linked to insecticide-binding sites in the voltage-gated sodium channel, most of them being in the trans-membrane segments 4 to 6 of the domain II region of the protein [11]. L1014F and M918T originally found in the housefly and respectively referred to as kdr and super-kdr mutations [12] are two of the most common of these mutations in insects [13]. The kdr mutation is associated to moderate resistance to DTT and pyrethroids. The super-kdr mutation is usually linked to the kdr mutation and increases by tenfold primary pyrethroid resistance due to kdr [13], [14]. The L1014F mutation is the only voltage-gated sodium channel mutation reported so far in the codling moth [15]. It was detected in few populations over the World [8], [16], [17]. A resistance ratio of about 80-fold to the pyrethroid insecticide deltamethrin is conferred by this recessive mutation in first-instar codling moth larvae [18], [19]. A low level of pyrethroid resistance in the codling moth is also attributed to enhanced detoxification activity notably due to the P450 cytochrome oxidases [18], [20].
The evolution of resistance in insect pest populations depends on both historic and current selective processes that should be understood to manage resistance [21]. To shed light on the evolutionary processes linked to the evolution of pyrethroid resistance in C. pomonella populations, we analysed genetic variations at the para sodium channel gene. We first report countries over the World where kdr resistance has been observed to establish origins of resistance alleles and identify factors that may affect their global spreading. Secondly we present a more detailed analysis on populations from South-eastern France to document the impact of local pyrethroid treatments on resistance evolution at the population level.
Materials and Methods
Sampling
The evolution of pyrethroid resistance conferred by the sodium channel gene was investigated on codling moth samples from 52 different apple commercial orchards in 19 countries (Table 1). Codling moths were collected as diapausing larvae using corrugated cardboard traps wrapped around the trunks of apple tree. Among these 52 codling moth population samples, seven populations were previously studied [16], [17]. Codling moth populations from 21 orchards showing high larva density in South-eastern France [22] were further analysed to determine the impact of current pyrethroid treatments and interaction between resistance mechanisms on genetic variation in the para sodium channel gene (Table 2). Pyrethroid treatments in these French orchards encompassed mainly class II pyrethroids (esfenvalerate, fluvalinate, deltamethrine).
Table 1. Origins of the codling moth samples, meteorology characteristic (annual mean of the daily minimal temperature in celsius degree, annual number of freezing days) and proportions in each country of alleles (77 and 112) and homozygous genotypes (77/77) detected with the PCR-RFLP test [16].
| Country | Year | Minimal Temperature | Freezing days | N | n | 112 | 77 | 77/77 |
| Armeniaa | 2002 | n.a. | n.a. | 30 | 1 | 0.00 | 1.00 | 1.00 |
| Argentinab | 2005 | 7.5 | 45 | 24 | 1 | 0.23 | 0.54 | 0.25 |
| France | 2006 | 9.1 | 56 | 771 | 21 | 0.40 | 0.24 | 0.08 |
| New Zealandb | 2005 | 8.7 | 5 | 18 | 1 | 0.61 | 0.17 | 0.00 |
| Turkey | 2010 | 11.8 | 0 | 42 | 1 | 0.56 | 0.14 | 0.05 |
| USA | 2008 | 1.3 | 177 | 217 | 5 | 0.54 | 0.09 | 0.01 |
| Bulgaria | 2007 | 5.3 | 100 | 60 | 3 | 0.54 | 0.06 | 0.02 |
| Uruguayb | 2005 | 11.8 | 0 | 17 | 1 | 0.32 | 0.06 | 0.00 |
| Switzerlanda | 2003 | 7.2 | 51 | 35 | 1 | 0.67 | 0.01 | 0.00 |
| Spain | 2007 | 10.2 | 21 | 28 | 2 | 0.60 | 0.00 | 0.00 |
| Italy | 2007 | 7.9 | 11 | 329 | 5 | 0.36 | 0.00 | 0.00 |
| Poland | 2009 | 6.1 | 64 | 10 | 1 | 0.61 | 0.00 | 0.00 |
| Czech Republic | 2005 | 4.2 | 109 | 31 | 1 | 0.68 | 0.00 | 0.00 |
| Greece | 2006 | 8.9 | 41 | 20 | 1 | 0.55 | 0.00 | 0.00 |
| Syria | 2006 | 14.3 | 0 | 53 | 2 | 1.00 | 0.00 | 0.00 |
| South-Africab | 2005 | 12.1 | 0 | 13 | 1 | 0.72 | 0.00 | 0.00 |
| Morocco | 2010 | 13.1 | 0 | 30 | 2 | 0.45 | 0.00 | 0.00 |
| Algeria | 2010 | 0.1 | 28 | 28 | 1 | 0.86 | 0.00 | 0.00 |
| Chilea | 2005 | n.a. | n.a. | 28 | 1 | 0.70 | 0.00 | 0.00 |
N and n respectively indicate the number of individuals and the number of orchards analysed per country. 77 and 77/77 respectively correspond to kdr allele [16] and to homozygous kdr genotype [18]. No meteorological data were available for the Armenian and Chilean locations (n.a.).
These samples correspond to the population samples A, C and 11 that were analysed in [16].
These samples correspond to the population samples Ar2, NZ1, Ur2, SA1 that were analysed in [17].
Table 2. Proportions of alleles (112 and 77) and of homozygous genotypes (77/77), and expected and observed heterozygosities (HE/HO) in codling moth samples from 21 commercial apple orchards in South-eastern France.
| Orchard | N | Protection | Pyrethroid treatment | Cytochrom P450 activity | 112 | 77 | 77/77 | HE/HO |
| 154 | 36 | Conventional | 5 | 77 | 0.26 | 0.64 | 0.42 | 0.52/0.53 |
| 149 | 20 | Conventional | 4 | 687 | 0.43 | 0.38 | 0.10 | 0.66/0.60 |
| 122 | 37 | Conventional | 4 | 513 | 0.18 | 0.23 | 0.05 | 0.57/0.57 |
| 75 | 43 | Conventional | 4 | 328 | 0.48 | 0.17 | 0.02 | 0.63/0.67 |
| 65 | 40 | Conventional | 4 | 652 | 0.48 | 0.14 | 0.02 | 0.61/0.58 |
| 55 | 39 | Conventional | 3 | 635 | 0.45 | 0.24 | 0.10 | 0.65/0.56 |
| 68 | 21 | Conventional | 3 | 507 | 0.60 | 0.10 | 0.00 | 0.55/0.57 |
| 132 | 49 | Conventional | 3 | 862 | 0.46 | 0.06 | 0.00 | 0.56/0.67 |
| 35 | 33 | Conventional | 2 | n.a. | 0.41 | 0.38 | 0.15 | 0.65/0.70 |
| 84 | 23 | Conventional | 2 | 141 | 0.30 | 0.37 | 0.04 | 0.68/0.87 |
| 17 | 27 | Conventional | 2 | 542 | 0.30 | 0.35 | 0.11 | 0.56/068 |
| 140 | 36 | Conventional | 2 | 208 | 0.32 | 0.31 | 0.11 | 0.67/0.61 |
| 134 | 16 | Conventional | 2 | 340 | 0.31 | 0.16 | 0.00 | 0.62/0.81 |
| 10 | 29 | Conventional | 1 | 406 | 0.57 | 0.17 | 0.03 | 0.59/0.66 |
| 42 | 43 | Conventional | 1 | n.a | 0.52 | 0.14 | 0.02 | 0.60/0.56 |
| 145 | 41 | Organic | 0 | 188 | 0.28 | 0.45 | 0.22 | 0.65/0.63 |
| 51 | 59 | Organic | 0 | 32 | 0.38 | 0.25 | 0.05 | 0.66/0.66 |
| 125 | 52 | Organic | 0 | 70 | 0.41 | 0.23 | 0.04 | 0.66/0.71 |
| 124 | 45 | Organic | 0 | 90 | 0.39 | 0.17 | 0.02 | 0.63/0.76 |
| 119 | 58 | Organic | 0 | 34 | 0.45 | 0.16 | 0.03 | 0.62/0.57 |
| 126 | 24 | Organic | 0 | 265 | 0.40 | 0.13 | 0.04 | 0.61/0.54 |
References to crop protection practices, numbers of annual pyrethroid treatments, and cytochrom P450 oxidase activity were reported for each orchard and linked population sample. N was the number of individuals analysed per orchard. HE and HO were calculated with all the three alleles (77, 101 and 112) detected with the PCR-RFLP. Values reported for cytochrom P450 oxidase activities were estimated as the average ECOD activity (pmol/min/individual) among the individuals collected at each orchard location. No ECOD measure was done on the individuals collected in orchards 35 and 134 (n.a.).
Detection of the Kdr Mutation
Total DNA was extracted from the head of each individual following Wash et al. [23] with 200 µl of 10% Chelex 100 (Biorad) solution and 6 µl (10 mg/ml) of proteinase K (Eurobio). Tissues were digested over night at 56°C. After boiling for 30 minutes, supernatant was used as DNA template for PCR reaction. A PCR-RFLP test slightly modified from Franck et al. [16] was used to detect the kdr mutation. It was developed based on sequence variations in the para sodium channel gene of susceptible and deltamethrin resistant strains [15]. PCR amplifications were carried out with a Mastercycler thermocycler (Eppendorf) in a 25 µl reaction volume containing 1X reaction buffer (10 mM Tris-HCl, pH = 9, 50 mM KCl, 1.5 mM MgCl2, and 0.1 mg/ml Bovine Serum Albumin), 200 µM of each dNTPs, 0.4 µM of each CpNa-F and CpNa-R primers (Table 3), 1 unit of Go Taq DNA Polymerase (Promega) and 2 µl of DNA template. The PCR conditions were: 3 minutes at 94°C followed by 35 cycles at 94°C for 30s, 55°C for 1 min, and 72°C of elongation for 1 min with a final extension step at 72°C for 2 min. PCR products were digested at 65°C for 16 hours with 2 unit of Tsp509I endonuclease and 1X of NEB1 Buffer (New England Biolabs) in 30 µl of reaction volume. Digested products were separated by electrophoresis on 6.5% polyacrylamide denaturing gel in a Li-Cor 4200 automatic DNA sequencer. The longest digested DNA fragments in acrylamide gels were visualised using 700 IRDye labelled CpNa-F primer with the SAGA software (Li-Cor Biosciences). Each revealed fragment length was defined as an allele. The test was performed on 1784 individuals: 165 individuals were re-analysed according to this modified protocol [16], [17] and 1619 individuals were newly investigated (Table 1).
Table 3. Primers used to amplify and to sequence domain II S4-S6 region of the codling moth para sodium channel gene.
| Primer | Sequence (5′-3′) |
| SKdr-F | GGCCGACACTTAATTTACTCATC |
| SKdr-R1 | TTCCCGAAAAGTTGCATACC |
| SKdr-R2 | GGGTTAACGAGCTAAACGTCCAA |
| SKdr-R3 | GCAATCCCACATGCTCTCTA |
| CpNa-F | TAGAGAGCATGTGGGATTGC |
| CpNa-R | AATTTCGTAGCCCTTGATCG |
| Kdr-F | GGTGGAACTTCACCGACTTC |
| Kdr-R | GCAAGGCTAAGAAAAGGTTAAG |
Detoxification Activity by the P450 Cytochrome Oxidases
Enhanced activity of the P450 cytochrome oxidases confers heritable metabolic resistance to pyrethroid insecticides [18]. We assessed the activity of the P450 cytochrome oxidases measuring 7-ethoxycoumarin-O-deethylation (ECOD) activity on 557 moths (out of 771) collected in 19 French orchards (out of 21) to shed light on putative interaction between this resistance mechanism and pyrethroid target mutations in the sodium channel gene. ECOD activity was individually measured on abdomen samples using 0.4 mM ethoxycoumarin in 100 µL Hepes buffer [16], [24]. After four hours of incubation at 30°C, the enzymatic reaction was stopped with 100 µL of glycine buffer (10−4 M), pH 10.4/ethanol (v/v) and fluorescence was measured with 380 nm excitation and 465 nm emission filters on a microplate reader (HTS 7000, Perkin Elmer). ECOD activity was estimated for each moth based on the amount of 7-hydroxycoumarine formed (pg/min).
DNA Sequencing in the Para Gene
DNA sequencing in the para gene (corresponding to trans-membrane segments 4 to 6 of the domain II region of the canal sodium protein, Figure 1) was performed on 50 codling moth individuals from various geographic origins and two Grapholita molesta (Lepidoptera: Tortricidae), collected in France and Brazil, to be used as an outgroup.
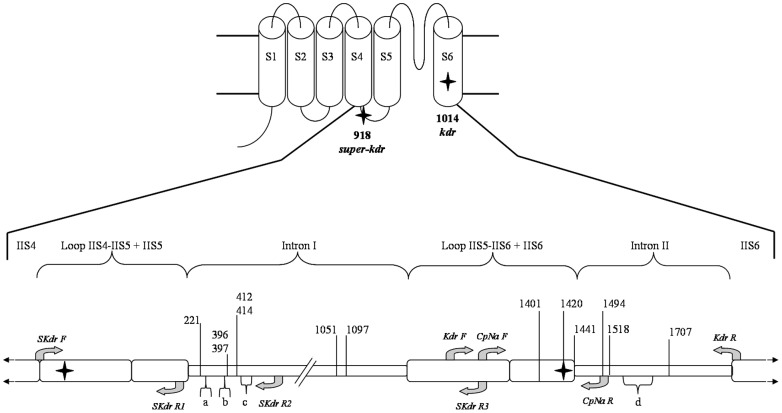
Figure 1. Trans-membrane segments in the domain II voltage-gated sodium channel with reference to codon position of the most frequent non-synonymous mutations and nucleotide variation in the codling moth sequences.
Polymorphic sites in the nucleotide sequences are indicated as numbers (13 substitutions) or letter (4 indels) using Syria1 sequence (1859 bp) as reference (Genbank accession number GU082334, Table 5). Arrows indicate the positions of the primers used for PCR-RFLP and sequencing analyses (Table 3).
The whole region was sequenced for 38 codling moths displaying different homozygous genotypes according to the PCR-RFLP test (Table 4). The whole region was amplified in two independents PCRs with respectively the SKdr-F/SKdr-R3 and the Kdr-F/Kdr-R primer pairs (Table 3). These PCR were performed in the same conditions as above but at annealing temperatures of 56°C and 54°C respectively. The PCR products were purified from agarose gel [25] then sequenced (GATC Biotech) using, as sequencing primers, SKdr-R1, SKdr-R2 and the four PCR primers described above (Table 3). For twelve additional codling moths collected in orchard treated with pyrethroid (Table 2), the first exon coding for the transmenbrane segments 4 and 5 was partially sequenced using the SKdrF and SKdr-R1 primers in order to check for the presence of the super-kdr mutation.
Table 4. Statistical results about six generalized linear models of the proportions of kdr allele and of kdr homozygote genotypes in the orchard population samples.
| Statistical models | Observed data | Statistical test | |||||
| Dependent variables | Covariables | Orchard origins | N | n | chi2 | (df) | P-value |
| Proportion of kdr allele | Pyrethroid treatments | France | 1114 | 19 | 0.16 | (1) | 0.687 |
| ECOD activity | 1.00 | (1) | 0.317 | ||||
| Pyrethroid × ECOD | 0.92 | (1) | 0.338 | ||||
| Proportion of kdr allele | Minimal temperature | World | 1830 | 30 | 5.05 | (1) | 0.025 |
| Proportion of kdr allele | Freezing days | World | 1830 | 30 | 4.57 | (1) | 0.033 |
| Proportion of kdr homozygote | Pyrethroid treatments | France | 557 | 19 | 0.09 | (1) | 0.768 |
| ECOD activity | 9.16 | (1) | 0.003 | ||||
| Pyrethroid × ECOD | 2.63 | (1) | 0.105 | ||||
| Proportion of kdr homozygote | Minimal temperature | World | 915 | 30 | 3.13 | (1) | 0.077 |
| Proportion of kdr homozygote | Freezing days | World | 915 | 30 | 3.54 | (1) | 0.060 |
N and n respectively indicate the number of individuals and the number of orchards observed for each statistical model.
Data Analysis
The genetic variation at the sodium channel detected with the PCR-RFLP test was first analysed. In each orchard population sample, observed (HO) and expected (HE) heterozygosities were calculated and departure of genotype frequencies from Hardy-Weinberg proportions tested using the Genepop software [26] considering either all the detected alleles or only the kdr and the susceptible allele groups. Generalised linear models were used (genmod procedure, SAS version 9.1) to explain the proportions of kdr allele, and of homozygous kdr genotypes in the orchard population samples. These proportions were modelled as binomial variables with a logit link function considering the orchard as a random variable. First, the proportions of kdr allele or of homozygous kdr genotypes were modelled for the French population samples as functions of two factors (ECOD activity in moths and number of pyrethroid treatments in orchard) and their interactions (Table 2). Second, the proportions of kdr allele or homozygous kdr genotypes were modelled considering all the sampled populations that displayed kdr polymorphism as functions of either the annual mean of the daily minimal temperatures or the annual number of freezing days at each orchard. Meteorological data were obtained from the National Climatic Data Center website (http://www.ncdc.noaa.gov/oa/ncdc.html) for each orchard location and sampling date.
Furthermore, sequence variation at the sodium channel gene was investigated. DNA sequences were manually aligned with the Bioedit software [27]. Recombination between DNA sequences was tested using six different methods implemented in the RDP3 software [28]: RDP [29], GENECONV [30], Chimaera [31], MaxChi [31], [32], BootScan [33], and SiScan [34]. Minimum evolution trees were computed with the MEGA software [35] using a p-distance between the sequences that takes into account both substitution and indel polymorphisms. To assess the reliability of the tree, standard error tests were performed for every interior branch by resampling variable sites (1,000 bootstraps).
Results
Detection and Distribution of the Kdr Mutation in Populations
To detect the kdr mutation (L1014F) in codling moth populations we amplified a 170 bp region with the CpNa-F and CpNa-R primers (Figure 1 and Table 3), then digested it with the Tsp509I endonuclease that specifically cut ↓AATT sites. Two out of five restriction sites in the 170 bp region were polymorphic (Figure 1, Table 4). This polymorphism was summed up by DNA fragments of three different detectable lengths (77, 101 and 112 bp), hereafter designed as three different alleles (Figure 2). Restriction at position 1417 generated the 77 bp fragment, which was interpreted as corresponding to the kdr allele [16]. Restrictions at positions 1441 and 1452 in intron II respectively generated 101 and 112 bp fragments, which were interpreted as corresponding to two different susceptible alleles.
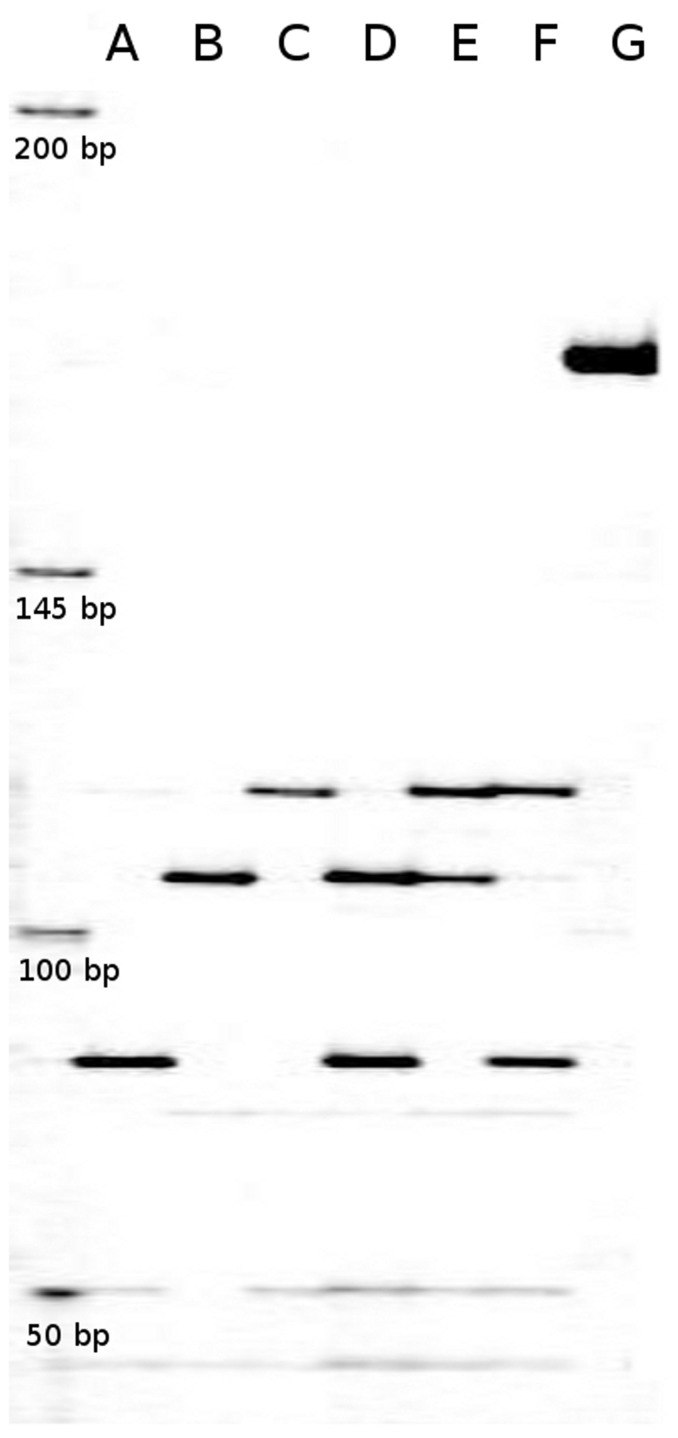
Figure 2. Detection of the kdr mutation by PCR-RFLP.
PCR were conducted with the CpNa-F and CpNa-R primers (Table 3), and then digested with Tsp509I (700 IRDye labelled CpNa-F primer). Lengths of the restricted fragments were determined by electrophoresis in a Li-Cor 4200 automatic DNA sequencer (6.5% polyacrylamide denaturing gel) and visualised using the SAGA software (Li-Cor Biosciences). The 77 bp fragment corresponds to the kdr allele. The 101 and 112 bp fragments correspond to two different susceptible alleles. A–F corresponds to the different PCR-RFLP genotypes: 77/77, 101/101, 112/112, 77/101, 101/112 and 77/112. G is the length of the non-restricted PCR product (170 bp). 50–350 pb sizing standard (Biosciences) is in the left well.
A total of 1784 codling moths were genotyped using this PCR-RFLP test (Table 1). The 77 allele was observed in population samples from all the continents except Africa and from 9 out of the 19 countries analysed. It was observed in all the samples from South-eastern France in variable proportions (Table 2). It was the only observed allele in the Armenian sample. The 101 and 112 alleles were both observed at relatively high proportions in all the other samples, except the one from Syria that was monomorphic for the 112 allele (Tables 1 and 2). No departure from Hardy-Weinberg equilibrium was detected in any sample from the 52 orchards analysed when the 101 and 112 susceptible alleles were grouped (Fisher’s exact test, P>0.28). Significant heterozygote excesses were detected in two French samples (Fisher’s exact test, P = 0.03 and P = 0.04 in the orchard sample 84 and 132, respectively) when all three alleles were considered (Table 2). The proportions of 77 allele and 77/77 genotype in the population samples from South-eastern France were slightly higher in orchards sprayed with pyrethroid insecticides (Tables 2 and 4), but these proportions did not depend on the number of pyrethroid treatments according to the generalized linear models (chi2<0.16, df = 1, P>0.68). A significant effect of ECOD activities was detected on the proportion of the homozygous 77/77 genotype (chi2 = 9.16, df = 1, P = 0.0025), but not on the proportion of 77 allele (chi2 = 1.00, df = 1, P = 0.317). Consistently, ECOD activities were lower in homozygous 77/77 genotypes (284 pg/min in average) than in other genotypes (337 pg/min in average). The proportions of the 77 allele and the 77/77 genotype were modelled according to climatic covariables using the 30 population samples over the World that displayed kdr polymorphism. The annual mean of the daily minimal temperatures and the annual number of freezing days in the sampled regions were highly correlated (number of freezing days = −16.6 minimal temperature +184, R2 = 0.897). Consequently, generalized linear models were performed independently with these two climatic variables (Table 4). The proportions of 77 allele (chi2 = 5.05, df = 1, P = 0.025) and, to a lesser extent, the proportions of 77/77 genotype (chi2 = 3.13, df = 1, P = 0.077) in the codling moth samples were positively correlated with temperature in the sampled regions. Consistently, very similar negative correlations were observed with the annual number of freezing days (Table 4).
DNA Sequencing in the Para Gene and Haplotype Divergences
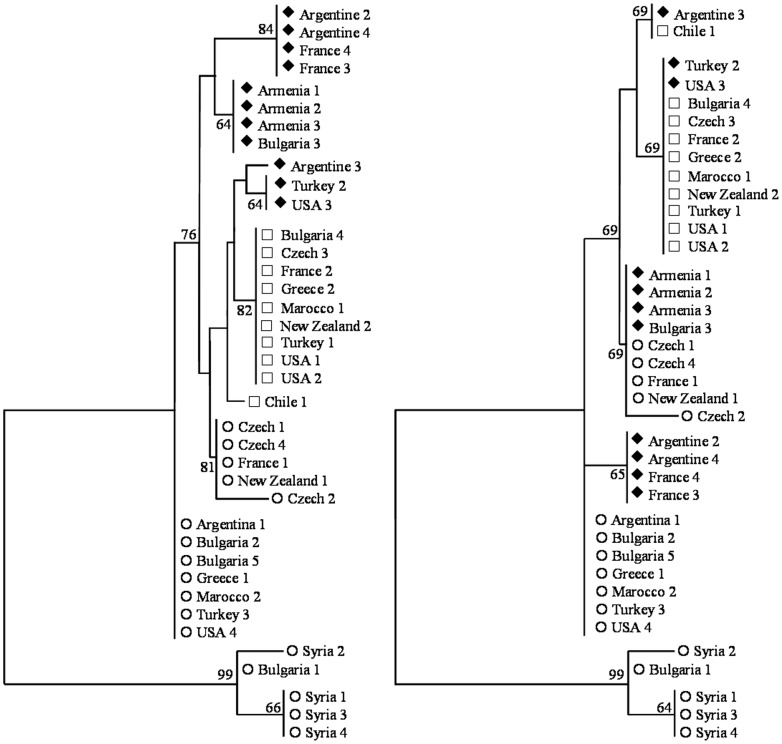
Partial DNA sequences of the para gene (domain II, trans-membrane segments 5 and 6, Figure 1) were obtained for two G. molesta (accession number GU082359 and GU082360) and 38 C. pomonella individuals (GU082334-GU082358 and JQ946336–JQ946348): These 38 sequenced codling moths displayed homozygous genotypes according to the PCR-RFLP test; eleven were 77/77, ten 101/101 and seventeen 112/112. Introns I and II were both shorter in G. molesta than in C. pomonella (Figure 3) and we were not able to align and compare G. molesta and C. pomonella intron sequences. Twelve different haplotypes were identified among the 38 codling moth sequences. The sequences differed by their lengths (1649 to 1756 bp) because of the presence of three indels in intron I and one in intron II (Figure 3, Table 5). In addition, 13 substitutions were observed. Only one substitution at position 1420 was non-synonymous (L1014F). All the eleven 77/77 genotypes sequenced displayed at this kdr locus the phenylalanine amino acid. No variation was detected in the first exon that encompasses the super-kdr locus (50 moths sequenced including 23 displaying the 77/77 genotype). No evidence of recombination was detected among the 12 observed haplotypes with any of the six recombination tests performed with the RDP3 package. Consequently, we did not take into account recombination in the phylogenetic analyses. Thirteen out of the 17 polymorphic sites in C. pomonella were parsimonious informative sites. Minimum evolution trees were computed with the p-distance between the C. pomonella sequences using polymorphism at all the variable sites or all the variable sites except the kdr mutation (Figure 4). Both data sets highly supported the presence of two main clades, which differed at 11 sites in average (approximately 0.7% divergence in their nucleotide sequences). The first clade encompassed three different 112 sequences from the Bulgarian and Syrian samples. The second clade encompassed nine different 77, 101 and 112 sequences from samples distributed all over the World. Sequences within this second clade were lastly structured according to their PCR-RFLP profiles and their geographic origin. The four different 77 sequences differed from each other at three informative sites (Table 4, positions 380, 1051 and 1441) and were distributed in a least two non related subclades in addition with 101 and 112 sequences respectively (Figure 4).
Figure 3. PCR product lengths obtained with the SKdr-F/SKdr-R3 (left) and the Kdr-F/Kdr-R (right) primer pairs, respectively.
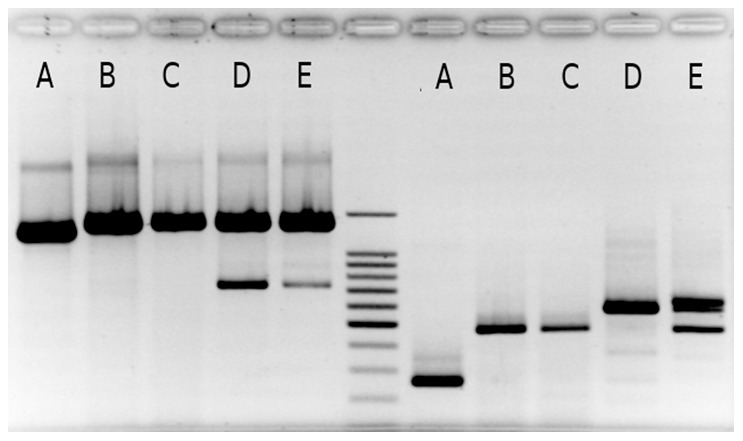
In Grapholita molesta, lengths were respectively 1245 and 283 bp (A). In Cydia pomonella, lengths were respectively 1363 and 460 pb in specimens from France (B), 1359 and 460 pb in specimens from USA (C), 1359 and 460 or 576 pb in specimens from Syria (D and E). The electrophoresis was run on a 2% agarose gel at 100 volts for 30 minutes using the RunOne™ Electrophoresis System (Embi Technology). 100 pb DNA ladder (Promega) was in the central well.
Table 5. Sequence variability in the codling moth voltage gated sodium channel gene with variable positions numbered according to Figure 1.
| Haplotype | 221 | 265 a | 380 b | 396 | 397 | 412 | 414 | 447 c | 1051 | 1097 | 1401 | 1420 kdr | 1441 | 1494 | 1518 | 1528 d | 1707 | PCR RFLP | IntronI | IntronII |
| Syria 1 | A | 0 | 0 | T | T | A | T | 0 | C | G | G | C | G | C | C | 0 | T | 112 | 1106 | 411 |
| Syria 2 | A | 0 | 0 | T | T | A | T | −34 | C | G | G | C | G | C | C | −116 | T | 112 | 1072 | 305 |
| Bulgaria 1 | A | 0 | 0 | T | T | A | T | 0 | C | G | G | C | G | C | C | −116 | T | 112 | 1106 | 305 |
| Armenia 1 | G | 0 | 0 | C | C | T | A | 0 | T | A | C | T | G | A | T | −116 | C | 77 | 1106 | 305 |
| Turkey 1 | G | 0 | 0 | C | C | T | A | 0 | T | A | C | C | A | A | T | −116 | C | 101 | 1106 | 305 |
| Turkey 2 | G | 0 | 0 | C | C | T | A | 0 | T | A | C | T | A | A | T | −116 | C | 77 | 1106 | 305 |
| Czech 1 | G | 0 | 0 | C | C | T | A | 0 | T | A | C | C | G | A | T | −116 | C | 112 | 1106 | 305 |
| Czech 2 | G | +7 | 0 | C | C | T | A | 0 | T | A | C | C | G | A | T | −116 | C | 112 | 1113 | 305 |
| Argentina 1 | G | 0 | 0 | C | C | T | A | 0 | C | A | C | C | G | A | T | −116 | C | 112 | 1106 | 305 |
| Argentina 2 | G | 0 | +4 | C | C | T | A | 0 | C | A | C | T | G | A | T | −116 | C | 77 | 1110 | 305 |
| Argentina 3 | G | 0 | 0 | C | C | T | A | 0 | C | A | C | T | A | A | T | −116 | C | 77 | 1106 | 305 |
| Chile 1 | G | 0 | 0 | C | C | T | A | 0 | C | A | C | C | A | A | T | −116 | C | 101 | 1106 | 305 |
Substitutions are indicated by the observed nucleotides and indels by the number of inserted or deleted base pairs using the Syria1 sequence as reference. Nucleotides in bold characters refer to Tsp509I restriction sites. The three last columns respectively refer to lengths in base pairs of the largest Tsp509I digested fragment, and of introns I and II. Twelve different haplotypes were recognized among 38 sequences. The distribution of these twelve haplotypes among countries was reported in figure 4.
Figure 4. Minimum evolution trees established with the p-distance among 38 partial DNA sequences of the codling moth para gene.
The trees were computed either with all the variable sites (left: 4 indels and 13 substitutions) or all the variable sites except the kdr locus (right: 4 indels and 12 substitutions). The 112 and 101 sequences (L1014) were represented by unfilled circles and squares, respectively. The 77 sequences (F1014) were represented by filled diamonds. Bootstraps values above 60% were reported.
Discussion
DNA sequences and SNPs analyses in a gene involved in insecticide resistance are complementary tools to shed light on recent evolutionary changes [13], [36]. Proximal evolutionary processes can be assessed by analysing the DNA sequence that contains the mutations involved in resistance. SNPs analyses are convenient molecular tools that allow following the dynamic of insecticide resistance in natural populations and understanding selective processes that may enhance or delay insecticide resistance evolution [21].
Although useful, SNP detection methods may fail to detect some genetic variations involved in insecticide resistance at a selected gene [37], [38]. The L1014F replacement in the voltage-gated sodium channel was primarily observed in a deltamethrin resistant codling moth strain [15]. In the present study, a rapid PCR-RFLP test was developed to monitor this kdr mutation in codling moth populations. However, at least two additional mutations were reported in insect pest populations at this locus: L1014S in Culex pipiens [39] and Anopheles gambiae [40] and L1014H in Heliothis virescens [41] and Musca domestica [42]. As for the F1014 variant, the S1014 but not the H1014 variants would have produced a 77 bp restriction fragment in C. pomonella with the developed PCR-RFLP test. However, it is unlikely that these two additional mutations are present in the codling moth: L1014F was the only non-synonymous variation in the para gene observed along trans-membrane segments 5 and 6 in domain II in individuals from various origins in the World. In absence of other proof, we assumed that L1014F was the only kdr mutation in this species.
A total of 1784 individuals collected all over the World were analysed using this PCR-RFLP test to estimate the distribution of kdr within and among populations. The 77 allele was observed almost worldwide and it is heterogeneously distributed among the codling moth populations. High proportions of 77/77 homozygous genotypes that are physiologically resistant to pyrethroid were only observed in Armenia, Argentina, Turkey and South-eastern France. These results confirm and extend previous observations [8], [16], [17], [43].
At the orchard level, neither kdr selection by current pyrethroid treatments nor kdr counter-selection in absence of pyrethroid treatment was evident. The proportions of kdr allele were not significantly correlated with the number of pyrethroid treatments in the French apple orchards. Distributions of kdr genotypes did not significantly depart from Hardy-Weinberg proportions in any orchard population samples. The low impact of pyrethroid treatments observed on kdr proportions at the orchard level seems to be a general feature whatever the within-year generation of the codling moth [44]. These results contrast with observations in Haematobia irritans or in Musca domestica populations that clearly showed seasonal variations in the proportions of kdr allele as a function of pyrethroid treatments [45], [46]. Three non exclusive hypotheses may explain such lack of structure of kdr in codling moth populations according to current insecticide applications. First, resistance management guidelines recommend alternation of pyrethroids with other insecticides among codling moth generations. Non-continuous use of pyrethroids largely limits the selection of sodium channel target mutations. This could explain why the super-kdr mutation is apparently absent in codling moth populations, a result also found in wild populations of horn flies [47]. Second, the usage of a large spectrum of insecticides selected various resistance mechanisms in the codling moth populations. Metabolic resistance associated with enhanced activity of the P450 cytochrome oxidases is largely spread over the World [16], [17] and confers cross-resistance to numerous insecticides including pyrethroids [8], [20]. In the present study, the activity of the P450 cytochrome oxidases was negatively correlated with the proportion of homozygous kdr genotypes. Metabolic resistance should be sufficient for the codling moth to resist pyrethroid treatments and it could limit selection of sodium channel target mutations in absence of strong pyrethroid selection [48]. Third, insignificant fitness cost associated with kdr was measured in laboratory codling moth strains [49], which could explain the maintenance of high kdr proportions in absence of pyrethroid selection as observed in populations from organic orchards in South-eastern France (the granulosis virus was the only insecticide used to control codling moth in these orchards). However, fitness costs are difficult to predict when several resistance mechanisms interact [50] and may depend on the environmental conditions [51].
The lack of population structure of the kdr genotypes detected at the orchard level contrasts with the high structure observed among populations from different orchards at larger geographic scale. First, the selection of different resistance mechanisms among countries can partially explain regional kdr structure. Interestingly, the Armenian populations which were the only populations that fixed the kdr allele in the present study were also those in which metabolic resistance conferred by the cytochrome P450 oxidases were insignificant [8], [16]. This is an additional argument supporting that kdr resistance evolved in interaction with other resistance mechanisms. Second, kdr proportions were negatively correlated with temperature in codling moth populations displaying kdr polymorphism in agreement with the hypothesis that fitness cost associated with sodium channel target mutations depends on temperature [51]. Consequently, fitness cost associated with kdr could be not equally distributed geographically and differently expressed along seasons in C. pomonella as previously noted in house fly populations [42], [52]. It is to note that such temperature-dependent cost could also explain the latitudinal variation in the proportion of kdr allele previously observed in codling moth populations from France [16]. Finally, in the absence of strong resistance cost, ancient insecticide treatments may explain current kdr distribution [53]. Resistance to DTT was reported in some codling moth populations in the early 1950s [54]. The first cases of resistance to pyrethroid in the 1990s in some codling moth populations from France were observed less than five years after the beginning of treatments with these insecticides [7]. DTT treatments may have initiated the selection of the kdr mutation in the codling moth, which was secondarily selected by pyrethroid as suggested by observations in Plutella xylostella [55]. Consequently, any differences among countries in DTT and pyrethroid usage during the last sixty years may also explain current differences in the proportions of kdr allele among codling moth populations at regional scales.
Variation in the para introns and exons was investigated in few insect pests – Bemisia tabaci [56], Musca domestica [42], Mysus persicae [57] and Anopheles gambiiae [40] – to shed light on the history of mutation events associated to insecticide resistance at this gene. In each species, several mutations, which likely arose independently in different populations, were linked with pyrethroid resistance. In contrast with these studies, only one substitution at the only kdr locus (L1014F) was observed in the codling moth. However, variations in para introns differentiated several haplotypes for both 1014L and 1014F variants. Phylogenetic relationships among these haplotypes confirmed the existence of two highly differentiated clades in C. pomonella, which is in agreement with previous analyses based on mitochondrial DNA sequences [58]. The four kdr haplotypes were all observed in only one clade (the most cosmopolitan one) but in several slightly differentiated sub-clades. In absence of recombination evidence, this result supports the hypothesis of independent and convergent L1014F mutation events. A subclade encompassed kdr haplotypes only observed in Armenia and Bulgaria, which suggests that one mutation event may have occurred somewhere in Eastern Europe. The origins of the kdr mutations observed in America and Western Europe remain more dubious. Among the two different kdr haplotypes observed in Argentina, one was identical to a French haplotype. This suggests that the kdr mutation in the Argentine population may have two independent origins and that one origin would be shared with the French population. This similarity supports the idea that in some situations local selection of kdr resistance may have followed dispersal events in C. pomonella as postulated to explain the unique origin of A2 esterase over-production in Culex pipiens [59]. Previous observations also pointed at the importance of commercial exchanges on the dispersal of codling moth larvae among continents [16]. To prevent the spread of insecticide resistance, pest control programs should think management both at local and global levels [21], [60].
Acknowledgments
We thank the EPI team for its help collecting codling moth larvae in the Basse-Durance Valley, France. We thank Maritza Reyes, Wilson Barros, Jim Walker, Claudio Ioriatti, Damian Gorzka, Costas Voudouris, John Margaritopoulos, Frantisek Marec, Oldrich Pultar, Alan Knight, Azya Ter-Hovhannesyan, Nadia Lombarkia, Salma Iraqui, Jesus Avilla, Marcella Rodriguez, Mohanad Ismail, and Recep Ay who kindly provided the codling moth samples from the other countries. We also thank Juliette Goussopoulos and Mathilde Willerval for their help extracting DNA and performing biochemistry analyses. Finally, we thank Benoît Moury for his suggestion about testing recombination and anonymous reviewers for their relevant comments and their corrections.
Funding Statement
This study was partially funded by the French program ECOGER “Ecco des vergers”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1. Shel’Deshova GG (1967) Ecological factors determining distribution of the codling moth Lapspeyresia pomonella L. in the northern and southern hemispheres. Entomological Review 46: 349–361. [Google Scholar]
- 2.Thwaite WG, Williams DG, Hately AM (1993) Extent and significance of Azinphos-Methyl resistance in codling moth in Australia. Pest Control and Sustainable Agriculture: 166–168.
- 3. Fuentes-Contreras E, Reyes M, Barros W, Sauphanor B (2007) Evaluation of azinphos-methyl resistance and activity of detoxifying enzymes in codling moth (Lepidoptera : Tortricidae) from central Chile. Journal of Economic Entomology 100: 551–556. [DOI] [PubMed] [Google Scholar]
- 4. Soleño J, Anguiano L, de D’Angelo AP, Cichon L, Fernandez D, et al. (2008) Toxicological and biochemical response to azinphos-methyl in Cydia pomonella L. (Lepidoptera : Tortricidae) among orchards from the Argentinian Patagonia. Pest Management Science 64: 964–970. [DOI] [PubMed] [Google Scholar]
- 5. Varela LG, Welter SC, Jones VP, Brunner JF, Riedl H (1993) Monitoring and characterization of insecticide resistance in codling moth (Lepidoptera, Tortricidae) in 4 Western States. Journal of Economic Entomology 86: 1–10. [Google Scholar]
- 6. Reuveny H, Cohen E (2004) Evaluation of mechanisms of azinphos-methyl resistance in the codling moth Cydia pomonella (L.). Archives Of Insect Biochemistry And Physiology 57: 92–100. [DOI] [PubMed] [Google Scholar]
- 7. Sauphanor B, Bovier JC, Brosse V (1998) Spectrum of insecticide resistance in Cydia pomonella (Lepidoptera : Tortricidae) in southeastern France. Journal of Economic Entomology 91: 1225–1231. [Google Scholar]
- 8. Reyes M, Franck P, Charlemillot P-J, Ioriatti C, Olivares J, et al. (2007) Diversity of insecticide resistance mechanisms and spectrum in European populations of the codling moth, Cydia pomonella . Pest Management Science 63: 890–902. [DOI] [PubMed] [Google Scholar]
- 9. Sauphanor B, Brosse V, Bouvier JC, Speich P, Micoud A, et al. (2000) Monitoring resistance to diflubenzuron and deltamethrin in French codling moth populations (Cydia pomonella). Pest Management Science 56: 74–82. [Google Scholar]
- 10. Zlotkin E (1999) The insect voltage-gated sodium channel as target of insecticides. Annual Review of Entomology 44: 429–455. [DOI] [PubMed] [Google Scholar]
- 11. O’Reilly AO, Khambay BPS, Williamson MS, Field LM, Wallace BA (2006) Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochemical Journal 396: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson MS, Martinez-Torres D, Hick CA, Castells N, Devonshire AL (1996) Analysis of sodium channel gene sequences in pyrethroid-resistant houseflies. In: Brown TM, editor. Molecular Genetics and Evolution of Pesticide Resistance. Washington: American Chemical Society. 53–61.
- 13. Soderlund DM, Knipple DC (2003) The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochemistry and Molecular Biology 33: 563–577. [DOI] [PubMed] [Google Scholar]
- 14. Vais H, Williamson MS, Devonshire AL, Usherwood PNR (2001) The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels. Pest Management Science 57: 877–888. [DOI] [PubMed] [Google Scholar]
- 15. Brun-Barale A, Bouvier J-C, Pauron D, Bergé J-B, Sauphanor B (2005) Involvement of a sodium channel mutation in pyrethroid resistance in Cydia pomonella L., and development of a diagnostic test. Pest Management Science 61: 549–554. [DOI] [PubMed] [Google Scholar]
- 16. Franck P, Reyes M, Olivares J, Sauphanor B (2007) Genetic architecture in codling moth populations: comparison between microsatellite and insecticide resistance markers. Molecular Ecology 16: 3554–3564. [DOI] [PubMed] [Google Scholar]
- 17. Reyes M, Franck P, Olivares J, Margaritopoulos J, Knight A, et al. (2009) Worldwide variability of insecticide resistance mechanisms in the codling moth, Cydia pomonella L. (Lepidoptera: Tortricidae). Bulletin of Entomological Research 99: 359–369. [DOI] [PubMed] [Google Scholar]
- 18. Bouvier JC, Bues R, Boivin T, Boudinhon L, Beslay D, et al. (2001) Deltamethrin resistance in the codling moth (Lepidoptera : Tortricidae): inheritance and number of genes involved. Heredity 87: 456–462. [DOI] [PubMed] [Google Scholar]
- 19. Sauphanor B, Brosse V, Monier C, Bouvier JC (1998) Differential ovicidal and larvicidal resistance to benzoylureas in the codling moth, Cydia pomonella . Entomologia Experimentalis et Applicata 88: 247–253. [Google Scholar]
- 20. Bouvier JC, Cuany A, Monier C, Brosse V, Sauphanor B (1998) Enzymatic diagnosis of resistance to deltamethrin in diapausing larvae of the codling moth, Cydia pomonella (L.). Archives of Insect Biochemistry and Physiology 39: 55–64. [Google Scholar]
- 21. Labbe P, Lenormand T, Raymond M (2005) On the worldwide spread of an insecticide resistance gene: a role for local selection. Journal of Evolutionary Biology 18: 1471–1484. [DOI] [PubMed] [Google Scholar]
- 22. Ricci B, Franck P, Toubon J-F, Bouvier J-C, Sauphanor B, et al. (2009) The influence of landscape on insect pest dynamics: a case study in southeastern France Landscape Ecology. 24: 337–349. [Google Scholar]
- 23. Walsh PS, Metzger DA, Higuchi R (1991) Chelex (R)100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10: 507. [PubMed] [Google Scholar]
- 24. De Sousa G, Cuany A, Brun A, Amichot M, Rahmani R, et al. (1995) A microfluorometric method for measuring Ethoxycoumarin-o-deethylase activity on individual Drosophila melanogaster abdomens: interest for screening resistance in insect populations. Analytical Biochemistry 229: 86–91. [DOI] [PubMed] [Google Scholar]
- 25.Joly E (1996) Purification of DNA fragments from agarose gels using glass beads. In: Harwood AJ, editor. Basic DNA and RNA Protocols. Berlin: Springer Verlag. 237–240. [DOI] [PubMed]
- 26. Rousset F (2008) Genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Molecular Ecology Resources 8: 103–106. [DOI] [PubMed] [Google Scholar]
- 27. Hall TA (1999) Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- 28. Heath L, van der Walt E, Varsani A, Martin DP (2006) Recombination patterns in aphthoviruses mirror those found in other picornaviruses. Journal of Virology 80: 11827–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin D, Rybicki E (2000) RDP: detection of recombination amongst aligned sequences. Bioinformatics 16: 562–563. [DOI] [PubMed] [Google Scholar]
- 30. Padidam M, Sawyer S, Fauquet CM (1999) Possible emergence of new geminiviruses by frequent recombination. Virology 265: 218–225. [DOI] [PubMed] [Google Scholar]
- 31. Posada D, Crandall KA (2001) Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proceedings of the National Academy of Sciences of the United States of America 98: 13757–13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maynard Smith J (1992) Analysing the mosaic structure of genes. Journal of Molecular Evolution 34: 126–129. [DOI] [PubMed] [Google Scholar]
- 33. Martin DP, Posada D, Crandall KA, C W (2005) A modified BOOTSCAN algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Research and Human Retroviruses 21: 98–102. [DOI] [PubMed] [Google Scholar]
- 34. Gibbs MJ, Armstrong JS, Gibbs AJ (2000) Sister-Scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 16: 573–582. [DOI] [PubMed] [Google Scholar]
- 35. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 36. Lynd A, Weetman D, Barbosa S, Egyir Yawson A, Mitchell S, et al. (2010) Field, genetic, and modeling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Molecular Biology and Evolution 27: 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, et al. (1998) Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. . Insect Molecular Biology 7: 179–184. [DOI] [PubMed] [Google Scholar]
- 38. Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, et al. (2000) Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Molecular Biology 9: 491–497. [DOI] [PubMed] [Google Scholar]
- 39. Martinez-Torres D, Chevillon C, Brun-Barale A, Berge JB, Pasteur N, et al. (1999) Voltage-dependent Na+ channels in pyrethroid-resistant Culex pipiens L mosquitoes. Pesticide Science 55: 1012–1020. [Google Scholar]
- 40.Pinto J, Lynd A, Vicente JL, Santolamazza F, Randle NP, et al. (2007) Multiple origins of knockdown resistance mutations in the Afrotropical mosquito vector Anopheles gambiae. Plos One 2. [DOI] [PMC free article] [PubMed]
- 41. Park Y, Taylor MFJ (1997) A novel mutation L1029H in sodium channel gene hscp associated with pyrethroid resistance for Heliothis virescens (Lepidoptera: Noctuidae). Insect Biochemistry and Molecular Biology 27: 9–13. [DOI] [PubMed] [Google Scholar]
- 42. Rinkevich FD, Zhang L, Hamm RL, Brady SG, Lazzaro BP, et al. (2006) Frequencies of the pyrethroid resistance alleles of Vssc1and CYP6D1 in house flies from the eastern United States. Insect Molecular Biology 15: 157–167. [DOI] [PubMed] [Google Scholar]
- 43. Voudouris CC, Sauphanor B, Franck P, Reyes M, Mamuris Z, et al. (2011) Insecticide resistance status of the codling moth Cydia pomonella (Lepidoptera: Tortricidae) from Greece. Pesticide Biochemistry and Physiology 100: 229–238. [Google Scholar]
- 44. Franck P, Timm AE (2010) Population genetic structure of Cydia pomonella: a review and case study comparing spatiotemporal variation. Journal of Applied Entomology 134: 191–200. [Google Scholar]
- 45. Cao XM, Song FL, Zhao TY, Dong YD, Sun CX, et al. (2006) Survey of deltamethrin resistance in house flies (Musca domestica) from urban garbage dumps in Northern China. Environmental Entomology 35: 1–9. [Google Scholar]
- 46. Guerrero FD, Alison MW, Kammlah DM, Foil LD (2002) Use of the polymerase chain reaction to investigate the dynamics of pyrethroid resistance in Haematobia irritans irritans (Diptera : Muscidae). Journal of Medical entomology 39: 747–754. [DOI] [PubMed] [Google Scholar]
- 47. Jamroz RC, Guerrero FD, Kammlah DM, Kunz SE (1998) Role of the kdr and super-kdr sodium channel mutations in pyrethroid resistance: correlation of allelic frequency to resistance level in wild and laboratory populations of horn flies (Haematobia irritans). Insect Biochemistry and Molecular Biology 28: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 48. Brooke BD (2008) kdr: can a single mutation produce an entire insecticide resistance phenotype? Transactions of the Royal Society of Tropical Medicine and Hygiene 102: 524. [DOI] [PubMed] [Google Scholar]
- 49. Boivin T, Chabert d’Hieres C, Bouvier JC, Beslay D, Sauphanor B (2001) Pleiotropy of insecticide resistance in the codling moth, Cydia pomonella . Entomologia Experimentalis et Applicata 99: 381–386. [Google Scholar]
- 50. Berticat C, Bonnet J, Duchon S, Agnew P, Weill M, et al. (2008) Costs and benefits of multiple resistance to insecticides for Culex quinquefasciatus mosquitoes. BMC Evolutionary Biolology 8: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Foster SP, Young S, Williamson MS, Duce I, Denholm I, et al. (2003) Analogous pleiotropic effects of insecticide resistance genotypes in peach-potato aphids and houseflies. Heredity 91: 98–106. [DOI] [PubMed] [Google Scholar]
- 52. Rinkevich FD, Hamm RL, Geden CJ, Scott JG (2007) Dynamics of insecticide resistance alleles in house fly populations from New York and Florida. Insect Biochemistry and Molecular Biology 37: 550–558. [DOI] [PubMed] [Google Scholar]
- 53. Davies ET, O’Reilly AO, Field LM, Wallace B, Williamson MS (2008) Perspective knockdown resistance to DDT and pyrethroids: from target-site mutations to molecular modelling. Pest Management Science 64: 1126–1130. [DOI] [PubMed] [Google Scholar]
- 54. Smith LC (1955) DDT-resistant codling moth. A report on the 1954/55 control trials. Journal of the Department of Agriculture, Victoria 59: 12–15. [Google Scholar]
- 55. Kwon DH, Choi BR, Park HM, Lee SH, Miyata T, et al. (2004) Knockdown resistance allele frequency in field populations of Plutella xylostella in Korea. Pesticide Biochemistry and Physiology 80: 21–30. [Google Scholar]
- 56. Alon M, Benting J, Lueke B, Ponge T, Alon F, et al. (2006) Multiple origins of pyrethroid resistance in sympatric biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochemistry and Molecular Biology 36: 71–79. [DOI] [PubMed] [Google Scholar]
- 57. Anstead JA, Williamson MS, Denholm I (2005) Evidence for multiple origins of identical insecticide resistance mutations in the aphid Myzus persicae . Insect Biochemistry and Molecular Biology 35: 249–256. [DOI] [PubMed] [Google Scholar]
- 58. Meraner A, Brandstätter A, Thaler R, Aray B, Unterlechner M, et al. (2008) Molecular phylogeny and population structure of the codling moth (Cydia pomonella) in Central Europe: I. Ancient clade splitting revealed by mitochondrial haplotype markers. Molecular Phylogenetics and Evolution 48: 825. [DOI] [PubMed] [Google Scholar]
- 59. Guillemaud T, Rooker S, Pasteur N, Raymond M (1996) Testing the unique amplification event and the wordwide migration hypothesis of insecticide resistance genes with sequence data. Heredity 77: 535–543. [DOI] [PubMed] [Google Scholar]
- 60. Denholm I, Devine GJ, Williamson MS (2002) Insecticide resistance on the move. Science 297: 2222–2223. [DOI] [PubMed] [Google Scholar]