Abstract
Objective
To estimate the effectiveness of anterior cervical discectomy with arthroplasty (ACDA) compared to anterior cervical discectomy with fusion (ACDF) for patient-important outcomes for single-level cervical spondylosis.
Data sources
Electronic databases (MEDLINE, EMBASE, Cochrane Register for Randomized Controlled Trials, BIOSIS and LILACS), archives of spine meetings and bibliographies of relevant articles.
Study selection
We included RCTs of ACDF versus ACDA in adult patients with single-level cervical spondylosis reporting at least one of the following outcomes: functionality, neurological success, neck pain, arm pain, quality of life, surgery for adjacent level degeneration (ALD), reoperation and dysphonia/dysphagia. We used no language restrictions. We performed title and abstract screening and full text screening independently and in duplicate.
Data synthesis
We used random-effects model to pool data using mean difference (MD) for continuous outcomes and relative risk (RR) for dichotomous outcomes. We used GRADE to evaluate the quality of evidence for each outcome.
Results
Of 2804 citations, 9 articles reporting on 9 trials (1778 participants) were eligible. ACDA is associated with a clinically significant lower incidence of neurologic failure (RR = 0.53, 95% CI = 0.37–0.75, p = 0.0004) and improvement in the Neck pain visual analogue scale (VAS) (MD = 6.56, 95% CI = 3.22–9.90, p = 0.0001; Minimal clinically important difference (MCID) = 2.5. ACDA is associated with a statistically but not clinically significant improvement in Arm pain VAS and SF-36 physical component summary. ACDA is associated with non-statistically significant higher improvement in the Neck Disability Index Score and lower incidence of ALD requiring surgery, reoperation, and dysphagia/dysphonia.
Conclusions
There is no strong evidence to support the routine use of ACDA over ACDF in single-level cervical spondylosis. Current trials lack long-term data required to assess safety as well as surgery for ALD. We suggest that ACDA in patients with single level cervical spondylosis is an option although its benefits and indication over ACDF remain in question.
Introduction
Rationale
Cervical spondylosis is a common cause of radiculopathy and/or myelopathy resulting in significant disability [1]. In patients that do not respond adequately to conservative management, anterior cervical discectomy with fusion (ACDF) is performed to achieve neural decompression, maintain cervical lordosis and provide segmental stabilization. ACDF halts neurological deterioration and relieves radicular symptoms in patients with myelopathy and radiculopathy, respectively. However, fusion results in increased biomechanical forces at the adjacent (mobile) level and may thus accelerate symptomatic degenerative progression [2]; some of these patients may require further surgery at the adjacent level.
Anterior cervical discectomy with arthroplasty (ACDA) is an alternative surgical option that could preserve segmental mobility at the diseased level and theoretically decrease the incidence of adjacent level degeneration (ALD). The key difference in this procedure compared to an ACDF is a wider decompression (i.e. generous bilateral foraminotomies) including resection of the uncovertebral joints bilaterally. Further, patients are commonly prescribed non-steroidal anti-inflammatory medication to prevent heterotopic ossification in addition to postoperative pain control. Heterotopic ossification is most commonly described as a complication of large joint arthroplasty and is the main cause of the prosthesis to lose function [3]. Its prevalence in cervical arthroplasty is 58.2% (95% CI = 29.7–86.8%) 12 months after surgery [3]. In addition, ACDA is a technically more difficult operation to perform compared to an ACDF.
If ALD is truly decreased, this procedure may result in decreased disability, decreased incidence of reoperation and increased quality of life while achieving similar rates of neurological success. If not, the use of ACDA increases health care costs without any additional neurological benefit [4] and a potential of greater harm if performed by a non-expert surgeon. Further, the long-term risks associated with ACDA may not be as well delineated compared to the more commonly performed ACDF.
Although several randomized clinical trials (RCTs) have compared ACDF to A [5]–[9], it remains unclear whether ACDA results in improved patient-important outco [4], [10] and whether or not its widespread use should be advocated. A systematic review found that ACDA results in modest clinical benefits with respect to neck pain, arm pain and quality of life compared to ACDF at 12 month follow-up, most of which were not sustained at 2 year follow-up [4]. A recent review of 3 United States Food and Drug Administration cervical arthroplasty trials concluded that ACDA may be associated with a higher rate of neurological success and lower prevalence of ALD 2 years following surgery [10].
There are no systematic reviews that have assessed publication bias, evaluated the risk of bias of included trials, interpreted the results with respect to clinical significance, evaluated the quality of the evidence using the GRADE approach [11] (this is a systematic and explicit method to evaluate the quality of the evidence), and reported review findings in concordance with PRISMA guidelines [12]. This review will improve upon the methodological shortcomings of the previous studies as well as include recently published trials.
Objective
We systematically reviewed all randomized clinical trials comparing the relative effects of ACDF to ACDA for single-level cervical spondylosis on patient-important outcomes.
Methods
Protocol and registration
We developed a protocol prior to conduct of the review but did not register it.
Eligibility criteria
Eligible studies had to include adult patients (greater than 50% over 19 years of age at the time of inclusion), with single-level cervical spondylosis (i.e. C3-T1) causing radiculopathy and/or myelopathy, who have undergone single-level ACDF or ACDA. Our outcomes of interest were the following: functionality, pain, quality of life, surgery for ALD, reoperations, and dysphonia/dysphagia. We only included RCTs. We excluded articles that were duplicate reports of an earlier trial, post-hoc analysis of RCT data or those in which we were unable to obtain the full-text article.
Information sources
We searched MEDLINE (2002-January 2012), Embase (2002-January 2012), Cochrane Central Register of Controlled Trials (Issue 1 of 12, Jan 2012), BIOSIS (2002-January 2012) and LILACS (2002-January 2012). We restricted the search to humans and adults (19 years of age and older) but not to any specific language(s). We limited the search to 2002 onwards because the earliest trial included in a previously published systematic review was published in 2004 [13]. We imported all search results into Endnote X5 for removal of duplicates, and title and abstract screening.
We hand searched reference lists of included articles and conference abstracts for the 2011 AANS/CNS Section on Disorders of the Spine and Peripheral Nerves and Eurospine 2011 meeting. We translated non-English papers. The first author (A.F.) designed and conducted the search strategy with reference to a systematic review on this topic [4] and reference to a highly sensitive search strategy for identifying randomized trials [14].
Search
We used the following search terms in Ovid MEDLINE(R) and Embase: ‘cervical spondylosis’, ‘cervical vertebrae’, ‘prosthesis’, ‘discectomy’, ‘arthroplasty’, ‘spinal fusion’, ‘randomized-controlled trial’, ‘random allocation’ and ‘clinical trials’ (Appendix S1). We used the term “cervical arthroplasty” to search the Cochrane Central trials registry, BIOSIS and LILACS.
Study selection
Title and abstract screening
Three reviewers (A.F., S.E. and G.M.I.) screened independently and in duplicate, the title and abstracts of identified citations for potential eligibility; we obtained the full text of these citations.
Full text review
Using a standardized form, the same reviewers independently and in duplicate applied the eligibility criteria to full text articles. We checked agreement and resolved disagreements through discussion. We calculated kappa scores to measure the degree of agreement. If articles reported on the same trial, we included the article with the most recent results or the greater number of patients. We performed calibration exercises and maintained a full list of excluded articles with reasons for exclusion.
We performed title and abstract screening, full text review and data abstraction in ‘RefWorks’ and ‘Endnote X5’ softwares.
Grading the quality of evidence
Two reviewers (A.F. and G.M.I.) independently and in duplicate, applied GRADE to eligible trials. The instructional manual in the ‘GRADEprofiler’ software version 3.6 was utilized to guide ratings. We downgraded the quality of evidence only by 1 for each component. Risk of bias was assessed as serious for subjective outcomes (i.e. neck pain and arm pain) when blinding was not performed. Inconsistency was determined by an I2 value of greater than 40% which could not be explained by our predefined subgroup analysis. We marked down for imprecision if the estimate crossed the nil effect point, unless the 95% CI did not cross the MCID (for continuous outcomes). We marked down for publication bias if this was suspected by visual inspection of the funnel plot.
Data collection process
We developed and pilot-tested a data extraction form on an electronic spreadsheet. One reviewer (A.F.) extracted data from the included trials while a second reviewer (A.M.) checked the extracted data for accuracy. We resolved disagreements through discussion.
Data items
Reviewers extracted the following information from each included trial: (1) characteristics of the trial (including number of trial centers, year of trial, type of RCT, trial location, length of follow-up and eligibility criteria); (2) characteristics of trial participants (number and mean age of participants in each trial arm); (3) name of prosthetic device utilized and whether the surgical procedure was described; (4) Outcomes of interest; (5) Cochrane risk of bias characteristics as well as other characteristics that may lead to bias (A priori registration of protocol, expertise based trial design, funding sources, method of statistical analysis (i.e. intention to treat or per protocol analysis) and affiliation of the authors with the medical device company).
Risk of bias in individual studies
To ascertain the validity of the included randomized trials, reviewers independently determined the adequacy of randomization; concealment of allocation; blinding of participants, providers, outcome assessors, data collectors and data analysts; the extent of loss to follow-up; freedom from selective outcome reporting; and freedom from other bias [15] (this was used to assess whether the trial authors were affiliated or the trial was funded with the prosthesis company) [16]. We used a ‘Modification of Cochrane Tool to assess risk of bias in randomized trials’ where a forced decision regarding bias must be made into ‘probably no’ or ‘probably yes’ for items that are thought to be of unclear risk [17], [18] (Appendix S1). We judged trials with more than 2 and more than 4 high risk components as moderate risk and high risk, respectively.
Summary measures
For continuous data, we calculated the pooled mean difference (MD) and its 95% CI using the change from baseline scores, standard deviation (SD) and total number of participants in each treatment arm. If the SD was not reported, we imputed this from a reported p value, confidence interval (CI) or standard error. In cases where none of these were available, we used the mean SD from other trials. For binary outcomes, we calculated the relative risk (RR) and its 95% CI using the number of events and total participants in each treatment arm. To facilitate interpretability, we converted RR ratios to absolute risk reductions (ARR) and number needed to treat (NNT). We selected values for minimal clinically important differences (MCID) through a literature review. MCID values were selected based on the methodological rigour of the study and the similarity of the patients to this review.
Planned method of analysis
Any kind of variability across trials in a systematic review is termed heterogeneity. Variability may result from clinical diversity (i.e. the participants or interventions differ across trials) or methodological diversity (i.e. methodological design and risk of biases differ across trials). When the statistical tests for heterogeneity (variability in the treatment effects between trials) is significant, this is unlikely to be attributed to chance alone [19]. We explored heterogeneity using the I2 statistic. This statistic aims to assess the impact of the heterogeneity on the meta- analysis [19]. We considered an I2 score of 0% to 40% as “heterogeneity might not be important”; 30% to 60% as “may represent moderate heterogeneity*; 50% to 90% as “may represent substantial heterogeneity” and; 75% to 100% as “considerable heterogeneity” [19]. We explored heterogeneity greater than 30% by performing a priori specified subgroup analyses: type of prosthesis used and the length of follow-up (24 months versus less than 24 months). We utilized a random effects (inverse variance) model to account for heterogeneity amongst trials.
Risk of bias across studies
We assessed the possibility of publication bias by evaluating funnel plots. A funnel plot is a scatter plot of the intervention effect estimates from individual trials against a measure of its precision [20]. In the absence of publication bias, the plot will resemble an inverted funnel. Although there are several reasons for asymmetric funnel plots, its presence generally indicates publication bias or is due to exaggeration of treatment effects in small, low quality trials [20].
Additional analyses
We performed sensitivity analyses for any continuous outcome with a statistically significant result (and greater than the MCID where applicable) for which SD was estimated; in these cases, we assumed the highest and lowest SD from other trials to determine the robustness of our conclusions.
Results
Study selection
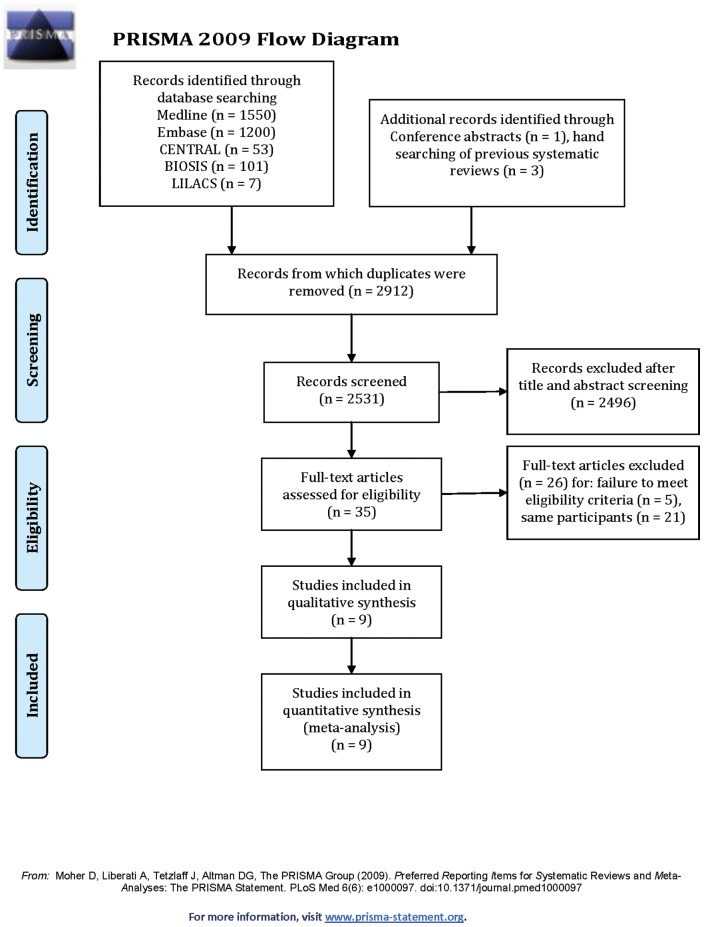
Figure 1 illustrates the study flow. We identified a total of 9 articles reporting on 9 trials (1778 participants) for inclusion (Table 1). Appendix S1 presents the list of excluded articles with reasons for exclusion. We achieved excellent agreement for screening of full text articles (Kappa = 0.92, SE 0.08; 95% CI = 0.78–1.07).
Figure 1. PRISMA 2009 Flow Diagram.
Table 1. Patient and study characteristics of the 9 included studies.
| Source | Study location | Age range (years) | Main inclusion criteria | No. of patients (ACDA group) | No. of patients (ACDF group) | Prosthetic device | Outcomes reported | Minimal follow-up duration (months) | No. of centers | Duration of study | Type of RCT/Expertise based design? | Superiority, non-inferiority or equivalence trial | Protocol registered? | Surgical procedure described? |
| Burkus et al. 2010[23] | USA | >18 | Single level symptomatic DDD, C3-7, NDI scores ≥30 | 144 | 127 | Prestige | a; b; c; d; e; f; g; h | 60 | 31 | 2002–2004 | Parallel/Yes | Not mentioned | No | Yes |
| Coric et al. 2011[6] | USA | 18–60 | Single level symptomatic DDD, C3-7, NDI scores ≥40 | 136 | 133 | Kineflex-C | a; c; d**; e**; f; g; h; i | 24 | 21 | N/A | Parallel/No | Non-inferiority | Yes | Yes |
| Delamarter et al. 2010[25] | USA | 18–60 | Single level symptomatic DDD, C3-7, NDI scores ≥15 | 103 | 106 | ProDisc-C | a; b; c; d; e; g; h | 48 | 13 | 2003–2004 | Parallel/No | Non-inferiority | No | Yes |
| McAfee et al. 2011* [21] | USA | 18–65 | Single level symptomatic DDD, C3-T1, NDI ≥30 | 188 | 151 | PCM, Bryan, Prestige and ProDisc-C* | a; c | 24 | N/A | N/A | Parallel/N/A | Not mentioned | No | No |
| McAfee et al. 2010[20] | USA | 18–65 | Single level symptomatic DDD, C3-T1, NDI ≥30 | 151 | 100 | PCM | f | 24 | 5 | N/A | Parallel/N/A | Not mentioned | No | No |
| Nabhan et al. 2007[24] | Germany | 20–60 | Single level symptomatic (Radiculopathy only) DDD, C3-7 | 20 | 21 | ProDisc-C | d; e | 36 | 1 | 2004–2005 | Parallel/No | Not mentioned | No | Yes |
| Nabhan et. al 2011[26] | Germany | N/A | Symptomatic cervical (radiculopathy only) DDD | 10 | 10 | ProDisc-C | a; d; e | 12 | 1 | 2006–2007 | Parallel/No | Not mentioned | No | Yes |
| Sasso et. al 2011[5] | USA | ≥21 | Single level cervical symptomatic DDD | 181 | 138 | Bryan | a; b; c; d; e; g; h; | 48 | 30 | 2002–2004 | Parallel/No | Superiority | Yes | No |
| Wang et. al 2008[22], [25] | China | 30–50 | Single level symptomatic DDD, C3-7 | 28 | 31 | Bryan | a; d; e | 24 | 1 | 2003–2005 | Parallel/No | N/A | No | Yes |
This article is a meta-analysis and the only study that reports the results of the PCM disc trial. Only data regarding the PCM disc trial was extracted.
Neck and Arm VAS pain score is combined.
Outcomes reported: a = NDI; b = SF-36 PCS; c = Neurological Success; d = Neck VAS pain score; e = Arm VAS pain score; f = dysphagia/dysphonia; g = Operations for ALD; h = Repeat operations; i = length of hospital stay.
LOCF – Last outcome carried forward.
Study characteristics
Tables 1 and Figure 11 present the study and participant characteristics of the included trials.
Risk of bias within studies
Appendix S1 presents the Cochrane risk of bias assessment of the included articles. There were 3 trials with high risks of bias [20]–[22], 3 trials with moderate risk of bias [5], [23], [24], and 3 trials with low risk of bias [6], [25], [26] (Appendix S1).
Results of individual studies and synthesis of results
Functionality
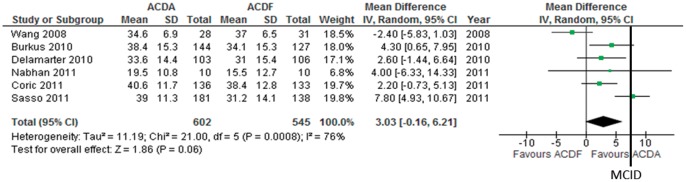
Six trials consisting of 1147 pariticpants report on NDI change (continuous outcome) following surgery to measure functionality. We imputed the SD using the p value in 1 trial [23], the 95% CI in 1 trial [5], the SD of baseline and end scores in 3 trials [22], [25], [26] and the mean SD in 1 trial [6]. Pooled analysis shows that ACDA is associated with a greater improvement in NDI compared to ACDF: MD (95% CI) = 3.03 (−0.16 to 6.21), p = 0.06 (Figure 2). The upper and lower limits of the CI are smaller than the minimally clinically important difference (MCID) of 7.5 [27] and 8.5 [28] identified in the literature. There is considerable heterogeneity (I2 = 76%) which can not be explained using our predefined subgroup analysis. The quality of evidence for this outcome is low.
Figure 2. Neck disabiltiy index improvement in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
Neurological Success
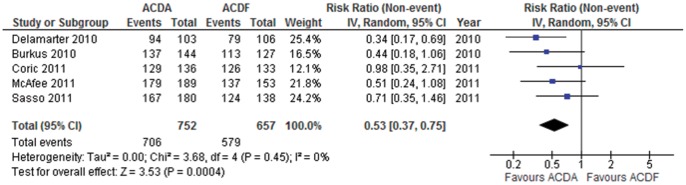
Five trials consisting of 1409 participants report on neurological success (dichotomous outcome). Pooled analysis shows that ACDA is associated with a higher incidence of neurological success compared to ACDF: RR (95% CI) = 0.53 (0.37 to 0.75), p = 0.0004 (Figure 3). Heterogeneity amongst the trials might not be important (I2 = 0%). This translates into an ARR of 5.8% (NNT = 17). The quality of evidence for this outcome is moderate.
Figure 3. Neurological success in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
Neck pain
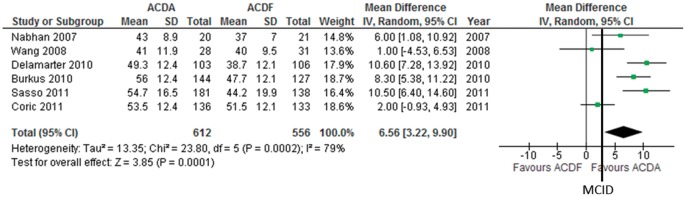
Six trials consisting of 1168 participants report on neck pain using the Visual Analogue Scale (VAS) (continuous outcome). We imputed unreported SD using the 95% CI in 1 trial [5], the SD of baseline and end scores in 2 trials [22], [24] and the mean SD in 3 trials [6], [23], [25]. One trial reports neck and arm pain together [25]. Pooled analysis shows that ACDA is associated with a greater improvement in neck pain compared to ACDF: MD (95% CI) = 6.56, (3.22 to 9.90), p = 0.0001 (Figure 4). This effect is greater than the MCID of 2.5 [27]. There is moderate heterogeneity (I2 = 79%) which can not be explained using our predefined subgroup analysis. The quality of evidence for this outcome is very low.
Figure 4. Neck visual analogue scale pain score improvement in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
Arm pain
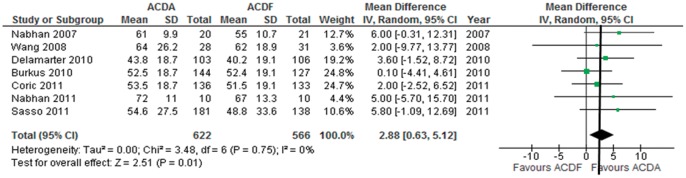
Seven trials consisting of 1188 paritcipants report on arm pain using the VAS (continuous outcome). The SD was imputed using the 95% CI in 1 trals [5], the SD of baseline and end scores in 2 trials [24], [26] and the mean SD in 3 trials [6], [23], [25]. One trial reports neck and arm pain together [25]. Pooled analysis shows that ACDA is associated with a greater improvement in arm pain on the VAS compared to ACDF: MD (95% CI) = 2.88 (0.63 to 5.12), p = 0.01 (Figure 5). However, the 95% CI of the summary effect crosses the MCID threshold of 2.5 [27]. Heterogeneity amongst the trials might not be important (I2 = 0%). The quality of evidence for this outcome is low.
Figure 5. Arm visual analogue pain score improvement in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
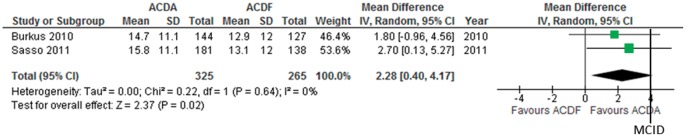
Quality of life
Two trials consisting of 590 participants report on the SF-36 physical component score (PCS) change (continuous outcome) following surgery to measure quality of life. The SD is not available in one trial but was estimated using the SD in the other trial [23]. Pooled analysis shows that ACDA is associated with a greater improvement in SF-36 PCS compared to ACDF: MD (95% CI) = 2.28 (0.40 to 4.17), p = 0.02 (Figure 6). However, the 95% CI of the summary effect spans the MCID threshold of 4.1 [27]. Heterogeneity amongst the trials might not be important (I2 = 0%). The quality of evidence for this outcomes is moderate.
Figure 6. SF-36 physical component summary score improvement in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
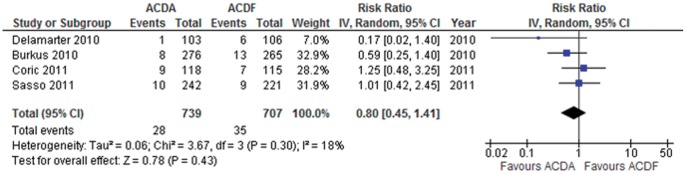
Surgery for adjacent level disease
Four trials consisting of 1446 participants report data on surgery for ALD (dichotomous outcome). Pooled analysis shows that participants that undergo an ACDA are at a non-statistically significant lower risk to undergo surgery for ALD in comparison to those that undergo ACDF: RR (95% CI) = 0.80 (0.45 to 1.41) p = 0.43 (Figure 7). This translates to an ARR of 1.2% (NNT = 83). Heterogeneity amongst the trials might not be important (I2 = 18%). The quality of evidence for this outcome is low.
Figure 7. Surgery for ALD in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
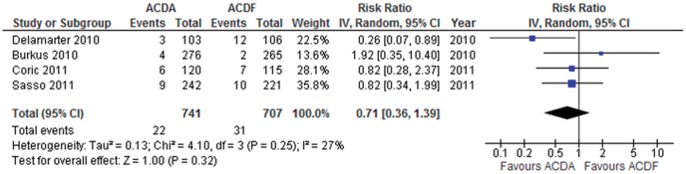
Reoperation
Four trials consisting of 1448 participants reported on reoperation (dichotomous outcome). Pooled analysis shows that participants that undergo an ACDA are at a non-statistically significant lower risk to undergo a reoperation in comparison to those that undergo ACDF: RR (95% CI) = 0.71 (0.36 to 1.39) p = 0.32 (Figure 8). This translates to an ARR of 1.4% (NNT = 71). Heterogeneity amongst the trials might not be important (I2 = 27%). The quality of evidence for this outcome is low.
Figure 8. Reoperation in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
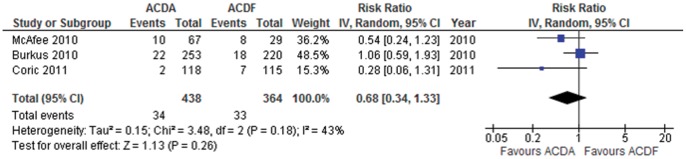
Dysphonia/Dysphagia
Three trials consisting of 802 participants report on surgery for dysphonia/dysphagia. The trial by McAfee et al. only includes participants with dysphagia [20]. Pooled analysis shows that participants that undergo an ACDA are at a non-statistically significant lower risk to develop dyphagia/dyphonia in comparison to those that undergo ACDF: RR (95% CI) = 0.68 (0.34 to 1.33), p = 0.26 (Figure 9). This translates to an ARR of 1.3% (NNT = 77). Heterogeneity amongst the trials may be moderate (I2 = 43%) and is not explained using our predefined subgroup analysis. The quality of evidence for this outcome is very low.
Figure 9. Dysphonia/dysphagia in participants undergoing ACDA vs. ACDF for single level cervical spondylosis.
CI indicates confidence interval.
Risk of bias across studies
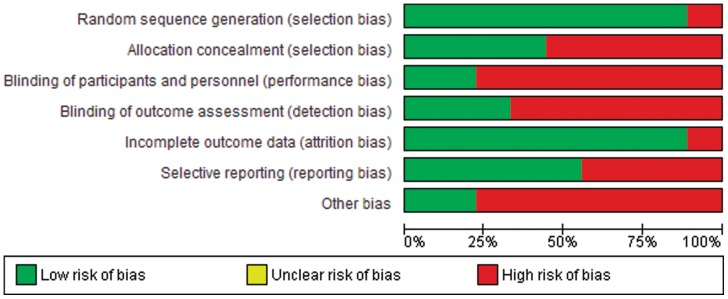
The trials generally had a high risk of bias for lack of blinding and affiliation with the sponsoring implant manufacturing company, moderate risk of bias for poor allocation concealment, lack of blinding of outcome assessors and selective reporting bias, and low risk of bias for randomization sequence generation and incomplete outcome data (Figure 10). Due to the small number of trials for each outcome, we could not reliably detect publication bias. However, funnel plots were assymmetric, raising suspicion for publication bias, for ALD requiring surgery and reoperation.
Figure 10. Cochrane risk of bias across studies.
Additional analysis
Sensitivity analysis
Statistical heterogeneity for functionality is eliminated if the trial by Wang et al. is removed. This is the only Chinese trial and only trial that favours ACDF for functionality. Removal of this trial still results in no clinically significant improvement in functionality in comparison to ACDF.
Sensitivity analysis assuming the highest and lowest SD for estimated SD data for Neck pain VAS score improvement results in a MD (95% CI) = 6.52, (3.24 to 9.80), p<0.0001 and MD (95% CI) = 6.58, (3.08 to 10.07), p = 0.0002, respectively. The upper and lower threshold of the 95% CI under both assumptions is still greater than the MCID threshold of 2.5 [27].
Discussion
Summary and quality of evidence using GRADE
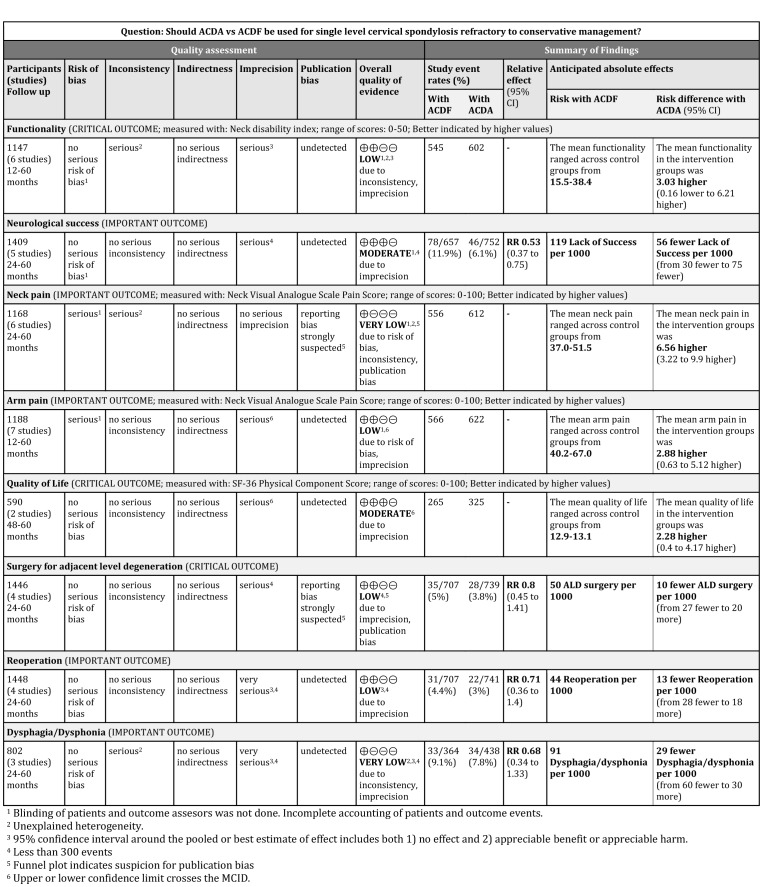
ACDA is associated with a clinically significant greater improvement in neck pain and higher incidence of neurologic success. ACDA is associated with a statistically significant but not clinically significant greater improvement in arm pain and quality of life. ACDA is associated with a non-statistically significant greater improvement in functionality, and lower incidence of ALD requiring surgery, reoperation and dysphagia/dysphonia. The quality of evidence was assessed using GRADE (Figure 11).
Figure 11. Grade profile for ACDA vs. ACDF for single level cervical spondylosis.
Neck pain and neurological success
It is unclear why ACDA results in a greater improvement in neck pain and neurological success as these are thought to be related to adequate neural decompression and stabilization, respectively. Perhaps, a wider lateral decompression that is required for ACDA results in a greater amount of neural decompression; this is irrespective of the spacer device used. The greater neurological success and decreased neck pain associated with ACDA should be cautiously interpreted given that the patients and outcome assessors were generally not blinded [4].
Functionality and surgery for adjacent level degeneration
The two most critical outcomes when considering the use of ACDA over ACDF is functionality and surgery for ALD. We obtained adequate power to conclude that there is no clinically important benefit of ACDA over ACDF. We were underpowered to determine whether ACDA results in a lower incidence of surgery for ALD. The point estimate equates to a NNT of 83. The NNT for the lower boundary of the CI equates to 37 corresponding to the most benefit one would expect to obtain. However, there are two important caveats: 1) ALD is a time dependent complication requiring studies of longer follow-up duration to determine any benefit for ACDA. Interestingly, the trial by Coric et al [6] that most strongly favoured ACDF is also the one with the shortest followup duration (i.e. 24 months) (Figure 7). A hypothesis for future studies is that any benefits of ACDA over ACDF in preventing ALD may be better appreciated in the long-term; and 2) None of the trials defined the criteria for surgical intervention for ALD a priori. Given the paucity of long-term data, there is no evidence that ACDA results in increased complications compared to ACDF. Therefore, ACDA remains an option for symptomatic single-level spondylosis.
Comparison to other systematic reviews/meta-analysis
There are several systematic reviews and meta-analyses on this topic, some suggesting a clinical benefit of ACDA over ACDF [21], [29]–[32] while others suggested no clinical benefit of ACDA over ACDF [4], [33]. However, these systematic reviews suffer from a number of methodological limitations. Only two reviews searched for non-English articles [4], [13] and only one translated non-English articles [13]. There is evidence that trials with positive results are more likely to be published in English language journals [34]. This is particularly relevant as the only trial that found greater NDI improvement in the ACDF arm was the Chinese trial by Wang et al [22].
Cepoui-Martin et al. only qualitatively described the data [33] due to methodological flaws and poor reporting of the original trials. Although this, more conservative, approach may be appropriate, we obtained enough information and imputed data when it was unavailable to perform a meta-analysis. We tested our assumptions using sensitivity analysis. The authors of this review conclude moderate to strong evidence supporting the efficacy of ACDA but are not transparent on how GRADE was applied to formulate these recommendations. This is important as the application of GRADE has a subjective component. We graded the quality of evidence as ‘very low’ to ‘moderate’ and were transparent about our decision-making (Figure 11).
Yu et al. pooled trial results using fixed effects and concluded that neurological success, repeat operation and neck pain favour ACDA [32]. Random effects were only utilized when I2 was greater than 50%. Since we are pooling results from several prosthetic devices which are thought to work through different mechanisms, it is much more likely that the underlying effect is not fixed and therefore a more conservative, random effects model should be utilized.
Two reviews quantitatively pooled trials to enhance power for obtaining a statistically significant result without conducting systematic searches [21], [31]. Authors from one of these reviews had a priori knowledge that all selected trials favoured arthroplasty [21]. This approach is extremely susceptible to providing misleading results. In this review, NDI success is defined as a greater than 15 point increase; no reference is provided for a scientific basis of choosing this threshold [21].
We agree with Botelho et al. that there is a paucity of data regarding the incidence of ALD with ACDA [13]. Most trials report ALD requiring surgery which may be prone to bias if the criteria for ALD surgery are not defined a priori. The systematic review by Jiang et al. conclude that there is a lower rate of ALD surgery with ACDA [30]. However, they have included a non-randomized study [35] in their analysis, whose removal would lead to a borderline significant result.
Strengths
There are several strengths to this meta-analysis including a rigorous search strategy, no language limitations, article screening and methodological assessments performed in duplicate, abstracted data verified by a second reviewer and utilization of the GRADE approach to summarize findings and judge the quality of evidence. In addition, this is the first systematic review on this topic to incorporate MCID in interpreting findings. This approach focuses on clinically important differences as opposed to statistically important differences.
Limitations
There are several limitations to this meta-analysis: 1) There is a variable length of follow-up across trials. This is particularly important for evaluating surgery for ALD as this outcome is time-dependent; 2) There may be a prosthetic device that is superior to others with respect to functionality and decreased incidence of ALD. Combining data across trials may fail to identify this; 3) Reporting quality was generally poor across trials; therefore, it is unclear if a lack of difference between the 2 interventions is due to poor methodological quality of the trial or a true lack of difference in effect; and 4) We were unable to obtain data for several relevant abstracts that we identified despite contacting authors.
Ongoing studies
We identified several ongoing trials pertaining to this topic: DISCOVER™ (NCT00432159, NCT00735176), GRANVIA®-C (NCT01518582), Mobi-C (NCT00554528) and The NeoDisc™ (NCT00478088). The eventual addition of these trials to the current body of evidence is likely to improve our confidence in the conclusions and ability to make informed treatment recommendations.
Conclusions
Implications for practice
We suggest that ADCA in adult patients with single level cervical spondylosis is an option. The indication and benefits over ACDF remain in question. In addition, long-term safety data of this device is not available. We invite clinicians to consider patient's values and preferences in selecting the surgical treatment.
Implications for research
The quality of current evidence varied from ‘very low’ to ‘moderate’ (Figure 11). Future trials should register their protocols, not be funded or clearly be independent from influence by implant manufacturing companies [15], be adequately powered to assess all patient important outcomes, utilize an expertise-based design, safeguard against biases, centrally adjudicate indications for surgery for ALD and reoperation, fully account for all trial participants and report long-term follow-up utilizing the CONSORT guidelines.
Supporting Information
Medline and Embase search strategy.
(DOCX)
Funding Statement
The authors have no funding or support to report.
References
- 1. Carette S, Fehlings MG (2005) Clinical practice. Cervical radiculopathy. N Engl J Med 353: 392–399. [DOI] [PubMed] [Google Scholar]
- 2. Reitman CA, Hipp JA, Nguyen L, Esses SI (2004) Changes in segmental intervertebral motion adjacent to cervical arthrodesis: a prospective study. Spine (Phila Pa 1976) 29: E221–226. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Wang X, Bai W, Shen X, Yuan W (2012) Prevalence of heterotopic ossification after cervical total disc arthroplasty: a meta-analysis. Eur Spine J 21: 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartels RH, Donk R, Verbeek AL (2010) No justification for cervical disk prostheses in clinical practice: a meta-analysis of randomized controlled trials. Neurosurgery 66: 1153–1160; discussion 1160. [DOI] [PubMed]
- 5. Sasso RC, Anderson PA, Riew KD, Heller JG (2011) Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am 93: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 6. Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, et al. (2011) Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine 15: 348–358. [DOI] [PubMed] [Google Scholar]
- 7. Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, et al. (2009) Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J 9: 275–286. [DOI] [PubMed] [Google Scholar]
- 8. Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fessler RG, et al. (2009) Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976) 34: 101–107. [DOI] [PubMed] [Google Scholar]
- 9. Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA (2007) Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 6: 198–209. [DOI] [PubMed] [Google Scholar]
- 10.Upadhyaya CD, Wu JC, Trost G, Haid RW, Traynelis VC, et al.. (2011) Analysis of the three United States Food and Drug Administration investigational device exemption cervical arthroplasty trials. J Neurosurg Spine. [DOI] [PubMed]
- 11. Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A (2011) GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 13. Botelho RV, Moraes OJ, Fernandes GA, Buscariolli Ydos S, Bernardo WM (2010) A systematic review of randomized trials on the effect of cervical disc arthroplasty on reducing adjacent-level degeneration. Neurosurg Focus 28: E5. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre C, Clarke MJ (2001) Identifying randomised trials. In: Egger M, Smith D, Altman DG, editors. Systematic reviews in healthcare. 2nd edition ed. London: BMJ Publishing Group.
- 15. Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, et al. (2004) Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ 170: 477–480. [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Sterne JAC (2011) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011): The Cochrane Collaboration, 2011.
- 17. Akl EA, Sun X, Busse JW, Johnston BC, Briel M, et al. (2012) Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol 65: 262–267. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Busse JW (2011) Modification of Cochrane Too to assess risk of bias in randomized trials.
- 19.Deeks JJ, Higgins JPT, Altman DG (2011) Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011). Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. ed: The Cochrane Collaboration, 2011.
- 20. McAfee PC, Cappuccino A, Cunningham BW, Devine JG, Phillips FM, et al. (2010) Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial. 23: 1–8. [DOI] [PubMed] [Google Scholar]
- 21.McAfee PC, Reah C, Gilder K, Eisermann L, Cunningham B (2011) A Meta-Analysis of Comparative Outcomes Following Cervical Arthroplasty or Anterior Cervical Fusion: Results from Four Prospective Multi-center Randomized Clinical Trials and up to 1226 Patients. Spine (Phila Pa 1976). [DOI] [PubMed]
- 22. Wang Y, Cai B, Zhang XS, Xiao SH, Wang Z, et al. (2008) Clinical outcomes of single level Bryan cervical disc arthroplasty: a prospective controlled study. 46: 328–332. [PubMed] [Google Scholar]
- 23. Burkus JK, Haid RW, Traynelis VC, Mummaneni PV (2010) Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: results from a prospective randomized controlled clinical trial. 13: 308–318. [DOI] [PubMed] [Google Scholar]
- 24. Nabhan A, Steudel WI, Nabhan A, Pape D, Ishak B (2007) Segmental kinematics and adjacent level degeneration following disc replacement versus fusion: RCT with three years of follow-up. Journal of long-term effects of medical implants 17: 229–236. [DOI] [PubMed] [Google Scholar]
- 25. Delamarter RB, Murrey D, Janssen ME, Goldstein JA, Zigler J, et al. (2010) Results at 24 months from the prospective, randomized, multicenter Investigational Device Exemption trial of ProDisc-C versus anterior cervical discectomy and fusion with 4-year follow-up and continued access patients. 4: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nabhan A, Ishak B, Steudel WI, Ramadhan S, Steimer O (2011) Assessment of adjacent-segment mobility after cervical disc replacement versus fusion: RCT with 1 year's results. 20: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carreon LY, Glassman SD, Campbell MJ, Anderson PA (2010) Neck Disability Index, short form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J 10: 469–474. [DOI] [PubMed] [Google Scholar]
- 28. Young IA, Cleland JA, Michener LA, Brown C (2010) Reliability, construct validity, and responsiveness of the neck disability index, patient-specific functional scale, and numeric pain rating scale in patients with cervical radiculopathy. Am J Phys Med Rehabil 89: 831–839. [DOI] [PubMed] [Google Scholar]
- 29. Anderson PA, Puschak TJ, Sasso RC (2009) Comparison of short-term SF-36 results between total joint arthroplasty and cervical spine decompression and fusion or arthroplasty. Spine (Phila Pa 1976) 34: 176–183. [DOI] [PubMed] [Google Scholar]
- 30. Jiang H, Zhu Z, Qiu Y, Qian B, Qiu X, et al. (2012) Cervical disc arthroplasty versus fusion for single-level symptomatic cervical disc disease: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 132: 141–151. [DOI] [PubMed] [Google Scholar]
- 31. Upadhyaya CD, Wu JC, Trost G, Haid RW, Traynelis VC, et al. (2012) Analysis of the three United States Food and Drug Administration investigational device exemption cervical arthroplasty trials. J Neurosurg Spine 16: 216–228. [DOI] [PubMed] [Google Scholar]
- 32. Yu L, Song Y, Yang X, Lv C (2011) Systematic review and meta-analysis of randomized controlled trials: comparison of total disk replacement with anterior cervical decompression and fusion. Orthopedics 34: e651–658. [DOI] [PubMed] [Google Scholar]
- 33.Cepoiu-Martin M, Faris P, Lorenzetti D, Prefontaine E, Noseworhty T, et al.. (2011) Artificial Cervical Disc Arthroplasty (ACDA): a systematic review. Spine (Phila Pa 1976). [DOI] [PubMed]
- 34. Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, et al. (1997) Language bias in randomised controlled trials published in English and German. Lancet 350: 326–329. [DOI] [PubMed] [Google Scholar]
- 35. Robertson JT, Papadopoulos SM, Traynelis VC (2005) Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine 3: 417–423. [DOI] [PubMed] [Google Scholar]
- 36. Anderson PA, Sasso RC, Riew KD (2008) Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. 33: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 37. Coric D, Finger F, Boltes P (2006) Prospective randomized controlled study of the Bryan Cervical Disc: early clinical results from a single investigational site. 4: 31–35. [DOI] [PubMed] [Google Scholar]
- 38. Garrido BJ, Taha TA, Sasso RC (2010) Clinical outcomes of Bryan cervical disc arthroplasty a prospective, randomized, controlled, single site trial with 48-month follow-up. 23: 367–371. [DOI] [PubMed] [Google Scholar]
- 39. Hacker RJ (2005) Cervical disc arthroplasty: a controlled randomized prospective study with intermediate follow-up results. Invited submission from the joint section meeting on disorders of the spine and peripheral nerves, March 2005. 3: 424–428. [DOI] [PubMed] [Google Scholar]
- 40. Peng-Fei S, Yu-Hua J (2008) Cervical disc prosthesis replacement and interbody fusion: a comparative study. 32: 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sasso RC, Metcalf NH, Hipp JA, Wharton ND, Anderson PA (2011) Sagittal alignment after Bryan cervical arthroplasty. Spine (Phila Pa 1976) 36: 991–996. [DOI] [PubMed] [Google Scholar]
- 42. Sasso RC, Best NM, Metcalf NH, Anderson PA (2008) Motion analysis of bryan cervical disc arthroplasty versus anterior discectomy and fusion: Results from a prospective, randomized, multicenter, clinical trial. 21: 393–399. [DOI] [PubMed] [Google Scholar]
- 43. Sasso RC, Smucker JD, Hacker RJ, Heller JG (2007) Clinical outcomes of BRYAN Cervical Disc arthroplasty: A prospective, randomized, controlled, multicenter trial with 24-month follow-up. 20: 481–491. [DOI] [PubMed] [Google Scholar]
- 44. Sasso RC, Smucker JD, Hacker RJ, Heller JG (2007) Artificial disc versus fusion: a prospective, randomized study with 2-year follow-up on 99 patients. 32: 2933–2932. [DOI] [PubMed] [Google Scholar]
- 45. Xu JX, Zhang YZ, Shen Y, Ding WY (2009) Effect of modified techniques in bryan cervical disc arthroplasty. 34: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 46. Guyer R, Lauryssen C, Blementhal S (2008) A prospective randomized comparison of cervical total disc replacement to anterior cervical fusion. Abstractbook Spineweek Geneva C73: 45–46. [Google Scholar]
- 47. Davis R (2011) Comparison of the Mobi C cervical artificial disc to anterior cervical discectomy and fusion in the treatment of symptomatic cervical degenerative disc disease at two levels. 11: 135S. [Google Scholar]
- 48.Hisey MS, Bae HW, Davis R, Gaede S, Hoffman G, et al.. (2011) Reduction in the incidence of adjacent segment degeneration at 2 years: results of a multicenter, prospective, randomized, controlled trial comparing Mobi C cervical artificial disc to anterior cervical discectomy and fusion. Eurospine 2011.
- 49. Nunley P, Jawahar A, Bae H, Davis R, Hisey M, et al. (2011) A prospective randomized controlled trial to assess the efficacy of the Mobi-Cbeta artificial cervical disc in the management of intractable cervical myelo-radiculopathy at one or two contiguous level. 11: 47S–48S. [Google Scholar]
- 50. Park DK, Lin EL, Phillips FM (2011) Index and adjacent level kinematics after cervical disc replacement and anterior fusion: In vivo quantitative radiographic analysis. 36: 721–730. [DOI] [PubMed] [Google Scholar]
- 51. Howell K, Phillips F, Cappuccino A, Geisler F, Chaput C, et al. (2011) A prospective, randomized clinical investigation of the porous coated motion (PCM) artificial cervical disc: Two-year results from the US IDE study. 11: 18S. [Google Scholar]
- 52. Porchet F, Metcalf NH (2004) Clinical outcomes with the Prestige II cervical disc: preliminary results from a prospective randomized clinical trial. 17: E6. [DOI] [PubMed] [Google Scholar]
- 53. Riina J, Patel A, Dietz JW, Hoskins JS, Trammell TR, et al. (2008) Comparison of single-level cervical fusion and a metal-on-metal cervical disc replacement device. 37: E71–77. [PubMed] [Google Scholar]
- 54. Anakwenze OA, Auerbach JD, Milby AH, Lonner BS, Balderston RA (2009) Sagittal cervical alignment after cervical disc arthroplasty and anterior cervical discectomy and fusion: results of a prospective, randomized, controlled trial. 34: 2001–2007. [DOI] [PubMed] [Google Scholar]
- 55. Auerbach JD, Anakwenze OA, Milby AH, Lonner BS, Balderston RA (2011) Segmental contribution toward total cervical range of motion: A comparison of cervical disc arthroplasty and fusion: A comparison of cervical disc arthroplasty and fusion. 36: E1593–E1599. [DOI] [PubMed] [Google Scholar]
- 56. Nabhan A, Ahlhelm F, Pitzen T, Steudel WI, Jung J, et al. (2007) Disc replacement using Pro-Disc C versus fusion: a prospective randomised and controlled radiographic and clinical study. 16: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nabhan A, Steudel WI, Nabhan A, Pape D, Ishak B (2007) Segmental kinematics and adjacent level degeneration following disc replacement versus fusion: RCT with three years of follow-up. Journal of long-term effects of medical implants 17: 229–236. [DOI] [PubMed] [Google Scholar]
- 58. Nabhan A, Ahlhelm F, Shariat K, Pitzen T, Steimer O, et al. (2007) The ProDisc-C prosthesis: clinical and radiological experience 1 year after surgery. 32: 1935–1941. [DOI] [PubMed] [Google Scholar]
- 59.Marzluff J, McConnell J, Tomaras C, Peppelman W, Volcan I, et al.. (2010) 2-Year Multicenter Follow-up in a Prospective Randomized Clinical Trial: Comparison of a Cervical Artificial Disc to an ACDF Treatment. NASS Conference.
- 60. Coric D, Cassis J, Carew JD, Boltes MO (2010) Prospective study of cervical arthroplasty in 98 patients involved in 1 of 3 separate investigational device exemption studies from a single investigational site with a minimum 2-year follow-up: Clinical article. 13: 715–721. [DOI] [PubMed] [Google Scholar]
- 61. Jawahar A, Cavanaugh DA, Kerr EJ 3rd, Birdsong EM, Nunley PD (2010) Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: results of 93 patients in three prospective randomized clinical trials. 10: 1043–1048. [DOI] [PubMed] [Google Scholar]
- 62. Utter PA, Jawahar A, Cavanaugh DA, Kerr EJ, Nunley PD (2011) Does total disc arthroplasty in cervical spine reduce the incidence of symptomatic adjacent segment disease? Analysis from multiple randomized trials. 153: 1886–1887. [Google Scholar]
- 63. Abibtol JJ, Baldwin NG, Youssef JA, Wright NM (2008) Cervicore disc replacement vs fusion for cervical nerve root compression: functional and occupational outcomes from a prospective, randomized multicenter trial. Abstractbook Spineweek Geneva C74: 46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Medline and Embase search strategy.
(DOCX)