Abstract
We recently reported isolation of viable rat amniotic fluid-derived stem (AFS) cells [1]. Here, we tested the therapeutic benefits of AFS cells in a rodent model of ischemic stroke. Adult male Sprague-Dawley rats received a 60-minute middle cerebral artery occlusion (MCAo). Thirty-five days later, animals exhibiting significant motor deficits received intravenous transplants of rat AFS cells or vehicle. At days 60–63 post-MCAo, significant recovery of motor and cognitive function was seen in stroke animals transplanted with AFS cells compared to vehicle-infused stroke animals. Infarct volume, as revealed by hematoxylin and eosin (H&E) staining, was significantly reduced, coupled with significant increments in the cell proliferation marker, Ki67, and the neuronal marker, MAP2, in the dentate gyrus (DG) [2] and the subventricular zone (SVZ) of AFS cell-transplanted stroke animals compared to vehicle-infused stroke animals. A significantly higher number of double-labeled Ki67/MAP2-positive cells and a similar trend towards increased Ki67/MAP2 double-labeling were observed in the DG and SVZ of AFS cell-transplanted stroke animals, respectively, compared to vehicle-infused stroke animals. This study reports the therapeutic potential of AFS cell transplantation in stroke animals, possibly via enhancement of endogenous repair mechanisms.
Introduction
Stroke is the fourth leading cause of death and the leading cause of disability in the United States [3]. To date, the only FDA-approved drug for ischemic stroke is tissue plasminogen activator (tPA). Due to the limited therapeutic window (4.5 hours from disease onset to tPA administration) and the risks associated with tPA (i.e., hemorrhagic transformation), only about 3 percent of ischemic stroke patients benefit from tPA therapy [4], [5]. In an effort to increase the therapeutic window, novel treatment strategies target a longer delay post-stroke, specifically the restorative phase which begins days to weeks post-stroke [1], [6], [7], [8].
Due to their ability to release anti-inflammatory cytokines that can potentially modify the hostile environment associated with the secondary cell death of the ischemic brain, stem cells have emerged as a potential therapeutic agent for stroke. The positive effects obtained by transplantation of AFS cells are ascribed to the grafted cells’ production of trophic factors and cytokines, as well as the increase in the levels of neurotrophic factors and reduced inflammatory response within the ischemic region in response to the administration of AFS cells. Additionally, the benefits of AFS cells may be attributable to its inhibition of apoptosis and oxidative stress, in tandem with stimulation of angiogenesis, neurogenesis, and synaptogenesis [1], [6], [7], [8]. Although stem cells can be isolated from many sources, including bone marrow, fetal and embryonic tissues, amnion-derived stem cells are an attractive source of stem cells because of many logistical and ethical advantages. Amnion-derived stem cells can be isolated from the tissue and the fluid [1], [6], [7], [8]. Harvesting these cells poses minimal risk of harming the fetus. Unlike amniotic tissue-derived stem cells, the AFS cells can be isolated from amniocentesis around 15–20 weeks gestation, whereas amniotic tissue-derived stem cells are harvested after childbirth. Extraction of AFS cells prior to delivery allows for the cells to be cultured, and in the event of childbirth-associated disorders (e.g., cerebral palsy) the stem cells can be amplified in advance and transplanted upon disease diagnosis within hours after birth. This efficient amplification process may be difficult with amniotic tissue-derived cells. AFS cells are isolated during an earlier phase of pregnancy, have a higher proliferative capacity, and their properties may more closely mimic embryonic stem cells compared to amniotic tissue-derived stem cells [1], [6], [7], [8]. Another advantage of AFS cells, compared to amniotic tissue-derived cells, is that the sterility of these cells is likely to be satisfied with AFS cells extracted via amniocentesis, but may be compromised when stem cells are harvested from the amnion tissue during child delivery.
AFS cells can differentiate into multiple lineages [9], [10], [11], [12], [13], [14], [15]. Although the term “fluid” has been ascribed to AFS cells, cells isolated during amniocentesis contain a variety of stem cells originating from extra-embryonic and embryonic tissues [12]. The properties of AFS cells vary with gestational age [1], [6], [7], [8]. The versatility and plasticity properties of AFS cells fall somewhere in between the pluripotent embryonic stem cells and the multipotent adult stem cells [16], [17]. AFS cells have a high renewal capacity and can be expanded for over 250 doublings without any detectable loss of chromosomal telomere length [16]. The population doubling time for our AFS cells is approximately 30–36 hours. Taken together, these data provide support to the notion that the amniotic fluid is a rich and promising source of stem cells for clinical applications.
Transplantation of AFS cells has been explored in neurological disorders [1], [6], [7], [8]. Under standard neuronal induction protocols for stem cells, AFS cells possess preferential dopaminergic phenotypic commitment, making them a potentially valuable source of stem cells to treat Parkinson’s disease. In the same vein of specific fate commitment, AFS cells from second trimester amniotic fluid show the capacity to differentiate into all three germ layers and expressed Oct-4, Nanog, and SSEA-4 [18], which are pluripotent embryonic stem cell markers [1], [6], [7], [8]. These findings suggest that the amniotic fluid may be an attractive source of stem cells for neurological disorders.
Transplantation of stem cells thus stands as a promising therapy for stroke; however, few studies focusing on AFS cells as a donor cell source for transplantation in stroke have been conducted. AFS cells delivered intracerebroventricularly in mice, three days after receiving a 60-minute middle cerebral artery occlusion (MCAo), attenuated short-term memory impairment and improved sensorimotor ability, somatosensory functions, and motor coordination seven days post-MCAo [19]. Although this study reports the beneficial effects of AFS cell transplantation in a mouse model of MCAo, histological analysis was not conducted and the animals were euthanized at an early time point following MCAo. Chronic stroke studies are needed to determine the long-term efficacy of AFS cell transplantation, as well as to elucidate the mechanism underlying their therapeutic effects. Here we analyzed the effects of intravenously transplanted AFS cells in MCAo animals using cognitive and motor tests and subsequent histological analysis of the brain for determination of therapeutic benefits and mechanism of action associated with this cell therapy for stroke.
Materials and Methods
Subjects
All experiments were conducted in accordance with the National Institute of Health Guide and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use committee of the University of South Florida, Morsani College of Medicine. Rats were housed two per cage in a temperature- and humidity-controlled room that was maintained on 12/12 hour-light/dark cycles. They had free access to food and water.
Surgical procedures
Ten weeks old male Sprague-Dawley rats (n = 30) were subjected to stroke (n = 17) or sham surgery (n = 13) anesthetized by a mixture of 1–2% isoflurane in nitrous oxide/oxygen (69%/30%) via face mask. Body temperature was maintained at 37°C±0.3°C during the surgical procedures. The midline skin incision was made in the neck with subsequent exploration of the right common carotid artery (CCA), the external carotid artery, and internal carotid artery. A 4-0 monofilament nylon suture (27.0–28.0 mm) was advanced from the CCA bifurcation until it blocked the origin of the middle cerebral artery (MCA). Animals were allowed to recover from anesthesia during MCA occlusion (MCAo). After 60 minutes of transient MCAo, animals were re-anesthetized with 1–2% isoflurane in nitrous oxide/oxygen (69%/30%) using a face mask and reperfused by withdrawal of the nylon thread. Animals receiving the sham operation were anesthetized with 1–2% isoflurane in nitrous oxide/oxygen (69%/30%) via face mask. A midline incision was made in the neck and the right CCA was isolated. The animals were then closed and allowed to recover from anesthesia. We have standardized the MCAo model, with stroke animals showing at least 80% reduction in regional cerebral blood flow during the occlusion period as determined by laser Doppler (Perimed). To further ensure similar degree of stroke insults, physiological parameters including PaO2, PaCo2, and plasma pH measurements were monitored, and we found no significant differences in our stroke animals. Animals that did not display the 70% swing bias were excluded (n = 2). All animals were euthanized on day 63 post-MCAo for subsequent immunohistochemical investigations (n = 14). One animal died immediately after reperfusion, thus a motality rate of approximately 6% was observed post-MCAo in this study. The total number of animals in each group was as follows: n = 6 for the vehicle-infused stroke animals and n = 8 for the AFS cell-transplanted stroke animals. All data corresponding to the deceased animal and the animals excluded from the study based on a lack of swing bias were removed. A schematic diagram of experimental design is shown (Fig. 1).
Figure 1. Experimental design is shown.
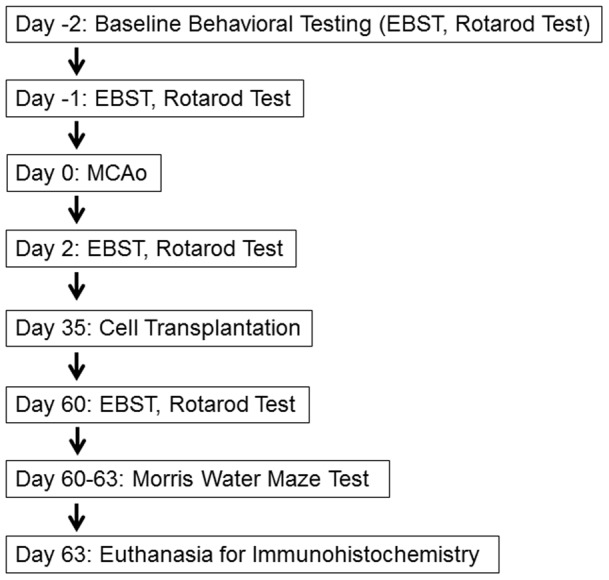
Rats were subjected to a 60 minute transient MCAo and received intravenous transplants of AFS cells or vehicle. After behavioral evaluations, all rats were euthanized for immunohistochemical evaluations. EBST: elevated body swing test; MCAo: middle cerebral artery occlusion.
Isolation of AFS Cells
Amniotic fluid samples were obtained from timed pregnant Sprague-Dawley rats at gestation age 16–18 weeks. The study has been approved by the Ethics Committee for Biomedical Research of the "G. d'Annunzio" University, Chieti. For each sample, 2–3 ml of amniotic fluid, corresponding to a cell number ranging from 2×103 to 2×106 were centrifuged for 10 minutes at 1800 rpm. Pellets were resuspended in Iscove's modified Dulbecco's medium supplemented with 20% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma), 2 mM L-glutamine, 5 ng/ml basic fibroblast growth factor (FGF2) and incubated at 37°C with 5% humidified CO2. After 7 days, non-adherent cells were removed and the adherent cells allowed to growth in the same medium, which was changed every 4 days. When culture reached confluency (about 20 days after the primary culture), cells were treated with 0,05% trypsin and 0,02% EDTA, then counted and replaced in 25 cm2 culture flasks.
Intravenous administration of AFS cells or vehicle
On day 35 post-MCAo, rats were anesthetized with 1–2% isoflurane in nitrous oxide/oxygen (69%/30%) using a face mask. Transplantation of AFS cells (1 million viable cells in 1 ml of sterile saline; n = 8) or infusion of vehicle (equivalent volume of sterile saline; n = 6) or was performed intravenously via the jugular vein.
Behavioral testing
Each rat was subjected to a series of behavioral tests to reveal motor, neurological, and cognitive performance of animals prior to MCAo, post-MCAo, and following transplantation. The tests included the elevated body swing test (EBST; motor test) and the rotarod test, both performed prior to and after MCAo, at days −1, 2 and 60 post-MCAo. The Morris Water Maze (MWM) test was performed on days 60, 61, 62, and 63 post-MCAo. EBST is a measure of asymmetrical motor behavior that does not require animal training or drug injection [20]. The rats were held, in the vertical axis, approximately 1 inch from the base of its tail and then elevated to an inch above the surface on which it has been resting. The frequency and direction of the swing behavior were recorded for over 20 tail elevations. A swing was counted when the head of the rat moved more than 10 degrees from the vertical axis to either side. Rats with unilateral ischemic stroke exhibited significant biased swing activity to the right (ipsilateral side), while those with no unilateral ischemic stroke displayed equal number of swings to the right and left. The total number of swings made to the biased side was divided by 20 to get percentages of the swings. For the criterion of successful MCAo model completion, biased swing behavior was set at 70% or higher. Rotarod test was also performed to evaluate the degree of hemiparesis and coordinated movements. Prior to MCAo surgery, all rats were pre-trained to stay on the accelerating rotarod (Accuscan, USA) at a constant speed of 8 rpm until they could remain on the rotarod for 100 seconds. After 3 consecutive days of pretraining, we performed three trials in which the rotational speed was gradually increased from 4 to 40 rpm within 5 minutes. The longest time the rats remained on the rotarod was measured as the baseline. Thereafter, the data at day 2 and day 60 post-MCAo were presented as percentages of the longest time on the rotarod of three trials (for each time point) relative to the baseline [21]. Finally, to reveal the cognitive effects of AFS cell transplants, the hippocampal dependent Morris water maze (MWM) task was used to evaluate the effects of AFS cell grafts on MCAo injured young rats (about 18 weeks old old and approximately 300 grams at this time of cognitive testing) on day 63 post-MCAo. All rats were placed in a tank that measured 1.5 meter in diameter with a 10 cm-diameter platform centered at 30 cm from the wall and submerged 1 cm below the surface of water. The water temperature was kept at 25°C. The performance on the MWM was measured over four days of training, with four trials per day. The platform was placed in any of four positions/quadrants: North, South, East, and West. Every animal had an assigned platform position, yet the starting zone (dropping zone) was randomly changed per trial. Once the animals found the escape platform, they were allowed to remain on the platform for 30 seconds between trials and then were transferred to a warm resting cage before the next trial. If any animal did not find its target platform within 60 seconds, the animal was guided to its target platform, and remained on the platfrom for 30 seconds. There was an approximate 30 minute delay between trials for the same rat. A probe trial, where the platform is missing, was evaluated to test consolidation of spatial memory of the two groups. The probe trial was conducted 24 hours after four days of acquisition of learning or training days. During the probe trial, the platform was taken out of the maze and every rat was allowed to freely swim for a period of 60 seconds. Using a computer tracking software (Noldus), the cumulative distance to target quadrant (also called cumulative search error), percent time spent in target quadrant, and the total distance swam, were assessed. The Morris water maze allows the investigator to analyze basic parameters such as place or spatial learning. The acquisition of learning is when the animal must learn how to use distal cues in order to navigate a path to find the hidden platform. During MWM testing, starting locations are randomly selected and they are different every time the animal is placed in the water [22], [23]. Also, it has been shown that MWM learning is not dependent on locomotor ability, because this does not affect swimming speed [23]. The cumulative search error shows the ability to learn the use of distal cues to find the platform. Also, it has been demonstrated that this type of measurement helps to distinguish spatial learning impairments from non-hippocampal dependent functions [22]. To asses learning ability, cumulative search error was calculated using the average distance to the target platform in meters multiplied by the time to the target. Also, total distance swam was calculated as a measure of learning. If animals fail to perform well in this test, it can be taken as learning disability and not as memory impairments. In our test, we showed that both groups of animals, namely vehicle-infused stroke animals and AFS cell-transplanted stroke animals, learned how to use the distal cues to find the hidden platform. Twenty-four hours after the acquisition of learning, a probe trial was given to all the animals to evaluate their consolidation of reference memory. The swimming performance and learning can further be dissociated during probe trials. During probe trial, memory measurements are insensitive to swimming speeds because only the percent time spent in target quadrant is calculated [22], [23]. Spatial retention or spatial memory was assessed during the probe trial by comparing the percent time spent in target platform to adjacent non-target quadrants [22].
Brain tissue fixation and sectioning
Under deep anesthesia, rats were sacrificed on day 63 post-MCAo for immunohistochemical investigations (n = 6 for the vehicle-infused stroke animals and n = 8 for the AFS cell-transplanted stroke animals). Breifly, animals were perfused through the ascending aorta with 200 ml of cold phosphate buffered saline (PBS), followed by 200 ml of 4% paraformaldehyde (PFA) in phosphate buffer (PB). Brains were removed and post-fixed in the same fixative for 24 hours followed by 30% sucrose in PB until completely submerged. Six series of coronal sections were cut at a thickness of 40 μm with a cryostat and stored at −20°C.
Immunohistochemistry
Every sixth 40 μm thick coronal tissue section beginning at AP −1.70 and ending at +0.20 relative to the bregma was isolated for morphological analysis of the SVZ [24]. Sections corresponding to 1.8 mm, 2.3 mm 2.8 mm, 3.3 mm, 3.8 mm, and 4.3 mm posterior to the bregma were randomly selected for quantitative analysis of the DG [24]. Free floating sections were washed three times for five minutes in PBS. For Ki67 and MAP2 staining, samples were blocked for 60 min at room temperature with 5% normal goat serum (Invitrogen) in PBS containing 0.1% Tween 20 (PBST) (Sigma). Sections were then incubated overnight at 4°C with rabbit polyclonal anti-Ki67 (1∶100; Abcam, ab15580) and mouse monoclonal anti-MAP2 (1∶500; Abcam, ab11267) with 5% normal goat serum. The sections were washed five times for ten minutes in PBST and then soaked in 5% normal goat serum in PBST containing corresponding secondary antibodies, goat anti-rabbit IgG-Alexa 488 (green) and goat anti-mouse IgG-Alexa 594 (red) (1∶500; Invitrogen), for 90 minutes. Finally, sections were washed five times for ten minutes in PBST and three times for five minutes in PBS, then processed for Hoechst 33258 (bisBenzimideH 33258 trihydrochloride, Sigma) for 30 min, washed in PBS, and cover-slipped with Fluoromount (Sigma). Control studies included exclusion of primary antibody substituted with 5% normal goat serum in PBS. No immunoreactivity was observed in these controls.
Measurement of infarct volumes
A different set of serial sections corresponding to the same animal were stained with H&E for infarct volume calculations. Six coronal slices between the anterior edge and posterior edge of the infarct were collected and processed for hematoxylin and eosin (H&E) staining from each brain perfused at day 63 post-MCAo. Sections were cut at a thickness of 40 μm by a cryostat. Every sixth coronal tissue section, beginning at AP −1.70 and ending at AP +0.20 anterior to the bregma [24], were randomly selected for measurement of infarct. Brain sections were observed by a microscope equipped with a digital camera. The infarct volume of brain damage was measured in each slice and quantified by a computer assisted image analysis system (NIH Image Software, USA) and calculated by the following formula: [(area of the damaged region in each section) x 0.040] (mm3). Infarct volume was then expressed as a percentage of the ipsilateral hemisphere compared to the contralateral hemisphere.
Statistical analysis
The data were evaluated statistically using analysis of variance (ANOVA) and subsequent post hoc Scheffe's or Bonferonni’s test for behavior. In addition, for analysis of the MWM data, cumulative search error during acquisition of learning was evaluated as a measure of spatial learning. The four trials per day were organized into blocks and analyzed using unpaired Student’s t-tests. Mann-Whitney's U test was used for immunohistochemical investigations. Statistical significance was preset at p<0.05.
Results
AFS cell transplantation ameliorates motor deficits caused by MCAo
Significant treatment effects were detected in motor behavior as revealed by repeated measures of ANOVA (F1,12 = 157.96, p<0.0001). EBST revealed that all animals exhibited no detectable bias in swing activity at baseline (p>0.05) (Fig. 2A), but displayed significant bias in swing activity at day 2 post-MCAo (p<0.0001) (Fig. 2A). EBST at day 60 post-MCAo revealed that the swing bias was significantly decreased in the AFS cell-transplanted stroke animals compared to the vehicle-infused stroke animals (p<0.0001) (Fig. 2A).
Figure 2. Motor deficits were ameliorated in the AFS cell-transplanted stroke animals.

Results revealed that all animals exhibited no detectable bias in swing activity at baseline (p>0.05) (A) and all animals learned to balance on the rotating rod for 60 seconds (p>0.05) (B). EBST revealed a significant biased swing activity on day 2 post-MCAo. EBST conducted on day 60 post-stroke detected a significant decrease in swing bias in the AFS cell-transplanted stroke animals compared to the vehicle-infused stroke animals (*p<0.0001) (A). Rotarod test revealed significant deterioration in motor coordination on day 2 post-MCAo, but on day 60 post-MCAo revealed that the AFS cell-transplanted stroke animals exhibited significantly increased time spent balancing on the rotating rod compared to the vehicle-infused stroke animals (*p<0.0001) (B). Bars represent the mean ± SEM.
Similarly, significant treatment effects were detected in motor coordination as revealed by repeated measures of ANOVA (F1,12 = 48.19, p<0.0001). Rotarod testing conducted prior to MCAo showed there was no significant difference between the two groups of animals, in that all animals learned to balance on the rotating rod for about 60 seconds (p>0.05) (Fig. 2B). However, at day 2 post-MCAo, rotarod testing revealed a significant decrease in the time spent balancing on the rotating rod in both groups (p<0.0001) (Fig. 2B). Rotarod testing conducted at day 60 post-MCAo revealed that the AFS cell-transplanted stroke animals exhibited significantly increased time spent balancing on the rotating rod compared to the vehicle-infused stroke animals (p<0.0001) (Fig. 2B).
AFS cell transplantation attenuates cognitive impairments caused by MCAo
Both groups of rats were tested for spatial memory using a standard MWM design. In this behavioral paradigm, the rats were trained for four trials a day for four consecutive days to find the hidden escape platform. During the MWM task, there were no differences in performance during acquisition of learning task (p>0.05) (Fig. 3A, B). Additionally, there were no disparities in terms of motor function such as swimming capabilities, indicating they equally escaped from the water by finding the platform during the 60 second learning trials. There are four quadrants in the morris water maze, chance performance is equal to 25%. In the probe trial (Fig. 3C) a dashed line has been added at 25% to represent chance [25], [26]. During the probe trial, ANOVA revealed significant treatment effects (F1,12 = 7.586, p<0.001) with a posthoc pairwise test detecting significantly improved performance of AFS cell-transplanted stroke animals compared to vehicle-infused stroke animals in reference memory (Bonferroni’s test, p<0.05) (Fig. 3). We routinely maintain a sham control group as a reference condition on which to assess the experimental treatment groups. This sham group was age-matched, the same gender, and the same strain as the experimental treatment groups, and subjected to the same surgical maneuvers except for the major operation, which in this case was the stroke insult. In addition, our laboratory and others have historically shown the performance of young sham animals in both cumulative search error and distance swam. Sham data were provided as a reference control group (n = 20) for the present vehicle-infused stroke animals and AFS cell-transplanted stroke animals in order to show that MCAo had an impact on cognitive function [22], [23], [27]–[31].
Figure 3. Reference memory, but not spatial navigation, is rescued by AFS cell transplantation.
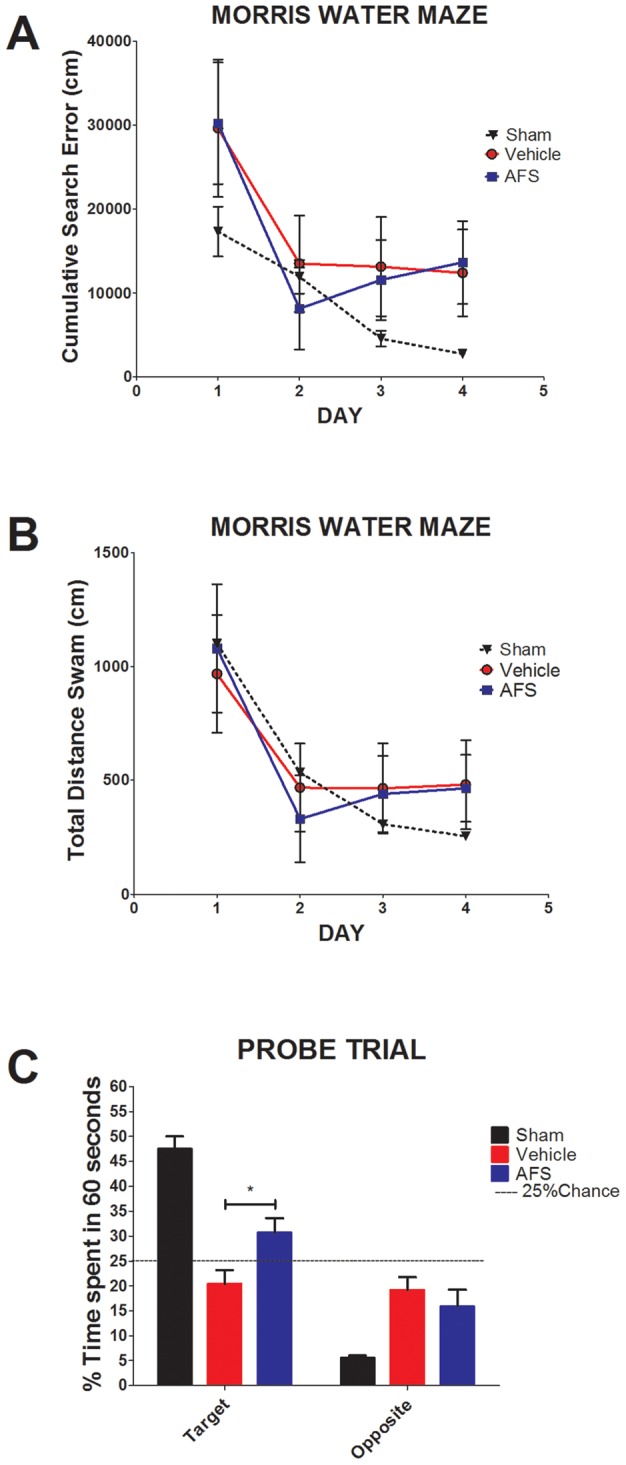
Results revealed that there were no significant differences in cumulative search error and total distance swam between the two treatment groups (A,B), indicating that spatial navigation was not improved in the AFS cell-transplanted stroke animals compared to the vehicle-infused stroke animals (p>0.05) (A). In contrast, reference memory was significantly improved in AFS cell-transplanted stroke animals compared to vehicle-infused stroke animals (C). Twenty-four hours after training in the MWM, rats were tested in a probe trial in which the platform was missing. Results revealed that AFS cell-transplanted stroke animals spent significantly more time in the target quadrant compared to vehicle-infused stroke animals (*p<0.05). Bars represent the mean ± SEM.
AFS cell transplantation decreases infarct volume caused by MCAo
H&E staining revealed that the AFS cell-transplanted stroke animals exhibited significantly decreased infarct volumes compared to the vehicle-infused stroke animals (Fig. 4A, B, and C). There was approximately a 21% difference between the infarct volumes of the AFS cell-transplanted stroke animals and the vehicle-infused stroke animals, which equated to about a 92% reduction in infarct volume in the AFS cell-transplanted stroke animals (p<0.01) (Fig. 4D). This reduction in infarct volume may mediate the improved motor activity, motor coordindation, and memory performance.
Figure 4. Infarct volume was reduced by AFS cell transplantation.
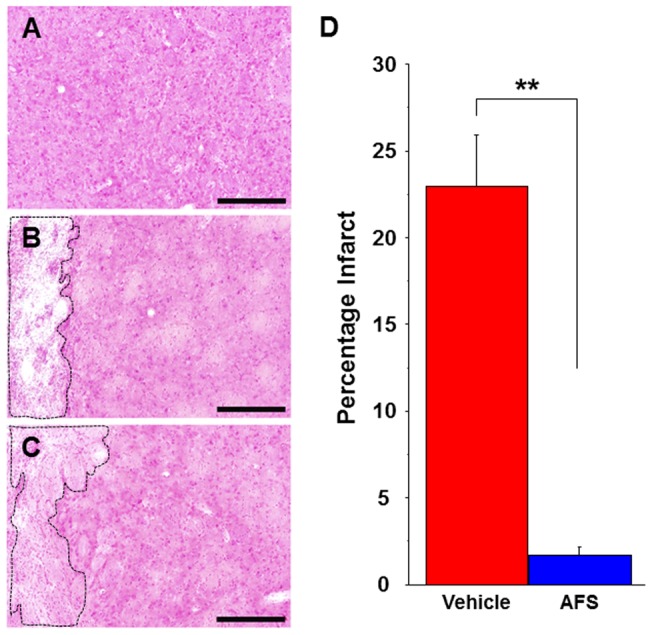
Stronger H&E staining is found in the intact striatum (A) compared to the vehicle-infused stroke animals (B) and AFS cell-transplanted stroke animals (C). Infarct volume is significanly reduced in the AFS cell-transplated stroke animals (C) compared to vehicle-infused stroke animals (B). The striatum in the AFS cell-transplated stroke animals is clearly preserved compared to that of vehicle-infused stroke animals (A-D). Quantitative analyses revealed that percentages of the infarct volumes of rats receiving AFS cell transplants are significantly reduced (**p<0.01) (D). Data are shown as percentages of the infarct volumes present in the ipsilateral hemisphere relative to the contralateral hemisphere. Bars represent the mean ± SEM. Scale bars = 200 µm. Black dotted box represents the infarct area.
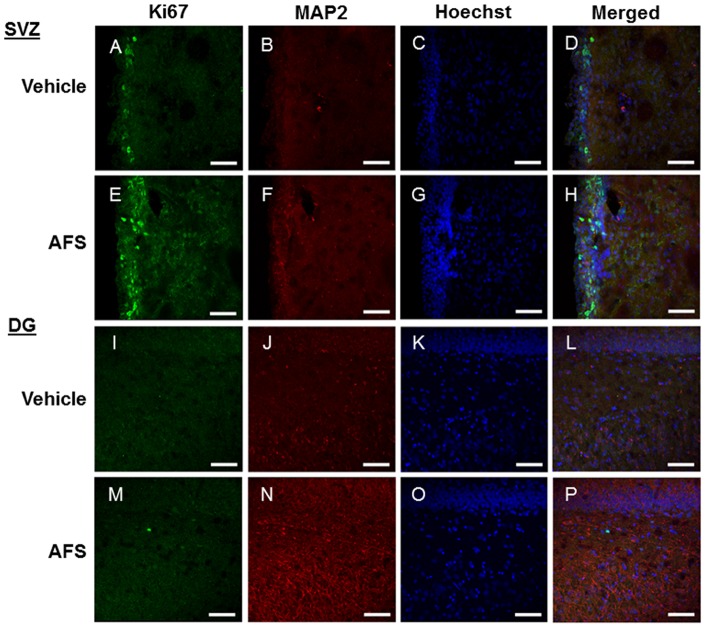
AFS cell transplantation after MCAo increases cell proliferation and decreases neuron loss in the sub-ventricular zone
Immunofluorescence revealed that the number of cells labeled with Ki67, a cell proliferation marker, was significantly upregulated in the subventrical zone (SVZ) of the AFS cell-transplanted stroke animals compared to the vehicle-infused stroke animals (p<0.05) (Fig. 5, 6A). Similarly, the number of MAP2 positive cells was significantly increased in AFS cell-transplanted stroke animals compared to the vehicle-infused stroke animals (p<0.01) (Fig. 5, 6B). A trend towards increased Ki67/MAP2-positive cells was observed in the SVZ of AFS cell-transplanted stroke animals compared to vehicle-infused stroke animals (p = 0.0946) (Fig. 5, 6C).
Figure 5. Enhanced endogenous cell proliferation and neuronal differentiation in the SVZ and DG following AFS cell transplantation.
Ki67 (A, E, I, M) and MAP2 (B, F, J, N) staining revealed an apparent increase in the number of positive cells in the SVZ (A-H) and DG (I-P) of the AFS cell-transplanted stroke animals (E-H, M-P) compared to the vehicle-infused stroke animals (A-D, I-L). Ki67 and MAP-2 double-positive cells are shown in panels: D, H, L, and P. Green: Ki67, Red: MAP2, Blue: Hoechst. Scale bar = 75 µm.
Figure 6. Quantification of cell proliferation and neuronal differentiation in the SVZ and DG.
The number of cells labeled with Ki67 (A) and MAP2 (B) were significantly increased in the SVZ and DG of AFS cell-transplated stroke animals compared to vehicle-infused stroke animals (*p<0.05, **p<0.01, ****p<0.0001) (A, B, D, E). While there was a trend towards increased Ki67/MAP2 double-positive cells in the SVZ (p = 0.0946) (C), the number of cells labeled with both Ki67 and MAP2 were significantly increased in the DG of AFS cell-transplated stroke animals compared to vehicle-infused stroke animals (***p<0.001) (F). Bars represent the mean ± SEM.
AFS cell transplantation after MCAo increases cell proliferation and decreases neuron loss in the dentate gyrus
The number of cells labeled with Ki67 was significantly increased in the dentate gyrus of the AFS cell-transplanted stroke animals compared to vehicle-infused stroke animals (p<0.05) (Fig 5, 6D). MAP2 was also significantly increased in the DG of the AFS cell-transplanted stroke animals compared to vehicle-infused stroke animals (p<0.0001) (Fig. 5, 6E). Similarly, the number of cells labeled with Ki67/MAP2 double-positive cells was significantly increased in the DG of AFS cell-transplanted stroke animals compared to vehicle-infused stroke animals (p<0.001) (Fig. 5, 6F).
Discussion
This study reports the therapeutic potential of AFS cell transplantation in stroke animals, characterized by attenuation of stroke-induced behavioral and histological deficits, possibly via enhancement of endogenous repair mechanisms. We demonstrate that intravenous AFS cell transplantation ameliorated both motor and cognitive impairment and was accompanied by reduction of infarct volume. These functional improvements in AFS cell-transplanted stroke animals coincided with increased cell proliferation and neuronal differentiation in the two neurogenic sites: SVZ and DG, suggesting the role of graft-induced host tissue repair in this brain remodeling process following stroke (Fig. 7).
Figure 7. Schematic diagram of proposed mechanism of action of AFS cell transplants in stroke.
This schematic diagram represents a speculative reparative mechanism underlying the functional recovery produced by AFS transplantation in stroke animals. Following AFS cell transplantation, neurotrophic factors are upregulated, which subsequently stimulates, endogenous cell proliferation, and neuronal differentiation to the injured area. These multi-pronged neurorestorative processes contribute to the rescue of the peri-infarct area leading to behavioral recovery.
In the clinic, stroke symptoms involve deficits in sensorimotor and cognitive functions [32], [33], [34]. To date, most stroke animal models have focused on motor impairments, neglecting the cognitive declines that proceed after the brain insult [35], [36], [37]. Studies on the pathology of ischemic stroke support the negative impact that this insult imposes to hippocampal functions. It is well known that learning and memory consolidation are part of brain cognitive functions. The hippocampus plays a pivotal role when encoding and retaining new memories [38]. Animal models of hippocampal ablation have helped to address the function of this brain structure, and its relationship with learning and memory formation [39], [40], [41]. Adult neurogenesis or the generation of new neurons as the brain matures, and its decline during aging correlates with the deterioration of cognitive functions [42], [43], [44]. The post-stroke neuropathological cascade of events affecting the hippocampal area implicates the down regulation of glutamate receptors, decreased neurogenesis, exacerbated glial activation and chronic inflammation [45], [46], [47]. In addition, there are in vivo stroke studies characterizing hippocampus-mediated cognitive impairments clearly delineating these ischemia-induced learning and memory deficits from sensorimotor and neurological dysfunctions [45], [47], [48].
Consequently, the present study expands experimental techniques to assess both motor and cognitive symptoms in an animal model of stroke to fully characterize the disease progression, as well as the therapeutic benefits of AFS cell transplantation. Our present observations on cognitive testing showed no difference in learning performance between the two treatment groups during MWM training, but the AFS cell-transplanted stroke animals exhibited a significantly improved reference memory during the MWM probe trial compared to the vehicle-infused stroke animals, which correlated with our results showing significant increase of neurogenesis relative to vehicle-infused stroke animals in the DG of the hippocampus of AFS cell-transplated stroke animals. In particular, the AFS cell-transplanted stroke animals spent a significantly longer time exploring the target quadrant than the vehicle-infused stroke animals, but both groups spent a comparable amount of time of exploration inside the non-target/opposite quadrant. This increment in exploration time in target quadrant indicates that the AFS cell-transplanted stroke animals displayed a recovery of hippocampal dependent reference memory in that they discriminated between target and non-target quadrants. On the other hand, the vehicle-infused stroke animals exhibited a selective reference memory deficit in that they could not discriminate between target and non-target quandrants. Our data support the MWM test as a hippocampal dependent task for spatial memory and reference memory [49], [50], [51], [52], and long-term potentiation [2], [53], [54], [55]. These data allow us to advance the concept that transplanted AFS cells selectively aid in the recovery of the reference memory, but not task acquisition. The differences in reference memory between groups are not attributable to motor deficits or swimming abilities, as both groups showed a similar performance during neurological and motor testing (EBST and Rotarod), and during MWM training. Cognitive improvements of the transplanted group were also previously associated with the reduction of infarct volumes of the striatum [56], [57], [58], [59], a brain structure implicated in the cognitive functions of memory systems [60], [61], [62]. Taken together, these results reveal that transplanted AFS cells reduced the infarction of brain areas implicated in the performance of cognitive tasks, and enhanced the level of neurogenesis when compared to our vehicle-infused stroke animals.
AFS cells transplanted intracerebroventricularly three days post-MCAo attenuate cognitive deficits associated with stroke [19]. To date, stroke studies utilizing AFS cells have focused on the intracerebral cell transplantation route [19], [63]. A recent study examined the feasibility of intraperitoneal administration of AFS cells in newborn rats [64]. The present intravenous route of transplantation is a minimally invasive procedure and poses less risk to the patient compared to intracerebral transplantation. Due to the acute nature of stroke, a peripheral injection route is preferred so that therapeutics can be administered quickly after the onset of a stroke. Here, AFS cells were transplanted intravenously, enhancing the clinical relevance of this study. A clinical trial, from Celgene Cellular Therapeutics, that utilizes placenta/amnion-derived stem cells transplanted intravenously in stroke patients is currently underway [65].
The present observations of transplant-mediated recovery of motor and cognitive functions mirror the findings by Rehni and colleagues [19]. However, the present study expanded the short timeline of seven days in that previous study to 63 days. The guidelines of Stem cell Therapeutics as an Emerging Paradigm for Stroke (STEPS) require that stem cell therapies be tested in multiple strains of adult and aged male and female rats to evaluate safety and efficacy [35], [36], [37]. The STEPS criteria also emphasize the need for long term (at least one month) testing after the administration of neurorestorative therapies [35], [36], [37]. Long term testing is critical for evaluating the safety of stem cell administration, as well as determining the long-term efficacy of transplantation. A novel motor test called the rotarod slip test, which monitors the number of slips of the paralytic hind limb from a rotarod, is able to detect mild hindlimb paresis in the acute and sub-acute phase after [66]. We will incorporate this new rotorod slip test in future studies to characterize functional deficits in stroke, as well as transplant-mediated therapeutic benefits. The present behavioral tests have been accepted by the FDA as appropriate tests for translating stem cell products to the clinic.
The present study transplanted rat AFS cells into stroke rats, which supports the envisioned use of allogenic grafts in the clinic. The differentiation potential of AFS cells falls somewhere between the pluripotent embryonic stem cells and the multipotent adult stem cells, making the amniotic fluid an optimal source of stem cells [1], [12]. Furthermore, since AFS cells can be obtained during a routine amniocentesis isolation of AFS cells does not harm the developing fetus [1], [67]. Our lab has a long-standing interest in intravenous stem cell transplantation for neurological disorders, in particular stroke [68], [69], [70], [71] and the use of amniotic tissue and fluid [1], [6], [7], [8].
Histologic analysis revealed that AFS cell-transplanted stroke animals exhibited a reduction in infarct areas compared to the vehicle-infused stroke animals. his robust reduction in cerebral infarcts was likely due to transplanted AFS cells increasing cell proliferation in tandem with a decrease in neuronal loss in both the SVZ and the hippocampus [2]. As the two neurogenic niches in the brain, the SVZ and DG, are critical to the repair of damaged brain tissue [72], [73], [74], [75]. The results suggest that AFS cell transplantation might have boosted endogenous repair mechanisms by maximizing the potential of these two neurogenic sites to confer a host brain remodeling process. The lack of labeled AFS cells is a limitation to this study, which prevented examination of the fate of the transplanted cells. Such cell labeling process (e.g., viral vector or DiL dye labeling) may change the cell phenotype and alter the cell’s functional capabilities [76], [77], [78]. With the present demonstration of functional effects of the transplanted AFS cells, future studies will evaluate the similar therapeutic benefits, as well as fate of transplanted labeled and non-labeled AFS cells.
In summary, intracerebral administration of AFS cells ameliorate in the short-term the behavioral deficits associated with stroke [19]. That AFS cells when transplanted intravenously on day 35 similarly attenuated motor and cognitive impairments accompanied by reduced histological deficits and increased cell proliferation and differentiation in the host brain (Fig. 7) represent highly innovative observations. These findings directly advance our basic scientific knowledge about a potent mechanism of brain repair in stroke, and provide pivotal guidance into the translational applications of cell therapy to stroke patients.
Funding Statement
CVB is supported in part by SanBio Inc., Celgene Cellular Therapeutics, KMPHC and NeuralStem Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Antonucci I, Stuppia L, Kaneko Y, Yu S, Tajiri N, et al. (2011) Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell transplantation 20: 789–795. [DOI] [PubMed] [Google Scholar]
- 2. Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39. [DOI] [PubMed] [Google Scholar]
- 3. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. (2012) Executive summary: heart disease and stroke statistics–2012 update: a report from the american heart association. Circulation 125: 188–197. [DOI] [PubMed] [Google Scholar]
- 4. Graham GD (2003) Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke; a journal of cerebral circulation 34: 2847–2850. [DOI] [PubMed] [Google Scholar]
- 5. Yip TR, Demaerschalk BM (2007) Estimated cost savings of increased use of intravenous tissue plasminogen activator for acute ischemic stroke in Canada. Stroke; a journal of cerebral circulation 38: 1952–1955. [DOI] [PubMed] [Google Scholar]
- 6. Kaneko Y, Hayashi T, Yu S, Tajiri N, Bae EC, et al. (2011) Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: a new perspective to neuroprotection. Journal of pineal research 50: 272–280. [DOI] [PubMed] [Google Scholar]
- 7. Manuelpillai U, Moodley Y, Borlongan CV, Parolini O (2011) Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 32 Suppl 4S320–325. [DOI] [PubMed] [Google Scholar]
- 8. Yu SJ, Soncini M, Kaneko Y, Hess DC, Parolini O, et al. (2009) Amnion: a potent graft source for cell therapy in stroke. Cell transplantation 18: 111–118. [DOI] [PubMed] [Google Scholar]
- 9. Fauza D (2004) Amniotic fluid and placental stem cells. Best practice & research Clinical obstetrics & gynaecology 18: 877–891. [DOI] [PubMed] [Google Scholar]
- 10. In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, et al. (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102: 1548–1549. [DOI] [PubMed] [Google Scholar]
- 11. McLaughlin D, Tsirimonaki E, Vallianatos G, Sakellaridis N, Chatzistamatiou T, et al. (2006) Stable expression of a neuronal dopaminergic progenitor phenotype in cell lines derived from human amniotic fluid cells. Journal of neuroscience research 83: 1190–1200. [DOI] [PubMed] [Google Scholar]
- 12. Prusa AR, Hengstschlager M (2002) Amniotic fluid cells and human stem cell research: a new connection. Medical science monitor : international medical journal of experimental and clinical research 8: RA253–257. [PubMed] [Google Scholar]
- 13. Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M (2003) Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Human reproduction 18: 1489–1493. [DOI] [PubMed] [Google Scholar]
- 14. Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL, et al. (2006) Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biology of reproduction 74: 545–551. [DOI] [PubMed] [Google Scholar]
- 15. Tsai MS, Lee JL, Chang YJ, Hwang SM (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Human reproduction 19: 1450–1456. [DOI] [PubMed] [Google Scholar]
- 16. De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, et al. (2007) Isolation of amniotic stem cell lines with potential for therapy. Nature biotechnology 25: 100–106. [DOI] [PubMed] [Google Scholar]
- 17. Mauro A, Turriani M, Ioannoni A, Russo V, Martelli A, et al. (2010) Isolation, characterization, and in vitro differentiation of ovine amniotic stem cells. Veterinary research communications 34 Suppl 1S25–28. [DOI] [PubMed] [Google Scholar]
- 18. Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, et al. (2007) Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem cells and development 16: 931–952. [DOI] [PubMed] [Google Scholar]
- 19. Rehni AK, Singh N, Jaggi AS, Singh M (2007) Amniotic fluid derived stem cells ameliorate focal cerebral ischaemia-reperfusion injury induced behavioural deficits in mice. Behavioural brain research 183: 95–100. [DOI] [PubMed] [Google Scholar]
- 20. Borlongan CV, Sanberg PR (1995) Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. The Journal of neuroscience : the official journal of the Society for Neuroscience 15: 5372–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi K, Yasuhara T, Shingo T, Muraoka K, Kameda M, et al. (2008) Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain research 1234: 172–182. [DOI] [PubMed] [Google Scholar]
- 22. Gallagher M, Burwell R, Burchinal M (1993) Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behavioral neuroscience 107: 618–626. [DOI] [PubMed] [Google Scholar]
- 23. Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature protocols 1: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition: Academic press.
- 25. Clark RE, Broadbent NJ, Squire LR (2007) The hippocampus and spatial memory: findings with a novel modification of the water maze. The Journal of neuroscience : the official journal of the Society for Neuroscience 27: 6647–6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Norris CM, Foster TC (1999) MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiology of learning and memory 71: 194–206. [DOI] [PubMed] [Google Scholar]
- 27. Anisman H, McIntyre DC (2002) Conceptual, spatial, and cue learning in the Morris water maze in fast or slow kindling rats: attention deficit comorbidity. The Journal of neuroscience : the official journal of the Society for Neuroscience 22: 7809–7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bergado JA, Almaguer W, Rojas Y, Capdevila V, Frey JU (2011) Spatial and emotional memory in aged rats: a behavioral-statistical analysis. Neuroscience 172: 256–269. [DOI] [PubMed] [Google Scholar]
- 29. Clarke MS, Prendergast MA, Terry AV Jr (1999) Plasma membrane ordering agent pluronic F-68 (PF-68) reduces neurotransmitter uptake and release and produces learning and memory deficits in rats. Learning & memory 6: 634–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pisu MG, Dore R, Mostallino MC, Loi M, Pibiri F, et al. (2011) Down-regulation of hippocampal BDNF and Arc associated with improvement in aversive spatial memory performance in socially isolated rats. Behavioural brain research 222: 73–80. [DOI] [PubMed] [Google Scholar]
- 31. Acosta S, Jernberg J, Sanberg CD, Sanberg PR, Small BJ, et al. (2010) NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation research 13: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, et al. (2008) Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Annals of neurology 63: 236–246. [DOI] [PubMed] [Google Scholar]
- 33. Lin MS, Chiu MJ, Wu YW, Huang CC, Chao CC, et al. (2011) Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion and cerebral ischemia. Stroke; a journal of cerebral circulation 42: 2850–2854. [DOI] [PubMed] [Google Scholar]
- 34. Rush BK, McNeil RB, Gamble DM, Luke SH, Richie AN, et al. (2010) Behavioral symptoms in long-term survivors of ischemic stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 19: 326–332. [DOI] [PubMed] [Google Scholar]
- 35. Chopp M, Steinberg GK, Kondziolka D, Lu M, Bliss TM, et al. (2009) Who's in favor of translational cell therapy for stroke: STEPS forward please? Cell transplantation 18: 691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borlongan CV (2009) Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke; a journal of cerebral circulation 40: S146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke; a journal of cerebral circulation 40: 510–515. [DOI] [PubMed] [Google Scholar]
- 38. Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, et al. (2008) Spatial relational memory requires hippocampal adult neurogenesis. PloS one 3: e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, et al. (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America 103: 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12: 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drapeau E, Nora Abrous D (2008) Stem cell review series: role of neurogenesis in age-related memory disorders. Aging cell 7: 569–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, et al. (2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell stem cell 8: 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McDonald HY, Wojtowicz JM (2005) Dynamics of neurogenesis in the dentate gyrus of adult rats. Neuroscience letters 385: 70–75. [DOI] [PubMed] [Google Scholar]
- 45. Dhawan J, Benveniste H, Nawrocky M, Smith SD, Biegon A (2010) Transient focal ischemia results in persistent and widespread neuroinflammation and loss of glutamate NMDA receptors. NeuroImage 51: 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, et al. (2009) Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behavioral neuroscience 123: 224–230. [DOI] [PubMed] [Google Scholar]
- 47. Zvejniece L, Svalbe B, Liepinsh E, Pulks E, Dambrova M (2012) The sensorimotor and cognitive deficits in rats following 90- and 120-min transient occlusion of the middle cerebral artery. Journal of neuroscience methods 208: 197–204. [DOI] [PubMed] [Google Scholar]
- 48. Wattanathorn J, Jittiwat J, Tongun T, Muchimapura S, Ingkaninan K (2011) Zingiber officinale Mitigates Brain Damage and Improves Memory Impairment in Focal Cerebral Ischemic Rat. Evidence-based complementary and alternative medicine : eCAM 2011: 429505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JP, et al. (1997) Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behavioral neuroscience 111: 1184–1196. [DOI] [PubMed] [Google Scholar]
- 50. Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America 101: 14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gillani RL, Tsai SY, Wallace DG, O'Brien TE, Arhebamen E, et al. (2010) Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy. Behavioural brain research 208: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jurgens HA, Amancherla K, Johnson RW (2012) Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 32: 3958–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gusev PA, Alkon DL (2001) Intracellular correlates of spatial memory acquisition in hippocampal slices: long-term disinhibition of CA1 pyramidal cells. Journal of neurophysiology 86: 881–899. [DOI] [PubMed] [Google Scholar]
- 54. Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC (2002) Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. The European journal of neuroscience 16: 1593–1598. [DOI] [PubMed] [Google Scholar]
- 55. Xu J, Wang Y, Li N, Xu L, Yang H, et al. (2012) l-3-n-butylphthalide improves cognitive deficits in rats with chronic cerebral ischemia. Neuropharmacology 62: 2423–2428. [DOI] [PubMed] [Google Scholar]
- 56.Fukunaga A, Kawase T, Uchida K (2003) Functional recovery after simultaneous transplantation with neuro-epithelial stem cells and adjacent mesenchymal tissues into infarcted rat brain. Acta neurochirurgica 145: 473–480, discussion 480–471. [DOI] [PubMed]
- 57. Fukunaga A, Uchida K, Hara K, Kuroshima Y, Kawase T (1999) Differentiation and angiogenesis of central nervous system stem cells implanted with mesenchyme into ischemic rat brain. Cell transplantation 8: 435–441. [DOI] [PubMed] [Google Scholar]
- 58. Mimura T, Dezawa M, Kanno H, Yamamoto I (2005) Behavioral and histological evaluation of a focal cerebral infarction rat model transplanted with neurons induced from bone marrow stromal cells. Journal of neuropathology and experimental neurology 64: 1108–1117. [DOI] [PubMed] [Google Scholar]
- 59. Nishino H, Koide K, Aihara N, Kumazaki M, Sakurai T, et al. (1993) Striatal grafts in the ischemic striatum improve pallidal GABA release and passive avoidance. Brain research bulletin 32: 517–520. [DOI] [PubMed] [Google Scholar]
- 60. Ebrahimi A, Pochet R, Roger M (1992) Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neuroscience research 14: 39–60. [DOI] [PubMed] [Google Scholar]
- 61. Miyoshi E, Wietzikoski EC, Bortolanza M, Boschen SL, Canteras NS, et al. (2012) Both the dorsal hippocampus and the dorsolateral striatum are needed for rat navigation in the Morris water maze. Behavioural brain research 226: 171–178. [DOI] [PubMed] [Google Scholar]
- 62. Pisani A, Centonze D, Bernardi G, Calabresi P (2005) Striatal synaptic plasticity: implications for motor learning and Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society 20: 395–402. [DOI] [PubMed] [Google Scholar]
- 63. Liu T, Wu J, Huang Q, Hou Y, Jiang Z, et al. (2008) Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock 29: 603–611. [DOI] [PubMed] [Google Scholar]
- 64. Ghionzoli M, Cananzi M, Zani A, Rossi CA, Leon FF, et al. (2010) Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: implication for therapy. Pediatric surgery international 26: 79–84. [DOI] [PubMed] [Google Scholar]
- 65.Health USNIo (2012) Clinical Trials.
- 66. Tabuse M, Yaguchi M, Ohta S, Kawase T, Toda M (2010) A simple behavioral test for locomotor function after brain injury in mice. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 17: 1412–1416. [DOI] [PubMed] [Google Scholar]
- 67. Kalogiannidis I, Prapa S, Dagklis T, Karkanaki A, Petousis S, et al. (2011) Amniocentesis-related adverse outcomes according to placental location and risk factors for fetal loss after midtrimester amniocentesis. Clinical and experimental obstetrics & gynecology 38: 239–242. [PubMed] [Google Scholar]
- 68. Borlongan CV, Evans A, Yu G, Hess DC (2005) Limitations of intravenous human bone marrow CD133+ cell grafts in stroke rats. Brain research 1048: 116–122. [DOI] [PubMed] [Google Scholar]
- 69. Borlongan CV, Hadman M, Sanberg CD, Sanberg PR (2004) Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke; a journal of cerebral circulation 35: 2385–2389. [DOI] [PubMed] [Google Scholar]
- 70. Ou Y, Yu S, Kaneko Y, Tajiri N, Bae EC, et al. (2010) Intravenous infusion of GDNF gene-modified human umbilical cord blood CD34+ cells protects against cerebral ischemic injury in spontaneously hypertensive rats. Brain research 1366: 217–225. [DOI] [PubMed] [Google Scholar]
- 71. Yasuhara T, Borlongan CV, Date I (2006) Ex vivo gene therapy: transplantation of neurotrophic factor-secreting cells for cerebral ischemia. Frontiers in bioscience : a journal and virtual library 11: 760–775. [DOI] [PubMed] [Google Scholar]
- 72. Ekdahl CT, Kokaia Z, Lindvall O (2009) Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 73. Jezierski A, Gruslin A, Tremblay R, Ly D, Smith C, et al. (2010) Probing stemness and neural commitment in human amniotic fluid cells. Stem cell reviews 6: 199–214. [DOI] [PubMed] [Google Scholar]
- 74. Prasongchean W, Bagni M, Calzarossa C, De Coppi P, Ferretti P (2012) Amniotic fluid stem cells increase embryo survival following injury. Stem cells and development 21: 675–688. [DOI] [PubMed] [Google Scholar]
- 75. Zhang C, Chopp M, Cui Y, Wang L, Zhang R, et al. (2010) Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. Journal of neuroscience research 88: 3275–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Burns TC, Verfaillie CM, Low WC (2009) Stem cells for ischemic brain injury: a critical review. The Journal of comparative neurology 515: 125–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Clavel C, Verfaillie CM (2008) Bone-marrow-derived cells and heart repair. Current opinion in organ transplantation 13: 36–43. [DOI] [PubMed] [Google Scholar]
- 78. Hematti P, Hong BK, Ferguson C, Adler R, Hanawa H, et al. (2004) Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS biology 2: e423. [DOI] [PMC free article] [PubMed] [Google Scholar]