Abstract
Background
Pastoralists in low-income countries usually live in close proximity to their animals and thus represent an important repository of information about livestock disease. Since wild and domestic animals often mix freely whilst grazing, pastoralists are also able to observe first-hand the diseases that are present in wildlife and as such are key informants in disease outbreaks in sylvatic animals. We report here the findings of the first study of the knowledge and role of Masai pastoralists in mange in wildlife and livestock in Masai Mara, Kenya.
Methodology/Principal Findings
In this paper we describe the knowledge of mange accrued by 56 Masai pastoralists in Kenya and how they respond to it in both wildlife and livestock. In total, 52 (93%) pastoralists had a clear idea of the clinical appearance of mange, 13 (23%) understood its aetiology and 37 (66%) knew that mites were the causal agent. Thirty-nine (69%) believed that mange cross-infection between domestic and wild animals occurs, while 48 (85%) had observed mange in domestic animals including sheep (77%), goats (57%), dogs (24%) and cattle (14%). The pastoralists had also observed wild animals infected with mange, above all lions (19%), gazelles (14%), cheetahs (12%) and wildebeests (2%). In 68% of cases Masai pastoralists treat mange infection or apply control measures, most commonly via the topical use of acaricides (29%) and/or the reporting of the outbreak to the veterinary authorities (21%). In the period 2007–2011, Kenya Wildlife Service received 24 warnings of 59 wild animals with mange-like lesions from the Masai Mara pastoralist community. The reported species were cheetah, lion, wild dog, Thomson’s gazelle and wildebeest.
Conclusion
Masai pastoralists have good knowledge of mange epidemiology and treatment. Their observations and the treatments they apply are valuable in the control of this disease in both wild and domestic animals.
Introduction
Mange is a highly contagious skin disease caused by one or a combination of several species of mites [1]. Mites affect both domestic animals and humans, but also wildlife of zoonotic importance [2], [3]. The most common mite species in wild and domestic animals in Kenya is Sarcoptes scabiei. This parasite is a ubiquitous ectoparasite that infects more than 100 species of mammals worldwide [3], [4]. In humans it is known to cause considerable morbidity in a number of different counties [5], [6] and epidemics can be caused by contagion from a single case of scabies in crowded living conditions [7]. Sarcoptic mange may lead to considerable economic losses in domestic animals [8] with repercussions for the animal trade [9]. It also has devastating consequences for wild animals, above all in isolated populations [10], [11], a situation that is worsening due to the limitations of available chemotherapy [12]–[14].
An appropriate disease control program against mites should take into account the entire ecosystem and thus integrate measures targeting both wildlife and livestock [15]. Disease control in domestic animals may be able to interrupt mange transmission to wild animals and vice versa [14], [16].
Recently, attempts have been made to understand mange molecular epidemiology using genetic tools to differentiate between isolates from different hosts and geographical regions [10], [17]–[24]. The epidemiology of mange is still not well understood and seems to differ between animal species and areas of the world [25].
Although mange is well known in the Masai Mara ecosystem [26]–[29], no data is available regarding how much pastoralists know about this disease. As the pastoralists use a number of different methods of controlling disease in their livestock, it is imperative to gather information on how they control mange in this ecosystem. They usually live in close contact with their animals and are an important repository of information about the challenges their animals have to face. Since wild and domestic animals mix freely during grazing, they also have first-hand knowledge of wildlife. Most of the reports of mange-infected wildlife that reach the veterinary department of the Kenya Wildlife Service are received from tour operators and wildlife officers (Veterinary Field Reports). Reports from areas outside the protected areas are brought to the attention of wildlife officers by pastoralists who represent a valuable source of information regarding the presence of mange in wild animals. Thus, the aim of this study was to evaluate the knowledge and practices of Masai pastoralists regarding mange and the repercussions they have on disease management. To date no study has ever evaluated the extent to which local pastoralists’ knowledge and understanding of mange in wildlife and livestock might play a role in mange management and control.
Methods
Study Area: Masai Mara National Reserve, Kenya
This 1510 km2 National Reserve is situated in SW Kenya and is effectively the northern continuation of the Serengeti National Park in Tanzania. Rainfall increases along a southeast–northwest gradient and rainy seasons are markedly bimodal. The terrain of the reserve is primarily open grassland with seasonal watercourses. All members of the ‘Big Five’ group of game species (lion, leopard, African elephant, African buffalo and Black Rhinoceros) are present. The millions of wildebeest that dominate Masai Mara migrate northwards in July from the Serengeti plains in search of fresh pasture, before returning southwards in October. This great migration involves some 1,300,000 wildebeest, 500,000 Thomson’s gazelles, 97,000 topi, 18,000 elands, and 200,000 zebras. The migrants are followed along their annual circular route by predators, most notably lions and hyenas. Numerous other antelope species are also found in the National Reserve, including Grant’s gazelle, impalas, topi, elands, duikers, Coke’s hartebeests, zebras and Masai giraffes. The Masai people living around the Masai Mara National Reserve depend on livestock for their livelihoods. Pastoral livestock rearing is the dominant production system in this area, which is characterised by intensive wildlife-livestock-human interaction that includes the sharing of pasture and water (Fig. 1). The livestock species consist mainly of goats, camels, cattle and sheep.
Figure 1. Mixed wildlife and livestock grazing.
Study Population and Design
The register of the Kenya Wildlife Service (KWS) lists 150 male householder pastoralists (commonly referred to as Manyattas) aged over 18 years and living within five kilometres of the reserve boundary who essentially form the interface of human-wildlife-livestock interactions. All of the registered households own livestock. From this register, we randomly selected 56 respondents who were invited during visits to their dwellings or via mobile phone to participate in this study. We included only male heads of families as in traditional Masai culture women are not authorised to discuss livestock with visitors or traders without consulting their husbands. The ages of the chosen pastoralists ranged between 18 and 50 years old. Interviews were carried out over a period of one month (June and July 2008) and were conducted by two veterinarians from KWS who are experts on animal disease and proficient in the Masai language. Each interview (conducted in Swahili) lasted approximately 30 minutes. As most of the respondents had either little educational background or were illiterate, questions were read out aloud.
The questionnaire contained (i) structured questions with binary variables and (ii) semi-structured questions with both binary variables (‘yes’ or ‘no’) followed by an open question in which respondents were free to provide open responses (for more details, see supplementary material). The first set of questions aimed to assess pastoralists’ basic knowledge of mange. Respondents were asked if they had ever heard of a disease called mange in any animal, irrespective of whether it was a domestic or wild animal, and if they knew its origin. They were also asked to mention if they had ever heard of parasites known as mites. Another set of questions was used to investigate whether any of the respondent’s animals had ever suffered from mange, whether they were aware of any infected wild animals, and to what extent they were aware of the transmission of mange at the wildlife-livestock interface, above all in light of the fact that livestock and wild animals interact regularly during grazing or when drinking. Finally, participants were asked to mention what control and preventive measures could be used to combat mange and which methods they used (Table S1). Additionally, we documented all warnings of the appearance of mangy wild animals reported by pastoralists from Masai Mara to the Department of Veterinary and Capture Services of Kenya Wildlife Service that occurred between March 2007 and June 2011.
Capture of Mange-infected Animals and Parasite Identification
Most of the reported animals were captured by chemical immobilization through darting using etorphine hydrochloride (M99® 9.8 mg/ml, Novartis South Africa Pty Ltd, Isando, South Africa) combined with Xylazine hydrochloride (Ilium Xylazil-100 100 mg/ml, Troy Laboratories Pty Ltd, Smithfield, Australia). After sampling and treatment, the animals were revived with diprenorphine hydrochloride (M5050® 12 mg/ml, Novartis South Africa Pty Ltd, Isando, South Africa) and Atipamezole hydrochloride (Antisedan® 5 mg/ml, Pfizer laboratories Pty Ltd, Sandton, South Africa).
Affected areas of the skin were scraped with a scalpel until bleeding to obtain crusts for parasitological examination [30]. Scrapings were placed in universal bottles containing 70% ethanol and transported to the laboratory. All mites were identified on the basis of known morphological criteria [31], [32].
Mange Treatment
Infected animals were given 1% ivermectin (Kalamectin 1% w/v, Kela NV, St. Lenaartseweg, Belgium), administered sub-cutaneously. Dexamethasone (Glucortin-20® 2 mg/ml, Interchemie, Castenray, Netherlands), an anti-inflammatory and antipruritic drug, was also used. On affected areas of skin, a broad-spectrum oxytetracycline based antibiotic (Alamycin LA® 200 mg/ml, Norbrook Laboratories Ltd, Newry, North Ireland) was applied to prevent bacterial superinfections.
Ethics
The pastoralists in our study either personally gave their written consent to be included in the survey or, in the case of illiterate pastoralists or those with little educational background, the appropriate document was signed by their families on their behalf. The ethics committee of the Department of Veterinary and Capture Services of the Kenya Wildlife Service (KWS) approved the study and the animal capture protocols. KWS guidelines on Wildlife Veterinary Practice-2006 were followed. All KWS veterinarians follow the Veterinary Surgeons and Veterinary Para-Professionals Act 2011, Laws of Kenya, which regulates veterinary practices in Kenya.
Results
As defined by the study design, all interviewed pastoralists were male. The mean age was 31.3±8.75 years. Thirty-one (55%) of the respondents were unable to read or to understand the questionnaires and so were assumed to be illiterate. Of the 56 pastoralists interviewed, 52 (92%) had a clear idea of the clinical appearance of mange. However, only 37 (66%) of these pastoralists understood the aetiology of mange (the respondents confirmed that they knew the cause of mange, i.e. that it is caused by small microscopic parasites not visible to the naked eye that could burrow under the skin), while 13 (23%) knew that mites were the causal agent (i.e. they stated that ilpepedo, the local name for the mite, is the origin of mange in animals). Thirty-nine (69%) believed that the cross-infection of mange between domestic and wild animals could occur (Table 1). When asked if their domestic animals had ever been affected by mange, 48 (85%) of them answered affirmatively. The animals involved were most commonly sheep and goats (Table 2). Interestingly, the pastoralists had also observed wild animals infected with mange, above all lions, gazelles, and cheetahs (Table 2).
Table 1. Pastoralists’ knowledge of mange and treatment and control practices (n = 56).
| No. pastoralists | % | |
| Knowledge of mange | 52 | 93 |
| Knowledge of the aetiology of mange | 37 | 66 |
| Knowledge of mites | 13 | 23 |
| Belief in cross-infection between domestic animals and wildlife | 39 | 70 |
| Seen infected domestic and/or wildlife (for more details see Table 2) | 48 | 86 |
| Practiced treatment or control measures (see text for details) | 38 | 68 |
Table 2. Animal species that pastoralists identified as being affected by mange.
| Animal species | N | % |
| Sheep (Ovis aries) | 43 | 78 |
| Goats (Capra hircus) | 32 | 58 |
| Dogs (Canis lupus familiaris) | 14 | 24 |
| Lions (Panthera leo) | 11 | 20 |
| Thomson’s gazelle (Gazella thomsonii) | 8 | 15 |
| Cattle (Bos indicus) | 8 | 14 |
| Cheetah (Acinonyx jubatus) | 7 | 12 |
| Wildebeest (Connochaetes taurinus) | 2 | 2 |
| Wild dogs (Lycaon pictus)) | 1 | 0.5 |
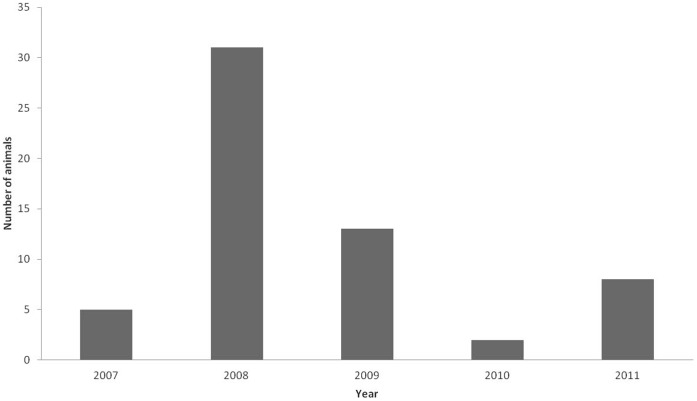
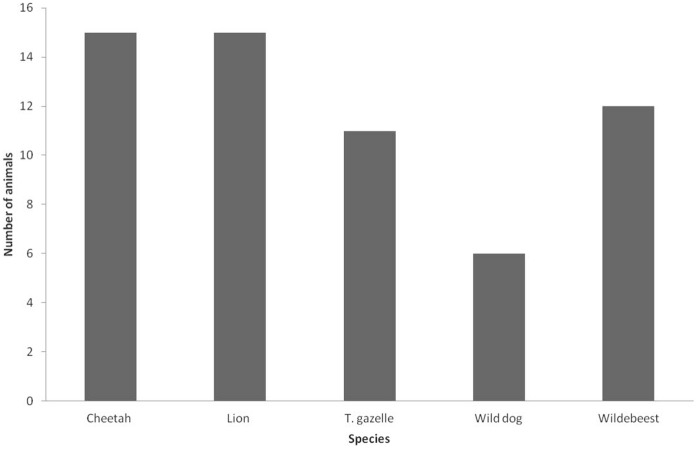
Between March 2007 and June 2011, the KWS department of Veterinary and Capture Services received 24 alerts about 59 wild animals with mange-like lesions from the Masai Mara pastoralist community, of which 19 were received by phone and five in person from pastoralists. Most reports occurred in 2008 (31/59; 52%), followed by 2009 with 13 reports (22%); there was a lower level of reporting in the other years (5/59; 8%), (2/59; 3%) and (8/59; 13%) in 2007, 2010 and 2011, respectively (Fig. 2). During the five-year period, the following wild species reported to have mange: cheetah, lion, wild dog, Thomson’s gazelle and wildebeest (Figure 3). Most reported cases were of cheetahs or lions (15/59; 25% each), followed by wildebeests (12/59; 20%), Thomson’s gazelles (11/59; 18%) and wild dogs (6/59; 10%).
Figure 2. Number of wild animals with mange reported by pastoralists per year (2007–2011).
Figure 3. Mange reports for each wild animal species reported by pastoralists (2007–2011).
Most of the pastoralists’ reports came from the Masai Mara National Reserve (68%), Mara Triangle (24%), Olare Orok conservancy (5%) or Ol Choro Oiroua conservancy (3%). Infected animals were captured and treated with ivermectin (Table 3). All the 59 mangy wild animals reported by the Masai people were confirmed as being infected by Sarcoptes mite via both the observation of clinical signs and the collection of adult Sarcoptes scabiei. Infection in domestic animals was also confirmed by clinical signs and the collection of Sarcoptes scabiei from cattle and goats, and Psoroptes ovis from sheep.
Table 3. Mange alerts reported by Masai people, the date of report, animal data and geographical locality, together with the mode of report and the Kenya Wildlife Service staff who received the alert.
| Date | Animalspecies | No. animals, ageclass and sex | Location | Reported to | Mode of reporting |
| March 2007 | Cheetah | 3 adult males | Maasai Mara National Reserve | KWS veterinaries | Telephone call |
| August 2007 | Cheetah | 1 adult male | Mara Triangle | KWS veterinaries | Telephone call |
| September 2007 | Cheetah | 1 adult female | Olare Orok WC | KWS veterinaries | Telephone call |
| January 2008 | T. gazelle | 2 adult males | Maasai Mara National Reserve (Talek) | KWS veterinaries | In person |
| January 2008 | T. gazelle | 4 Adult males | Maasai Mara National Reserve (Figtree) | KWS veterinaries | In person |
| January 2008 | T. gazelle | 4 Adult males | Maasai Mara National Reserve (Figtree) | KWS veterinaries | In person |
| April 2008 | T. gazelle | 1 adult male | Maasai Mara National Reserve (Talek) | KWS veterinaries | In person |
| July 2008 | Cheetah | 1 adult male | Olare Orok WC | KWS Rangers | Telephone call |
| August 2008 | Cheetah | 1 adult male | Olare Orok WC | KWS veterinaries | Telephone call |
| August 2008 | Cheetah | 3 adult males | Maasai Mara National Reserve | KWS Rangers | Telephone call |
| September 2008 | Lion | 2 cubs (female & male) | Maasai Mara National Reserve | KWS Rangers | Telephone call |
| October 2008 | Wildebeest | 12 calves (6 females & 6 males) | Mara Triangle | KWS veterinaries | Telephone call |
| October 2008 | Cheetah | 1 Adult female | Mara Triangle | KWS veterinaries | Telephone call |
| February 2009 | Wild dog | 1 Adult female | Maasai Mara National Reserve | KWS Rangers | In person |
| June 2009 | Wild dog | 5 Adult (2 males & 3 females) | Maasai Mara National Reserve (Figtree) | KWS veterinaries | Telephone call |
| August 2009 | Lion | 2 cubs Females | Maasai Mara National Reserve | KWS Rangers | Telephone call |
| September 2009 | Cheetah | 1 adult female | Maasai Mara National Reserve | KWS Rangers | Telephone call |
| December 2009 | Lion | 4 cubs (2 males & 2 females) | Maasai Mara National Reserve | KWS Rangers | Telephone call |
| June 2010 | Cheetah | 1 Adult male | Olchoro-oiroua | KWS veterinaries | Telephone call |
| August 2010 | Cheetah | 1 Adult male | Olchoro-oiroua | KWS veterinaries | Telephone call |
| Februrary 2011 | Cheetah | 1 adult male | Maasai Mara National Reserve | KWS veterinaries | Telephone call |
| February 2011 | Lion | 3 cubs (2 males & 1 female) | Maasai Mara National Reserve | KWS veterinaries | Telephone call |
| February 2011 | Lion | 1 adult female | Maasai Mara National Reserve | KWS veterinaries | Telephone call |
| June 2011 | Lion | 3 cubs (1 male & 2 females) | Maasai Mara National Reserve (Talek) | KWS Rangers | Telephone call |
Thirty-eight (68%) of the pastoralists used the following treatment or control measures when they found that their domestic animals were infected with mites: 16 (29%) sprayed or dipped their animals with acaricides, 12 (21%) reported the fact to the veterinary authorities, 12 (21%) injected teramycin, seven (13%) separated the affected individuals from the non-affected ones, four (8%) shaved the affected animals, three (5%) applied old engine oil, two (2%) injected penicillin and two (2%) injected ivermectin.
Discussion
Our study shows that in the Masai Mara ecosystem pastoralists have knowledge of mange as a disease. As indicated by previous reports, this disease poses a health risk to both domestic animal production and wildlife conservation in this area [26]–[29]. Although pastoralists have good knowledge of the disease and are aware of its presence, the majority did not understand its aetiology as a parasite-based disease. Up to 70% of the pastoralists thought that the disease was transmitted from domestic to wild animals and vice versa. This observation has a scientific basis since cross-infections of mange between wild and domestic animals are well documented [6]. More than 85% of pastoralists had affected animals in their herds and sheep, goats, dogs and cattle were identified with mange, which agrees with previous reports of infected domestic animals in Masai Mara [27], [32]–[34].
All of the 59 wild animals reported by Masai pastoralists with visible skin lesions and signs of pruritis that were captured and whose skin was scraped were found to be positive for mange by microscopy. This confirmed that the disease reported by pastoralists was mange. Although there were no false positives, there may have been some cases of infected wild animals that were not detected by the pastoralists because it is likely that some mangy wild animal carcasses were quickly scavenged. Additionally, predators may also preferentially hunt and kill animals infected by mange, as their flight ability may be less than that of healthy animals [26]–[29]. Hence we were not able to ascertain the true mortality and morbidity rate of the affected wildlife species in Masai Mara and so it is possible that the morbidity rate was higher than the reported 59 cases.
Nevertheless, the observation that all suspected cases had mites suggests that the pastoralist reporting system is highly precise since the Masai are able to observe infected animals with skin lesions and make accurate diagnosis of mange. However, this does not reflect the sensitivity of the system. The Masai people’s knowledge of mange and their accurate identification of the disease in wild animals is an essential element in the reporting of mangy wild animals in remote areas of Masai Mara where the veterinarians and rangers of the Kenya Wildlife Service are absent. Our data highlights for the first time the importance of the Masai people in mange surveillance and reporting and so the possibility of actively including this community in disease control protocols should be fully explored.
The increased use of mobile phones may also be a way of improving the reporting of affected wild animals since they can be employed to report disease in remote areas, thereby enhancing information flow to health authorities and quick response and control.
Around two out of three pastoralists employed several treatment, prevention or control methods when they suspected the presence of mange in their herds. The majority used effective methods such as spraying or dipping their animals with acaricides. Others reported the fact to the veterinary authorities or separated the affected animals. However, the administration of antibiotics is not effective against mange and can lead to antibiotic build-up and resistance in animal tissues. Interestingly, a few pastoralists even used ivermectin, the drug of choice for mange treatment [35]–[38]. Other control methods adopted by the pastoralists such as the use of old engine oil could have arisen empirically through trial and error, although its use as a treatment or control method in mange infection in animals is not evidence-based and may be detrimental to animal health. Hence, we recommend that competent veterinary authorities provide health education to enhance pastoralists’ knowledge of disease treatment options for domestic animals. This may increase the effectiveness of the treatment given by pastoralists to their animals and facilitate collaboration with authorities in the management and control of animal diseases. The ability of Masai pastoralists to identify and treat mange can be attributed to their long experience with this disease in their livestock and with the knowledge passed down over generations. The existence of a local Masai name for mange – olaldapash – highlights the fact that pastoralists have been aware of the presence of this disease in their herds for many years. Furthermore, mange has been reported to occur in cheetahs, wildebeests and Thompson’s gazelles in Masai Mara [28], [29].
The fact that all of the reported mange cases in wildlife species were confirmed to be positive by the observation of clinical signs and the collection of adult mites is a demonstration of the valuable understanding of and experience with this disease that exists amongst Masai pastoralists. Additionally, the majority of pastoralists administered correct treatment to their domestic animals.
Knowledge amongst Masai pastoralists of the agents that cause mange and its treatment is usually acquired informally, being passed down from one generation to the next. One of the reasons that their livestock herds survive is that Masai fathers teach their children from an early age all the necessary skills needed for managing and protecting their herds against mange and other common diseases. Masai children usually accompany their fathers when they care for their herds and this work-shadowing is probably the most important factor in the transfer of knowledge about mange between different generations of Masai people. One of the limitations of our study is that the questionnaire did not include questions relating to the way in which mange education was acquired. Further training and active involvement in treatment and control initiatives run by government veterinary authorities should improve Masai pastoralists knowledge. Pastoralists could potentially play important roles in disease reporting and control in both wild and domestic animals.
In conclusion, we have shown that Masai pastoralists have a good understanding of both the diagnosis of mange and the necessary measures to be taken in the event of an outbreak. Our findings give a clear indication of how Masai pastoralists could be used as key informants in the early detection – as well as in the control and prevention – of mange outbreaks in both wildlife and domestic animals in Masai Mara. An integrated approach to disease control involving veterinary authorities and local pastoralists, with emphasis on the correct treatment of domestic animals and active reporting of infected wild animals, should be considered as a way of effectively controlling mange transmission in the wildlife and livestock of the Masai Mara ecosystem.
Supporting Information
Questionnaire on knowledge of mange among Masai pastoralists.
(PDF)
Acknowledgments
The authors would like to thank the Director of KWS for allowing publication of this work. Special thanks are due to the staff of the KWS Veterinary and Capture Services Department for assisting in data collection and to Mike Lockwood for correcting the English of the paper. The authors would also like to thank the three anonymous reviewers for improving this article by repeating to provide us with constructive comments on earlier versions of the manuscript. Several members of the Sarcoptes World Molecular Network (Sarcoptes WMN) are thanked for their comments, which helped improve the manuscript.
Funding Statement
The research was supported by Kenya Wildlife Service, Projecto de Excelencia (Junta de Andalucia, Spain) and Juan de la Cierva Grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Muller GH, Kirk RW, Scott DW (1989) Small animal dermatology, 4th Edition. W.B. Saunders Company, Philadelphia, Pennsylvania, 1,007 pp.
- 2.Kahn CM, Line S, Allen DG, Anderson DP, Jeffcoh LB, et al. (2005) Acariasis mite infestation. In the Merck Veterinary Manual. 9th Edition. Published by Merck and Co Inc. Whitehouse Station, New Jersey U.S.A. Pages 742–749.
- 3.Bornstein S, Mörner T, Samuel WM (2001) Sarcoptes scabiei and sarcoptic mange. In: Samuel WM, Pybus MJ, Kocan AA (Eds) Parasitic diseases of wild mammals, 2PndP edn. Iowa State University Press, Ames. ISBN 0-8138-2978- X, 107–119.
- 4. Alasaad S, Walton S, Rossi L, Bornstein S, Abu-Madi M, et al. (2011) Sarcoptes-World Molecular Network (Sarcoptes-WMN): integrating research on scabies. Int J Infect Dis 15: 294–297. [DOI] [PubMed] [Google Scholar]
- 5. Walton SF, Holt DC, Currie BJ, Kemp DJ (2004) Scabies: New future for a neglected disease. Adv Parasitol 57: 309–376. [DOI] [PubMed] [Google Scholar]
- 6. Heukelbach J, Feldmeier H (2006) Scabies. Lancet 367: 1767–1774. [DOI] [PubMed] [Google Scholar]
- 7. Obasanjo OO, Wu P, Conlon M, Karanfil LV, Pryor P, Moler G, et al. (2001) An outbreak of scabies in a teaching hospital: lessons learned. Infection Control and Hospital Epidemiology 22: 13–18. [DOI] [PubMed] [Google Scholar]
- 8. Dagleish MP, Ali Q, Powell RK, Butz D, Woodford MH (2007) Fatal Sarcoptes scabiei infection of blue sheep (Pseudois nayaur) in Pakistan. Journal of Wildlife Diseases 43: 512–517. [DOI] [PubMed] [Google Scholar]
- 9. Alasaad S, Schuster RK, Gakuya F, Theneyan H, Jowers MJ, et al. (2012) Applicability of molecular markers to determine parasitic infection origins in the animal trade: A case study from Sarcoptes mites in wildebeest. Forensic Sci Med Pathol 8: 280–284. [DOI] [PubMed] [Google Scholar]
- 10. Pence DB. Ueckermann E (2002) Sarcoptic mange in wildlife. Revue Scientifique Et Technique 21: 385–398. [PubMed] [Google Scholar]
- 11. Soulsbury CD, Iossa G, Baker PJ, Cole NC, Funk SM, et al. (2007) The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mammal Rev 37: 278–296. [Google Scholar]
- 12. Curie BJ, Harumal P, McKinnon M, Walton SF (2004) First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei . Clin Inf Dis 39: 8–12. [DOI] [PubMed] [Google Scholar]
- 13. Bradberry SM, Cage SA, Proudfoot AT, Vale JA (2005) Poisoning due to pyrethroids. Toxicology Review 24: 93–106. [DOI] [PubMed] [Google Scholar]
- 14. Sanderson H, Laird B, Pope L, Brain R, Wilson C, et al. (2007) Assessment of the environmental fate and effects of ivermectin in aquatic mesocosms. Aquatic Toxicology 85: 229–240. [DOI] [PubMed] [Google Scholar]
- 15. Serrano E, Cross PC, Beneria M, Ficapal A, Curia J, et al. (2011) Decreasing prevalence of brucellosis in red deer via efforts to control disease in livestock. Epidemiology and Infection 139: 1626–1630. [DOI] [PubMed] [Google Scholar]
- 16. Polley L (2005) Navigating parasite webs and parasite flow: Emerging and re-emerging parasitic zoonoses of wildlife origin. International Journal for Parasitology 35: 1279–1294. [DOI] [PubMed] [Google Scholar]
- 17. Zahler M, Essig A, Gothe R, Rinder H (1999) Molecular analyses suggest monospecificity of the genus Sarcoptes (Acari: Sarcoptidae). International Journal for Parasitology 29: 759–766. [DOI] [PubMed] [Google Scholar]
- 18. Walton SF, Currie BJ, Kemp DJ (1997) A DNA fingerprinting system for the ectoparasite Sarcoptes scabiei . Molecular and Biochemical Parasitology 85: 187–196. [DOI] [PubMed] [Google Scholar]
- 19. Walton SF, Choy JL, Bonson A, Valle A, McBroom J, et al. (1999) Genetically distinct dog-derived and human-derived Sarcoptes scabiei in scabies-endemic communities in northern Australia. Am J Trop Med Hyg 61: 542–547. [DOI] [PubMed] [Google Scholar]
- 20. Walton SF, Dougall A, Pizzutto S, Holt D, Taplin D, et al. (2004) Genetic epidemiology of Sarcoptes scabiei (Acari: Sarcoptidae) in northern Australia. International Journal for Parasitology 34: 839–849. [DOI] [PubMed] [Google Scholar]
- 21. Alasaad S, Soglia D, Sarasa M, Soriguer RC, Pérez JM, et al. (2008) Skin-scale genetic structure of Sarcoptes scabiei populations from individual hosts: empirical evidence from Iberian ibex-derived mites. Parasitol Res 104: 101–105. [DOI] [PubMed] [Google Scholar]
- 22. Alasaad S, Soglia D, Spalenza V, Maione S, Soriguer RC, et al. (2009) Is ITS-2 rDNA suitable marker for genetic characterization of Sarcoptes mites from different wild animals in different geographic areas? Vet Parasitol 159: 181–185. [DOI] [PubMed] [Google Scholar]
- 23. Rasero R, Rossi L, Maione S, Sacchi P, Rambozzi L, et al. (2010) Host taxon-derived Sarcoptes mites in European wildlife animals, revealed by microsatellite markers. Biological Conservation 143: 1269–1277. [Google Scholar]
- 24. Alasaad S, Oleaga A, Casais R, Rossi L, Molinar-Min A, et al. (2011) Temporal stability in the genetic structure of Sarcoptes scabiei under the host-taxon law: empirical evidences from wildlife-derived Sarcoptes mite in Asturias, Spain. Parasit Vectors 4: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arlian LG (1989) Biology, host relations, and epidemiology of Sarcoptes scabiei . Annu Rev Entomol 34: 139–161. [DOI] [PubMed] [Google Scholar]
- 26. Gakuya F, Rossi L, Ombui J, Maingi N, Muchemi G, et al. (2011) The curse of the prey: Sarcoptes mite molecular analysis reveals potential prey-to-predator parasitic infestation in wild animals from Masai Mara, Kenya. Parasit Vectors 4: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngoru B, Mulama M (2002) Cheetah (Acinonyx jubatus) population status, problem and possible mitigation measures in Maasai Mara National Reserve and adjacent Group Ranches. Cheetah Conservation Project in Mara. A report to Kenya Wildlife Service, Nairobi, Kenya.
- 28.Mwanzia JM, Kock R, Wambua J, Kock N, Jarret O (1995) An outbreak of Sarcoptic Mange in the free-living cheetah (Acinonyx jubatus) in the Mara region of Kenya. In: Proceedings of American Association of Zoo Veterinarians and American Association of Wildlife Veterinarians Joint Conference, Omaha; Pages 105–112.
- 29.Gakuya F, Ombui J, Maingi N, Muchemi G, Ogara W, et al. (2012) Sarcoptic mange and cheetah conservation in Masai Mara (Kenya): Epidemiological study in a wildlife/livestock system. Parasitology [doi:10.1017/S0031182012000935]. [DOI] [PubMed]
- 30. Alasaad S, Rossi L, Soriguer RC, Rambozzi L, Soglia D, et al. (2009) Sarcoptes mite from collection to DNA extraction: the lost realm of the neglected parasite. Parasitol Res 104: 723–732. [DOI] [PubMed] [Google Scholar]
- 31. Fain A (1968) Étude de la variabilité de Sarcoptes scabiei avec une revisiondes Sarcoptidae. Acta zoologica et pathologica Antverpiensia 47: 1–196. [Google Scholar]
- 32. Sanders A, Froggatt P, Wall R, Smith KE (2000) Life-cycle stage morphology of Psoroptes mange mites. Medical and Veterinary Entomology 14: 131–141. [DOI] [PubMed] [Google Scholar]
- 33.Mugera GM, Bwangamoi O, Wandera JG (1979) Diseases caused by Ectoparasites I. In: Disease of cattle in Tropical Africa. Kenya Literature Bureau Nairobi; Pages 296–304.
- 34.Blood DC, Radostitis OM (1989) In:Veterinary Medicine. A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 7th Edition. Printed by the University printing House Oxford. Pages 1094–1099.
- 35. León-Vizcaíno L, Cubero MJ, González-Capitel E, Simón MA, Pérez L, et al. (2002) Experimental ivermectin treatment of sarcoptic mange and establishment of a mange-free population of Spanish ibex. J Wildl Dis 37: 775–785. [DOI] [PubMed] [Google Scholar]
- 36. Heukelbach J, Winter B, Wilcke T, Muehlen M, Albrecht S, et al. (2004) Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bull World Health Organ 82: 563–571. [PMC free article] [PubMed] [Google Scholar]
- 37. Munang’andu HM, Siamudaala VM, Matandiko W, Munyeme M, Chembensofu M, et al. (2020) Sarcoptes mite epidemiology and treatment in African buffalo (Syncerus caffer) calves captured for translocation from the Kafue game management area to game ranches. BMC Vet Res 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alasaad S, Ndeereh D, Rossi L, Bornstein S, Permunian R, et al. (2011) The opportunistic Sarcoptes scabiei: A new episode from giraffe in the drought-suffering Kenya. Vet Parasitol doi.org/10.1016/j.vetpar.2011.10.039. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questionnaire on knowledge of mange among Masai pastoralists.
(PDF)