Abstract
During kidney development, canonical Wnt signaling activates differentiation, while the transcription factor Six2 maintains the progenitor pool. These opposing signals help to regulate nephron formation and ensure the full complement of nephrons are formed. Since these two factors control differing fates in kidney mesenchyme, we hypothesized that overexpression of Wnt9b in Six2-expressing cells would disrupt kidney formation and may alter cell differentiation decisions in other tissues. We created a transgenic mouse that conditionally expressed the canonical Wnt ligand in the developing kidney, Wnt9b. The transgene is activated by cre recombinase and expresses GFP. We first tested its biological activity using Hoxb7-cre and found that transgenic Wnt9b was capable of inducing differentiation genes and of rescuing kidney development in Wnt9b−/− homozygous deficient mice. In contrast, expression of Wnt9b in cells using Six2-cre caused gastrointestinal distress and severe renal failure in adult mice. Transgenic kidneys had numerous cystic tubules and elevated creatinine values (0.652±0.044) compared to wild-type mice (0.119±0.002). These animals also exhibited a malformed pyloric sphincter, duodenogastric reflux, and a transformation of the distal stomach into proximal fate. The gene expression changes observed for the Wnt9b:EGFP transgene were compared to a stabilized β-catenin allele to determine that Wnt9b is activating the canonical Wnt pathway in the tissues analyzed. These results demonstrate that expression of Wnt9b in Six2-positive cells disrupts cell fate decisions in the kidney and the gastrointestinal tract.
Introduction
The canonical Wnt signaling pathway is a key regulator of cell fate, cell proliferation, and cell adhesion during development and throughout adulthood. Numerous positive and negative mediators have been identified that work together to achieve tight regulation of β-catenin signaling. The central paradigm is that cytoplasmic β-catenin is sequestered in a destruction complex, phosphorylated by GSK3β, and targeted to the ubiquitin destruction pathway in the absence of a Wnt signal. Wnt binding inhibits phosphorylation and destruction of β-catenin, allowing it to translocate to the nucleus to affect target gene expression with TCF/LEF factors (reviewed by MacDonald et al. [1]). Numerous mouse models have been created to study this pathway including both gain and loss of function alleles of Wnts, Wnt receptors, β-catenin, and the TCF/LEF transcriptional regulators. These models have uncovered essential roles for canonical Wnt signaling in bone, hair, intestine, blood, and cancer [2]. An emerging theme from these studies is that many biological processes are acutely sensitive to the strength of canonical Wnt signaling.
This tight regulation of canonical Wnt signaling is essential for kidney development. During the initial stages of kidney formation, Wnt9b is secreted from the ureteric bud and signals to progenitor cells in the cap mesenchyme to induce nephron differentiation. Treatment with lithium chloride or activation of β-catenin can induce nephron differentiation in the absence of the ureteric bud and Wnt9b, suggesting that an increase in canonical Wnt signaling is sufficient for this inductive event [3], [4]. Some cap mesenchyme cells proliferate to maintain the progenitor cells that are required for subsequent iterations of this process [5]. This balance between progenitor maintenance and the induction of differentiation is of paramount importance to ensure that the kidney forms the full complement of nephrons [5], [6], [7], [8], [9].
Several factors expressed in the cap mesenchyme have been shown to antagonize Wnt-stimulated differentiation including the transcription factor Six2. Deletion of Six2 results in ectopic differentiation, depletion of cap mesenchyme progenitors, and kidney agenesis [9]. Although the exact mechanism is not yet known, a hypothesis has been proposed whereby high levels of canonical Wnt signaling drive the commitment to differentiation whereas high Six2 activity in the same cells maintains the progenitor fate [7]. We have created a new mouse model to conditionally overexpress Wnt9b to activate the canonical Wnt signaling pathway. We have used this model to test whether increased Wnt9b activity is sufficient to disrupt the balance between progenitors and differentiation in the cap mesenchyme.
A second site where both Six2 activity and Wnt signaling play an important role is the pyloric sphincter. The pyloric sphincter is formed at the distal end of the stomach and functions as a valve to enable proper digestion of food prior to its entry into the duodenum. Dysfunction of the sphincter can result in reflux of duodenal contents into the stomach posing an increased risk of gastric metaplasia and cancer [10], [11]. This region is also the site of congenital anomalies including the rare disorder primary duodenogastric reflux and the more common birth defect pyloric stenosis [12]. Six2 deficiency leads to agenesis of the pyloric sphincter and antagonizing Wnt activity with Sfrp1 and Sfrp2 is required to pattern the stomach [13], [14]. This led us to hypothesize that canonical Wnt activity must be tightly regulated in the pylorus similar to what has been proposed in the kidney. To test this we have used Six2-cre to activate the Wnt9b transgene in Six2 expressing domains in kidney and stomach.
The conditional Wnt9b transgenic allele reported here expresses Wnt9b and GFP when activated by cre recombinase. It is biologically active and capable of rescuing kidney formation in Wnt9b−/− embryos. Overexpression of Wnt9b in the kidney ureteric bud is capable of inducing genes associated with differentiation of cap mesenchyme, but does not undergo mesenchymal-to-epithelial transition (MET) to produce morphologically distinct vesicle structures. Wnt9b transgene activation in Six2-positive cells causes kidney cysts and a transformation of the distal stomach regions into proximal stomach fate. Comparison of Wnt9b transgenics to an allele that produces stabilized β-catenin protein demonstrates dose-responsive gene changes and suggests that these strains represent an allelic series that should be valuable for modulating canonical Wnt signaling in other tissues.
Results
Transgenic mice express Wnt9b when activated by cre recombinase
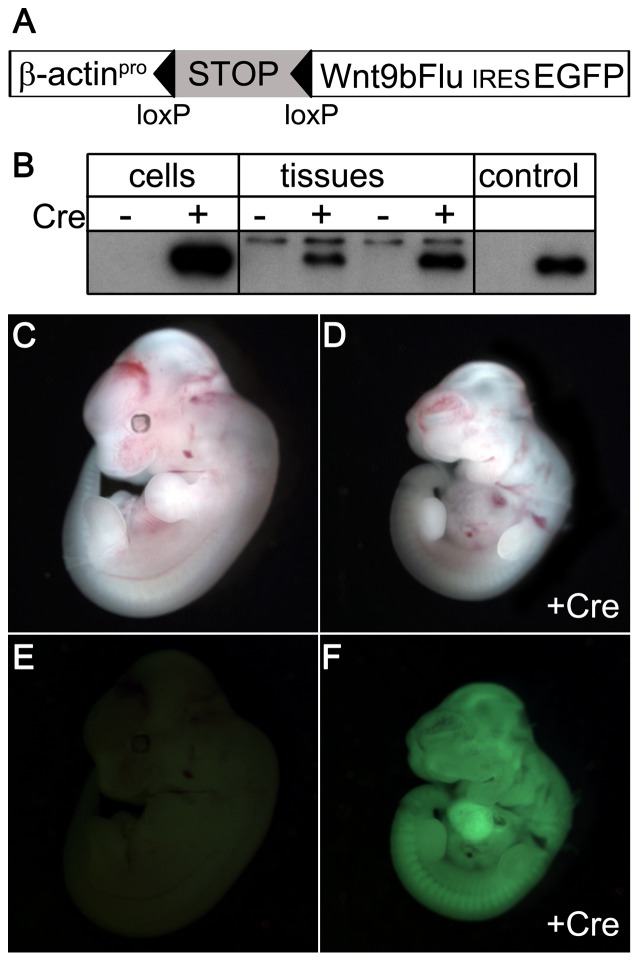
The Wnt9b transgene was constructed with a lox-STOP cassette [15] to create a conditionally active Wnt9b that depends on cre recombinase for expression (Fig. 1A). Once activated, the transgene-expressed Wnt9b is distinguishable from endogenous Wnt9b because it contains a C-terminal influenza hemagglutinin epitope tag and transgenic cells will exhibit GFP fluorescence due to the IRES-EGFP cassette. To test the fidelity of the transgene, we examined cre-dependent expression in cultured cells and in vivo in embryos (Fig. 1B). Epitope-tagged Wnt9b was detectable in lysates of cells co-transfected with the transgene and a cre plasmid, and not in transfections of the transgene alone. Similarly, lysates from embryos that were positive for both Wnt9b and β-actin-cre transgenes contained Wnt9b-FLU whereas single Wnt9b transgenics did not. GFP fluorescence was observed in Wnt9b/β-actin-cre double positive embryos and not in Wnt9b littermates (Fig. 1C–F). The expression level of transgenic Wnt9b correlated with intensity of GFP fluorescence for each founder line. (Fig. 1D). Four independent founder lines were unable to produce live β-actin-cretg/+, Wnt9btg/+ double heterozygous pups, demonstrating that ubiquitous elevated Wnt9b expression is embryonic lethal. Analysis of embryonic stages revealed that β-actin-cretg/+, Wnt9btg/+ double heterozygous embryos were detected at less than expected Mendelian frequencies at E11–E12. A single double heterozygous embryo was observed from a founder with very high expression (Fig. 1A, n = 23, expected frequency 25%) and 16% were observed from a different founder (n = 25). Wnt9b/ß-actin-Cre double transgenic embryos had smaller hearts with pooling of blood, suggesting cardiac insufficiency as the cause of death.
Figure 1. Transgene and GFP expression depends on cre in cells and tissues.
(A) Schematic of the transgene construct showing the β-actin promoter (β-actinpro), the stop cassette (STOP) flanked by lox P sites (triangles), the epitope-tagged Wnt9b cDNA (Wnt9bFLU), an internal ribosomal entry site (IRES) and green fluorescent protein cDNA (GFP). (B) Epitope-tagged Wnt9b protein is expressed when the transgene is introduced into Cos-1 cells (lanes 1, 2) and E11.5 transgenic embryos (lanes 3–6) only when cre recombinase is present (cre +). There is a higher molecular weight background band present in the tissue lysates that is not altered by the presence of cre. Lane 7 is empty. A positive control vector that lacks the lox-STOP cassette expresses Wnt9b-FLU in the absence of cre (lane 8). Brightfield (C–D) and fluorescence (E, F) images of Wnt9b transgenic E11.5 embryos with (D,F) and without (C,E) β-actin-cre demonstrate GFP expression and delayed embryogenesis for the Wnt9b tg/+, β-actin-cretg/+ embryos.
Transgenic Wnt9b is biologically active
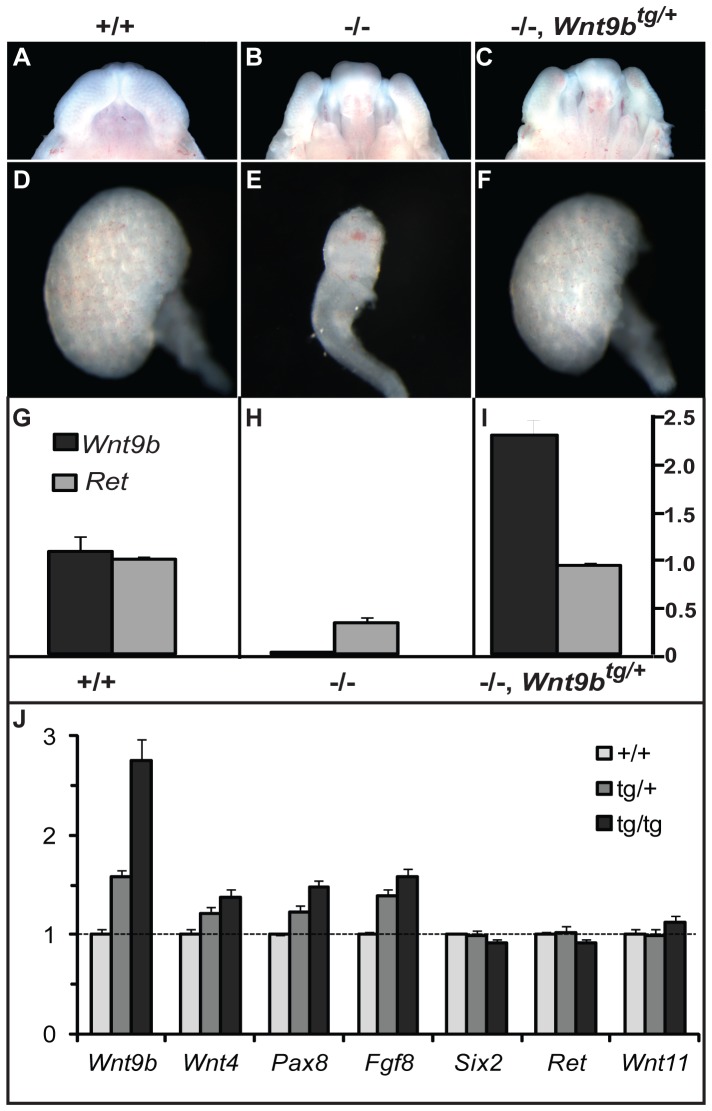
To determine if transgenic Wnt9b is biologically active, we tested if it was capable of replacing wild-type Wnt9b during kidney development. Wnt9b−/− embryos exhibit high penetrance cleft palate and renal agenesis phenotypes (Fig. 2). Activation of transgenic Wnt9b in the ureteric bud with Hoxb7-cre rescued kidney formation, but not palate closure at E14.5 in all Hoxb7-cretg/+, Wnt9b −/−, Wnt9btg/+ embryos (n = 7). This kidney-specific rescue confirms that transgenic Wnt9b expression is dependent on cre activation, because the palate does not express Hoxb7-cre. Quantitative PCR of wild-type, Wnt9b−/− and Wnt9b−/−, Wnt9btg/+ transgenic kidneys revealed that transgenic Wnt9b was expressed at 2.3- fold higher levels than the wild-type gene, while expression of Ret was unchanged (Fig. 2 G–I). The ability of transgenic Wnt9b to signal to the mesenchyme to replace wild-type Wnt9b activity confirms that it is biologically active in vivo.
Figure 2. Transgenic Wnt9b is biologically active and can induce expression of renal vesicle genes.
(A–F) Transgenic Wnt9b can rescue kidney formation in Wnt9b−/− E14.5 embryos. Wnt9b−/− embryos exhibit cleft palate (B) and kidney agenesis (E). Kidney-specific expression of the Wnt9b transgene with Hoxb7-cre rescues kidney formation (F), but not palate closure (C) in the Wnt9b −/− mutant. (G–I) Quantitative PCR analysis shows that the Wnt9b transgene is expressed 2.3-fold higher than wild-type Wnt9b. J) Wnt9b and the renal vesicle genes (Wnt4, Pax8, Fgf8) are increased in a dose-dependent fashion in wild-type (Hoxb7-cretg/+), hemizygous (Hoxb7-cretg/+, Wnt9btg/+), and homozygous (Hoxb7-cretg/+, Wnt9btg/tg) E12.5 embryonic kidneys (p<0.05 for all pairwise comparisons except Wnt4 hemizygous vs. homozygous (p = 0.08). This is consistent with Wnt9b's role in signaling from the ureteric bud to induce renal vesicle differentiation in the mesenchyme. A gene expressed in the metanephric mesenchyme and not in renal vesicles (Six2) is unaffected by the Wnt9b transgene. Ureteric bud markers Ret and Wnt11 are also unchanged.
A wealth of data has shown that Wnt9b and β-catenin signaling are critical inductive signals that initiate differentiation of cap mesenchyme into epithelialized renal vesicles [4], [8], [16]. Wnt9b produced in the ureteric bud activates the canonical Wnt signaling pathway in the adjacent mesenchyme and results in the expression of genes that regulate mesenchymal to epithelial transition (MET). Therefore, we tested whether overexpression of Wnt9b in the ureteric bud would signal to the mesenchyme to activate MET gene expression and to promote increased induction of nephrons. Wnt9b was overexpressed in the ureteric bud using Hoxb7-cre:EGFP. E12.5 embryos were genotyped by quantitative PCR analysis to determine if they were hemizygous or homozygous for the Wnt9b transgene and the kidneys were collected for gene expression analysis. We detected a dose-dependent increase in Wnt9b that was accompanied by a proportional activation of expression of the Wnt9b target genes, Wnt4, Pax8, and Fgf8 in three independent pools of transgenic kidneys (Fig. 2J). Six2 is expressed in undifferentiated (cap) mesenchyme and is downregulated as vesicle differentiation ensues [17]. Six2 mRNA expression is unaffected in Hoxb7-cre, Wnt9b double transgenics. Similarly, the ureteric bud genes, Ret and Wnt11, are not significantly altered by overexpression of Wnt9b. The specific upregulation of genes in the mesenchyme confirms that transgenic Wnt9b is capable of signaling from the ureteric bud to induce genes important for MET, however, no histological evidence of ectopic epithelialized renal vesicles was evident (data not shown). This suggests that upregulation of Wnt9b in the ureteric bud is sufficient for gene activation in the mesenchyme, but not for morphological differentiation of renal vesicles.
Misexpression of Wnt9b disrupts kidney function and pyloric sphincter formation by activating canonical β-catenin signaling
The paradigm emerging in the kidney is that there is a delicate balance between progenitor self renewal and activation of differentiation [6], [7], [9]. The Wnt9b/β-catenin pathway is required for differentiation and Six2 suppresses differentiation at the initial step of commitment. Therefore, we hypothesized that the ability of Six2 to promote fate decisions opposite from those activated by high β-catenin activity could be a common theme in development. We tested this possibility by activation of transgenic Wnt9b with Six2-cre. The double transgenics express Wnt9b in the same cells that express Six2 and could disrupt processes that are coordinately regulated in the kidney and other tissues. Six2-cretg/+, Wnt9btg/+ mice exhibited distress at weaning and body weight measurements were reduced by 39% at four weeks (14.5±3.4 grams, p<0.001) compared to cre-negative Wnt9btg/+ littermates (23.6±0.7 grams). Phenotypic analysis revealed that this reduction in body weight was due to malformations in the gastrointestinal system and the kidney, two sites of Six2-cre expression (Fig. 3).
Figure 3. Whole mount expression pattern of Six2-cre in E12.5 kidney and stomach.
(A) Six2-cre (GFP, green) is expressed in the cap metanephric mesenchyme (mm) surrounding the ureteric bud tips. Wnt9b expression and canonical Wnt signaling is highest in the ureteric bud which is labeled with cytokeratin (red). (B) In the stomach, Six2-cre is expressed in the hindstomach and pyloric region. Canonical Wnt signaling activity is downregulated in this region [13].
Numerous cysts of varying sizes were visible in the cortex and medulla of the double heterozygous kidneys (Fig. 4, n = 11/11). The cysts were predominantly located in the cortex and outer medulla and were of proximal tubule origin as demonstrated by LTL-staining. The cystic disease was first evident soon after birth (P7–10) in the proximal segments of the nephron (data not shown). These double heterozygous animals exhibited elevated serum creatinine levels in (0.652±0.044, p<0.005) compared to control littermates (0.119±0.002) as early as ∼3 weeks of age, indicating severe kidney failure. Older animals with advanced disease also contained cysts derived from more distal segments including the loop of Henle (Tamm-Horsfall-positive). In all animals with advanced kidney failure examined, we observed many collecting duct-derived cysts that expressed Aquaporin 2 or bound D. Biflorus-lectin. Since Six2-cre is not expressed in the collecting duct, this dilatation is likely secondary to dilatation in more proximal segments. The presence of kidney cysts in Wnt9b transgenic animals strengthens the conclusion that disruption of the Wnt signaling system causes cystic disease [7], [18], [19].
Figure 4. Kidney cysts in double transgenics can be found throughout all segments of the nephron.
(A–D) H&E staining of adult kidneys demonstrates numerous cystic dilatations in the cortex and outer medulla in Six2-cretg/+, Wnt9btg/+ double transgenics and a normal kidney architecture in Wnt9btg/+ controls. (E–H) Cysts were derived from proximal tubules (LTL-positive) and distal loop of Henle (THP-positive). DBA-positive and Aquaporin 2- positive collecting duct cysts were also identified in smaller number. Since Six2-cre is not expressed in the collecting duct, these cysts are produced through signaling from neighboring cells or secondary to dilatation of more proximal segments.
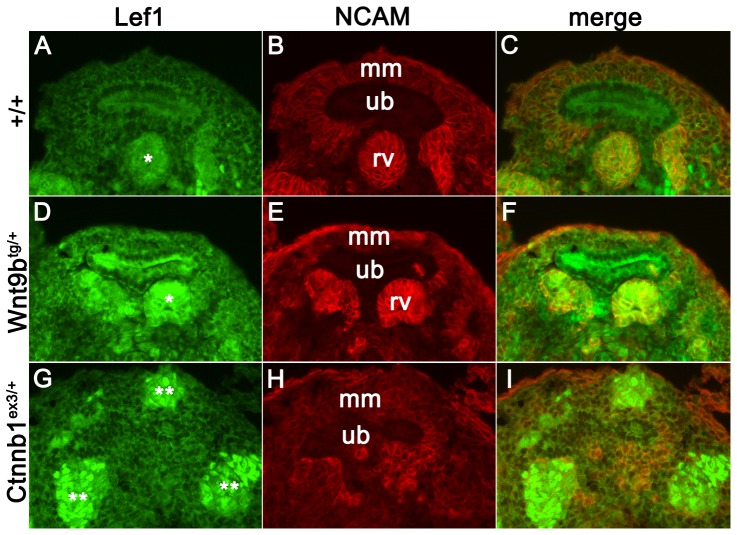
Although kidney cysts were not present until after birth, upregulation of canonical Wnt signaling was apparent at E12.5 when Six2-cre is initially expressed. Lef1 protein expression was increased in pre-tubular aggregates and renal vesicle structures in the double transgenics and was further increased in the numerous ectopic vesicles formed in stabilized β-catenin kidneys (N = 3, Fig. 5). The dose-responsive increase in Lef1 expression supports published data that Wnt9b is acting though the canonical Wnt signaling pathway in the developing kidney [7]. The lack of ectopic vesicle structures in the Wnt9b transgenics suggests that the Wnt9b allele exhibits a moderate effect on β-catenin signaling when compared to the stabilized β-catenin allele.
Figure 5. Lef1 expression exhibits dose-dependent effects in the Wnt9b and stabilized β-catenin alleles.
(A–C) Lef1 expression (green) is low in the self-renewing cap metanephric mesenchyme cells (mm) and upregulated in the differentiating renal vesicle structures (rv). NCAM expression is also increased as the cap mm differentiates, but is not expressed in the ureteric bud (ub). (D–I) Wnt9b transgenic kidneys have increased Lef1 staining which is further upregulated in the ectopic vesicles seen in the stabilized β-catenin allele. Notably, not all ectopic vesicles in the stabilized β-catenin allele are NCAM positive, suggesting abnormal specification of these differentiated structures.
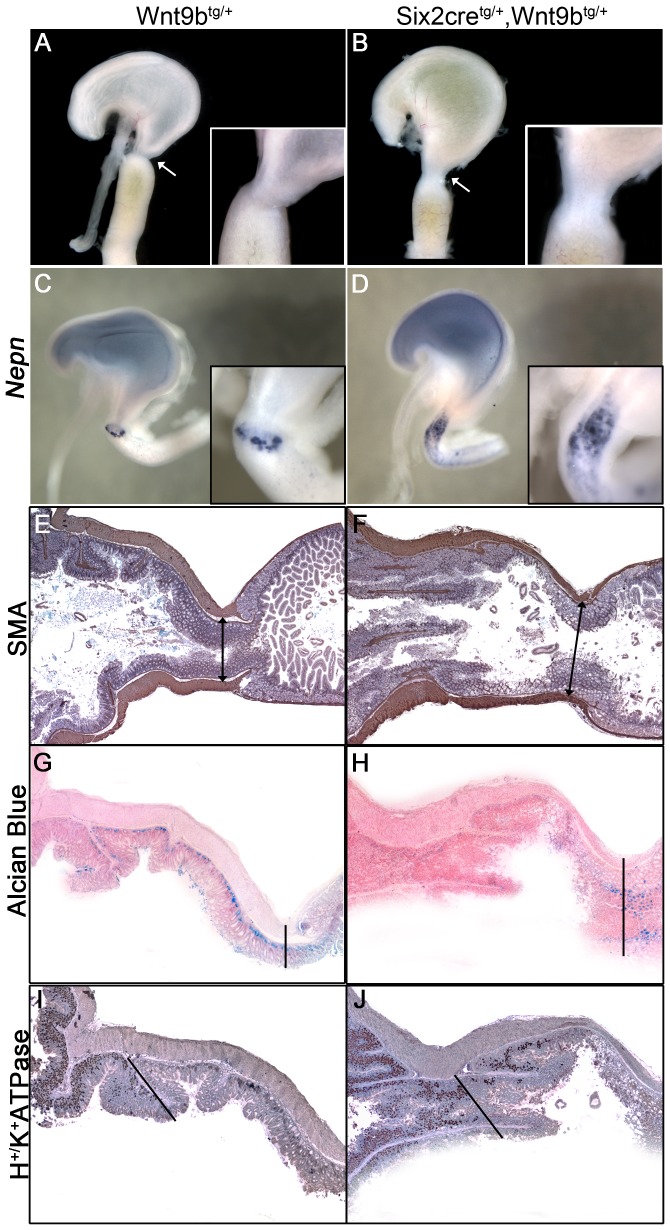
The second phenotype contributing to reduced body weight in Six2-cretg/+, Wnt9btg/+ double heterozygotes was gastric distress. Yellow amniotic fluid was observed in the stomach of double transgenics and not in single transgenic littermates (n = 6, Fig. 6). This indicated that duodenogastric reflux was occurring and could be due to abnormal pyloric sphincter formation. The sphincter between the stomach and the duodenum was elongated and widened in the double transgenic (Fig. 6, inset). The TGFβ antagonist, Nephrocan, which is normally expressed in a very restricted pattern at E16.5, was expanded in double transgenics, suggesting that the sphincter region was not properly constrained. This elongated morphology was supported by immunohistochemical staining of the smooth muscle layer that forms the dense muscular wall surrounding the pylorus (Fig. 6 E, F). In single transgenic controls, the thickest musculature was found where the tissue exhibits the most constriction at the sphincter (line). In the double transgenics, thick muscularity was observed, but this region was offset from the area that was the most constricted.
Figure 6. Duodenogastric reflux and pyloric sphincter abnormalities in Six2-cretg/+, Wnt9b tg/+ double heterozygotes.
(A, B) Yellow amniotic fluid refluxed into the stomach of E18.5 double heterozygotes, but was properly retained in the intestine in control animals. A lack of constriction (arrow) and an increased diameter (inset) suggests that the reflux is due to a malformed pyloric sphincter. (C, D) Whole mount staining for Nephrocan mRNA delineates a thin band demarking the sphincter at E16.5 that is expanded in the double heterozygotes. (E, F) Smooth muscle actin staining in sections from control adult stomach demonstrates a thick muscular layer that leads to the sphincter. Double heterozygotes exhibit a thickened muscular layer; however it does not coalesce at the point of highest constriction. Vertical lines mark the transition from antral stomach (left side) to duodenum (right side) in E–H. (G, H) Alcian blue stains mucosal cells in the antral stomach in control sections and these cells are absent in the double heterozygotes. Mucosal cells in the intestine are stained normally. (I, J) Staining for the H+/K+ ATPase detects parietal cells in the forestomach that are excluded from the distal stomach. These cells are found throughout the distal stomach and pylorus in double heterozygotes. Sections depicted in panels I and J highlight the transition between forestomach and distal stomach (diagonal line) and do not include the duodenum.
Mis-patterning defects were also observed by analyzing specific markers that distinguish between the proximal (H+/K+ ATPase) and the distal (Alcian blue) region of the stomach. The proximal stomach (corpus) contains H+/K+ ATPase-expressing parietal cells that are excluded from the antrum-pylorus region. Conversely, the antrum-pylorus contains mucosal cells that stain with Alcian Blue that are not found in the proximal region [20]. The double transgenics exhibited persistent H+/K+ ATPase staining and the absence of Alcian blue-positive mucosa in the antral stomach-pylorus indicating that the distal region is mis-patterned to proximal fate by Wnt9b expression. Alcian blue staining of goblet cells in the intestine was intact in both single and double transgenics because Six2-cre is not expressed in the duodenum. This mis-patterning suggests that, similar to the paradigm in the kidney, canonical β-catenin signaling specifies one fate, the forestomach region, and Six2 activity patterns the distal identity. This is supported by the expression domains of these genes since a canonical Wnt signaling reporter exhibits high activity only in the forestomach, and Six2 is confined to the distal stomach and pylorus [13], [14].
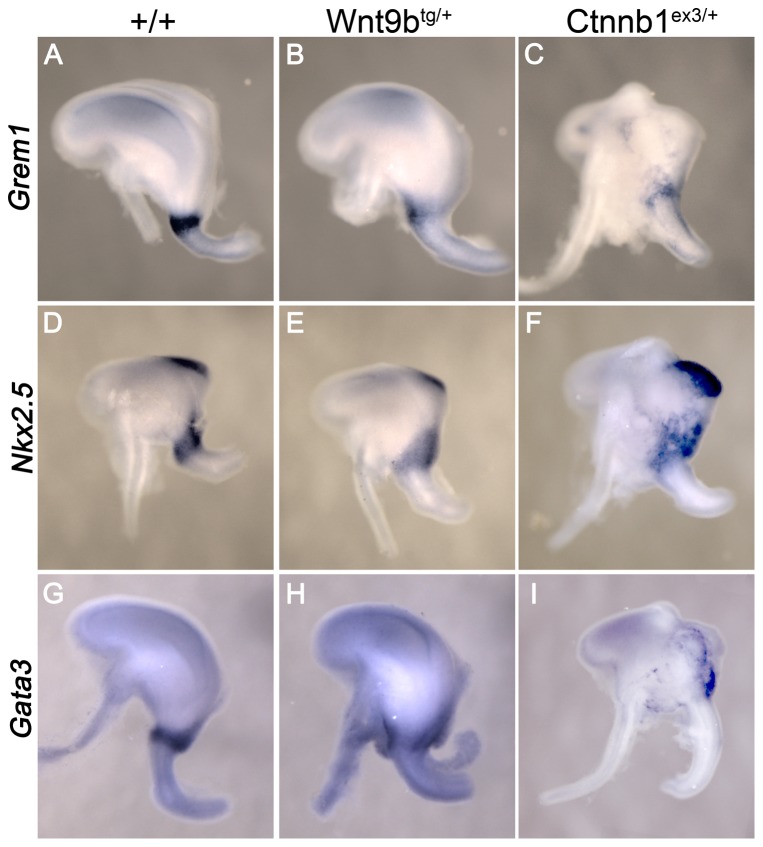
If canonical Wnt signaling is responsible for the gastroduodenal reflux in Six2-cretg/+, Wnt9btg/+ double transgenics, then the malformations observed should be recapitulated in a mouse model that expresses a stabilized β-catenin in the same domain (Ctnnb1ex3/+, [21]). After cre activation, this allele prevents the proteolytic degradation of β-catenin and would be expected to elevate canonical Wnt signaling to a higher degree than Wnt9b ligand alone. Whole mount in situ analysis of E12.5 stomachs revealed three genes, Grem1, Nkx 2.5, and Gata3, that are normally expressed in a thin band demarking the region that will become the sphincter (n = 8, Fig. 7). Ectopic expression of Wnt9b suppressed the BMP antagonist Grem1 and the transcription factor Gata3 in this region, and these genes were further downregulated when β-catenin signaling was increased. A dose-dependent expansion was observed for the transcription factor Nkx2.5 in both mutants. Genes that were localized exclusively to the intestine (villin) or to the forestomach (Bmp4) were not altered in either mutant (data not shown). Increased canonical Wnt signaling in the antral stomach and pylorus was confirmed by isolating this region and performing quantitative PCR. The canonical Wnt target genes that are upregulated in the stabilized β-catenin allele, Lef1 and Axin2, were also increased in the pyloric region of Six2-cretg/+, Wnt9btg/+ double transgenics [Lef1, 2.0±0.25 and Axin2, 1.4±0.06 (n = 6)]. Together, the dose-responsive gene expression changes observed in the pyloric region in the Wnt9b transgenics and the stabilized β-catenin embryos demonstrate that increased canonical Wnt signaling disrupts distal stomach and pyloric sphincter development.
Figure 7. Pyloric gene expression exhibits dose-dependent effects in the Wnt9b and stabilized β-catenin alleles.
Whole mount in situ analysis of Grem1, Nkx2.5, and Gata3 expression in E12.5 stomachs. All three genes are restricted to the pyloric sphincter in wild-type stomachs (Six2-cretg/+). Grem1 and Gata3 show decreased expression in Wnt9b transgenics (Six2-cretg/+, Wnt9btg/+) that is further downregulated in the stabilized β-catenin allele (Six2-cretg/+, Ctnnb1ex3/+). Nkx2.5 expression is expanded into the stomach in Wnt9b transgenics and further upregulated in the stabilized β-catenin allele. Nkx2.5 expression that can be seen at the upper right of the stomach is found in the spleen on the opposite side.
Discussion
These results describe the creation of a new mouse model that conditionally activates canonical Wnt signaling. The Wnt9b transgene is inducible upon exposure to cre recombinase and expresses GFP as a marker of induction. It also retains full biological activity since it can rescue Wnt9b−/− phenotypes in the kidney. These mice have been maintained in our colony for over a year and induce Wnt9b expression upon breeding to multiple different cre strains. We have utilized this novel strain to activate Wnt signaling in Six2-positive cells and found deleterious effects in the kidney and the stomach.
In the kidney, Wnt9b is secreted from the ureteric bud epithelium to induce the adjacent cap mesenchyme progenitor cells to initiate differentiation. In support of this model, our in vivo gain-of-function studies show that Wnt9b upregulates early markers of renal vesicle differentiation such as Wnt4, Fgf8 and Pax8. However, even though renal vesicle genes are induced by the Wnt9b transgene, we did not observe morphological evidence of increased or ectopic renal vesicle formation, as reported for Six2 mutants [9]. In addition to a Wnt inductive signal, factors that maintain the renal progenitor pool (e.g. Six2) must also be downregulated to fully elicit renal vesicle formation. Six2 expression was not altered in our Wnt9b transgenic and may thus explain why the increase in Wnt9b did not result in ectopic renal vesicles. Ectopic induction of renal vesicle markers is induced by activation of β-catenin in Six2-positive metanephric mesenchyme using the stabilized β-catenin allele [4], therefore, the difference between these two gain-of-function experiments may relate to the degree to which they activate canonical Wnt activity. In addition, it is likely that activation of canonical signaling by Wnt9b in metanephric mesenchyme induces feedback inhibition, as noted in other Wnt responsive tissues, thereby preventing induction of the full differentiation program [22]. Recent data support this idea of feedback inhibition since Wnt9b/β-catenin signaling is required not only for the differentiation of metanephric mesenchyme, but paradoxically the same signal is also required for maintenance or expansion of the renal progenitor pool [7]. We speculate that the stabilized β-catenin, acting downstream in the pathway, may bypass these regulatory mechanisms. These possibilities could be tested in future studies in that compare downstream gene activation in renal progenitor cells using the Wnt9b transgene and stabilized β-catenin gain-of-function mice.
Perturbations in the canonical Wnt signaling pathway have been shown to be important in the pathogenesis of renal cystic disease (reviewed by Patel et al. [23]). Transgenic expression of a stabilized β-catenin or deficiency of APC in renal tubular epithelia leads to renal cystic disease, supporting the conclusion that ectopic canonical Wnt signaling is sufficient for cystogenesis [18], [19]. Renal failure and cystic dilatation in Six2-cretg/+, Wnt9btg/+ double heterozygotes further supports the hypothesis that upregulated canonical Wnt signaling causes cysts. Disruption of non-canonical Wnt signaling (PCP) pathway is also important in renal cyst formation [24]. Thus, it is possible that cyst formation in our model is not simply due to activation of canonical signaling, but is the result of disruption of the relative balance between canonical and non-canonical signaling. In support of this idea, the genes that are mutated in the inherited forms of cystic disease (PKD1, PKD2, PKHD1, Inversin) localize to cilia and these organelles have been recently shown to be able to regulate the balance between canonical and non-canonical Wnt signaling [25], [26].
There was a severe kidney phenotype even though Wnt9b mRNA expression is increased ∼2-fold in our transgene, indicating that relatively low level, persistent activation of this pathway leads to renal dysfunction. This level of upregulation of Wnt9b in the ureteric bud using Hoxb7-cre did not produce cysts, suggesting that cystogenesis in the collecting ducts in Six2-cretg/+, Wnt9btg/+ double transgenics is likely a secondary effect of dilated tubular segments (data not shown). In support of this, in post-natal day 7–10 animals, cystic disease is evident in only tubular segments proximal to the collecting duct. Similar to polycystic kidney disease in patients, the transgenic mice developed severe renal failure, even though most nephrons do not develop cysts. This raises the question of whether renal tubules without overt cysts are abnormal and contribute significantly to progressive functional impairment in these disorders. Recent studies demonstrate that adult renal tubule epithelia express Wnt9b, Frizzled receptors and LRP5/6 co-receptors that are capable of mediating canonical Wnt signaling in response to acute ischemic injury [27]. In this setting, Wnt signaling promotes tubule repair and regeneration. However, Wnt signaling activity has also been implicated in promoting renal fibrosis after injury, such as in the UUO model [28]. Overall, these studies highlight the importance of regulating Wnt signaling in the adult kidney to balance beneficial responses, such as repair/regeneration, and maladaptive responses leading to fibrosis and chronic organ dysfunction.
The pyloric region of the stomach represents a critical boundary that separates stomach from intestine. Sphincter closure when a food bolus enters the stomach enables proper digestion before transit to the small intestine. The molecular basis for formation of this discrete region is not well understood, though some details are emerging. These results suggest that tight control of Wnt signaling is vital for development of the pylorus. Canonical Wnt activity is detected in the fundus and body (corpus) but excluded from the distal stomach and pylorus [13]. In Barx1−/− mice, the stomach is small, the antral-corpus boundary is blurred and there is agenesis of the pyloric sphincter. Elegant tissue recombination studies suggest that these phenotypes result from loss of expression of the Wnt antagonists Sfrp1 and Sfrp2. However, since Barx1 deficiency has global effects on foregut development, it was not clear if down-regulation of canonical Wnt activity is specifically required for formation of the pylorus. Our studies more directly tested this possibility by targeting ectopic Wnt activity to a restricted region just anterior to the pylorus with Six2-cre.
We found that ectopic Wnt activity specifically disrupts formation of the pyloric sphincter in the absence of global defects in stomach development. Thus, down-regulation of canonical Wnt activity is required to pattern the pyloric region that separates stomach from intestine. Ectopic expression of the Wnt9b ligand and stabilized β-catenin in transgenic mice could overcome the effects of secreted endogenous Wnt antagonists Sfrp1/2, consistent with the model proposed by Kim et al [13]. Similar to our results, in Six2 mutants there is reduced expression of Nkx2.5 and Gremlin, genes that are important for pyloric sphincter development in chick [14], [29]. This suggests that Six2 and Wnt regulate a common genetic cascade in the presumptive pyloric sphincter. We postulate that an important function of Six2 may be to interpret canonical Wnt signals in different developmental contexts.
Upregulated canonical Wnt signaling has also been shown to affect spleen and pancreas development [30]. In support of this, spleen and pancreas hypoplasia was also observed in Six2-cre, Wnt9b bi-transgenic mice (data not shown). Since Six2-cre is not expressed in these tissues, this suggests that signals from the posterior stomach mesenchyme can affect the spleen and pancreas during development. This would support the hypothesis that the pyloric region may act as an organizer for formation of these accessory organs. During development, spleen precursor cells transiently associate with the pyloric region, prior to their migration to the left of the stomach where they condense [31]. Although we did not detect Six2-cre expression in the spleen primordium or mesothelium, we cannot exclude that transient expression of Six2-cre in these regions is responsible for defects in the spleen and pancreas. Future studies are needed to directly test these possibilities.
In summary, we have developed a new mouse model that allows for temporal and spatial expression of Wnt9b. In the developing kidney, transgenic Wnt9b can induce early MET gene expression in Six2-positive renal progenitors, but it is not sufficient to activate the full differentiation program. Persistent expression of Wnt9b in Six2-positive cells leads to kidney cysts and severe organ failure. In the stomach, ectopic Wnt β-catenin activity in the pyloric region alters sphincter formation and distal stomach patterning. Together, these findings highlight the importance of tight control of Wnt activity and suggest that Six2 and β-catenin are involved in organ patterning in different contexts.
Materials and Methods
Ethics statement
The animal studies were approved by the animal care and use committee of Saint Louis University (protocol 1460) and the St. Louis VA (protocol 0305). All procedures involving animals were in compliance with institutional animal welfare regulations, standards and policies.
Mice
Wnt9b transgenic mice were crossed to each of three different cre lines [32]. 1) β-actin- cre was used to express Wnt9b ubiquitously (Fig. 1, [33]) 2) Hoxb7-cre:EGFP was used to overexpress Wnt9b in its endogenous location in the kidney, the ureteric bud (Fig. 2, [34]), and 3) the BAC Six2-cre:EGFP transgene was used to express Wnt9b exogenously in the stomach and the kidney (Figs. 3, 4, 5, [35]). Ctnnb1ex3/+ mice have been described previously [21] and were mated to the Six2-cre transgene to activate canonical Wnt signaling in the distal stomach. At least two independent founder lines were analyzed for all β-actin-cre and Six2-cre matings presented in this paper. The Hoxb7-cre phenotypes were verified for multiple founders in a previous publication [32]. Noon on the day of vaginal plug detection was considered E0.5.
Activation of Wnt9b-FLU expression in cells and tissues
Cos-1 cells (ATCC CRL-1650) were plated at a density of 2×105 cells per well in a six well plate and transfected using Fugene HD according to the manufacturer's instructions (Roche). 150 ng cre plasmid or control DNA was co-transfected with the Wnt9b transgene plasmid (2 µg/well). Cells were lysed at 48 hours in lysis buffer containing inhibitors (1% Triton X-100, 200 mm sucrose, 50 mmTris [pH 7.4], 150 mm NaCl, 1 mg/ml leupeptin, 2 mg/ml antipain, 10 mg/ml benzamidine, 1 mg/ml chymostatin, 1 mg/ml pepstatin, 24 mg/ml Pefabloc, 20 mM NaF and 2 mM sodium molybdate). A plasmid that contained a constitutively-expressed Wnt9b-FLU was used as a positive control. For tissue analysis, Wnt9btg/+ or Wnt9btg/+, β-actin-cretg/+ E11.5 embryos were homogenized in the same buffer. Protein lysates were quantitated and a 7.5% SDS-PAGE gel was loaded with 3 µg of cell lysates, 50 µg of tissue lysates and 10 µg positive control sample. Western Blotting using anti-FLU antibody (12CA5 1∶2000, Roche) was performed as described [36].
Quantitative PCR
E12.5 kidneys were collected from wild-type Hoxb7-cre tg/+, Wnt9btg/+, Hoxb7-cre tg/+ or Wnt9btg/tg, Hoxb7-cretg/+. Twelve kidneys per group were pooled and subjected to quantitative RT-PCR as described previously [36]. Relative expression of Wnt4, Pax8, Fgf8, Six2, Ret, Wnt9b and Wnt11 was calculated using the ΔΔCT method and normalized to the gene Rpl19 for three independent pools of each genotype. Sample sizes were smaller for the rescue experiment where two independent pools of two wild-type kidneys, two rescued kidneys, or four mutant rudiments were assayed for Wnt9b and Ret expression levels and normalized to Hprt expression. Statistical significance was determined by the students t-test.
Whole mount in situ hybridization
E12.5 stomachs were fixed in 4% PFA overnight at 4°C and stored in 100% methanol. In situ was performed as described using digoxigenin-labeled antisense probes for Grem1 (nucleotides 1 - 551), Nkx 2.5 (26 - 896), Gata3 (169 - 1000), Bmp4(10 - 888), and villin 1 (105 - 976) [32]. Tissues were photographed using a Leica DVC300 FX camera and M165 FC microscope.
Analysis of stomach phenotype
E17.5 and adult stomach tissues were fixed in 10% Formalin prior to dehydration and embedding in paraffin. Five micron sections were analyzed by immunohistochemistry using antigen retrieval as described previously [32]. Anti-smooth muscle actin (Millipore ASM-1, 1∶100) and anti-H+/K+ ATPase (Alexis Biochemical 2G11, 1∶500) were used to characterize the antral stomach and pyloris. Slides were counterstained with hematoxylin and mounted in Mowiol 4–88 (Polysciences). Sections were analyzed for the presence of mucosal cells by Alcian blue staining (1% Alcian blue in 3% Acetic Acid PH 2.5) for 30 minutes at room temperature. After washing in tap water, slides were counterstained in 0.1% Nuclear Fast Red Solution and mounted in Mowiol 4–88.
Kidney staining
Anti-THP (Santa Cruz H-135, 1∶250) and anti-Aquaporin 2 (Santa Cruz H-40, 1∶20) were used to localize Loop of Henle and collecting duct cysts on paraffin sections of bisected kidneys following antigen retrieval. LTL-biotin (Vector 1∶100) binding to proximal tubule cysts was detected by Vectastain ABC streptavidin-HRP (Vector Labs) and DAB (3,3-diaminobenzidine, Pierce) according to the manufacturer's recommended protocol. DBA-FITC (Vector, 1∶100) was used to directly label kidney collecting ducts and counterstained with Dapi. Photography of all tissue sections was performed on a Nikon 80I epifluorescence microscope using MetaMorph software.
Acknowledgments
The authors wish to thank the Washington University St. Louis Developmental Biology Pathology core for providing histology services. Rich DiPaulo (Saint Louis University) provided anti-H+/K+ ATPase antibody. Andy McMahon (Harvard) supplied Six2-cre transgenic mice. Monique Heitmeier helped with body weight and creatinine measurements. Abdullah Said performed genotyping. We also thank Darcy Denner, Sara Hirsch, and Jeannine Basta for feedback and helpful suggestions.
Funding Statement
This work was supported by National Institutes of Health grant R01 DK067222 (http://nih.gov/) and the American Heart Association Established Investigator 0840117N (http://www.heart.org) to MR. SK was supported by a Saint Louis University Presidential Research Fund Award (www.slu.edu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480. [DOI] [PubMed] [Google Scholar]
- 3. Kuure S, Popsueva A, Jakobson M, Sainio K, Sariola H (2007) Glycogen synthase kinase-3 inactivation and stabilization of beta-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. JAmSocNephrol 18: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 4. Park JS, Valerius MT, McMahon AP (2007) Wnt/{beta}-catenin signaling regulates nephron induction during mouse kidney development. Development 134: 2533–2539. [DOI] [PubMed] [Google Scholar]
- 5. Couillard M, Trudel M (2009) C-myc as a modulator of renal stem/progenitor cell population. Dev Dyn 238: 405–414. [DOI] [PubMed] [Google Scholar]
- 6. Boyle SC, Kim M, Valerius MT, McMahon AP, Kopan R (2011) Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development 138: 4245–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karner CM, Das A, Ma Z, Self M, Chen C, et al. (2011) Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138: 1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt-Ott KM, Masckauchan TN, Chen X, Hirsh BJ, Sarkar A, et al. (2007) beta-catenin/TCF/Lef controls a differentiation-associated transcriptional program in renal epithelial progenitors. Development 134: 3177–3190. [DOI] [PubMed] [Google Scholar]
- 9. Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, et al. (2006) Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25: 5214–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaezi MF, Richter JE (1996) Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology 111: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 11. Vaezi MF, Singh S, Richter JE (1995) Role of acid and duodenogastric reflux in esophageal mucosal injury: a review of animal and human studies. Gastroenterology 108: 1897–1907. [DOI] [PubMed] [Google Scholar]
- 12. Hermans D, Sokal EM, Collard JM, Romagnoli R, Buts JP (2003) Primary duodenogastric reflux in children and adolescents. Eur J Pediatr 162: 598–602. [DOI] [PubMed] [Google Scholar]
- 13. Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA (2005) The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell 8: 611–622. [DOI] [PubMed] [Google Scholar]
- 14. Self M, Geng X, Oliver G (2009) Six2 activity is required for the formation of the mammalian pyloric sphincter. Dev Biol 334: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Novak A, Guo C, Yang W, Nagy A, Lobe CG (2000) Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28: 147–155. [PubMed] [Google Scholar]
- 16. Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. DevCell 9: 283–292. [DOI] [PubMed] [Google Scholar]
- 17. Mugford JW, Yu J, Kobayashi A, McMahon AP (2009) High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. DevBiol 333: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, et al. (2005) Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem 280: 3938–3945. [DOI] [PubMed] [Google Scholar]
- 19. Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, et al. (2001) Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972–5981. [DOI] [PubMed] [Google Scholar]
- 20. Karam SM, Leblond CP (1992) Identifying and counting epithelial cell types in the “corpus” of the mouse stomach. Anat Rec 232: 231–246. [DOI] [PubMed] [Google Scholar]
- 21. Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, et al. (1999) Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 18: 5931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jho EH, Zhang T, Domon C, Joo CK, Freund JN, et al. (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel V, Chowdhury R, Igarashi P (2009) Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens 18: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, et al. (2009) Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41: 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kishimoto N, Cao Y, Park A, Sun Z (2008) Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell 14: 954–961. [DOI] [PubMed] [Google Scholar]
- 26. Lancaster MA, Schroth J, Gleeson JG (2011) Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol 13: 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, et al. (2010) Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Surendran K, Schiavi S, Hruska KA (2005) Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384. [DOI] [PubMed] [Google Scholar]
- 29. Smith DM, Nielsen C, Tabin CJ, Roberts DJ (2000) Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut-foregut boundary. Development 127: 3671–3681. [DOI] [PubMed] [Google Scholar]
- 30. Heller RS, Dichmann DS, Jensen J, Miller C, Wong G, et al. (2002) Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn 225: 260–270. [DOI] [PubMed] [Google Scholar]
- 31. Li X, Udager AM, Hu C, Qiao XT, Richards N, et al. (2009) Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn 238: 3205–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiefer SM, Robbins L, Stumpff KM, Lin C, Ma L, et al. (2010) Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development 137: 3099–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewandoski M, Meyers EN, Martin GR (1997) Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol 62: 159–168. [PubMed] [Google Scholar]
- 34. Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, et al. (2004) Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. DevBiol 276: 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, et al. (2008) Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiefer SM, Robbins L, Barina A, Zhang Z, Rauchman M (2008) SALL1 truncated protein expression in Townes-Brocks syndrome leads to ectopic expression of downstream genes. HumMutat 29: 1133–1140. [DOI] [PubMed] [Google Scholar]