Abstract
Plants release volatiles induced by herbivore feeding that may affect the diversity and composition of plant-associated arthropod communities. However, the specificity and role of plant volatiles induced during the early phase of attack, i.e. egg deposition by herbivorous insects, and their consequences on insects of different trophic levels remain poorly explored. In olfactometer and wind tunnel set-ups, we investigated behavioural responses of a specialist cabbage butterfly (Pieris brassicae) and two of its parasitic wasps (Trichogramma brassicae and Cotesia glomerata) to volatiles of a wild crucifer (Brassica nigra) induced by oviposition of the specialist butterfly and an additional generalist moth (Mamestra brassicae). Gravid butterflies were repelled by volatiles from plants induced by cabbage white butterfly eggs, probably as a means of avoiding competition, whereas both parasitic wasp species were attracted. In contrast, volatiles from plants induced by eggs of the generalist moth did neither repel nor attract any of the tested community members. Analysis of the plant’s volatile metabolomic profile by gas chromatography-mass spectrometry and the structure of the plant-egg interface by scanning electron microscopy confirmed that the plant responds differently to egg deposition by the two lepidopteran species. Our findings imply that prior to actual feeding damage, egg deposition can induce specific plant responses that significantly influence various members of higher trophic levels.
Introduction
A major challenge in ecology is to understand how phenotypic plasticity of plant traits affects the complexity and dynamics of plant-associated communities. Plants are at the base of food webs, which are defined as networks of feeding connections within an ecological community [1]. Insect herbivores are the most abundant and diverse attackers of plants and induce defensive traits that influence consumers at higher trophic levels [2], [3]. Upon attack by insects, plants emit a blend of volatile organic compounds that affect interactions with organisms belonging to the arthropod community of the plant [4]–[8]. These herbivore-induced plant volatiles (HIPVs) can consist of hundreds of compounds, such as terpenoids, green leaf volatiles and benzenoids and have been shown to act as repellents and/or attractants for herbivores and their natural enemies [4], [5], [8]. HIPVs can provide specific information on the status of the plant to various community members both below- and aboveground, including carnivores, herbivores, pollinators, or neighbouring plants [4], [9]–[12]. Thus, HIPV-mediated effects on different trophic levels imply an extensive effect of plants in structuring associated communities [4], [10], [13].
Although the majority of the about 300,000 described herbivorous insect species [3] deposit their eggs on plant tissues, plant responses elicited by egg deposition, i.e. in the initial phase of herbivore colonization, are still not widely accepted to play a significant role in plant-insect interactions [14]. Yet, an increasing number of studies demonstrates that insect egg deposition can modify (a) the plant’s internal chemistry, with direct consequences for eggs or subsequently feeding herbivores [15]–[19] or (b) the plant’s surface chemistry directly affecting egg survival or indirectly by arresting egg parasitoids, tiny parasitic wasps that kill insect eggs [20]–[28]. Moreover, egg deposition by herbivorous insects has been shown to change plant volatile emission, i.e. oviposition-induced plant volatiles (OIPVs), utilized by parasitoids during host location [29]–[37]. The emission of OIPVs was initially found to require cell damage inflicted by the attacking insects either by wounding caused by the ovipositing female or adult feeding [6], [14], [38]. However, recent studies have indicated that mere egg deposition itself, without wounding, can also enhance or reduce volatile emission with consequences for insect preferences [36], [37], [39].
In the Brassicaceae plant family, egg deposition has been demonstrated to induce resistance responses at the transcriptional level that affect herbivores and parasitoid wasps that attack eggs [27], [40]–[42]. Deposition of eggs by cabbage white butterflies (Pieris spp.) on black mustard plants, Brassica nigra, triggers the formation of a necrotic zone at the base of the eggs resembling a hypersensitive response (HR) or programmed cell death, that can lead to egg desiccation and mortality [43]. Moreover, it provokes gene expression changes similar to pathogen-induced HR in Arabidopsis [42]. Egg parasitoids of the genus Trichogramma are arrested on the leaf surface of Brussels sprouts plants (B. oleracea var. gemmifera) when induced by Pieris brassicae or P. rapae eggs [27], [40], [44]. Here, a butterfly anti-sex pheromone released with the egg-associated secretion was shown to quantitatively change plant surface chemistry [27], [28], most likely epicuticular wax composition, as has been reported for other Brassicaceae [45], [46].
We study oviposition-induced responses in B. nigra, an annual wild crucifer native to Europe. This plant species contains high concentrations of glucosinolates as defensive compounds that reduce herbivore growth and survival [47]. Generalist insects like the cabbage moth Mamestra brassicae suffer from the toxic breakdown products of glucosinolates, whereas specialists like the larval stages of the Large Cabbage White butterfly P. brassicae are adapted to them [48]. Both herbivores lay eggs in clutches on cultivated and wild brassicaceous plant species, such as B. nigra, with M. brassicae moths having a much larger host plant range than P. brassicae butterflies [49], [50]. The generalist wasp Trichogramma brassicae is known to parasitize eggs of a wide range of lepidopteran species, including P. brassicae and M. brassicae [51]. Cotesia glomerata is a fairly specialized gregarious endoparasitoid that attacks young instars of Pieris spp. in Eurasia.
The aim of this study was to investigate a) the effects of egg deposition on plant volatile-mediated interactions with insects at different trophic levels (figure 1) and b) the specificity of the plants’ response to egg deposition by using two different herbivores. We tested the response of the specialist butterfly P. brassicae and two parasitoids to volatiles of B. nigra plants induced by egg deposition by the specialist butterfly and a generalist moth M. brassicae. The behavioural differences were linked to modifications in the composition of volatile blends using gas chromatography coupled with mass spectrometry (GC-MS); cryo-scanning electron microscopy was used to study the bonding region between eggs of the two herbivores and the plant surface.
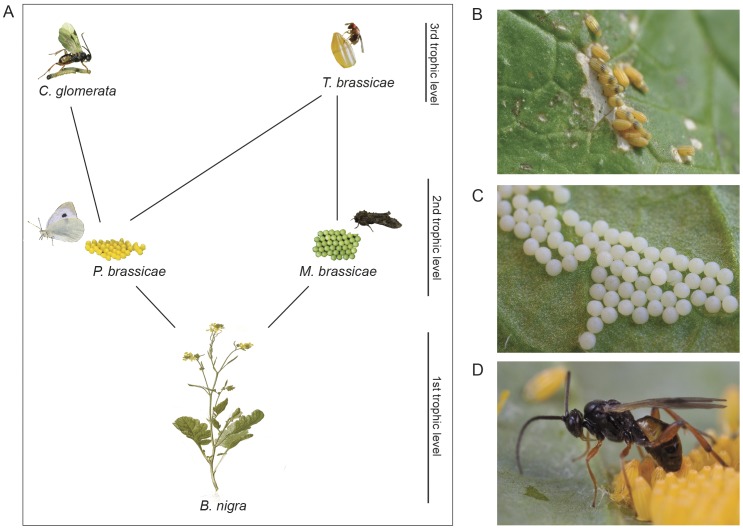
Figure 1. Studied insect community of B. nigra.
(A) Tritrophic system consisting of the Brassicaceae-specialist Pieris brassicae and the generalist moth Mamestra brassicae lay eggs in clusters on B. nigra. The egg parasitoid Trichogramma brassicae attacks eggs of both. The larval parasitoid Cotesia glomerata attacks young caterpillar stages of P. brassicae. (B) P. brassicae clutch on B. nigra expressing a strong necrotic zone, i.e. hypersensitive response (HR) (Photo credits: D. Lucas-Barbosa), (C) M. brassicae egg clutch on B. nigra without necrosis (Photo credits: Nina E. Fatouros, www.bugsinthepicture.com), (D) C. glomerata wasp on P. brassicae eggs parasitizing a neonate that just hatched (Photo credits: N. E. Fatouros, www.bugsinthepicture.com).
Results
Formation of Necrotic Tissue and Effects on Eggs and Egg Parasitoid
At 24 hours after oviposition (hao) by P. brassicae, plants start to express a necrotic zone below the egg clutches (i.e. hypersensitive response, HR+) that sometimes led to egg desiccation or egg drop-off at 72 hao (Figure 1B). All P. brassicae egg-infested plants were, therefore, examined for HR and separated from non-HR expressing plants (HR−). On the 10 plants on which eggs were counted, 91% of the eggs did not develop into larvae on HR+ plants, whereas 99% of the eggs hatched on HR− plants (P<0.001, 2×2 contingency test using Chi2). From these 10 plants, 50% developed HR. In contrast to eggs of P. brassicae, eggs of the moth M. brassicae did not induce any HR response in B. nigra; no necrosis was observed after egg deposition (Figure 1C).
Trichogramma brassicae wasps can successfully parasitize and complete their development inside eggs on plants that have expressed HR. The proportion of eggs that was parasitized by T. brassicae was not affected by plant phenotype, i.e. occurrence of HR (GLM; χ2 1 = 0.47, P = 0.49), but was marginally affected by the age of the eggs, i.e. 24 h or 72 h old (GLM; χ2 1 = 3.91, P = 0.053). Older eggs tended to be less parasitized than younger ones. The interaction between plant phenotype and egg age was not significant (GLM; χ2 1 = 0.34, P = 0.56). Similarly, there was no effect of plant phenotype on the number of wasp offspring that emerged from parasitized host eggs (GLM; χ2 1 = 0.01, P = 0.91), but there was an effect of egg age (GLM; χ2 1 = 6.70, P = 0.01). Less offspring emerged from 72 h old eggs than from 24 h old eggs. The effect of egg age was not influenced by the plant’s phenotype (GLM; χ2 1 = 1.15, P = 0.28).
Attraction of Egg Parasitoids
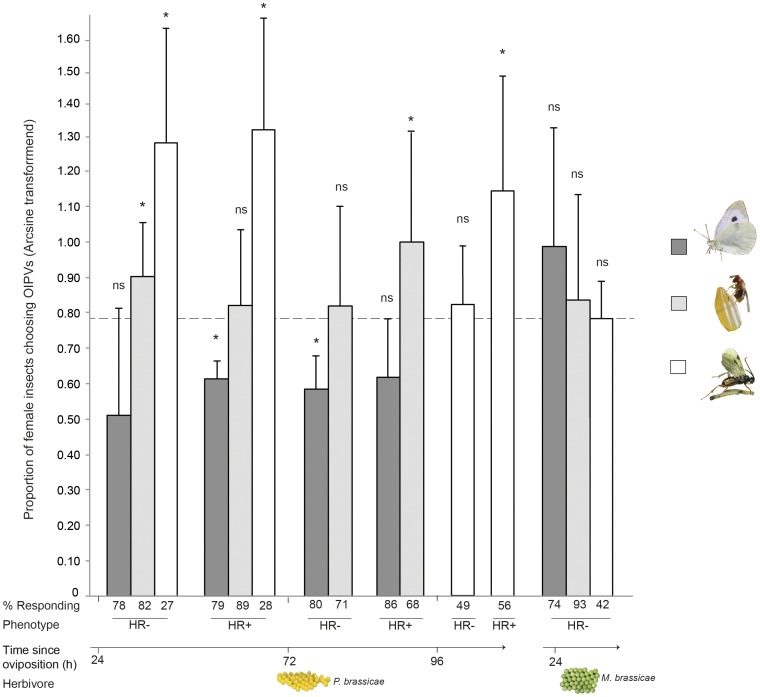
In a dynamic Y-tube olfactometer set-up, the distribution of naïve T. brassicae wasps did not differ from 50∶50 in a control test with an uninfested plant in both odour containers (t-test: t11 = 0.27, P = 0.79). The wasps did not discriminate between clean air and volatiles from uninfested B. nigra plants (t-test: t11 = −0.10, P = 0.92) or clean air and volatiles from plants infested with P. brassicae eggs less than 6 hao (t-test: t9 = −0.23, P = 0.82). However, wasps were attracted to volatiles from B. nigra plants infested with P. brassicae eggs 24 hao when tested against clean air, irrespective of HR (t-test: HR−: t9 = 5.1, P = 0.001; HR+: t9 = 4.2, P = 0.002).
The distribution of naive T. brassicae wasps choosing egg-induced or non-induced clean plants was marginally affected by the interaction between plant phenotype and egg age (GLM; χ2 1 = 4.14, P = 0.059, none of the main effects was significant). Wasps significantly preferred volatiles from plants (HR−) infested with P. brassicae eggs (24 hao) when tested against clean control plants (Figure 2; t-test: t9 = 2.54, P = 0.03). However, they did not discriminate systemically (S) induced volatiles from HR− plants from which leaves with 24 h old eggs had been removed before testing, when tested against uninfested plants (t-test: t9 = −0.45, P = 0.68). Wasps did not respond to volatiles from HR+ plants 24 hao (Figure 2; t-test: t9 = 0.81, P = 0.44) or HR− plants 72 hao neither locally (Figure 2; t-test: t9 = 0.90, P = 0.38) nor systemically (t-test: t9 = −1.00, P = 0.37) induced. Yet, volatiles from HR+ plants 72 hao significantly attracted wasps locally (Figure 2; t-test: t11 = 2.47, P = 0.03) and systemically induced (t-test: t9 = 2.78, P = 0.04).
Figure 2. Proportions (±SD) of female insects choosing oviposition-induced plant volatiles (OIPVs) of B. nigra plants.
Plants were infested with eggs of P. brassicae or M. brassicae. Columns represent arcsine of the proportion of choice for OIPVs by gravid P. brassicae females tested in a flight chamber (dark grey), T. brassicae egg parasitoids tested in a Y-tube olfactometer (light grey), and C. glomerata larval parasitoids tested in a windtunnel (white). All experiments were conducted in a two-choice situation between plants infested with eggs of different ages (24 h, 72 h, 96 h), and clean plants. The dashed line indicates arcsine (0.5) = no preference. Numbers below the columns represent the percentage of female insects making a choice. *P<0.05, one-sample t-test. Each treatment combination was replicated with at least four plant pairs. Different phenotypes: Hypersensitive response (HR), HR−: no necrotic zone observed, HR+: necrotic zone.
T. brassicae discriminated between volatiles of M. brassicae egg-infested B. nigra plants 24–36 hao and clean air (T-test: t11 = 6.61, P<0.001). However, when the same plants were tested against volatiles of uninfested plants, the wasps did not display a preference (Figure 2; t-test: t11 = 0.91, P = 0.38). At 48–60 hao, the wasps did not discriminate between volatiles emitted by plants infested with moth eggs and clean air (T-test: t9 = −1.81, P = 0.10).
Attraction of Larval Parasitoids
In a wind tunnel, the distribution of naive C. glomerata female wasps choosing volatiles of egg-induced or non-induced clean plants was only affected by the age of the eggs (GLM; χ2 1 = 8.19, P = 0.008) and not by the occurrence of HR (Figure 2; GLM; χ2 1 = 1.99, P = 0.17). At 24 hao, C. glomerata wasps discriminated between OIVPs and volatiles emitted by non-induced control plants, regardless of the occurrence of HR (Figure 2; t-test: HR−: t7 = 4.16, P = 0.004; HR+: t6 = 4.27, P = 0.005), whereas at 96 hao, the preference for OIPVs was less pronounced and was only significant in plants that had developed HR+ (Figure 2; t-test: HR−: t4 = 0.72, P = 0.51; HR+: t8 = 2.93, P = 0.019). However, the interaction between plant phenotype and egg age was statistically not significant (GLM; χ2 1<0,01, P = 0.95).
Cotesia glomerata did not discriminate between volatiles from plants infested with eggs of the non-host M. brassicae and uninfested plants 24–36 hao in a wind tunnel set-up (Figure 2; t-test: t8 = 0,22, P = 0.83). This wasp was not arrested by, and did not show any interest in, M. brassicae eggs.
Avoidance Behaviour of Gravid Butterflies
In a flight chamber set-up, the distribution of gravid P. brassicae butterflies first landing on egg-induced or non-induced clean plants was not affected by the age of the eggs (GLM; χ2 1 = 0.08, P = 0.77), the occurrence of HR (GLM; χ2 1 = 0.81, P = 0.37) nor by the interaction of egg age and plant phenotype (Figure; GLM; χ2 1 = 0.01, P = 0.90). Gravid female butterflies tended to first land on plants without eggs, regardless of the age and phenotype of the plants (Figure 2). However, P. brassicae butterflies did not discriminate between moth egg-infested plants and control plants 24–36 hao (Figure 2; t-test: t5 = −1.26, P = 0.27).
Specificity of OIPV Emission
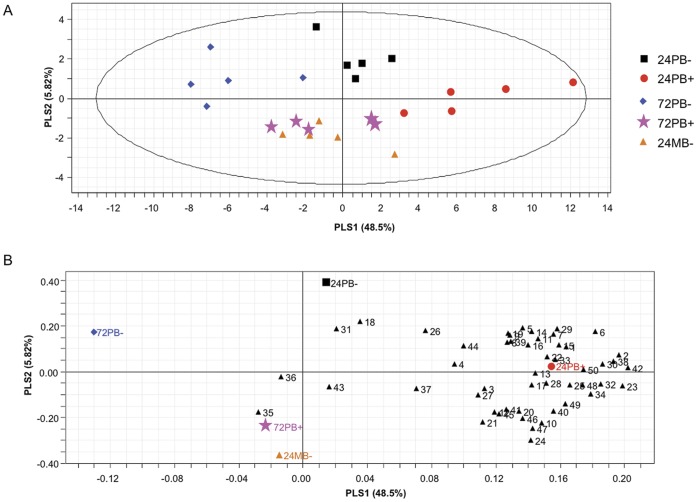
The headspace of uninfested B. nigra plants was compared with the headspace of P. brassicae egg-infested (24 and 72 hao, HR− and HR+) and M. brassicae moth egg-infested (24–36 hao, HR−) plants (Table 1). In total, 50 plant-related compounds were detected (present in more than 50% of the replicates of at least the control treatment). A Projection to Latent Structures Discriminant Analysis (PLS-DA) including volatiles of the five different egg treatments of B. nigra resulted in a model with two significant principal components (Figure 3A; 2 PLS-DA principal components, R2Xcum = 0.485, R2Ycum = 0.196, Q2cum = 0.159) and separated the five treatments to a large extent. Figure 3B shows the contribution of the emitted compounds to the two principal components. Oviposition by P. brassicae significantly suppressed the emission of the majority of compounds in HR− plants at 24 h (Table 1; 34 compounds suppressed, P<0.03, sign test) and 72 h (Table 1; 44 compounds suppressed, P = 0.001, sign test) compared to uninfested plants. Interestingly, HR+ plants carrying eggs of P. brassicae showed an enhanced emission 24 hao compared to uninfested plants (Table 1; 33 compounds enhanced, P = 0.05, sign test), whereas at 72 hao the number of compounds showing enhanced emission by HR+ plants was lower (Table 1; 20 compounds enhanced, P = 0.20, sign test) and not different to uninfested plants. In HR− plants, the emission rate of 22 compounds was significantly reduced at 72 hao (Table 1). Different forms of the sesquiterpene silphiperfolene (7-α-H-silphiperfol-5-ene, presilphiperfol-7-ene, 7-β-H-silphiperfol-5-ene and silphiperfol-6-ene) were identified for the first time in a Brassica species. The total emission of the four silphiperfolenes increased significantly 24 h after P. brassicae oviposition (HR−: P = 0.05, HR+: P = 0.02, Mann-Whitney U-test) as well as the emission of the monoterpene (E)-β-ocimene (HR−: P = 0.01, HR+: P = 0.03, figure 3, table 1). At 72 h after P. brassicae oviposition, there was a significant increase in emission of the monoterpene isomenthone (P = 0.01) and the sesquiterpene α-funebrene (P = 0.02) in HR+ plants (Figure 3, table 1).
Table 1. Volatile emissiona by Brassica nigra HR+ (+) or HR− (−) plants in response to eggs of Pieris brassicae (PB) and Mamestra brassicae (MB) sampled at 24 or 72 h after oviposition.
| Treatment → | Uninfested | 24PB− | 24PB+ | 72PB− | 72PB+ | 24MB− | |
| ID | Compound ↓ | (N = 25) | (N = 5) | (N = 5) | (N = 5) | (N = 5) | (N = 5) |
| Aliphatic | |||||||
| 1 | 2-Methylpropanal | 11.56±2.2 | 7.97±1.5 | 17.93±4.8 | 3.57±0.8* | 6.63±1.0 | 4.47±1.3 |
| 2 | 2-Methyl-2-propenal | 23.78±4.6 | 12.19±0.5* | 34.63±9.7 | 5.36±1.3 | 8.06±1.2 | 9.26±1.5 |
| 3 | Ethyl acetate | 134.44±25.9 | 2.92±0.9 | 21.81±8.0 | 3.69±1.2 | 4.29±1.5 | 12.75±10.4 |
| 4 | 2-Methyl-1-propanol | 4.41±0.8 | 3.82±1.2 | 6.49±2.8 | 1.42±0.4 | 4.18±1.5 | 1.48±0.4 |
| 6 | 2-Butenal | 10.70±2.1 | 7.28±1.1 | 17.93±5.9 | 3.25±0.6* | 4.23±0.6 | 3.85±0.4 |
| 7 | 3-Methylbutanal | 12.33±2.4 | 8.16±1.8 | 17.44±3.1 | 5.63±1.8 | 6.64±1.4 | 4.45±0.6 |
| 8 | 2-Methylbutanal | 8.37±1.6 | 5.46±2.1 | 11.08±2.9 | 4.85±2.4 | 4.50±1.1 | 2.89±0.3 |
| 9 | 1-Methoxy-2-propanol | 134.94±26.0 | 192.25±81.7 | 244.20±78.9 | 41.31±10.6 | 87.75±53.5 | 63.17±14.1 |
| 10 | 1-Penten-3-ol | 189.90±36.5 | 64.13±9.9 | 396.15±115.0 | 34.91±15.8* | 253.93±134.9 | 187.68±61.9 |
| 11 | 2-Pentanone | 43.81±8.4 | 47.31±19.5 | 39.54±11.6 | 8.06±2.0* | 15.20±2.9 | 18.20±5.5 |
| 12 | 3-Pentanone | 53.60±10.3 | 36.34±22.2 | 68.65±29.7 | 8.25±2.0* | 78.72±43.8 | 37.48±12.4 |
| 15 | 4-Methyl-2-pentanone | 7.08±1.4 | 3.92±1.3 | 6.57±1.9 | 1.34±0.3 | 2.23±0.3 | 2.41±1.1 |
| 17 | (E)-2-Pentenal | 2.53±0.5 | 1.10±0.3 | 3.15±1.4 | 0.31±0.2* | 1.28±0.5 | 1.67±0.7 |
| 18 | 2,4-Pentanedione | 55.07±10.6 | 112.50±89.9 | 45.09±14.0 | 2.45±1.2 | 16.10±7.7 | 11.76±3.6 |
| 19 | 4-Methyl-3-penten-2-one | 68.92±13.3 | 64.25±35.2 | 37.59±7.7 | 8.67±2.5* | 23.77±11.2 | 15.84±5.8 |
| 20 | (Z)-3-Hexen-1-ol | 215.11±41.4 | 44.56±12.6 | 179.94±74.5 | 18.05±6.4* | 43.50±18.3 | 174.64±109.8 |
| 23 | 6-Methyl-2-heptanone | 29.31±5.6 | 17.21±2.5 | 34.10±4.7 | 6.63±0.7* | 15.83±2.8 | 16.36±2.8 |
| 24 | (Z)-3-Hexen-1-yl acetate | 573.69±110.4 | 105.04±18.8 | 422.09±111.9 | 45.69±12.3* | 171.72±65.4 | 478.74±173.1 |
| 28 | Methyl 2-ethylhexanoate | 2.81±0.5 | 1.47±0.7 | 3.69±1.1 | 1.29±0.5* | 1.98±0.7 | 0.87±0.5 |
| 38 | Undecan-2-one | 13.61±2.6 | 11.20±1.0 | 20.18±4.3 | 3.94±0.6* | 8.54±1.6 | 7.93±0.8 |
| Aromatic | |||||||
| 30 | o-Cresol | 36.76±7.1 | 33.26±15.6 | 49.88±7.9 | 7.51±1.2* | 17.50±2.7 | 18.05±3.4 |
| 33 | 2-Phenylacetonitrile (benzyl cyanide) | 8.91±1.7 | 4.82±0.6 | 10.27±4.4 | 1.91±0.3* | 4.39±0.8 | 3.31±0.9 |
| 47 | Lilial | 18.95±3.6 | 5.10±0.6 | 20.95±3.6 | 2.71±0.5* | 20.12±9.6 | 14.48±8.4 |
| 49 | 2-Ethylhexyl salicylate | 5.76±1.1 | 4.92±3.4 | 15.31±6.3 | 1.73±1.4 | 5.74±3.0 | 10.85±4.8 |
| Terpenoids | |||||||
| 22 | α-Pinene | 55.24±10.6 | 22.78±7.1 | 109.40±44.9 | 11.74±3.3 | 12.47±4.4 | 24.32±12.7 |
| 25 | 3-Carene | 48.16±9.3 | 17.96±8.9 | 75.99±22.3 | 5.72±1.5* | 13.73±4.7 | 25.93±13.3 |
| 26 | (S)-Limonene | 68.75±13.2 | 29.85±11.9 | 76.41±25.6 | 14.19±5.4 | 10.70±3.8 | 39.98±32.5 |
| 27 | α -Phellandrene | 10.15±2.0 | 10.19±4.8 | 11.52±3.0 | 1.83±0.8* | 11.75±7.0 | 10.99±2.6 |
| 29 | (E)-β-Ocimene | 5.97±1.1 | 22.69±6.6* | 82.28±42.2* | 4.55±2.1 | 5.42±2.0 | 3.68±2.0 |
| 32 | p-Mentha-1,5,8-triene | 8.91±1.7 | 3.60±0.9 | 14.75±3.2 | 1.63±0.7* | 3.01±0.7 | 4.36±1.3 |
| 34 | Isopulegon | 2.75±0.5 | 1.20±0.3 | 4.04±1.0 | 0.55±0.1* | 1.20±0.2 | 1.42±0.4 |
| 35 | p-Menthan-3-one | 4.62±0.9 | 1.67±0.7 | 4.02±1.9 | 27.09±25.8 | 23.60±21.9 | 2.53±1.6 |
| 36 | Isomenthone | 2.50±0.5 | 2.36±0.3 | 3.30±0.2 | 10.65±10.0 | 10.79±8.8* | 1.44±0.7 |
| Treatment → | Uninfested | 24PB− | 24PB+ | 72PB− | 72PB+ | 24MB− | |
| ID | Compound ↓ | (N = 25) | (N = 5) | (N = 5) | (N = 5) | (N = 5) | (N = 5) |
| 37 | Menthol | 25.64±4.9 | 14.95±4.4 | 31.32±7.1 | 47.74±42.9 | 53.01±40.8 | 14.64±2.8 |
| 39 | 7-α-H-Silphiperfol-5-ene | 88.22±17.0 | 151.09±61.2 | 363.17±116.3 | – | 13.70±13.5 | 15.88±15.9 |
| 40 | Presilphiperfol-7-ene | 13.36±2.6 | 15.13±7.6 | 83.12±34.4 | 0.06±0.1 | 6.81±6.8 | 0.61±0.6 |
| 41 | 7-β-H-Silphiperfol-5-ene | 32.16±6.2 | 46.07±17.4 | 143.24±55.6 | – | 4.82±4.8 | 5.11±5.1 |
| 43 | Silphiperfol-6-ene | 10.09±1.9 | 17.14±9.2 | 40.07±15.8 | – | 2.42±2.4 | 1.81±1.8 |
| 44 | α-Funebrene | 10.80±2.1 | 29.44±23.7 | 8.42±8.4 | 4.81±2.1 | 11.26±4.5* | 18.37±10.6 |
| 45 | Longifolen | 13.10±2.5 | 6.66±2.1 | 17.41±3.1 | 4.02±1.3 | 20.06±13.4 | 6.71±2.2 |
| 48 | Guaiazulene | 10.04±1.9 | 5.57±1.7 | 11.40±3.2 | 2.29±0.7* | 3.17±1.4 | 5.00±1.1 |
| 50 | Cembrene | 16.90±3.3 | 14.07±8.7 | 54.27±16.6 | 1.28±0.8 | 15.73±3.5 | 9.78±1.5 |
| N and/or S containing | |||||||
| 16 | 1,2-Dimethyldisulfide | 66.36±12.8 | 39.86±8.1 | 420.67±341.7 | 33.40±20.2 | 19.96±2.1 | 22.90±5,2* |
| 21 | Allyl isothiocyanate | 393.18±75.7 | 63.65±38.0 | 782.07±391.7 | 53.90±20.8* | 147.79±50.1 | 641.32±565.5 |
| 46 | 2-Phenylethyl isothiocyanate | 13.86±2.7 | 3.06±1.0 | 20.23±7.8 | 1.06±0.4* | 10.81±2.9 | 3.92±0.9* |
| Cyclic/Heterocyclic | |||||||
| 5 | Tetrahydrofuran | 2.49±0.5 | 1.96±0.2 | 4.66±1.3 | 1.37±0.4 | 1.07±0.2 | 1.65±0.8 |
| 13 | Methylcyclohexane | 11.56±2.2 | 5.20±0.8 | 19.64±8.4 | 3.79±1.7 | 4.08±1.2 | 6.73±2.3 |
| 14 | Pyrazine | 9.15±1.8 | 7.32±2.4 | 12.03±2.5 | 3.58±1.4 | 3.28±1.0 | 3.38±0.5* |
| 31 | 2,2,6-Trimethyl-4-methylene-2H-pyran | 21.35±4.1 | 55.11±47.3 | 12.36±2.9 | 3.43±1.9 | 6.20±2.3 | 9.69±5.5 |
| 42 | 4-(3-Cyclohexen-1-yl)-3-buten-2-one | 4.65±0.9 | 5.58±1.8 | 9.73±4.0 | 1.51±0.8 | 1.19±0.7 | 4.60±1.2 |
Volatile emissions are given in mean peak area ± SEM/g fresh weight of foliage divided by 105 with the number of replicates between brackets.
values with asterisk indicate significant differences in emission quantities between oviposition-induced B. nigra and uninfested control for each treatment (Mann-Whitney U-test).
Figure 3. Projection to Latent Structures Discriminant Analysis (PLS-DA) on the volatile compounds emitted by egg-infested B. nigra.
HR+ (+) or HR− (−) plants were infested by eggs of Pieris brassicae (PB) or Mamestra brassicae (MB) sampled 24 or 72 h after oviposition. (A) Score plot visualizing the grouping pattern of the samples according to the first two PLS components with the explained variance in brackets. The ellipse defines Hotelling’s T2 confidence region (95%). (B) Loading plot of the first two principal components shows the contribution of each of the compounds to the two PLS-DA components. Markers of the 5 different treatments shown in the score plot are given. Hypersensitive response type, (−): no necrotic zone observed, (+): with necrotic zone. Compound numbers: (1) 2-Methylpropanal, (2) 2-methyl-2-Propenal, (3) Ethyl acetate, (4) 2-Methyl-1-propanol, (5) Tetrahydrofuran, (6) 2-Butenal, (7) 3-Methylbutanal, (8) 2-Methylbutanal, (9) 1-Methoxy-2-propanol, (10) 1-Pentene-3-ol, (11) 2-Pentanone, (12) 3-Pentanone, (13) Methylcyclohexane, (14) Pyrazine, (15) 4-Methyl-2-pentanone (16) 1,2-Dimethyldisulfide, (17) (E)-2-Pentenal, (18) 2,4-Pentanedione, (19) 4-Methyl-3-pentene-2-one, (20) (Z)-3-Hexen-1-ol, (21) Allyl isothiocyanate, (22) α-Pinene, (23) 6-Methyl-2-heptanone, (24) (Z)-3-Hexen-1-yl acetate, (25) 3-Carene, (26) (S)-Limonene, (27) α-Phellandrene, (28) Methyl-2-ethylhexanoate, (29) (E)-β-Ocimene, (30) o-Cresol, (31) 2,2,6-Trimethyl-4-methylene-2H-pyran, (32) p-Mentha-1,5,8-triene, (33) 2-Phenylacetonitrile, (34) Isopulegon, (35) p-Menthan-3-one, (36) Isomenthone, (37) Menthol, (38) Undecan-2-one, (39) 7-α-H-Silphiperfol-5-ene, (40) Presilphiperfol-7-ene, (41) 7-β-H-Silphiperfol-5-ene, (42) 4-(3-cyclohexen-1-yl)-3-Buten-2-one, (43) Silphiperfol-6-ene, (44) α-Funebrene, (45) Longifolen, (46) 2-Phenylethyl isothiocyanate, (47) Lilial, (48) Guaiazulene, (49) 2-Ethylhexyl salicylate, (50) Cembrene. Significantly increased terpenoids in volatile blends of P. brassicae egg-infested plants compared to uninfested plants are in bold (*P<0.05, Mann-Whitney U-test).
Oviposition by M. brassicae moths significantly suppressed the emission of the majority of compounds compared to uninfested plants (Table 1; 43 compounds suppressed, P<0.001, sign test). The emission of terpenes did not change after M. brassicae moth oviposition; however, there was a significant reduction in the emission of three compounds, i.e. 1,2-dimethyldisulfide (P = 0.02), and 2-phenylethyl isothiocyanate and pyrazine (Table 1; both: P = 0.05).
Specificity of Changes in Plant Surface Structure
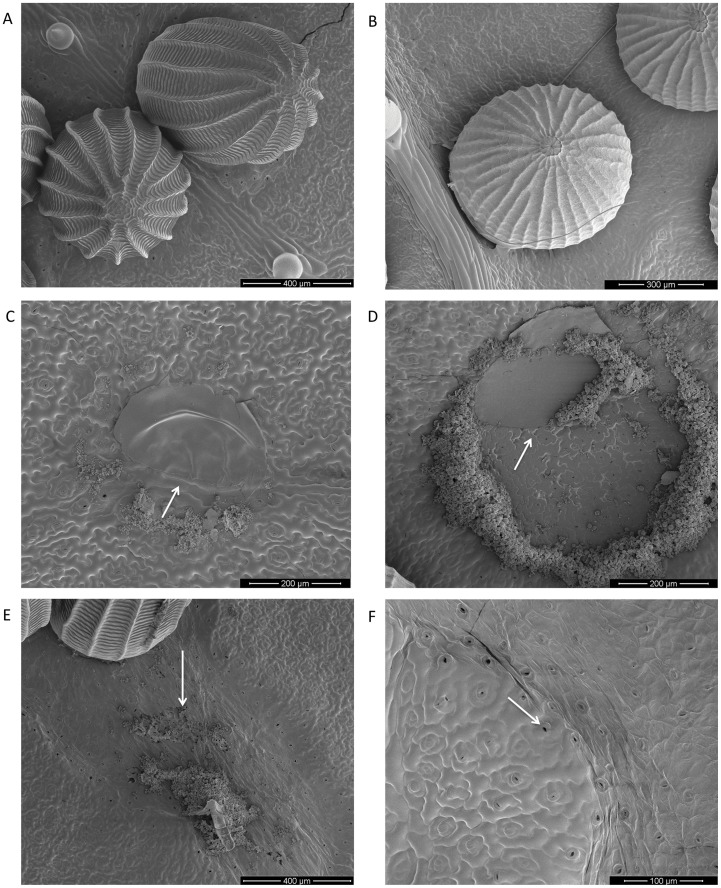
Pieris brassicae butterflies and M. brassicae moths carefully deposit their eggs on B. nigra plants without any visible damage to the surface in the vicinity of the eggs (Figure 4A–B). Egg cement is produced by the accessory reproductive gland and attaches the eggs of P. brassicae and M. brassicae to the substrate (Figure 4C–D). After freezing, we observed that moth eggs detached more easily from the plant surface than eggs of P. brassicae. Egg secretion of P. brassicae partly peeled off after egg removal, covering the surface of HR− B. nigra with a thick layer. Epidermal cell morphology and stomata are not visible under the egg cement of P. brassicae (Figure 4C). Part of the thin egg secretion of M. brassicae moths seems to be peeled off after egg removal or is missing. The cell layer below the egg cement seems to be intact and stomata are half opened (Figure 4D). In HR+ plants, necrotic tissue develops within 72 h and is only induced by P. brassicae eggs. Here, egg removal leads to detachment of surface layers of dead cells together with the egg secretion (Figure 4E). Interestingly, stomata are open adjacent to cells at the boundary of the necrotic zone, supposedly in the programmed cell death phase (Figure 4F).
Figure 4. Cryo-SEM micrographs of B. nigra leaf surfaces and adhering herbivore eggs and egg – leaf contact regions.
(A–E) abaxial site of B. nigra leaves. (A) Eggs of P. brassicae 72 hao with surrounding leaf surface of HR+ B. nigra and trichomes. (B) Eggs of M. brassicae (48–60 h old) with surrounding leaf surface of HR− B. nigra. (C) Contact region after P. brassicae egg removal (72 hao) on HR− B. nigra consisting of accessory reproductive gland (ARG) secretion functioning as cement (arrow). (D) Contact region after M. brassicae egg removal consisting of a part of ARG cement and healthy leaf cells with open stomata (arrow). (E) Necrotic zone on HR+ B. nigra leaf induced by P. brassicae eggs 72 hao, with some eggs removed. ARG-derived cement is removed together with parts of the cell layer (arrow) and open stomata at the border between dead and living cells. (F) Adaxial site of necrotic zone of HR+ B. nigra with open stomata (arrow) between healthy cells and necrotic zone on right side. Hypersensitive response type, HR−: no necrotic zone observed, HR+: with necrotic zone. Scale bars are given in lower right corners.
Discussion
Our study revealed that plant volatiles induced in the early phase of colonization by insect herbivores, before actual feeding starts, mediate interactions between a range of insect community members at different trophic levels: egg and larval parasitoids are attracted and the specialist herbivore prefers plants that are free of eggs. Moreover, we show that the plant and associated insects respond differently to egg deposition by two herbivores, the specialist butterfly P. brassicae and the generalist moth M. brassicae. Oviposition by the abundant specialist pine sawflies Diprion pini and Neodiprion sertifer on Pinus sylvestris induced the emission of pine volatiles that attracted the specialized egg parasitoid Chrysonotomyia ruforum, whereas eggs of the less abundant pine sawfly Gilpinia pallida did not induce such a response [32].
OIPVs may provide an effective defence against the attacking herbivore: egg parasitoids kill a certain proportion of the host eggs; and the remaining proportion of eggs yields caterpillars that may be attacked by larval parasitoids. Gravid butterfly females avoid plants infested by eggs of conspecifics by using OIPVs. Herbivore-induced plant volatiles have been shown to mediate interactions with other herbivores [52], [53], but to the best of our knowledge this has not been reported for OIPVs. In combination with limited feeding damage, odours of plants carrying few eggs were shown to be avoided by gravid elm leaf beetles [54]. Recently, Bruce et al. [39] showed that the spotted stemborer Chilo partellus avoided oviposition on egg-induced African forage grass (Brachiaria brizantha), but the role played by visual and contact cues here was not determined.
Furthermore, we here show that parasitism of P. brassicae eggs by T. brassicae wasps on HR+ and HR− plants was equally successful, which means that there is no conflict between the induced hypersensitive response and the performance and attraction of the egg parasitoid. While eggs of P. brassicae induce HR in about 50% of the observed B. nigra plants, eggs of M. brassicae moths did not induce the formation of necrotic tissue. Eggs of cabbage white butterflies, moths and beetles have been shown to induce the formation of necrotic tissue leading to increased egg mortality on different plant species, including the wild crucifer Sinapis arvensis (F. G. Pashalidou, personal observations), potato [22] and Physalis plants [20]. A whole-genome transcriptomic study with Arabidopsis confirmed that oviposition by Pieris sp. triggers a defensive response with strong similarities to microbial-induced HR, i.e. up-regulation of pathogenesis-related genes, callose accumulation, and production of reactive oxygen species [42].
For successful settlement of an herbivorous insect, it is crucial that eggs are deposited with a proper adherence. How firmly eggs are attached to the leaf surface may affect different cells that are able to perceive information about when an egg has been laid [38]. Epicuticular waxes lead to hydrophobic surfaces which can prevent insect egg attachment [55]. Pieris brassicae is specialized to deposit eggs on leaf surfaces typical of species in the Brassicaceae plant family. A water-soluble yellow phenolic compound of the egg cement probably moistens the surface and increases the strength of adhesion [56]. Generalist herbivores such as M. brassicae moths are expected to be less adapted to certain plant surfaces; their eggs seem less firmly attached to the hydrophobic B. nigra surface. From the plant’s plants perspective, we expect less selection pressure on B. nigra to respond to eggs of the less abundant generalist M. brassicae because their larvae perform poorly on wild crucifers containing high levels of secondary compounds, i.e., glucosinolates [57], and tend to leave such plants quickly after emergence (J.A. Harvey and F.G. Pashalidou, personal observations). Egg deposition by Spodoptera frugiperda moths was shown to suppress HIPV emission in maize while eggs were in close contact with the plant cuticle, accompanied with accessory gland secretion. A possible explanation for the HIPV suppression was that the S. frugiperda egg masses are dense and cover parts of the photosynthetic tissue, thus inhibiting the volatile emission [58]. Indeed, oviposition was shown to reduce photosynthesis, which several workers have suggested is caused by the coverage of photosynthetic tissue and/or physiological mechanisms, i.e. reduced CO2 diffusion in the mesophyll or water deficiency [59], [60]. Unlike with M. brassicae eggs, stomata were closed underneath the eggs of P. brassicae and gas/water exchange probably inhibited in B. nigra (Figure 4C–D). However, although eggs of M. brassicae are covering a slightly larger part of the leaf surface than P. brassicae eggs (Figure 1B–C), it is unlikely that this would lead to significant differences in the volatile emission demonstrated here. The observed attraction to volatiles from M. brassicae egg-infested plants by T. brassicae at 24 hao when tested against clean air is probably caused by the moths’ sex pheromone adsorbed to the plant surface, previously shown to attract Trichogramma wasps 24 h after release [61].
Chemical analysis of volatile blends revealed reduced emissions for the majority of chemical compounds in the plant treatments with eggs. Usually, insect herbivory leads to an increase in the emission of plant volatiles that attract carnivorous natural enemies [9], [62]. A reduced emission induced by egg deposition has recently been demonstrated in other plant species as well [39], [58]. Only HR+ plants 24 h after P. brassicae oviposition showed an increased emission, probably due to the initiation of necrosis. A significant induction of some terpenoids might contribute to the specificity of P. brassicae egg-induced volatile blends that are attractive or repellent to the tested insects. For example, the emission of (E)-β-ocimene was enhanced in B. nigra 24 h after P. brassicae oviposition. This monoterpene has been shown to be highly inducible by herbivory [5], [8], [63]. In their study on African grass, Bruce et al. [39] demonstrated similar effects: oviposition by C. partellus reduces the plant volatile emission of the main compound, the green-leaf volatile (Z)-3-hexenyl acetate, and increases the emission of minor compounds, i.e. terpenoids, causing an increased attraction of the larval parasitoid Cotesia sesamiae. The same wasps were shown to be attracted also to synthetic terpenoids [36].
Whether the attraction to OIPVs by different parasitoid species and avoidance by herbivores is adaptive for the plant and eventually leads to enhanced plant fitness remains to be proven. Kessler & Heil [64] argued that HIPV-mediated reduction in herbivory may not result in increased plant fitness because most natural enemies do not immediately kill the herbivore, plants have a high tolerance to herbivory, and HIPV are part of a network with many more functions. However, the results of our study suggest a benefit for B. nigra resulting from the release of OIPVs. Idiobiont parasitoids that immediately kill the host such as Trichogramma spp. are likely to have a greater impact on plant fitness than parasitism by koinobiont parasitoids, which allow the parasitized host to continue to feed. Trichogramma wasps have been demonstrated to be significant mortality factors for eggs of Pieris species in the field. In an on-going field survey of a Dutch B. nigra population, 30–40% of the collected Pieris eggs were found to be parasitized by Trichogramma spp. (N.E. Fatouros, unpublished data) [65]. Feeding by Pieris caterpillars can have detrimental effects on flowering brassicaceous plant species: P. brassicae caterpillars have been shown to move to and preferentially feed on the flowers of B. nigra plants a few days after hatching [66], [67]. Moreover, the multifunctional effects of OIPVs released by B. nigra on different members of the insect community demonstrated here is beneficial to the plant: direct (egg-killing HR and avoidance by female butterflies) and indirect (parasitoid attraction) defence traits against Pieris butterflies work in concert which seems to lead to high Pieris egg mortality rates under natural conditions (N.E. Fatouros, unpublished data) [65].
Both parasitoid species studied here discriminated between volatiles induced by eggs of their host P. brassicae and uninfested B. nigra plants, but not between plant volatiles induced by eggs of the moth M. brassicae and uninfested B. nigra. For C. glomerata, M. brassicae cannot serve as a host and, therefore, it may be adaptive for the wasps to discriminate between host- and non-host induced plant volatile blends. Studies on brassicaceous plant species demonstrated that naïve C. glomerata failed to discriminate between HIPV blends from host and non-host insects [68] or from different host instars [69]. Volatiles emitted by B. oleracea plants damaged by different herbivores were shown to be very similar [9], [12]. So far, a single study revealed that parasitoids can innately use HIPV blends to discriminate between host and non-host herbivores [70]. Approaching host-infested plants in an early stage of host development might help Cotesia wasps to find host patches and avoid to fly to patches of older host larvae, which are unsuitable for development [71]. Recent studies on different maize varieties and a grass species induced by eggs of the stemborer moth C. partellus confirmed an attraction to OIPVs by a larval parasitoid [36], [37], [39].
Our data reveal an effect of an induced plant response on members of the insect community at different trophic levels during the pre-feeding phase of herbivore colonization. The synergistic effect of OIPVs attracting different parasitoid species and causing avoidance by herbivores might lead to an effective reduction of fitness loss caused by a common insect herbivore of brassicaceous plant species. Our findings thus suggest that studies on plant defences induced by herbivores should consider the first phase of herbivore attack before feeding damage has occurred, because of it significant impact on multi-trophic interactions. As a follow-up, we are currently investigating the role of OIPVs under natural conditions to fully understand the consequences of plant-mediated effects of insect egg deposition for the structure and dynamics of arthropod communities.
Materials and Methods
Plants and Insects
Black mustard plants (B. nigra L.) were grown in a greenhouse (18±5°C, 50–70% r.h., L16:D8). Seeds originated from the Centre for Genetic Resources (CGN, Wageningen, The Netherlands). This accession (feral population, collected in 1975 from the Peloponesus, Greece) had been multiplied by exposing them to pollinators in a common garden experiment in the surroundings of Wageningen, The Netherlands [66]. Plants of 3 to 5 weeks old were used in the experiments. All used insects were collected in the surroundings of Wageningen, The Netherlands. No specific permits were required for their collection. The collection sites were not privately owned or protected in any way and field samplings did not involve endangered or protected species. Mated females of P. brassicae (Lepidoptera: Pieridae) were obtained by pairing a virgin male and a virgin female butterfly one day after eclosion. Two days after mating, P. brassicae females were used in the experiments. Female M. brassicae L. (Lepidoptera: Noctuidae) moths were placed together with a B. nigra plant in a cage to allow egg deposition. Both herbivorous insects were reared on Brussels sprouts plants (B. oleracea var. gemmifera cv. Cyrus) in a climate room (21±1°C, 50–70% rh, L16:D8). Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae) was reared in eggs of the moth Ephestia kuehniella (Koppert, Berkel en Rodenrijs, The Netherlands) in a climate chamber (25±1°C, 50–70% rh, L16:D8). Only mated, 2–5 days old, wasps were used in the experiments. The larval parasitoid Cotesia glomerata L. (Hymenoptera: Braconidae) was reared in P. brassicae caterpillars, feeding on Brussels sprouts plants in a greenhouse (see above). Only mated, 2–8 days old female wasps were used in the experiments. None of the wasps used in the experiments have had previous contact with any plant material or host residues and the wasps are referred to as naïve.
Plant Treatments
For bioassays with egg-infested plants, test plants were placed into a cage with more than 100 P. brassicae adults (female: male ratio 1∶1) to allow deposition of eggs onto the plants. Plants were exposed for no more than 15 min to the butterflies, to obtain 2–3 egg clutches. After this exposure time, egg-infested plants were tested immediately or kept in a greenhouse compartment (21±2°C, 70% r.h., L16:D8) either overnight (24 h) or for 48 to 72 h following egg deposition. Thus, the duration of induction in response to egg deposition was less than 6 to 96 h. Around 120 hao eggs started to hatch. Plants used as controls were kept under the same conditions as the treated plants but had not been in contact with P. brassicae or any other insect. To test for a systemic induction of volatiles, butterflies were allowed to oviposit on the lower leaves and the upper leaves were covered with a mesh bag that prevented oviposition. Bags were removed afterwards. Prior to bioassays, leaves with eggs were removed. Leaves at similar stem positions were removed from control plants. Plants with 2–5 M. brassicae egg clutches were obtained by exposing plants to M. brassicae females during the scotophase. These plants were incubated for an additional one or two days in a greenhouse compartment. Thus, eggs were 24–36 or 48–60 h old when the plants were used in the bioassay.
Egg-induced Necrosis
All egg-induced plants were checked for the formation of necrotic tissue, referred to as hypersensitive response (HR) 24 h and 72 h after oviposition. The strength of HR was recorded and the plants were categorized into HR− (no necrotic zone observed) and HR+ (necrotic zone +/− eggs fallen off). Plants were kept under greenhouse conditions (22±2°C, 70% r.h., L16: D8). From 10 plants, the number of plants with necrosis was noted and the number of eggs was counted directly after oviposition and after 5 days.
Egg Parasitoid Performance
To investigate whether the performance of T. brassicae in eggs deposited on plants that respond with HR is affected, we infested at least five different plants with P. brassicae eggs and offered them 24 h or 72 h after oviposition to T. brassicae. Previous research showed that Trichogramma wasps were able to parasitize P. brassicae eggs 0–72 hao [26]. An egg-carrying leaf of an HR+ or HR− plant was excised and a piece of it carrying 8 eggs (about 2 cm2) was offered to a 2–3 days old inexperienced female T. brassicae wasp in a glass tube. After 48 h, wasps as well as hatching eggs were discarded. Successful parasitism was checked after 7 days and emerging offspring were counted 12 days after oviposition. In total, 15 female wasps were tested for each treatment.
Dynamic Y-tube Olfactometer
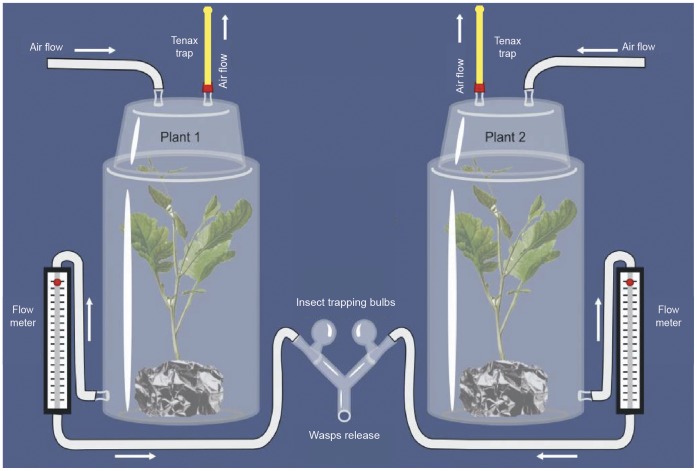
Bioassays with T. brassicae wasps were conducted in a dynamic airflow Y-tube olfactometer, a modified version of the six-arm olfactometer developed by Turlings et al. [72] (Figure 5). This olfactometer was adapted for small wasps like Trichogramma sp.; wasps were released in groups collected in so-called insect trapping bulbs (Figure 5). Pressurized air was filtered through activated charcoal and approximately 150 mg of Tenax-TA 25/30 mesh (Grace-Alltech) before entering the system. Subsequently, air was humidified by passing through a bottle containing 50 mL of tap water. A flow meter-controlled (Brooks Instrument B.V., Veenendaal, NL) airflow of 400 mL min−1 was admitted into the system. The airflow was split into two and each subflow was led into a glass container (45 mm high, 200 mm diameter) holding an odour source through an inlet situated on the lid. These containers were sealed airtight using a Viton O-ring and a metal clamp. Air from each odour container was subsequently led into one of the arms of a glass Y-tube olfactometer (stem 9 cm, arms 8 cm, ID 1 cm). All glass parts were connected using Teflon tubing. The airflow was set at 100 mL min−1 in each arm using flow meters. Experiments were carried out between 10∶00 and 16∶00 h in the laboratory at 21±2°C using a T5-growth light with a spectrum that is close to sunlight. Light bulbs (4×24 W) were situated above the olfactometer and the containers with the odour sources. Just before placing a plant in the odour containers, the pot of the plant was removed and the roots and soil were tightly covered with aluminium foil.
Figure 5. Overview of the Y-tube olfactometer with simultaneous volatile trapping.
Wasps were released in groups and collected in insect trapping collection bulbs. Volatiles were trapped simultaneously or after a bioassay with Tenax TA tubes. Illustration credits: I. Figueroa.
Ten adult females of T. brassicae were released simultaneously and their preference for one of the two odour sources was recorded. Wasps, which were attracted to light, were trapped in two round trapping bulbs connected to the Y-tube near the end of each arm. After 30 minutes, the wasps collected in each of the trapping bulbs were counted. When a wasp did not make a choice within 30 minutes, it was recorded as a “no response” and excluded from the statistical analysis. In total, 100–120 wasps were tested with 5–6 different plants per odour source combination with two replicates per experimental day. Each wasp was used only once. To exclude any bias, the position of the odour sources was exchanged after every trial.
Flight Chamber Experiments
Butterfly odour preferences were tested in a two-choice situation in a flight tent as described by Gols et al. [70]. A female butterfly was released 80 cm away from the uninfested and egg-infested plant, which were placed 55 cm apart. Eggs were removed just prior to testing. After releasing the butterfly, first landing and oviposition was recorded, after which the observation was ended and the female and her eggs were removed immediately. Females that did not respond within 15 min were recorded as “no response” and excluded from the analysis. Plants were switched after 3 consecutive butterfly observations with a total of max. 10 responding females per set of plants. Plants had been infested with eggs for either 24 h or 72 h (for P. brassicae) or 24–36 h (for M. brassicae). Per treatment, 43–85 butterflies were tested and 4–6 sets of plants were used.
Wind Tunnel Experiments
Attraction of C. glomerata wasps was conducted in a wind tunnel set-up described in detail by Geervliet et al. [73]. Females were released individually 70 cm down-wind from the two plants, one egg-infested plant and an uninfested control plant. The plant on which the female landed for the first time within 10 min following release was recorded. Non-responding wasps, i.e. those females that did not land within 10 min were counted but excluded from the statistical analysis. Each wasp was used only once. During bioassays, plants were switched after every second wasp tested. The number of tested wasps ranged between 60 and 105 wasps per treatment, tested on at least 5 different days with 5 new sets of plants.
Headspace Collection of Volatiles
When testing the response of T. brassicae wasps to B. nigra volatiles using the Y-tube olfactometer (see above), we simultaneously or afterwards collected volatiles from the headspace of the same plant (s) (Figure 5). Volatiles were collected by sucking air with odours out of a glass jar at a rate of 80 mL min−1 for 4 h through a stainless steel cartridge filled with 200 mg Tenax TA (20/35 mesh; CAMSCO, USA). A pump (PAS-500 SPECTREX, US) was directly connected to the cartridge steel tube with Tenax TA onto the outlet for sucking the air out of the glass jar. In total, 5 plant pairs (control vs. treatment) were sampled per treatment on 5 different days. Aerial parts of the plant were weighed after volatile collection (balance Mettler-Toledo B.V., NL).
Chemical Analysis
Thermo Trace Ultra gas chromatography (GC) coupled with Thermo Trace DSQ quadruple mass spectrometer (MS) (Thermo Fisher Scientific Waltham, USA) were used for separation and detection of plant volatiles. Prior to release of volatiles, Tenax TA cartridges were dry-purged under a stream of nitrogen at 20 ml min−1 for 10 min at ambient temperature in order to remove moisture. The collected volatiles were released thermally from the Tenax TA in an Ultra 50∶50 thermal desorption unit (Markes, Llantrisant, UK) at 250°C for 10 min under a helium flow of 20 ml−1 while re-collecting the volatiles in a thermally cooled universal solvent trap at 10°C using Unity (Markes, Llantrisant, UK). Once the desorption process was completed, the cold trap was heated fast at 40°C s−1 to 280°C and was kept for 5 min at 280°C, while the volatiles were released to a ZB-5MSi capillary column with dimensions 30 m L×0.25 mm I.D.×1.00 µm F.T. (Phenomenex, Torrance, CA, USA), in a split mode at a split ratio of 5∶1 for further separation. The GC oven was operated at an initial temperature of 40°C and was immediately raised at 8°C min−1 to 280°C and held there for 4 min under a helium flow of 1 mL min−1 in constant flow mode. The DSQ MS was operated in scan mode with a mass range of 35–350 amu at 5.38 scans s−1 and ionization was performed in EI mode at 70 eV. MS transfer line and ion source were set at 275 and 250°C, respectively.
Identification of compounds was based on comparison of mass spectra with those in the NIST 2005 and Wageningen Mass Spectral Database of Natural Products MS libraries. Experimentally obtained linear retention indices (LRI) were also used as additional criterion for confirming the identity of compounds. Relative quantitation (peak areas of individual compounds) was carried out using a single (target) ion, in selected ion monitoring (SIM) mode. These individual peak areas of each compound were corrected for the aerial fresh weight of each plant sample and were used for further characterization of the different plant groups using statistical analysis.
Cryo-SEM Imaging
Abaxial and adaxial sites of fresh leaves with P. brassicae or M. brassicae eggs were fresh-frozen and analysed by field emission scanning microscopy (Magellan 400, FEI, Eindhoven, the Netherlands). Leaves were glued on a brass Leica sample holder by carbon glue (Leit- C, Neubauer Chemicalien, Germany), flash-frozen in liquid nitrogen and simultaneously fitted in a cryo-sample loading system (VCT 100). The Leica sample holder was transferred to a non-dedicated cryo-preparation system (MED 020/VCT 100, Leica, Vienna, Austria) onto a sample stage at −93°C. In this cryo-preparation chamber samples were freeze dried for 2 minutes at −93°C at 1.3×10−6 millibar to remove water vapor contamination from the surface of the sample. The sample was sputter-coated with a layer of 15 nm Tungsten at the same temperature. The samples were transferred in high vacuum into a field emission scanning microscope (Magellan 400, FEI, Eindhoven, the Netherlands) on the sample stage at −122°C at 4×10−7 millibar. The analysis was performed with SE at 1 and 2 kV, 13 pA. All images were recorded digitally.
The interface between the B. nigra surface and (1) 72 h old P. brassicae eggs (HR− and HR+) and (2) 48–60 h old M. brassicae eggs (HR−) was comparatively studied (a) in contact and (b) with eggs detached after being frozen.
Statistical Analysis
To analyse whether the distribution of behavioural choices of butterflies and wasps was affected by egg age and plant phenotype, a generalized linear model (GLM) with a logit link function and a binomial distribution for errors was used. The response of a number of animals tested with one set of plants served as experimental unit in the analyses. For each phenotype - -egg age - -animal species-combination at least five newly prepared plant combinations were used. The responses were analysed separately for the three animal species. When overdispersion was detected in the variance parameter, we corrected for this by allowing the variance functions of the binomial distribution to have a multiplicative overdispersion factor by dividing the square root of the deviance of the model by the degrees of freedom.
To determine whether there was a preference for an odour source within a treatment combination, we used one sample t-test on the proportion of wasps preferring egg induced volatiles in each replicate. Data were arcsine-transformed and tested against arcsine (0.5), i.e. no preference for either odour source. Non-responding wasp were excluded from the analyses (both GLM and t-tests).
Percentages of P. brassicae eggs hatching on HR+ and HR− plants were compared with a chi-square test using a 2×2 contingency table. Performance of T. brassicae on HR+/− plants in relation to egg age was also analysed using a GLM. The proportions of P. brassicae eggs that were parasitized by T. brassicae were analysed using the same GLM approach as for the behavioural responses. The offspring numbers were compared with a logarithm link function and a Poisson distribution for the errors.
Volatile compounds, measured as peak area divided by the fresh weight of a plant’s foliage were analysed using the software program SIMCA P+12.0 (Umetrics AB, Umeå, Sweden). A PLS-DA was used to determine whether samples belonging to specific groups (here treatments) could be separated based on quantitative differences in volatile emissions [74]. A Y-data matrix of dummy variables was included, which assigns a sample to its respective class. The PLS-DA extension of the SIMCA P+12.0 program used for this analysis approximates the point ‘swarm’ in X (matrix with volatile compounds) and Y in PLS components in such a way that maximum covariation between the components in X and Y is achieved. The results of the analysis were visualised in score plots, which reveal the sample structure according to the model components, and loading plots, which display the contribution of the volatile emission to these components, as well as the relationships among the variables. PLS-DAs were performed on full data sets including all volatile compounds and on restricted data sets containing compounds of which the VIP (Variable Importance in the Projection) values were greater than 1. Data were log-transformed, mean-centred, and scaled to unit variance before they were subjected to the analysis.
A Mann-Whitney-U-test was used to test differences in peak area per compound between treated and control plants. A sign test was used to determine whether the number of compounds emitted in larger or smaller amounts differed from a 50∶50 distribution.
Acknowledgments
The authors thank Irena Vafia, Lydia van Bruggen, and Ilich A. Figueroa for their help with conducting some behavioural assays, André Gidding, Léon Westerd and Frans van Aggelen for culturing insects and the experimental farm of Wageningen University (Unifarm) for rearing and providing plants. Adriaan van Aelst kindly helped with Cryo-SEM imaging. We thank Maarten A. Posthumus for chemical identification of some of the plant volatile compounds and Martine Kos for statistical advice.
Funding Statement
The Netherlands Organisation for Scientific Research (NWO/ALW VENI grants 863.05.020 to Dr. Huigens and 863.09.002 to Dr. Fatouros) is acknowledged for funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pimm SL, Lawton JH, Cohen JE (1991) Food web patterns and their consequences. Nature 350: 669–674. [Google Scholar]
- 2.Karban R, Baldwin IT (1997) Induced Responses to Herbivory. Chicago: The Univerity of Chicago Press.
- 3.Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-Plant Biology. Oxford: Oxford University Press.
- 4. Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15: 167–175. [DOI] [PubMed] [Google Scholar]
- 5. Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards for indirect plant defense. Can J Zool 88: 628–667. [Google Scholar]
- 6. Hilker M, Meiners T (2010) How plants “notice” attack by herbivores. Biol Rev 85: 267–280. [DOI] [PubMed] [Google Scholar]
- 7. Dudareva N, Negre F, Nagegowda DA, Orlova I (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25: 417–440. [Google Scholar]
- 8. Unsicker SB, Kunert G, Gershenzon J (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol 12: 479–485. [DOI] [PubMed] [Google Scholar]
- 9. Dicke M (2009) Behavioural and community ecology of plants that cry for help. Plant Cell Envir 32: 654–665. [DOI] [PubMed] [Google Scholar]
- 10. Kessler A, Halitschke R (2007) Specificity and complexity: the impact of herbivore-induced plant responses on arthropod community structure. Curr Opin Plant Biol 10: 409–414. [DOI] [PubMed] [Google Scholar]
- 11. Van der Putten WH, Vet LEM, Harvey JA, Wäckers FL (2001) Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16: 547–554. [Google Scholar]
- 12. Hare JD (2010) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Ann Rev Entomol 56: 161–180. [DOI] [PubMed] [Google Scholar]
- 13. Poelman EH, van Loon JJA, Dicke M (2008) Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant Sci 13: 534–541. [DOI] [PubMed] [Google Scholar]
- 14. Hilker M, Meiners T (2011) Plants and insect eggs: How do they affect each other? Phytochemistry 72: 1612–1623. [DOI] [PubMed] [Google Scholar]
- 15. Seino Y, Suzuki Y, Sogawa K (1996) An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (Horvath) (Homoptera: Delphacidae). Appl Ent Zool 31: 467–473. [Google Scholar]
- 16. Suzuki Y, Sogawa K, Seino Y (1996) Ovicidal reaction of rice plants against the whitebacked planthopper, Sogatella furcifera Horvath (Homoptera: Delphacidae). Appl Ent Zool 31: 111–118. [Google Scholar]
- 17. Beyaert I, Köpke D, Stiller J, Hammerbacher A, Yoneya K, et al. (2011) Can insect egg deposition ‘warn’ a plant of future feeding damage by herbivorous larvae? Proc Roy Soc Lond B 279: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruessow F, Gouhier-Darimont C, Buchala A, Metraux JP, Reymond P (2010) Insect eggs suppress plant defence against chewing herbivores. Plant J 62: 876–885. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Tooker JF, Luthe DS, De Moraes CM, Felton GW (2012) Insect eggs can enhance wound response in plants: a study system of tomato Solanum lycopersicum L. and Helicoverpa zea Boddie. PLoS ONE 7: e37420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petzold-Maxwell J, Wong S, Arellano C, Gould F (2011) Host plant direct defence against eggs of its specialist herbivore, Heliothis subflexa . Ecol Entomol 36: 700–708. [Google Scholar]
- 21. Desurmont GA, Weston PA (2011) Aggregative oviposition of a phytophagous beetle overcomes egg-crushing plant defences. Ecol Entomol 36: 335–343. [Google Scholar]
- 22. Balbyshev NF, Lorenzen JH (1997) Hypersensitivity and egg drop: A novel mechanism of host plant resistance to Colorado potato beetle (Coleoptera: Chrysomelidae). J Econom Entomol 90: 652–657. [Google Scholar]
- 23. Doss RP, Proebsting WM, Potter SW, Clement SL (1995) Response of NP mutant of pea (Pisum sativum L.) to pea weevil (Bruchus pisorum L.) oviposition and extracts. J Chem Ecol 21: 97–106. [DOI] [PubMed] [Google Scholar]
- 24. Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy SR, et al. (2000) Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. PNAS USA 97: 6218–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conti E, Salerno G, Leombruni B, Frati F, Bin F (2010) Short-range allelochemicals from a plant-herbivore association: A singular case of oviposition-induced synomone for an egg parasitoid. J Exp Biol 213: 3911–3919. [DOI] [PubMed] [Google Scholar]
- 26. Fatouros NE, Bukovinszkine’Kiss G, Kalkers LA, Soler Gamborena R, Dicke M, et al. (2005) Oviposition-induced plant cues: Do they arrest Trichogramma wasps during host location? Entomol Exp Appl 115: 207–215. [Google Scholar]
- 27. Fatouros NE, Broekgaarden C, Bukovinszkine’Kiss G, van Loon JJA, Mumm R, et al. (2008) Male-derived butterfly anti-aphrodisiac mediates induced indirect plant defense. PNAS, USA 105: 10033–10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fatouros NE, Pashalidou FG, Cordero WVA, van Loon JJA, Mumm R, et al. (2009) Anti-aphrodisiac compounds of male butterflies increase the risk of egg parasitoid attack by inducing plant synomone production. J Chem Ecol 35: 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colazza S, Fucarino A, Peri E, Salerno G, Conti E, et al. (2004) Insect oviposition induces volatile emission in herbaceous plants that attracts the egg parasitoid Trissolcus basalis . J Exp Biol 207: 47–53. [DOI] [PubMed] [Google Scholar]
- 30. Colazza S, McElfresh JS, Millar JG (2004) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis . J Chem Ecol 30: 945–964. [DOI] [PubMed] [Google Scholar]
- 31. Meiners T, Hilker M (1997) Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae). Oecologia 112: 87–93. [DOI] [PubMed] [Google Scholar]
- 32. Mumm R, Tiemann T, Varama M, Hilker M (2005) Choosy egg parasitoids: Specificity of oviposition-induced pine volatiles exploited by an egg parasitoid of pine sawflies. Entomol Exp Appl 115: 217–225. [Google Scholar]
- 33. Meiners T, Hilker M (2000) Induction of plant synomones by oviposition of a phytophagous insect. J Chem Ecol 26: 221–232. [Google Scholar]
- 34. Hilker M, Kobs C, Varama M, Schrank K (2002) Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J Exp Biol 205: 455–461. [DOI] [PubMed] [Google Scholar]
- 35. Conti E, Zadra C, Salerno G, Leombruni B, Volpe D, et al. (2008) Changes in the volatile profile of Brassica oleracea due to feeding and oviposition by Murgantia histrionica (Heteroptera: Pentatomidae). Eur J Entomol 105: 839–847. [Google Scholar]
- 36. Tamiru A, Bruce TJA, Woodcock CM, Caulfield JC, Midega CAO, et al. (2011) Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol Lett 14: 1075–1083. [DOI] [PubMed] [Google Scholar]
- 37. Tamiru A, Bruce TJ, Midega CA, Woodcock CM, Birkett MA, et al. (2012) Oviposition induced volatile emissions from African smallholder farmers’ maize varieties. J Chem Ecol 38: 231–234. [DOI] [PubMed] [Google Scholar]
- 38. Hilker M, Meiners T (2006) Early herbivore alert: Insect eggs induce plant defense. J Chem Ecol 32: 1379–1397. [DOI] [PubMed] [Google Scholar]
- 39. Bruce TJA, Midega CAO, Birkett MA, Pickett JA, Khan ZR (2010) Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol Lett 6: 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fatouros NE, Pashalidou FG, Aponte Cordero WV, van Loon JJA, Mumm R, et al. (2009) Anti-aphrodisiac compounds of male butterflies increase the risk of egg parasitoid attack by inducing plant synomone production. J Chem Ecol 35: 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bruessow F, Reymond P (2007) Oviposition-Induced Changes in Arabidopsis Genome Expression - Anticipating your Enemy? Plant Sig Behav 2: e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Little D, Gouhier-Darimont C, Bruessow F, Reymond P (2007) Oviposition by pierid butterflies triggers defense responses in Arabidopsis . Plant Physiol 143: 784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shapiro AM, Devay JE (1987) Hypersensitivity reaction of Brassica nigra L. (Cruciferae) kills eggs of Pieris butterflies (Lepidoptera, Pieridae). Oecologia 71: 631–632. [DOI] [PubMed] [Google Scholar]
- 44. Pashalidou FG, Huigens ME, Dicke M, Fatouros NE (2010) The use of oviposition-induced plant cues by Trichogramma egg parasitoids. Ecol Entomol 35: 748–753. [Google Scholar]
- 45.Colazza S, Peri E, Salerno G, Conti E (2010) Host Searching by Egg Parasitoids: Exploitation of Host Chemical Cues. In: Consoli FL, Parra JRP, Zucchi R, editors. Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma. Dordrecht: Springer. 97–147.
- 46.Blenn B, Bandoly M, Kueffner A, Otte T, Geiselhardt S, et al. (2012) Insect egg deposition induces indirect defense and epicuticular wax changes in Arabidopsis thaliana. J Chem Ecol: online published DOI 10.1007/s10886-10012-10132-10888. [DOI] [PubMed]
- 47. Gols R, Harvey JA (2009) Plant-mediated effects in the Brassicaceae on the performance and behaviour of parasitoids. Phytochem Rev 8: 187–206. [Google Scholar]
- 48. Poelman EH, Galiart R, Raaijmakers CE, van Loon JJA, van Dam NM (2008) Performance of specialist and generalist herbivores feeding on cabbage cultivars is not explained by glucosinolate profiles. Entomol Exp Appl 127: 218–228. [Google Scholar]
- 49. Harvey JA, Gols R (2011) Development of Mamestra brassicae and its solitary endoparasitoid Microplitis mediator on two populations of the invasive weed Bunias orientalis . Population Ecology 53: 587–596. [Google Scholar]
- 50. Harvey JA, Gols R (2011) Population-related variation in plant defense more strongly affects survival of an herbivore than its solitary parasitoid wasp. J Chem Ecol 37: 1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polaszek A (2010) Species Diversity and Host Associations of Trichogramma in Eurasia. In: Consoli FL, Parra JRP, Zucchi R, editors. Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma. Dordrecht: Springer. 237–266.
- 52. Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144. [DOI] [PubMed] [Google Scholar]
- 53. De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410: 577–580. [DOI] [PubMed] [Google Scholar]
- 54. Meiners T, Hacker NK, Anderson P, Hilker M (2005) Response of the elm leaf beetle to host plants induced by oviposition and feeding: the infestation rate matters. Entomol Exp Appl 115: 171–177. [Google Scholar]
- 55.Gorb S (2001) Attachment devices of insect cuticule. Dordrecht: Kluwer Academic Publishers.
- 56. Beament JWL, Lal R (1957) Penetration through the Egg-shell of Pieris brassicae (L.). Bulletin of Entomological Research 48: 109–125. [Google Scholar]
- 57. Gols R, Wagenaar R, Bukovinszky T, van Dam NM, Dicke M, et al. (2008) Genetic variation in defense chemistry in wild cabbages affects herbivores and their endoparasitoids. Ecology 89: 1616–1626. [DOI] [PubMed] [Google Scholar]
- 58. Penaflor MFGV, Erb M, Robert CAM, Miranda LA, Werneburg AG, et al. (2011) Oviposition by a moth suppresses constitutive and herbivore-induced plant volatiles in maize. Planta 234: 207–215. [DOI] [PubMed] [Google Scholar]
- 59. Schröder R, Forstreuter M, Hilker M (2005) A plant notices insect egg deposition and changes its rate of photosynthesis. Plant Physiol 138: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Velikova V, Salerno G, Frati F, Peri E, Conti E, et al. (2010) Influence of feeding and oviposition by phytophagous pentatomids on photosynthesis of herbaceous plants. J Chem Ecol 36: 629–641. [DOI] [PubMed] [Google Scholar]
- 61. Noldus LPJJ, Potting RPJ, Barendregt HE (1991) Moth sex pheromone adsorption to leaf surface: bridge in time for chemical spies. Physiol Entomol 16: 329–344. [Google Scholar]
- 62. Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178: 41–61. [DOI] [PubMed] [Google Scholar]
- 63. Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–331. [PMC free article] [PubMed] [Google Scholar]
- 64. Kessler A, Heil M (2011) The multiple faces of indirect defences and their agents of natural selection. Funct Ecol 25: 348–357. [Google Scholar]
- 65.Figueroa IA (2010) Attraction of Trichogramma wasps to Brassica nigra plants induced by lepidopteran eggs. Wageningen: Wageningen University. 20 p.
- 66. Smallegange RC, Van Loon JJA, Blatt SE, Harvey JA, Agerbirk N, et al. (2007) Flower vs. leaf feeding by Pieris brassicae: Glucosinolate-rich flower tissues are preferred and sustain higher growth rate. J Chem Ecol 33: 1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lucas-Barbosa D, Van Loon JJA, Gols R, Van Beek TA, Dicke M (2012) Reproductive escape: Brassica nigra plants respond to Pieris brassicae eggs by accelerating seed production. Funct Ecol: tentatively accepted.
- 68. Geervliet JBF, Vet LEM, Dicke M (1996) Innate responses of the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae) to volatiles from different plant-herbivore complexes. J Insect Behav 9: 525–538. [Google Scholar]
- 69. Mattiacci L, Dicke M (1995) The parsitoid Cotesia glomerata (Hymenoptera: Braconidae) discriminates between first and fifth larval instars of its host Pieris brassicae, on the basis of contact cues from frass, silk and herbivore-damaged leaf tissue. J Insect Behav 8: 485–498. [Google Scholar]
- 70. Gols R, Van Dam NM, Raaijmakers CE, Dicke M, Harvey JA (2009) Are population differences in plant quality reflected in the preference and performance of two endoparasitoid wasps? Oikos 118: 733–743. [Google Scholar]
- 71. Harvey JA (2000) Dynamic effects of parasitism by an endoparasitoid wasp on the development of two host species: implications for host quality and parasitoid fitness. Ecol Entomol 25: 267–278. [Google Scholar]
- 72. Turlings TCJ, Davison AC, Tamo C (2004) A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol Entomol 29: 45–55. [Google Scholar]
- 73. Geervliet JBF, Vet LEM, Dicke M (1994) Volatiles from damaged plants as major cues in long-range host-searching by the specialist parasitoid Cotesia rubecula . Entomol Exp Appl 73: 289–297. [Google Scholar]
- 74.Eriksson L, Johansson E, Kettaneh-Wold N, Trygg JCW, Wold S (2006) Multi- and Megavariate Data Analysis. Part 1: Basic Principles and Applications. Umea, Sweden: Umetrics AB.