Abstract
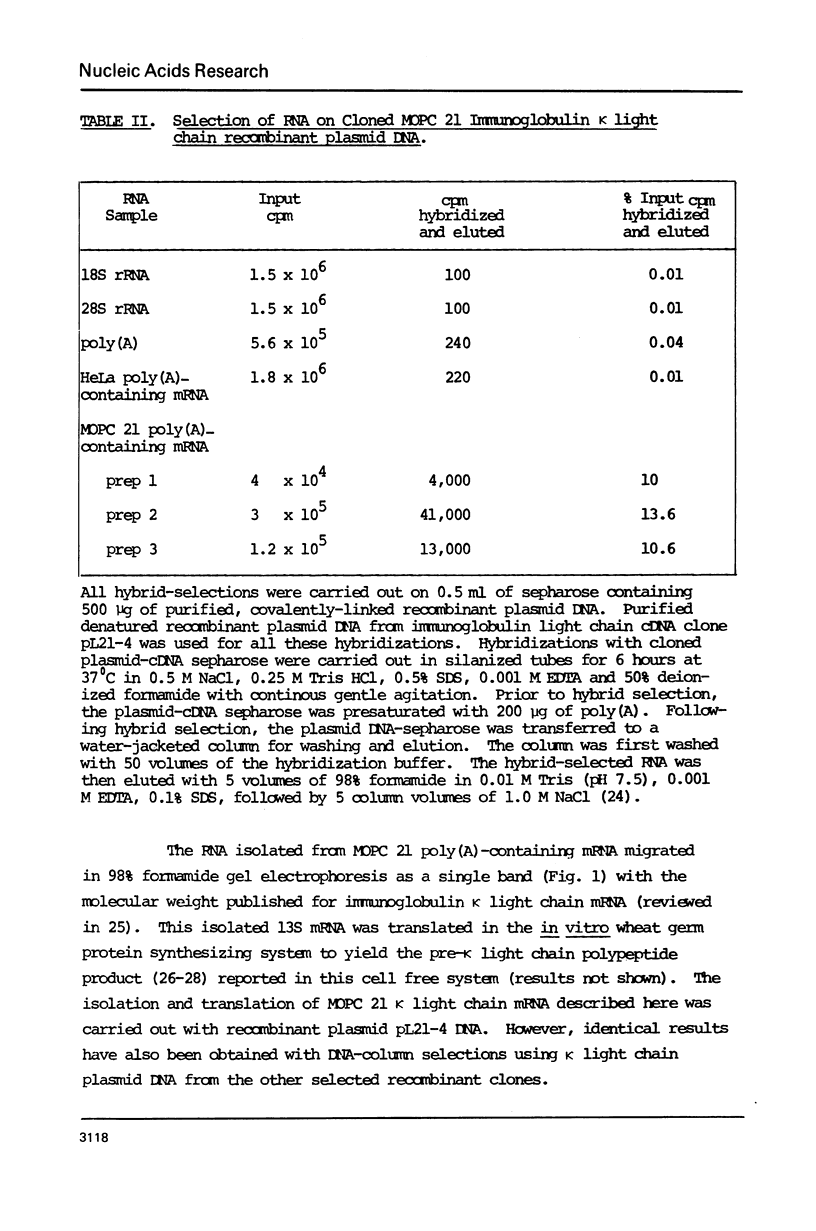
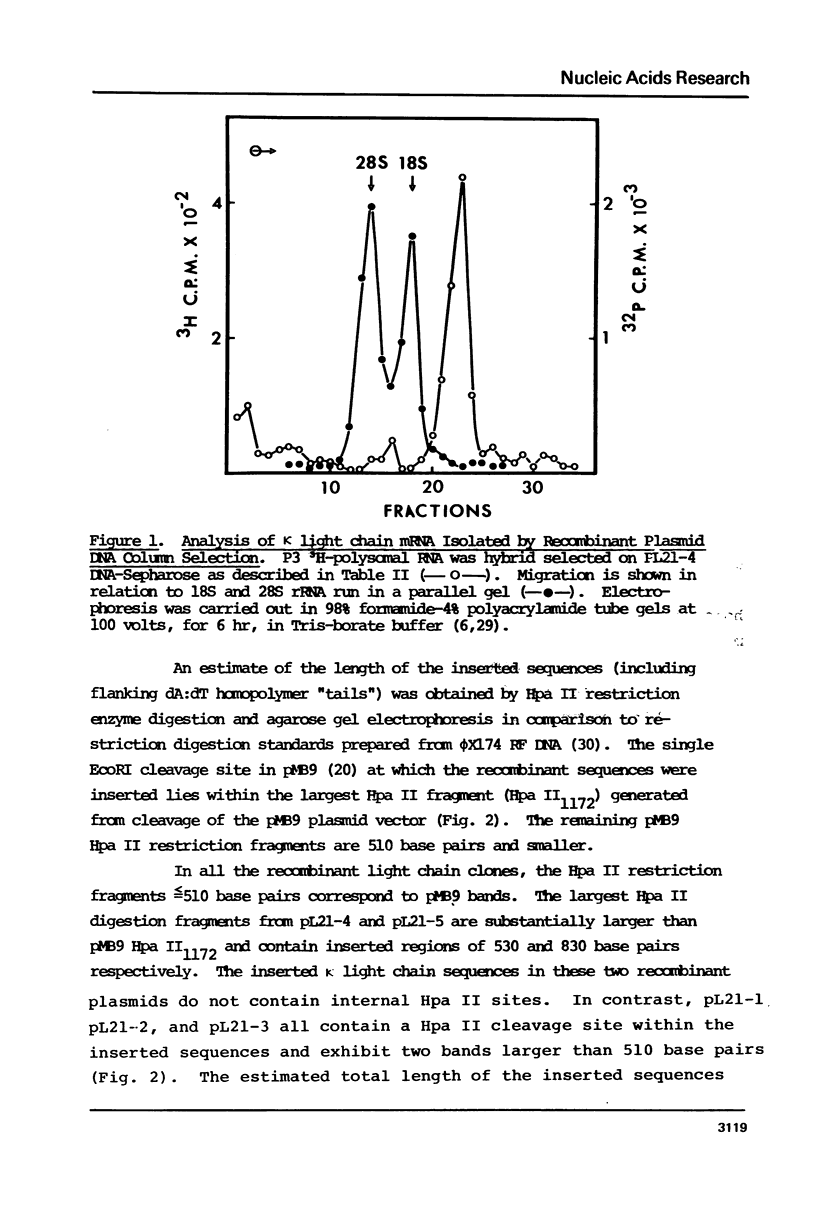
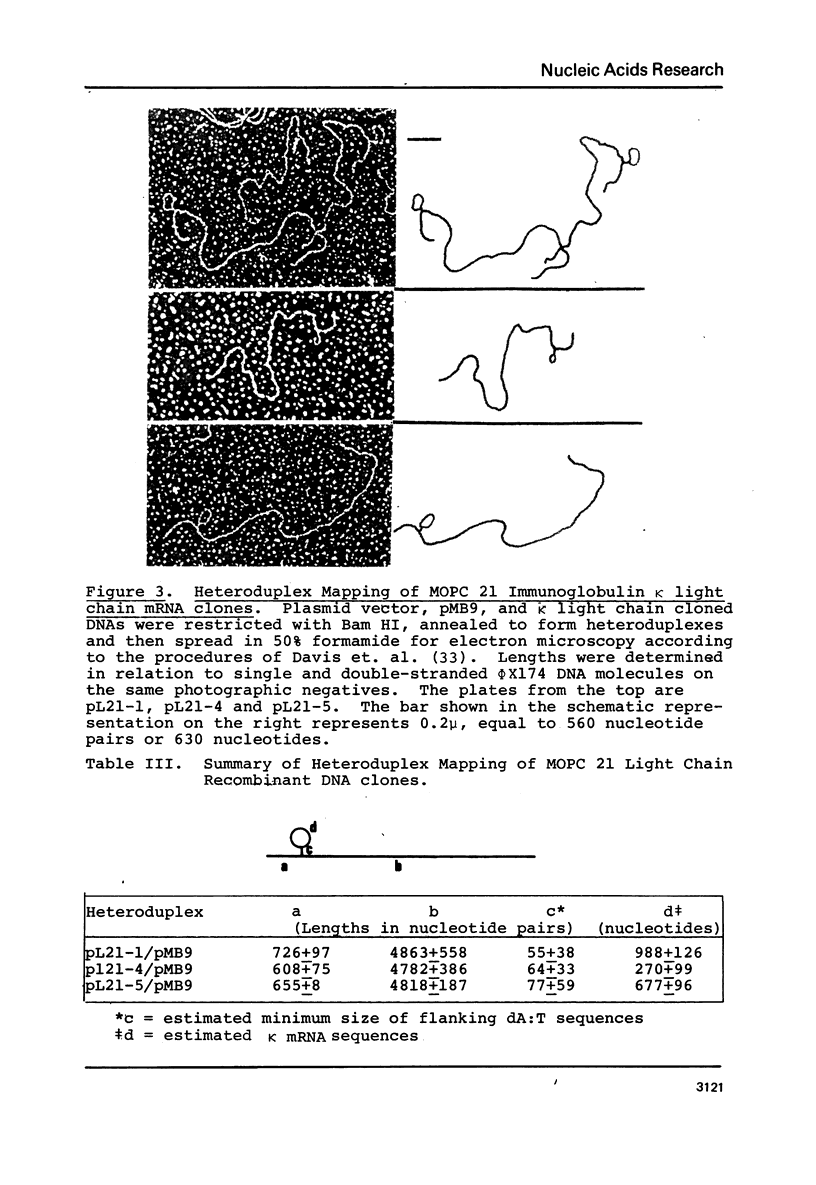
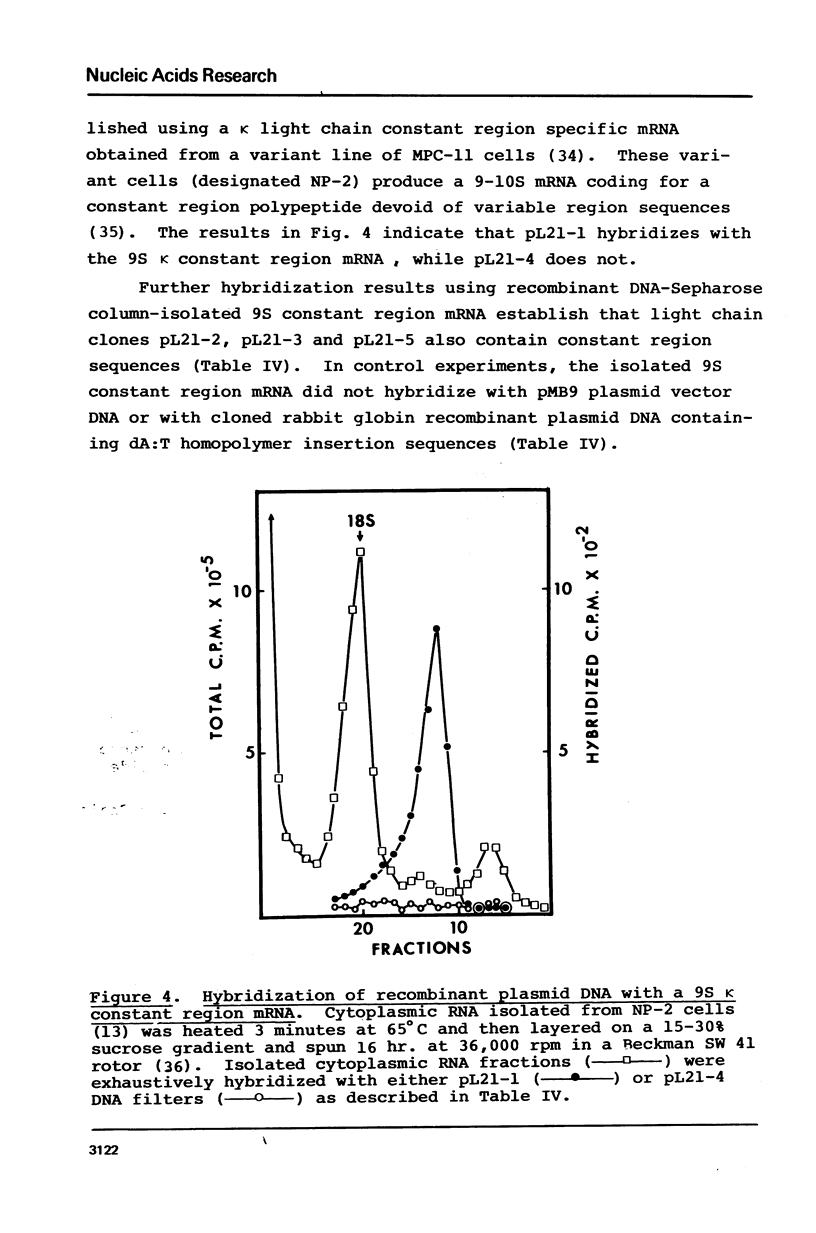
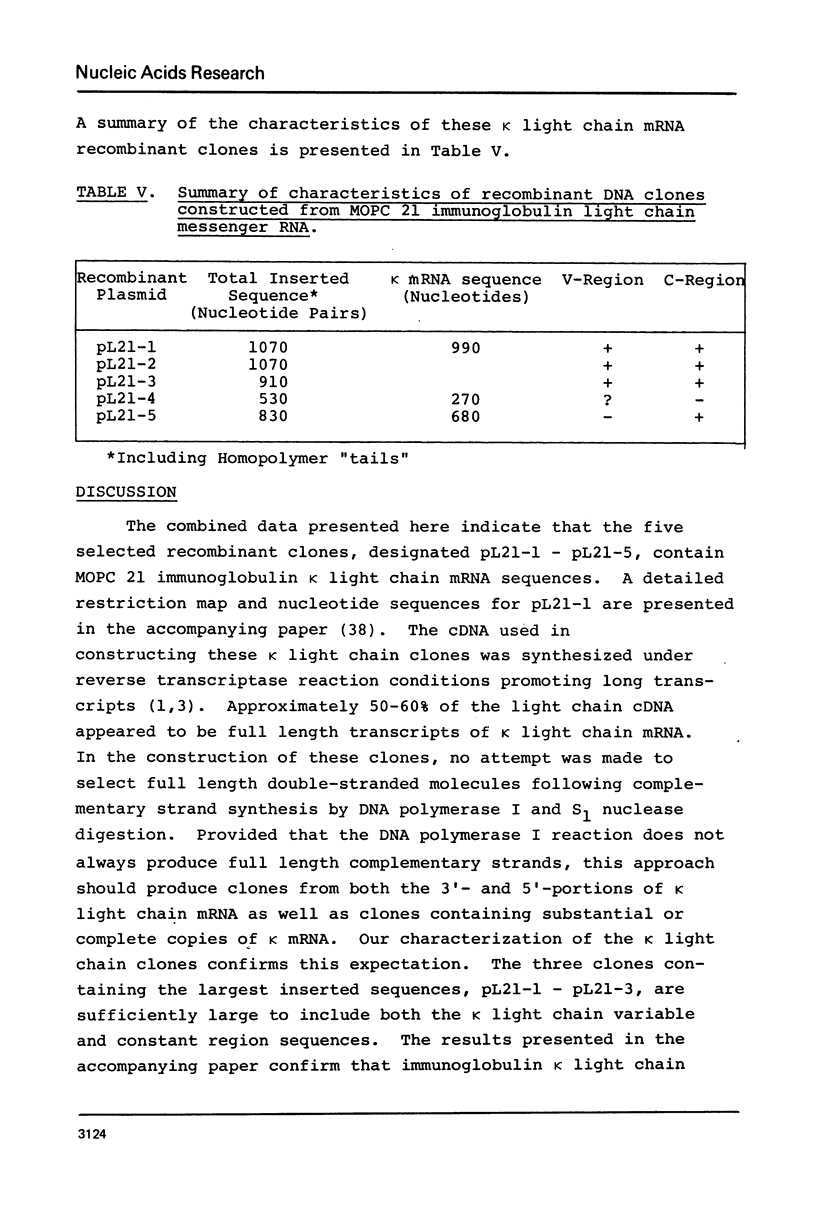
Recombinant DNA clones have been generated from mouse myeloma MOPC 21 immunoglobulin kappa light chain mRNA. Complementary DNA (cDNA) synthesized on kappa light chain mRNA by reverse transcriptase was made double stranded and inserted into the bacterial plasmid vector, pMB9. Approximately 70 tetracycline-resistant transformed colonies containing kappa light chain mRNA sequences were identified by colony hybridization. Five of these recombinant clones were selected and characterized. Three clones contain both kappa light chain constant and variable region sequences. Two of these three recombinant clones have been shown to include all of the kappa light chain constant and variable region coding sequences. Another of the five selected recombinant clones contain kappa light chain constant region sequences. The remaining characterized clone appears to be derived from sequences at the 5'-end of kappa light chain mRNA, possibly extending to the terminal cap structure.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Farace M. G., Aellen M. F., Briand P. A., Faust C. H., Vassalli P., Mach B. No detectable reiteration of genes coding for mouse MOPC 41 immunoglobulin light-chain mRNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):727–731. doi: 10.1073/pnas.73.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Prives C. L., Aviv H. Purification of SV-40 messenger RNA by hybridization to SV-40 DNA covalently bound to Sepharose. Biochemistry. 1975 Sep 23;14(19):4215–4220. doi: 10.1021/bi00690a010. [DOI] [PubMed] [Google Scholar]
- Gilmore-Hebert M., Wall R. Immunoglobulin light chain mRNA is processed from large nuclear RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):342–345. doi: 10.1073/pnas.75.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Paddock G. V., Wall R., Salser W. A general method for cloning eukaryotic structural gene sequences. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3146–3150. doi: 10.1073/pnas.73.9.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kuehl W. M., Kaplan B. A., Scharff M. D. Characterization of light chain and light chain constant region fragment mRNAs in MPC 11 mouse myeloma cells and variants. Cell. 1975 Jun;5(2):139–147. doi: 10.1016/0092-8674(75)90022-7. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Paddock G. V., Heindell H. C., Salser W. Nucleotide sequences from a rabbit alpha globin gene inserted in a chimeric plasmid. Science. 1977 Apr 8;196(4286):192–195. doi: 10.1126/science.847466. [DOI] [PubMed] [Google Scholar]
- Lobban P. E., Kaiser A. D. Enzymatic end-to end joining of DNA molecules. J Mol Biol. 1973 Aug 15;78(3):453–471. doi: 10.1016/0022-2836(73)90468-3. [DOI] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Poon R., Paddock G. V., Heindell H., Whitcome P., Salser W., Kacian D., Bank A., Gambino R., Ramirez F. Nucleotide sequence analysis of RNA synthesized from rabbit globin complementary DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3502–3506. doi: 10.1073/pnas.71.9.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Genetics of immunoglobulins in the mouse. Adv Immunol. 1967;7:91–145. doi: 10.1016/s0065-2776(08)60127-3. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Bacterial cloning of plasmids carrying copies of rabbit globin messenger RNA. Nature. 1976 Mar 18;260(5548):221–225. doi: 10.1038/260221a0. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Milstein C. Mouse immunoglobulin genes: studies on the reiteration frequency of light-chain genes by hybridisation procedures. Eur J Biochem. 1975 Mar 3;52(1):125–133. doi: 10.1111/j.1432-1033.1975.tb03980.x. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Stepwise biosynthesis in vitro of globin genes from globin mRNA by DNA polymerase of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3418–3422. doi: 10.1073/pnas.73.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W. A. DNA sequencing techniques. Annu Rev Biochem. 1974;43(0):923–965. doi: 10.1146/annurev.bi.43.070174.004423. [DOI] [PubMed] [Google Scholar]
- Salser W., Browne F., Clarke P., Heindell H., Higuchi R., Paddock G., Roberts J., Studnicka G., Zakar P. Determination of globin mRNA sequences and their insertion into bacterial plasmids. Prog Nucleic Acid Res Mol Biol. 1976;19:177–204. doi: 10.1016/s0079-6603(08)60918-6. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Baldi I. Electrophoretically homogeneous myeloma light chain mRNA and its translation in vitro. Biochem Biophys Res Commun. 1973 Mar 5;51(1):81–87. doi: 10.1016/0006-291x(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Reiteration frequency of immunoglobulin light chain genes: further evidence for somatic generation of antibody diversity. Proc Natl Acad Sci U S A. 1976 Jan;73(1):203–207. doi: 10.1073/pnas.73.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wall R., Lippman S., Toth K., Fedoroff N. A general method for the large-scale isolation of polysomes and messenger RNA applied to MOPC 21 mouse myeloma tumors. Anal Biochem. 1977 Sep;82(1):115–129. doi: 10.1016/0003-2697(77)90140-3. [DOI] [PubMed] [Google Scholar]
- Wood K. O., Lee J. C. Integration of synthetic globin genes into an E. coli plasmid. Nucleic Acids Res. 1976 Aug;3(8):1961–1971. doi: 10.1093/nar/3.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]





