Abstract
Dimercaptosuccinic acid (DMSA) coating improves the uptake efficiency presumably by engendering the Fe2O3-NPs. In the present study, we investigated the possible environmental safety concentrations of Fe2O3-NPs using different assay systems in nematode Caenorhabditis elegans with lethality, development, reproduction, locomotion behavior, pharyngeal pumping, defecation, intestinal autofluorescence and reactive oxygen species (ROS) production as the endpoints. After exposure from L4-larvae for 24-hr, DMSA coated Fe2O3-NPs at concentrations more than 50 mg/L exhibited adverse effects on nematodes. After exposure from L1-larvae to adult, DMSA coated Fe2O3-NPs at concentrations more than 500 μg/L had adverse effects on nematodes. After exposure from L1-larvae to day-8 adult, DMSA coated Fe2O3-NPs at concentrations more than 100 μg/L resulted in the adverse effects on nematodes. Accompanied with the alterations of locomotion behaviors, ROS production was pronouncedly induced by exposure to DMSA coated Fe2O3-NPs in the examined three assay systems, and the close associations of ROS production with lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, or intestinal autofluorescence in nematodes exposed to DMSA coated Fe2O3-NPs were confirmed by the linear regression analysis. Moreover, mutations of sod-2 and sod-3 genes, encoding Mn-SODs, showed more susceptible properties than wild-type when they were used for assessing the DMSA coated Fe2O3-NPs-induced toxicity, and the safety concentrations for DMSA coated Fe2O3-NPs should be defined as concentrations lower than 10 μg/L in sod-2 and sod-3 mutant nematodes.
Introduction
Iron oxide nanoparticles (NPs) hold immense potential in a vast variety of applications such as magnetic resonance imaging, targeted delivery of drugs or genes, tissue engineering, targeted destruction of tumor, magnetic transfections, chelation therapy and environmental catalysis [1]–[2]. Although, the potential benefits of Fe-NPs are considerable, exposure to Fe-NPs can result in the significant cytotoxicity such as inflammation, formation of apoptosis, impaired mitochondrial function, membrane leakage, oxidative stress, damage on cell function, DNA damage, chromosomal damage and chromosome condensation [2]–[4]. Besides the cytocotocity, the in vivo toxicity assays further indicated that Fe-NPs exposure caused lung inflammation associated with increased cytokine productions in lymph node cell cultures and decreased pulmonary immune response against sleep erythrocytes, disrupted embryo development, and induced oxidative injury in peritoneal macrophage in mice [5]–[7]. Fe-NPs exposure also resulted in the potential lung and systemic cumulative toxicity, and histopathological alterations of liver and spleen in rats [8]–[9]. Moreover, the ecotoxic investigations demonstrated that Fe-NPs exposure induced the lipid peroxidation in excised mussel gills, and caused the oxidative stress, disturbance of antioxidative balance, and some histopathological and morphological alterations in medaka [10]–[11]. The increased production of novel food additives in the form of Fe-NPs bring attention to the potential environmental health risks [12], and the recent increasing interest in the use of Fe-NPs for wastewater treatment [11] may further bring about the amplification of the possible environmental risk. However, present knowledge concerning the ecotoxic effects of Fe-NPs is very limited and merits to be documented more fully.
The model animal of nematode Caenorhabditis elegans, a free-living nematode with the abundance in soil ecosystems, is useful for environmental and toxicological studies of toxicants from whole-animal level down to single cell level [13]. C. elegans can be explored to assess the toxicities of both contaminated soil and contaminated river water or sediments [14]–[17]. Endpoints of lethality, development, reproduction, locomotion behavior, lifespan, pharyngeal pumping, defecation, intestinal autofluorescence, stress response, and oxidative stress can be used to evaluate the acute or chronic toxicity of environmental toxicants [18]–[31]. So far, C. elegans has been successfully used for the toxicity evaluation of different nano-materials, such as Al-NPs, Ti-NPs, Ce-NPs, Zn-NPs, Ag-NPs, Si-NPs, fullerene, and quantum dots [32]–[45]. It has been proven that C. elegans can be used to evaluate the toxicity of specific toxicants at environmental relevant concentrations [37], [39]. The mean lifespan of nematodes was significantly decreased even at the exposure level of 1 nM for CeO2-NPs [39]. The locomotion behavior, intestinal autofluorescence and reactive oxygen species (ROS) production were significantly altered in nematodes chronically exposed to 13 μg/L of Cr(VI) [46]. Nevertheless, no report was raised so far to investigate the ecotoxic effects of Fe-NPs using C. elegans as a toxicity assay system.
For uncoated Fe-NPs, it has been shown that they are NPs-specific cytotoxic [47]. To effectively use Fe-NPs in the medical treatment or magnetic resonance imaging, various organic coatings have been employed as a means of optimizing the delivery of Fe-NPs to or into the cells [48]–[49]. A simple dimercaptosuccinic acid (DMSA) coating can improve the uptake efficiency presumably by engendering the Fe-NPs with an anionic charge, resulting in nonspecific adsorption to the cell surface followed by endocytosis into the cells [50]. In the present study, we investigated the possible environmental safety concentrations of Fe2O3-NPs using different assay systems in nematode Caenorhabditis elegans.
Results
Toxicity evaluation in nematodes exposed to DMSA coated Fe2O3-NPs at the L4-larvae stage for 24-hr
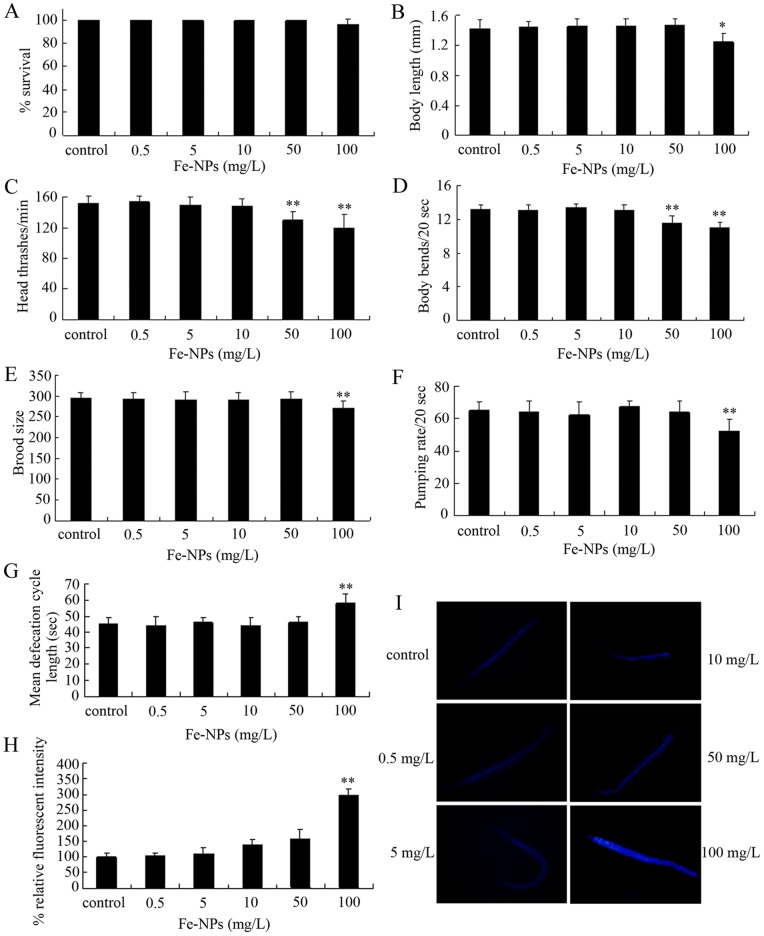
We first investigated the possible adverse effects of DMSA coated Fe2O3-NPs exposure at the L4-larvae for 24-hr on nematodes. Exposure to 0.5–100 mg/L of DMSA coated Fe2O3-NPs at the L4-larvae for 24-hr did not obviously influence the survival of nematodes (Fig. 1A). Similarly, exposure to 0.5–50 mg/L of DMSA coated Fe2O3-NPs did not significantly affect the body length of nematodes (Fig. 1B), exposure to 0.5–10 mg/L of DMSA coated Fe2O3-NPs did not noticeably influence the locomotion behavior as indicated by head thrash and body bend in nematodes (Fig. 1C and 1D), exposure to 0.5–50 mg/L of DMSA coated Fe2O3-NPs did not change the brood size of nematodes (Fig. 1E), exposure to 0.5–50 mg/L of DMSA coated Fe2O3-NPs did not obviously alter the pumping rate and defecation (Fig. 1F and 1G), and exposure to 0.5–50 mg/L of DMSA coated Fe2O3-NPs did not significantly induce the intestinal autofluorescence of nematodes (Fig. 1H and 1I). In contrast, exposure to 100 mg/L of DMSA coated Fe2O3-NPs significantly reduced the body length and brood size (Fig. 1B and 1C), decreased the pumping rate (Fig. 1F), increased the mean defecation cycle length (Fig. 1G), and induced the intestinal autolfuorescence of nematodes (Fig. 1H and 1I). Especially, exposure to 50–100 mg/L of DMSA coated Fe2O3-NPs significantly decreased both the head thrashes and the body bends in nematodes (Fig. 1C and 1D). Therefore, acute exposure to 50–100 mg/L of DMSA coated Fe2O3-NPs may exhibit adverse effects on nematodes.
Figure 1. Toxicity evaluation in nematodes exposed to DMSA coated Fe2O3-nanoparticles at the L4-larvae stage for 24-hr.
(A) Comparison of lethality in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (B) Comparison of body length in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (C) Comparison of head thrash in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (D) Comparison of body bend in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (E) Comparison of brood size in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (F) Comparison of pumping rate in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (G) Comparison of mean defecation cycle length in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (H) Comparison of intestinal autofluorescence in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (I) Pictures showing the intestinal autofluorescence in nematodes exposed to different concentrations of Fe2O3-nanoparticles. Bars represent mean ± S.E.M. * p<0.05, ** p<0.01.
Toxicity evaluation in nematodes exposed to DMSA coated Fe2O3-NPs from L1-larvae to adult
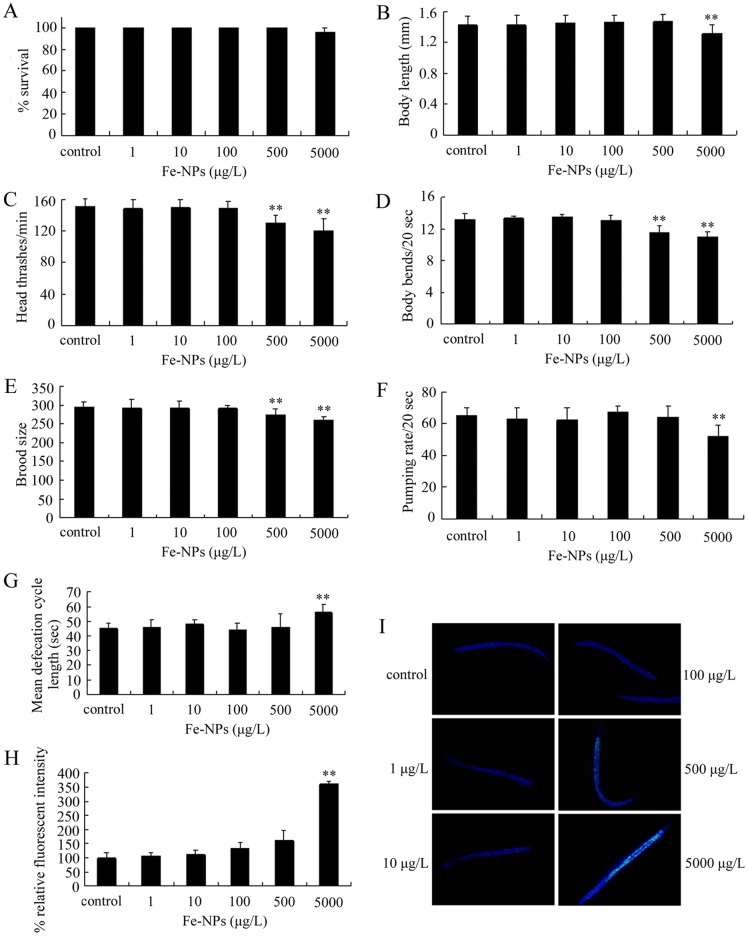
Previous studies indicated that exposure to environmental relevant concentrations of CeO2-NPs or Al2O3-NPs from L1-larvae to adult caused the adverse effects in nematodes, implying the sensitivity of L1-larvae to environmental toxicants [39], [43]. We next investigated the possible adverse effects of exposure to DMSA coated Fe2O3-NPs from L1-larvae to day 1 adult nematodes (approximately 3 days). As shown in Fig. 2, exposure to 1–5000 μg/L of DMSA coated Fe2O3-NPs did not obviously influence the survival of nematodes. Similarly, exposure to 1–500 μg/L of DMSA coated Fe2O3-NPs did not affect the body length, pumping rate, and defecation in nematodes, exposure to 1–100 μg/L of DMSA coated Fe2O3-NPs did not noticeably alter the head thrash, body bend, and brood size in nematodes, and exposure to 1–500 μg/L of DMSA coated Fe2O3-NPs also did not induce the significant intestinal autofluorescence in nematodes. In contrast, exposure to 5000 μg/L of DMSA coated Fe2O3-NPs significantly reduced the body length, decreased the pumping rate, increased the mean defecation cycle length, and induced the intestinal autofluorescence. Especially, exposure to 500–1000 μg/L of DMSA coated Fe2O3-NPs significantly decreased both the locomotion behavior and the brood size of nematodes. These data imply that, after exposure from L1-larvae to adult, DMSA coated Fe2O3-NPs with concentrations more than 500 μg/L may have adverse effects on nematodes.
Figure 2. Toxicity evaluation in nematodes exposed to DMSA coated Fe2O3-nanoparticles from L1-larvae to adult.
(A) Comparison of lethality in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (B) Comparison of body length in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (C) Comparison of head thrash in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (D) Comparison of body bend in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (E) Comparison of brood size in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (F) Comparison of pumping rate in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (G) Comparison of mean defecation cycle length in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (H) Comparison of intestinal autofluorescence in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (I) Pictures showing the intestinal autofluorescence in nematodes exposed to different concentrations of Fe2O3-nanoparticles. Bars represent mean ± S.E.M. * p<0.05, ** p<0.01.
Toxicity evaluation in nematodes exposed to DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult
C. elegans can be used for chronic toxicity evaluation [38], [42], [46], [51]. To combine the sensitive value of L1-larvae for toxicity evaluation with the previous chronic assay system in C. elegans, we further investigated the possible adverse effects of exposure to DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult on nematodes. As shown in Fig. 3, different from the toxicity evaluation above, we observed that exposure to 5000 μg/L of DMSA coated Fe2O3-NPs moderately but significantly decreased the survival of nematodes. Moreover, exposure to 500–5000 μg/L of DMSA coated Fe2O3-NPs significantly reduced the body length, decreased the pumping rate, prolonged the mean defecation cycle length, and induced the intestinal autofluorescence. Especially, exposure to 100–5000 μg/L of DMSA coated Fe2O3-NPs significantly decreased the locomotion behaviors and induced the intestinal autofluorescence of nematodes. Therefore, our data suggest that, after exposure from L1-larvae to day-8 adult, DMSA coated Fe2O3-NPs with concentrations more than 100 μg/L may exhibit adverse effects on nematodes.
Figure 3. Toxicity evaluation in nematodes exposed to DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult.
(A) Comparison of lethality in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (B) Comparison of body length in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (C) Comparison of head thrash in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (D) Comparison of body bend in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (E) Comparison of pumping rate in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (F) Comparison of mean defecation cycle length in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (G) Comparison of intestinal autofluorescence in nematodes exposed to different concentrations of Fe2O3-nanoparticles. (H) Pictures showing the intestinal autofluorescence in nematodes exposed to different concentrations of Fe2O3-nanoparticles. Bars represent mean ± S.E.M. * p<0.05, ** p<0.01.
ROS production in nematodes exposed to DMSA coated Fe2O3-NPs in different assay systems
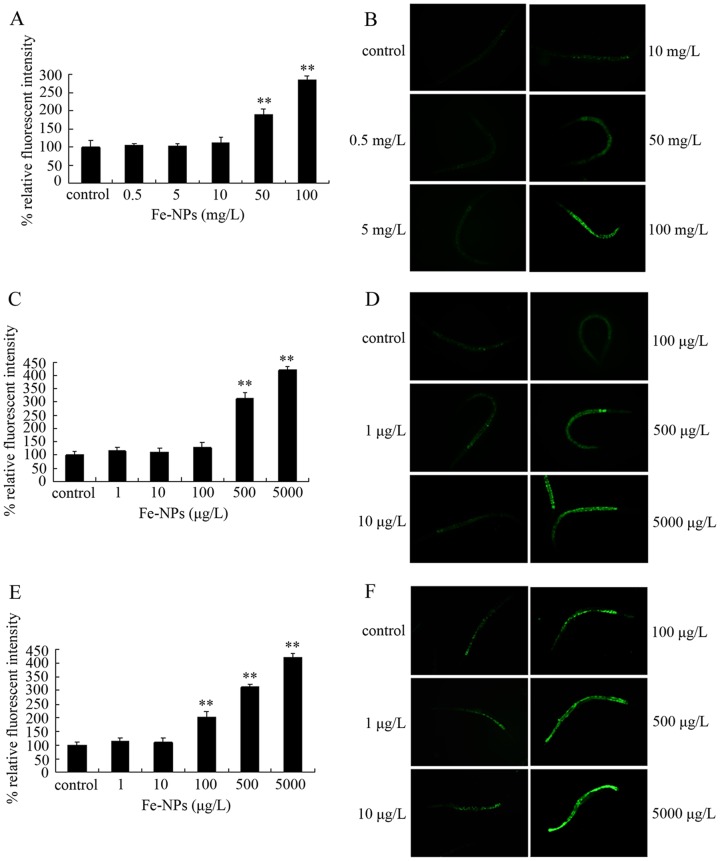
Considering the fact that the uncoated Fe2O3-NPs can induce the oxidative stress [4]–[5], [10]–[11], we examined whether exposure to DMSA coated Fe2O3-NPs will induce the oxidative stress by analyzing the ROS production in nematodes. After exposure from L4-larvae for 24-hr, 50–100 mg/L of DMSA coated Fe2O3-NPs induced the significant ROS production in nematodes (Fig. 4A and 4B). After exposure from L1-larvae to adult, 500–5000 μg/L of DMSA coated Fe2O3-NPs induced the significant ROS production in nematodes (Fig. 4C and 4D). Moreover, after exposure from L1-larvae to day-8 adult, we found that 100–5000 μg/L of DMSA coated Fe2O3-NPs induced the significant ROS production in nematodes (Fig. 4E and 4F).
Figure 4. ROS production in nematodes exposed to DMSA coated Fe2O3-nanoparticles.
(A) Comparison of ROS production in nematodes exposed to Fe2O3-nanoparticles at the L4-larvae stage for 24-hr. (B) Pictures showing the ROS production in nematodes exposed to Fe2O3-nanoparticles at the L4-larvae stage for 24-hr. (C) Comparison of ROS production in nematodes exposed to Fe2O3-nanoparticles from L1-larvae to adult. (D) Pictures showing the ROS production in nematodes exposed to Fe2O3-nanoparticles from L1-larvae to adult. (E) Comparison of ROS production in nematodes exposed to Fe2O3-nanoparticles from L1-larvae to day-8 adult. (F) Pictures showing the ROS production in nematodes exposed to Fe2O3-nanoparticles from L1-larvae to day-8 adult. Bars represent mean ± S.E.M. **p<0.01.
To examine the possible associations of ROS production with lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, or intestinal autolfuorescence in nematodes exposed to different concentrations of DMSA coated Fe2O3-NPs, the linear regression analysis was further performed. With growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, and intestinal autolfuorescence as the dependent variables and with ROS production as the independent variable, after DMSA coated Fe2O3-NPs exposure from L4-larvae for 24-hr, ROS production was significantly correlated with growth (R2 = 0.856, p<0.01), reproduction (R2 = 0.913, p<0.01), body bend (R2 = 0.730, p<0.05), head thrash (R2 = 0.795, p<0.05), pumping rate (R2 = 0.861, p<0.01), mean defecation cycle length (R2 = 0.954, p<0.01), and intestinal autofluorescence (R2 = 0.977, p<0.01) (Table S1). With growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, and intestinal autolfuorescence as the dependent variables and with ROS production as the independent variable, after DMSA coated Fe2O3-NPs exposure from L1-larvae to adult, ROS production was significantly correlated with growth (R2 = 0.688, p<0.05), reproduction (R2 = 0.881, p<0.01), body bend (R2 = 0.739, p<0.05), head thrash (R2 = 0.842, p<0.05), pumping rate (R2 = 0.863, p<0.01), mean defecation cycle length (R2 = 0.884, p<0.01), and intestinal autofluorescence (R2 = 0.993, p<0.01) (Table S1). With lethality, growth, locomotion behavior, pharyngeal pumping, defecation, and intestinal autolfuorescence as the dependent variables and with ROS production as the independent variable, after DMSA coated Fe2O3-NPs exposure from L1-larvae to day-8 adult, ROS production was significantly correlated with lethality (R2 = 0.920, p<0.01), growth (R2 = 0.883, p<0.01), body bend (R2 = 0.947, p<0.01), head thrash (R2 = 0.920, p<0.01), pumping rate (R2 = 0.828, p<0.05), mean defecation cycle length (R2 = 0.842, P<0.05), and intestinal autofluorescence (R2 = 0.781, p<0.05) (Table S1). Therefore, ROS production was significantly correlated with lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, or intestinal autofluorescence in nematodes exposed to DMSA coated Fe2O3-NPs in different toxicity evaluation assay systems.
Effects of sod-2 and sod-3 mutations on locomotion behavior and ROS production in nematodes exposed to DMSA coated Fe2O3-NPs
Previous studies indicated that mutations of some genes, such as sod-2 and sod-3 genes encoding Mn-SODs, exhibited more susceptible properties than wild-type nematodes for assessing the nanotoxicity [35], [42]. We further investigated the possible susceptible properties of sod-2 and sod-3 mutants to toxicity of DMSA coated Fe2O3-NPs. Based on the analysis above, we selected two relative sensitive endpoints, locomotion behavior and ROS production, for the further toxicity assay. In sod-2 and sod-3 mutants exposed to DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult, 10–5000 μg/L of DMSA coated Fe2O3-NPs significantly inhibited both the head thrashes and the body bends of nematodes (Fig. 5A and 5B). Further, the ROS productions were significantly induced in sod-2 and sod-3 mutants exposed to 10–5000 μg/L of DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult (Fig. 5C and 5D). More interestingly, we found that double mutations of sod-2 and sod-3 gens resulted in the more significant (p<0.01) decreases of head thrash and body bend, and increases of ROS production in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult compared with the adverse effects from single mutations of sod-2 or sod-3 gens (Fig. S1).
Figure 5. Effects of sod-2 and sod-3 mutations on locomotion behavior and ROS production in nematodes exposed to DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult.
(A) Effects of sod-2 and sod-3 mutations on head thrashes of nematodes exposed to DMSA coated Fe2O3-nanoparticles. (B) Effects of sod-2 and sod-3 mutations on body bends of nematodes exposed to DMSA coated Fe2O3-nanoparticles. (C) Effects of sod-2 and sod-3 mutations on ROS production in nematodes exposed to DMSA coated Fe2O3-nanoparticles. (D) Pictures showing the ROS production in nematodes exposed to Fe2O3-nanoparticles. Bars represent mean ± S.E.M. *p<0.05, **p<0.01.
Discussion
Surface-coated Fe2O3-NPs has been proposed for medical treatment or as a contrast agent in magnetic resonance imaging. The in vitro toxicity assay on Fe2O3-NPs has shown the low toxicity and no clear difference between different particle sizes [4]. Enhanced endocytosis by DMSA coating is an efficient method for intracellular delivery of Fe2O3-NPs. Previous study has shown little or no in vivo toxicity for DMSA [52]. In the current study, with lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, and intestinal autofluorescence as the endpoints, we investigated the adverse effects of exposure to DMSA coated Fe2O3-NPs for 24-hr on nematodes, and found that exposure to 50–100 mg/L of DMSA coated Fe2O3-NPs had adverse effects on nematodes (Fig. 1). Although exposure to DMSA coated Fe2O3-NPs at the examined concentrations did not obviously influence the survival of nematodes, exposure to 100 mg/L of DMSA coated Fe2O3-NPs altered the growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, and intestinal autofluorescence (Fig. 1). Especially, exposure to 50 mg/L of DMSA coated Fe2O3-NPs decreased the locomotion behavior of nematodes, implying the locomotion behavior is the most sensitive one among the used endpoints for assessing the toxicity of DMSA coated Fe2O3-NPs (Fig. 1). Therefore, after exposure from L4-larvae for 24-hr, only relatively high concentrations of DMSA coated Fe2O3-NPs can cause the adverse effects on nematodes. Previous in vitro toxicity assay on DMSA coated Fe2O3-NPs (5–12 nm) also indicated that exposure at concentrations of 1.5–15 mM resulted in a diminishing viability and capacity of PC12 cells to extend neuritis in response to their putative biological cue, i.e. nerve growth factor [3].
To evaluate the possible environmental safety concentrations for DMSA coated Fe2O3-NPs, we further explored two other toxicity assay systems. With lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, and intestinal autofluorescence as the endpoints, we investigated the adverse effects of exposure to DMSA coated Fe2O3-NPs from L1-larvae to adult on nematodes and found that DMSA coated Fe2O3-NPs with concentrations more than 500 μg/L exhibited adverse effects on nematodes (Fig. 2). Exposure to DMSA coated Fe2O3-NPs from L1-larvae to adult at the examined concentrations also did not obviously affect the survival of nematodes; however, exposure to 5000 μg/L of DMSA coated Fe2O3-NPs altered the growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, and intestinal autofluorescence and exposure to 500 μg/L of DMSA coated Fe2O3-NPs further suppressed the locomotion behavior of nematodes (Fig. 2). In contrast to these, with lethality, growth, locomotion behavior, pharyngeal pumping, defecation, and intestinal autofluorescence as the endpoints, we examined the adverse effects of exposure to DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult on nematodes, and found that DMSA coated Fe2O3-NPs with concentrations more than 100 μg/L resulted in the toxic effects on nematodes (Fig. 3). Different from the observations in other assay systems, exposure to 5000 μg/L of DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult reduced the survival rate of nematodes (Fig. 3). Moreover, exposure to 100 μg/L of DMSA coated Fe2O3-NPs from L1-larvae to day-8 adult decreased the locomotion behavior and induced the significant intestinal autofluorescence (Fig. 3). Among the used assay systems, the assay system of exposure from L1-larvae to day-8 adult combines both the value of assay system of exposure from L1-larvae to adult [39], [42] and the value of assay system of exposure from day-1 adult to day-8 adult [46]. With the aid of the above toxicity assay systems, we showed here that DMSA coated Fe2O3-NPs with concentrations lower than 100 μg/L should be relative safe in the environment. Nevertheless, different from some other NPs [53]–[54], so far we still do not know the exact environmental concentrations or predicated environmental concentrations of Fe2O3-NPs.
The environmental safety concentrations for Fe2O3-NPs revealed in this study are somewhat different from previous publications. In excised mussel gills, exposure to 1000 μg/L of Fe2O3-NPs (50 nm) induced the lipid peroxidation and impairment on lysosomal stability in circulating blood cells [11]. In addition, exposure to 500 μg/L of Fe-NPs (30 nm) with a biodegradable polymer modification caused the decrease of superoxide dismutase in medaka [10]. At least three possibilities will explain these differences. One possibility is that the size of Fe2O3-NPs used in this study is different from those used in previous studies [10]–[11]. Another possibility is that the assay system used in this study is different from those used in other organisms [10]–[11]. In addition, we still can not exclude such a possibility that C. elegans may be more sensitive than cell line or medaka while assessing the nanotoxicity, as implied in the studies on the Al2O3-NPs toxicity in C. elegans [42]–[43].
In C. elegans, sod-2 and sod-3 mutants are very sensitive to the oxidative stress [55]. Furthermore, our data here suggest that sod-2 and sod-3 mutants were more susceptible than wild-type to DMSA coated Fe2O3-NPs exposure-induced toxicity (Fig. 5). In sod-2 and sod-3 gene mutation backgrounds, the relative safety concentrations for DMSA coated Fe2O3-NPs should be defined as concentrations lower than 10 μg/L in the environment. Therefore, on the one hand, sod-2 and sod-3 mutants can be used to detect the possible potential toxicity from DMSA coated Fe2O3-NPs; on the other hand, under the specific sensitive mutation or physiological backgrounds, the environmental safety concentrations for specific nanomaterials should be carefully investigated and defined. More interestingly, the relative safety concentrations for DMSA coated Fe2O3-NPs in sod-2; sod-3 double mutants should be defined as concentrations even lower than those in sod-2 or sod-3 single mutants in the environment (Fig. S1).
Previous studies indicated that exposure to uncoated Fe2O3-NPs (30 nm) caused more H2O2, OH and O2 − free radicals in peritoneal macrophage of mice compared with control, exposure to uncoated Fe2O3-NPs (29 nm) induced oxidative DNA lesions in human cell line A549, exposure to uncoated Fe2O3-NPs (50 nm) resulted in the lipid peroxidation in excised mussel gills, and exposure to Fe-NPs (30 nm) with a biodegradable polymer modification induced the decrease of superoxide dismutase, increase of malondialdehyde, and reduced glutathione in medaka [4]–[5], [10]–[11]. In the present study, our data further demonstrated that, accompanied with the alterations of locomotion behaviors induced by exposure to DMSA coated Fe2O3-NPs, ROS production was pronouncedly induced by exposure to DMSA coated Fe2O3-NPs in the used three assay systems (Fig. 4). Moreover, the linear regression analysis confirmed the close association of the observed ROS production with the altered lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, or intestinal autofluorescence in nematodes exposed to DMSA coated Fe2O3-NPs in different toxicity evaluation assay systems (Table S1). These data also imply that ROS production is also a very sensitive endpoint used for environmental safety assessment of specific nanomaterials.
In summary, we examined the possible environmental safety concentrations for DMSA-coated Fe2O3-NPs using three different toxicity assay systems in nematodes, and indicated that DMSA coated Fe2O3-NPs with concentrations lower than 100 μg/L should be relative safe in the environment. In contrast, in sod-2 and sod-3 mutants, the environmental safety concentrations for DMSA coated Fe2O3-NPs should be defined as concentrations lower than 10 μg/L. Moreover, we provide some evidence to prove that the toxicity induced by DMSA coated Fe2O3-NPs is at least partially due to the formation of oxidative stress in nematodes.
Materials and Methods
Reagents and preparation of DMSA coated Fe2O3-NPs suspensions
DMSA coated Fe2O3-NPs was the gift from Prof. Yu Zhang [56]. The size of particles was 9 nm as measured by transmission electron microscopy (TEM, JEM 200 CX) (Fig. 6). We determined the crystal structure by powder X-ray diffraction (XRD, Rigaku, D/Max-RA, λ = 1.5405×10−10 m, CuK), measured the specific surface area by N2 sorption at 77 K using a NOVA 1000e instrument, tested the particle size distribution in K medium by dynamic light scattering (DLS) measurement (Brookhaven Instruments Corp., Holsvile, NY), and analyzed the zeta potential for Fe2O3-NPs with a Nano Zetasizer (Malvern Instrument Ltd., Malvern, UK). The detailed properties of Fe2O3-NPs are shown in Table S2. The prepared stock suspension concentrations of Fe2O3-NPs were 0.5, 5, 10, 50, and 100 mg/L, and 1, 10, 100, 500, and 5000 μg/L. Series of stock suspensions of Fe2O3-NPs were prepared in a K-medium [57], and dispersed for 20 min by probe sonication at 100 W and 40 kHz for 30 min to form the used suspensions. All the other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Figure 6. TEM image of 9 nm DMSA coated Fe2O3-nanoparticles.
Strain preparation
Nematodes used were wild-type N2, RB1072 [sod-2(ok1030)], VC433 [sod-3(gk235)], which were maintained on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 at 20°C [58]. Gravid nematodes were washed off the plates into centrifuge tubes, and were lysed with a bleaching mixture (0.45 M NaOH, 2% HOCl). Age synchronous populations of L1-larvae or L4 larvae nematodes were obtained by the collection as described [59]. The adult were washed with K medium (50 mM NaCl, 30 mM KCl, 10 mM NaOAc, pH 5.5) [57].
Exposures to different concentrations of Fe2O3-NPs were performed in three assay systems: (1) from the L4-larvae stage in K medium of 12-well sterile tissue culture plates for 24-hr, (2) from the L1-larvae to day 1 adult in K medium of 12-well sterile tissue culture plates, and (3) from L1-larvae to day-8 adult in K medium of 12-well sterile tissue culture plates (chronic exposure). All the exposures were performed at 20°C incubator in the presence of food. Five hundred microliters of exposure suspension solution was added into each well of the 12-well sterile tissue culture plates. The exposure suspension solutions were freshly prepared prior to use. For the chronic exposure assay, 5′-fluoro-2′-deoxyuridine (FUdR), an inhibitor of DNA synthesis, was used to prevent production of offspring from reproducing without otherwise interfering with the organism's post-maturational development [60] by adding at a final concentration of 25 μM when the examined nematodes developed into the L4-larvae stage. For chronic exposure, the exposed nematodes were transferred from day 1 to day 8 to a new well with Fe2O3-NPs suspension solution with food each day in order to ensure the food supply.
Lethality, growth, reproduction, metabolism, and locomotion behavior
We used the percentage of survival animals to evaluate lethality of nematodes. Following exposure, inactive ones were scored under a dissecting microscopy and nematodes were judged to be dead if they did not respond to stimulus using a small, metal wire. Ten replicates were performed, and three hundred of nematodes were examined for each replicate. We used the body length to assess growth of nematodes. The body length was determined by measuring the flat surface area of nematodes using the Image-Pro® Express software. Ten replicates were performed. We used the brood size to evaluate reproduction of nematodes. To assay the brood size, number of the offspring at all stage beyond the egg was counted. Ten replicates were performed. To assay the pumping rate, nematodes were placed onto NGM plates with food, and left undisturbed for 1-hr before measuring. Pharyngeal pumping was counted for 1 min under DIC optics with a Ziess axioscope. To assay the mean defecation cycle length, individual animal was examined for a fixed number of cycles, and a cycle period was defined as the interval between the initiations of two successive posterior body-wall muscle contraction steps. Thirty replicates were performed. We used the head thrash, and body bend to evaluate locomotion behavior of nematodes [61]. To assay the head thrash, every examined nematode was transferred into a microtiter well containing 60 µL of K medium on the top of agar without food, and head thrashes were counted for 1-min after a 1-min recovery period. A thrash was defined as a change in the direction of bending at the mid body. To assay the body bend, nematodes were picked onto a second plate without food and scored for the number of body bends in an interval of 20 sec. A body bend was counted as a change in the direction of the part of nematodes corresponding to the posterior bulb of the pharynx along the y axis, assuming that the nematode was traveling along the x axis. Fifty replicates were examined per treatment.
Intestinal autofluorescence
Intestinal autoflorescence caused by lysosomal deposits of lipofuscin can accumulate over time in aging nematodes [62]. Images were collected for endogenous intestine fluorescence using a 525-nm bandpass filter and without automatic gain control in order to preserve the relative intensity of different animal's fluorescence. We used the Magnafire® software (Olympus, Irving, TX, USA) to analyze the fluorescence of color images taken for the documentation of results. Lipofuscin levels were measured using ImageJ Software (NIH Image) by determining average pixel intensity in each animal's intestine. Twenty nematodes for each treatment were counted.
ROS production
To quantify whether Fe2O3-NPs treatment activated the oxidative damage, the ROS production was assayed. The examined nematodes were transferred to M9 buffer containing 1 µM CM-H2DCFDA to pre-incubate for 3-h at 20°C, and then mounted on agar pads for the examination with a laser scanning confocal microscope (Leica, TCS SP2, Bensheim, Germany) at 488 nm of excitation wavelength and 510 nm of emission filter. Relative fluorescent intensities of the intestine were semi-quantified. The semiquantified ROS was expressed as relative fluorescent units (RFU). Twenty nematodes for each treatment were counted.
Statistical analysis
All data were expressed as means ± standard error of the mean (S.E.M.). Statistical analysis was performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was used to determine the significance of differences between the groups. Probability levels of 0.05 and 0.01 were considered statistically significant. Associations of ROS production with lethality, growth, reproduction, locomotion behavior, pharyngeal pumping, defecation, and intestinal autolfuorescence were assessed with linear regression analysis.
Supporting Information
Effects of double mutations of sod-2 and sod-3 genes on locomotion behavior and ROS production in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. (A) Effects of double mutations of sod-2 and sod-3 genes on head thrash in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. (B) Effects of double mutations of sod-2 and sod-3 genes on body bend in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. (C) Effects of double mutations of sod-2 and sod-3 genes on ROS production in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. Bars represent mean ±S.E.M. **p<0.01.
(DOC)
Associations of ROS production with lethality, growth, reproduction, locomotion behavior, metabolism, and intestinal autofluorescence in nematodes exposed to DMSA coated Fe2O3-NPs as assayed by linear regression analysis.
(DOC)
Physicochemical properties of Fe2O3-nanoparticles.
(DOC)
Acknowledgments
The nematode strains used in this study were provided by the Caenorhabditis Genetics Center (funded by the NIH National Center for Research Resource, USA). We thank Prof. Yu Zhang (College of Biological Science and Medical Engineering, Southeast University) for providing the DMSA coated Fe2O3-NPs.
Funding Statement
This work was supported by grants from the National Basic Research Program of China (No. 2011CB933404), the National Natural Science Foundation of China (No. 81172698), and the Postdoctoral Foundation in Jiangsu Province of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hood E (2004) Nanotechnology: looking as we leap. Environ Health Perspect 112: A470–749. [Google Scholar]
- 2. Singh N, Jenkins GJS, Asadi R, Doak SH (2010) Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev 1: 5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pisanic II TR, Blackwell JD, Shubayev VI, Finones, Jin S (2007) Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28: 2572–2581. [DOI] [PubMed] [Google Scholar]
- 4. Karlsson HL, Gustafsson J, Cronholm P, Moller L (2009) Size-dependent toxicity of metal oxide particles – a comparison between nano- and micrometer size. Toxicol Lett 188: 112–118. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Tang M, Zhang T, Yang L, Xia T, et al. (2007) Oxidative injury of mgahetic ferric oxide nanoparticles to peritoneal macrophage in mice. J Clin Rehab Tissue Engineer Res 11: 2575–2577. [Google Scholar]
- 6. Noori A, Pativar K, Modaresi M, Messripour M, Yousefi MH, et al. (2011) Effect of magnetic iron oxide nanoparticles on pregnancy and testicular development of mice. African J Biotechnol 10: 1221–1227. [Google Scholar]
- 7.Ban M, Langonne I, Huguet N, Goutet M (2012) Effect of submicro and nano-iron oxide particle on pulmonary immunity. Toxicol Lett doi: 10.1016/j.toxlet.2012.02.004. [DOI] [PubMed]
- 8. Zhu M-T, Feng W-Y, Wang Y, Wang B, Wang M, et al. (2009) Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol Sci 107: 342–351. [DOI] [PubMed] [Google Scholar]
- 9. Katsnelson BA, Degtyareva TD, Minigalieva II, Privalova LI, Kuzmin SV, et al. (2011) Subchronic systemic toxicity and bioaccumulation of Fe3O4 nano- and microparticles following repeated intraperitoneal administration to rats. Int J Toxicol 30: 59–68. [DOI] [PubMed] [Google Scholar]
- 10. Li H, Zhou Q, Wu Y, Fu J, Wang T, Jiang G (2009) Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol Environ Safety 72: 684–692. [DOI] [PubMed] [Google Scholar]
- 11. Kadar E, Lowe DM, Sole M, Fisher AS, Jha AN, et al. (2010) Uptake and biological response to nano-Fe versus soluble FeCl3 in excised mussel gills. Anal Bioanal Chem 396: 657–666. [DOI] [PubMed] [Google Scholar]
- 12. Fidler MC, Walczyk T, Davidsson L, Zeder C, Sakaguchi N, et al. (2004) A micronized, dispersible ferric pyrophosphate with high relative bioavailability in man. British J Nutr 91: 107–112. [DOI] [PubMed] [Google Scholar]
- 13. Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, et al. (2008) Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci 106: 5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X-Y, Shen L-L, Yu H-X, Wang D-Y (2008) Toxicity evaluation in a paper recycling mill effluent by coupling bioindicator of aging with the toxicity identification evaluation method in nematode Caenorhabditis elegans . J Environ Sci 20: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 15. Heininger P, Höss S, Claus E, Pelzer J, Trauspurger W (2007) Nematode communities in contaminated river sediments. Environ Pollut 146: 64–76. [DOI] [PubMed] [Google Scholar]
- 16. Harmon SM, Wyatt DE (2008) Evaluation of post-Katrina flooded soils for contaminats and toxicity to the soil invertebrates Eisenia fetida and Caenorhabditis elegans . Chemosphere 70: 1857–1864. [DOI] [PubMed] [Google Scholar]
- 17. Höss S, Jänsch S, Moser T, Junker T, Römbke J (2009) Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicol Environ Safety 72: 1811–1818. [DOI] [PubMed] [Google Scholar]
- 18. Williams PL, Dusenbery DB (1988) Using the nematode, Caenorhabditis elegans, to predict mammalian acute lethality to metallic salts. Toxicol Ind Health 4: 469–478. [DOI] [PubMed] [Google Scholar]
- 19. Mutwakil MHAZ, Reader JP, Holdich DM, Smithurst PR, Candido EPM, et al. (1997) Use of stress-inducible transgenic nematodes as biomarkers of heavy metal pollution in water samples from an English river system. Arch Environ Contam Toxicol 32: 146–153. [DOI] [PubMed] [Google Scholar]
- 20. Sochová I, Hofman J, Holoubek I (2006) Using nematodes in soil ecotoxicology. Environ Int 32: 374–383. [DOI] [PubMed] [Google Scholar]
- 21. Harada H, Kurauchi M, Hayashi R, Eki T (2007) Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol Environ Safety 66: 378–383. [DOI] [PubMed] [Google Scholar]
- 22. Hu Y-O, Wang Y, Ye B-P, Wang D-Y (2008) Phenotypic and behavioral defects induced by iron exposure can be transferred to progeny in Caenorhabditis elegans . Biomed Environ Sci 21: 467–473. [DOI] [PubMed] [Google Scholar]
- 23. Pei B, Wang S, Guo X, Wang J, Yang G, et al. (2008) Arsenite-induced germline apoptosis through a MAPK-dependent, p53-independent pathway in Caenorhabditis elegans. Chem. Res. Toxicol. 21: 1530–1535. [DOI] [PubMed] [Google Scholar]
- 24. Xing X-J, Wang D-Y (2009) The lethality toxicities induced by metal exposure during development in nematode Caenorhabditis elegans . Bull Environ Contam Toxicol 83: 530–536. [DOI] [PubMed] [Google Scholar]
- 25. Jadhav KB, Rajini PS (2009) Evaluation of sublethal effects of dichlorvos upon Caenorhabditis elegans based on a set of endpoints of toxicity. 23: 9–17. [DOI] [PubMed] [Google Scholar]
- 26. Boyd WA, McBride SJ, Rice JR, Snyder DW, Freedman JH (2010) A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol Appl Pharmacol 245: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helmcke KJ, Aschner M (2010) Hormetic effect of methylmercury on Caenorhabditis elegans . Toxicol Appl Pharmacol 248: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang D-Y, Liu P-D, Yang Y-C, Shen L-L (2010) Formation of combined Ca/Cd toxicity on lifespan of nematode Caenorhabditis elegans. Ecotoxicol Environ Safety. 73: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 29. Wang D-Y, Wang Y, Shen L-L (2010) Confirmation of the combinational effects of calcium with other metals in a paper recycling mill effluent on nematode lifespan with the toxicity identification evaluation method. J Environ Sci 22: 731–737. [DOI] [PubMed] [Google Scholar]
- 30. Ye B-P, Rui Q, Wu Q-L, Wang D-Y (2010) Metallothioneins are required for formation of cross-adaptation response to neurobehavioral toxicity from lead and mercury exposure in nematodes. PLoS ONE 5: e14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu P-D, He K-W, Li Y-X, Wu Q-L, Yang P, et al. (2012) Exposure to mercury causes formation of male-specific structural deficits by inducing oxidative damage in nematodes. Ecotoxicol Environ Safety 79: 90–100. [DOI] [PubMed] [Google Scholar]
- 32. Ma H, Bertsch PM, Glenn TC, Kabengi NJ, Williams PL (2009) Toxicity of manufactured zinc oxide nanoparticles in the nematode Caenorhabditis elegans . Environ Toxicol Chem 28: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 33. Wang H, Wick RL, Xing B (2009) Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans . Environ Pollut 157: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 34. Pluskota A, Horzowski E, Bossinger O, von Mikecz A (2009) In Caenorhabditis elegans nanoparticle-bio-interactions become transparent: silica-nanoparticles induce reproductive senescence. PLoS ONE 4: e6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roh J, Sim SJ, Yi J, Park K, Chung KH, et al. (2009) Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol 43: 3933–3940. [DOI] [PubMed] [Google Scholar]
- 36. Meyer JN, Lord CA, Yang XY, Turner EA, Badireddy AR, et al. (2010) Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans . Aquat Toxicol 100: 140–150. [DOI] [PubMed] [Google Scholar]
- 37. Wu S, Lu J-H, Rui Q, Yu S-H, Cai T, et al. (2011) Aluminum nanoparticle exposure in L1 larvae results in more severe lethality toxicity than in L4 larvae or young adults by strengthening the formation of stress response and intestinal lipofuscin accumulation in nematodes. Environ Toxicol Pharmacol 31: 179–188. [DOI] [PubMed] [Google Scholar]
- 38. Yu S-H, Rui Q, Cai T, Li Y-X, Wang D-Y (2011) Close association of intestinal autofluorescence with the formation of severe oxidative damage in intestine of nematodes chronically exposed to Al2O3-nanoparticle. Environ Toxicol Pharmacol 32: 233–241. [DOI] [PubMed] [Google Scholar]
- 39. Zhang H, He X, Zhang Z, Zhang P, Li Y, et al. (2011) Nano-CeO2 exhibits adverse effects at environmental relevant concentrations. Environ Sci Technol 45: 3725–3730. [DOI] [PubMed] [Google Scholar]
- 40. Ma H, Kabengi NJ, Bertsch PM, Unrine JM, Glenn TC, et al. (2011) Comparative phototoxicity of nanoparticulate and bulk ZnO to a free-living nematode Caenorhabditis elegans: the importance of illumination mode and primary particle size. Environ Pollut 159: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 41. Qu Y, Li W, Zhou Y, Liu X, Zhang L, et al. (2011) Full assessment of fate and physiological behavior of quantum dots utilizing Caenorhabditis elegans as a model organism. Nano Lett 11: 3174–3184. [DOI] [PubMed] [Google Scholar]
- 42. Li Y-X, Yu S-H, Wu Q-L, Tang M, Pu Y-P, et al. (2012) Chronic Al2O3-nanoparticle exposure causes neurotixic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode Caenorhabditis elegans . J Hazard Mater 219-220: 221–230. [DOI] [PubMed] [Google Scholar]
- 43.Li Y-X, Yu S-H, Wu Q-L, Tang M, Wang D-Y (2012) Transmissions of serotonin, dopamine and glutamate are required for the formation of neurotoxicity from Al2O3-NPs in nematode Caenorhabditis elegans. Nanotoxicology doi: 10.3109/17435390.2012.689884. [DOI] [PubMed]
- 44. Cha YJ, Lee J, Choi SS (2012) Apoptosis-mediated in vivo toxicity of hydroxylated fullerene nanoparticles in soil nematode Caenorhabditis elegans . Chemosphere 87: 49–54. [DOI] [PubMed] [Google Scholar]
- 45. Lim D, Roh JY, Eom HJ, Choi JY, Hyun J, et al. (2012) Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans . Environ Toxicol Chem 31: 585–592. [DOI] [PubMed] [Google Scholar]
- 46. Wu Q-L, Qu Y-Y, Li X, Wang D-Y (2012) Chromium exhibits adverse effects at environmental relevant concentrations in chronic toxicity assay system of nematode Caenorhabditis elegans . Chemosphere 87: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 47. Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, et al. (2006) In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 40: 4374–4381. [DOI] [PubMed] [Google Scholar]
- 48. Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, et al. (2001) Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Nanotechnol 19: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 49. Sonvico F, Mornet S, Vasseur S, Dubernet C, Jaillard D, et al. (2005) Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: synthesis, physicochemical characterization, and in vitro experiments. Bioconjug Chem 16: 1181–1188. [DOI] [PubMed] [Google Scholar]
- 50. Wilhelm C, Billotey C, Royer J, Pons JN, Bacri JC, et al. (2003) Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating. Biomaterials 24: 1001–1011. [DOI] [PubMed] [Google Scholar]
- 51. Shen L-L, Xiao J, Ye H-Y, Wang D-Y (2009) Toxicity evaluation in nematode Caenorhabditis elegans after chronic metal exposure. Environ Toxicol Pharmacol 28: 125–132. [DOI] [PubMed] [Google Scholar]
- 52. Aposhian HV, Aposhian MM (1990) Meso-2,3-dimercaptosuccinic acid: chemical, pharmacological and toxicological properties of an orally effective metal chelating agent. Annu Rev Pharmacol Toxicol 30: 279–306. [DOI] [PubMed] [Google Scholar]
- 53. Tiede K, Hassellöv M, Breitbarth E, Chaudhry Q, Boxall ABA (2009) Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineering nanoparticles. J Chromatography A 1216: 503–509. [DOI] [PubMed] [Google Scholar]
- 54. O'Brien N, Cummins E (2010) Ranking initial environmental and human health risk resulting from environmentally relevant nanomaterials. J Environ Sci Health Part A 45: 992–1007. [DOI] [PubMed] [Google Scholar]
- 55. Van Raamsdonk JM, Meng Y, Camp D, Yang W, Jia X, et al. (2010) Decreased energy metabolism extends life span in Caenorhabditis elegans without reducing oxidative damage. Genetics 185: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang S, Chen X, Gu C, Zhang Y, Xu J, et al. (2009) The effect of iron magnetic nanoparticles on smooth muscle cells. Nanoscale Res Lett 4: 70–77. [Google Scholar]
- 57. Williams PL, Dusenbery DB (1990) Aquatic toxicity testing using the nematode Caenorhabditis elegans . Environ Toxicol Chem 9: 1285–1290. [PubMed] [Google Scholar]
- 58. Brenner S (1974) The genetics of Caenorhabditis elegans . Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Donkin SG, Dusenbery DB (1993) A soil toxicity test using the nematode Caenorhabditis elegans and an effective method of recovery. Arch Environ Contam Toxicol 25: 145–151. [Google Scholar]
- 60. Michell DH, Stiles JW, Santelli J, Sanadi DR (1979) Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol 34: 28–36. [DOI] [PubMed] [Google Scholar]
- 61. Wang D-Y, Xing X-J (2008) Assessment of locomotion behavioral defects induced by acute toxicity from heavy metal exposure in nematode Caenorhabditis elegans . J Environ Sci 20: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 62. Shen L-L, Hu Y-O, Cai T, Lin X-F, Wang D-Y (2010) Regulation of longevity by genes required for the functions of AIY interneuron in nematode Caenorhabditis elegans . Mech Ageing Dev 131: 732–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of double mutations of sod-2 and sod-3 genes on locomotion behavior and ROS production in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. (A) Effects of double mutations of sod-2 and sod-3 genes on head thrash in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. (B) Effects of double mutations of sod-2 and sod-3 genes on body bend in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. (C) Effects of double mutations of sod-2 and sod-3 genes on ROS production in nematodes exposed to 10 μg/L of DMSA coated Fe2O3-nanoparticles from L1-larvae to day-8 adult. Bars represent mean ±S.E.M. **p<0.01.
(DOC)
Associations of ROS production with lethality, growth, reproduction, locomotion behavior, metabolism, and intestinal autofluorescence in nematodes exposed to DMSA coated Fe2O3-NPs as assayed by linear regression analysis.
(DOC)
Physicochemical properties of Fe2O3-nanoparticles.
(DOC)