Abstract
In a randomized controlled trial, we administered seasonal trivalent inactivated influenza vaccine (TIV) or placebo to subjects 6–15 years of age in two consecutive years. Receipt of TIV in year 2 induced seroprotection in most subjects. Among 39 children who received TIV in the second year, receipt of TIV in the first year was associated with lower antibody titer rises in the second year to seasonal influenza A(H1N1) and A(H3N2) strains for which the vaccine strains remained unchanged. Antibody response to a different influenza B strain in the second year was unaffected by receipt of TIV in the first year.
Keywords: vaccination, influenza, antibody response, children
INTRODUCTION
In a cohort of school-age children who were randomized to receive seasonal trivalent inactivated influenza vaccine (TIV) or placebo in 2008–2009 and again in 2009–2010 (1,2), we investigated whether antibody response to TIV could be affected by vaccination in the preceding year (3,4).
METHODS
Participants and follow up
In 2008, 119 children 6–15 years old were enrolled to a randomized controlled trial (1). They were randomized to receive TIV or placebo in the ratio 3:2 in November-December 2008. After completing follow-up between August-October 2009, 64 children rejoined the study and were again randomized to receive either TIV or placebo in the ratio 3:2 (2). Allocation of TIV/placebo in year 2 was independent of allocation in year 1.
Serum specimens were collected immediately before and 1 month after vaccination, in the middle (April-May) and at the end of the follow-up period (August-December) each year. The subjects and their household members were monitored for acute upper respiratory tract infections (URTIs) by daily symptom diaries, bi-weekly telephone interviews and reminders to report acute URTIs to a hotline as soon after onset. Nose and throat swabs were collected from all household members once any individual reported any 2 of fever ≥37.8°C, chills, headache, sore throat, cough, presence of phlegm, coryza or myalgia. The study was approved by the University of Hong Kong Institutional Review Board.
Vaccines
Seasonal TIV (0.5ml VAXIGRIP, Sanofi Pasteur) was used in both years. The 2008–2009 TIV contained the A/Brisbane/59/2007(H1N1)-like, A/Brisbane/10/2007(H3N2)-like, and B/Florida/4/2006-like strains. The 2009–10 TIV used contained the same seasonal A (H1N1) and A(H3N2) strains, and a B/Brisbane/60/2008-like strain. Children in the placebo group received intramuscular injection (deltoid muscle) of 0.5ml saline. Vaccines and placebos were identically packaged and administered using a 5/8” needle.
Laboratory methods
Sera were tested using hemagglutination-inhibition (HI) assays for antibody to the vaccine strains A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007-like virus A/Uruguay/716/2009 (H3N2), B/Florida/4/2006 and B/Brisbane/60/2008, and the circulating strain A/California/7/2009 (pandemic H1N1) using standard methods as previously described (1,2,5). A viral microneutralization (VN) rather than HI assay was used to measure antibody responses to the pandemic A(H1N1) strain in year 1 (1). Tests were done at serial doubling dilutions from an initial dilution of 1:10. Pooled nose and throat swabs were tested for influenza A and B viruses by reverse-transcription polymerase-chain-reaction (RT-PCR) (1,2,5).
Statistical methods
The reciprocal of antibody titers and the geometric rise (ratio of post-vaccination to pre-vaccination titer) before and 1 month after vaccination in the first and second year were compared using Wilcoxon signed-rank test and Wald test after log transformation (6). Antibody titers <1:10 were imputed as 1:5. Sensitivity analyses were done using antibody titer thresholds of 1:40 and 1:160, and 4 and 8-fold geometric titer rises as endpoints. Fisher’s exact test and chi-squared tests were used to compare groups. A multivariable log-linear regression model was used to estimate the effects of various factors on the logarithm of the ratio of post-vaccination to pre-vaccination titer in year 2, including receipt of TIV in year 1, age, sex, infection in year 1 based on a ≥4-fold rise in antibody titers during follow-up or confirmed by RT-PCR, and calendar time of the post-vaccination serum draw. Following the principle of intention-to-treat, multiple imputation was used to allow for a small amount of missing data (7). Statistical analyses were conducted in R version 2.10.1.
RESULTS
Most (86%) of the 64 subjects were 9–16 years of age, and 55% of subjects were male. There were 3 children with chronic health conditions (allergic rhinitis). No children received the monovalent pandemic A(H1N1) influenza vaccine during the study period.
Among the 39 subjects randomized to receive TIV in year 2, 23 subjects had previously been randomized to receive TIV in year 1 and 16 to placebo. Among these 39 subjects, there were no significant differences in sex or mean age between those who had received TIV or placebo in year 1. The intervals between vaccination in year 2 and post-vaccination blood collection were similar between children who were vaccinated in both years (median 28 days, range 27–49 days) and those who received placebo in year 1 (median 28 days, range 28–41 days) (Wilcoxon signed-rank test, p=0.28).
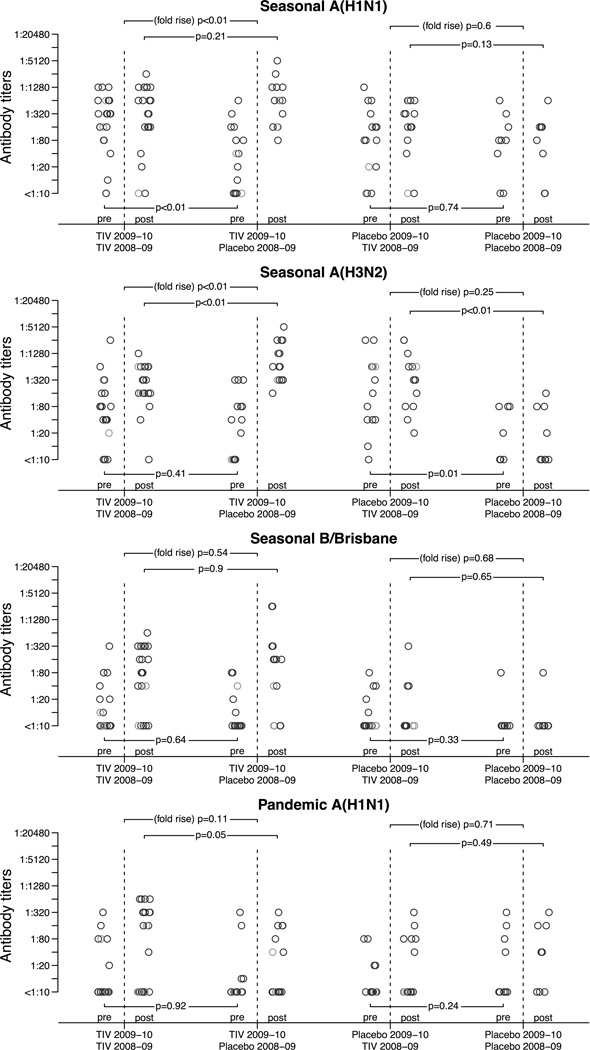
TIV in year 1 elicited good antibody responses to the seasonal strains (Table 1). Before receipt of TIV in year 2, compared to those who received placebo the subjects who had received TIV in year 1 had significantly higher antibody titers against seasonal A(H1N1), but there was no significant difference in titers against seasonal A(H3N2), B or pandemic A(H1N1) (Table 1, Figure 1). Prior receipt of TIV rather than placebo in year 1 was associated with significantly lower ratio of post-vaccination to pre-vaccination titer against the seasonal A(H1N1) and A(H3N2) strains (both p<0.01) following receipt of TIV in year 2, but no significant differences in the ratio of post-vaccination to pre-vaccination titer to both influenza B strains and the pandemic A(H1N1) strains. Most subjects attained antibody titers at or above 1:160 against seasonal A(H1N1) and A(H3N2) one month after receipt of TIV in year 2 regardless of their vaccination status in the first year (Table 1). Post-vaccination antibody titers against pandemic A(H1N1) were higher in subjects who had previously received TIV rather than placebo in year 1 (p=0.06). There were no significant changes in antibody titer in 25 children following receipt of placebo in year 2 (Figure 1, Table 2). Results were consistent using post-vaccination titer of 1:40 and 1:160, 4 and 8-fold geometric rises as endpoints (Table 1, Table 2).
Table 1.
Comparison of geometric mean and geometric mean ratio of antibody titers (GMT) before and 1 month after receipt of trivalent inactivated influenza vaccine (TIV) or placebo in year 1 (2008–2009) and year 2 (2009–2010) in 39 children who received TIV in year 2 (2009–2010).
| Group | n | Pre-dose 1 | p-valuea | Post-dose 1 | p-valuea | Pre-dose 2 | p-valuea | Post-dose 2 | p-valuea | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seasonal A(H1N1)b | |||||||||||
| Geometric mean titer | TIV-TIVc | 23 | 20 | 0.20 | 1714 | <0.01 | 192 | <0.01 | 270 | 0.12 | |
| PL-TIVd | 16 | 37 | 62 | 28 | 562 | ||||||
| Proportion titer ≥40 | TIV-TIVc | 23 | 0.48 | 0.33 | 0.91 | 0.63 | 0.87 | 0.03 | 0.87 | 0.44 | |
| PL-TIVd | 16 | 0.69 | 0.81 | 0.50 | 0.99 | ||||||
| Proportion titer≥160 | TIV-TIVc | 23 | 0.09 | 0.66 | 0.91 | <0.01 | 0.74 | 0.01 | 0.83 | 0.64 | |
| PL-TIVd | 16 | 0.19 | 0.44 | 0.25 | 0.93 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
TIV-TIVc | 23 | 85.7 | <0.01 | 1.4 | <0.01 | |||||
| PL-TIVd | 16 | 1.7 | 19.9 | ||||||||
| Proportion ratio ≥4 | TIV-TIVc | 23 | 0.83 | <0.01 | 0.17 | <0.01 | |||||
| PL-TIVd | 16 | 0.19 | 0.69 | ||||||||
| Proportion ratio ≥8 | TIV-TIVc | 23 | 0.83 | <0.01 | 0.09 | <0.01 | |||||
| PL-TIVd | 16 | 0.12 | 0.62 | ||||||||
| Seasonal A(H3N2)b | |||||||||||
| Geometric mean titer | TIV-TIVc | 23 | 18 | 0.02 | 859 | 0.01 | 45 | 0.33 | 243 | <0.01 | |
| PL-TIVd | 16 | 103 | 140 | 26 | 819 | ||||||
| Proportion titer ≥40 | TIV-TIVc | 23 | 0.39 | 0.14 | 0.91 | 0.63 | 0.65 | 0.54 | 0.95 | 0.84 | |
| PL-TIVd | 16 | 0.69 | 0.81 | 0.50 | 1.00 | ||||||
| Proportion titer≥160 | TIV-TIVc | 23 | 0.22 | 0.48 | 0.87 | 0.06 | 0.30 | 0.65 | 0.86 | 0.35 | |
| PL-TIVd | 16 | 0.38 | 0.56 | 0.19 | 1.00 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
TIV-TIVc | 23 | 47.0 | <0.01 | 5.4 | <0.01 | |||||
| PL-TIVd | 16 | 1.4 | 31.6 | ||||||||
| Proportion ratio ≥4 | TIV-TIVc | 23 | 0.91 | <0.01 | 0.65 | 0.09 | |||||
| PL-TIVd | 16 | 0.12 | 0.94 | ||||||||
| Proportion ratio ≥8 | TIV-TIVc | 23 | 0.78 | <0.01 | 0.34 | <0.01 | |||||
| PL-TIVd | 16 | 0.06 | 0.88 | ||||||||
| Seasonal Bb | |||||||||||
| Geometric mean titer | TIV-TIVc | 23 | 292 | 0.04 | 1444 | <0.01 | 11 | 0.57 | 73 | 1.00 | |
| PL-TIVd | 16 | 118 | 123 | 9 | 73 | ||||||
| Proportion titer ≥40 | TIV-TIVc | 23 | 0.96 | 1.00 | 1.00 | 0.41 | 0.22 | 0.86 | 0.78 | 0.80 | |
| PL-TIVd | 16 | 0.94 | 0.94 | 0.19 | 0.69 | ||||||
| Proportion titer≥160 | TIV-TIVc | 23 | 0.74 | 0.23 | 0.96 | <0.01 | 0.04 | 0.85 | 0.49 | 0.88 | |
| PL-TIVd | 16 | 0.50 | 0.50 | 0.00 | 0.56 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
TIV-TIVc | 23 | 4.9 | <0.01 | 6.5 | 0.76 | |||||
| PL-TIVd | 16 | 1.0 | 8.0 | ||||||||
| Proportion ratio ≥4 | TIV-TIVc | 23 | 0.61 | <0.01 | 0.65 | 0.83 | |||||
| PL-TIVd | 16 | 0.00 | 0.56 | ||||||||
| Proportion ratio ≥8 | TIV-TIVc | 23 | 0.35 | 0.01 | 0.60 | 1.00 | |||||
| PL-TIVd | 16 | 0.00 | 0.56 | ||||||||
| Pandemic A(H1N1)b | |||||||||||
| Geometric mean titer | TIV-TIVc | 23 | 7 | 0.93 | 8 | 0.03 | 11 | 0.66 | 65 | 0.06 | |
| PL-TIVd | 16 | 6 | 6 | 9 | 20 | ||||||
| Proportion titer ≥40 | TIV-TIVc | 23 | 0.00 | 1.00 | 0.00 | 1.00 | 0.22 | 0.75 | 0.63 | 0.44 | |
| PL-TIVd | 16 | 0.00 | 0.00 | 0.12 | 0.45 | ||||||
| Proportion titer≥160 | TIV-TIVc | 23 | 0.00 | 1.00 | 0.00 | 1.00 | 0.09 | 0.88 | 0.58 | 0.11 | |
| PL-TIVd | 16 | 0.00 | 0.00 | 0.12 | 0.26 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
TIV-TIVc | 23 | 1.3 | 0.06 | 6.1 | 0.09 | |||||
| PL-TIVd | 16 | 1.0 | 2.3 | ||||||||
| Proportion ratio ≥4 | TIV-TIVc | 23 | 0.04 | 1.00 | 0.55 | 0.31 | |||||
| PL-TIVd | 16 | 0.00 | 0.32 | ||||||||
| Proportion ratio ≥8 | TIV-TIVc | 23 | 0.00 | 1.00 | 0.46 | 0.63 | |||||
| PL-TIVd | 16 | 0.00 | 0.32 | ||||||||
P-values comparing between TIV-TIV and PL-TIV were obtained by the Chi-square test, Fisher’s Exact test, Wilcoxon-signed rank test or Wald test where appropriate.
Antibody titers measured by hemagglutination inhibition assays to A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007-like virus A/Uruguay/716/2009 (H3N2), B/Florida/4/2006, A/California/7/2009 (H1N1) viruses in year 1;A/Brisbane/59/2007 (H1N1), A/Uruguay/716/2009 (H3N2), B/Brisbane/60/2008, A/California/7/2009 (H1N1) viruses in year 2 (see methods for details of strains used).
The TIV-TIV group consists of subjects who received TIV in 2008–2009 and 2009–10. The 2008–09 TIV (year 1) included A/Brisbane/59/2007-like (H1N1), A/Brisbane/10/2007-like (H3N2) and B/Florida/4/2006-like strains. The 2009–10 TIV (year 2) included A/Brisbane/59/2007-like (H1N1), A/Brisbane/10/2007-like (H3N2) and B/Brisbane/60/2008 strains.
The PL-TIV group consists of subjects who received placebo in 2008–2009 and TIV in 2009–10.
Figure 1.
Table 2.
Comparison of geometric mean and geometric mean ratio of antibody titers (GMT) before and one month after receipt of trivalent inactivated influenza vaccine (TIV) or placebo in year 1 (2008–2009) and year 2 (2009–2010) in 25 children who received placebo in year 2 (2009–2010).
| Group | n | Pre-dose 1 | p-valuea | Post-dose 1 | p-valuea | Pre-dose 2 | p-valuea | Post-dose 2 | p-valuea | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seasonal A(H1N1)b | |||||||||||
| Geometric mean titer | PL-TIVc | 16 | 19 | 0.32 | 789 | <0.01 | 77 | 0.79 | 174 | 0.17 | |
| PL-PLd | 9 | 37 | 47 | 63 | 69 | ||||||
| Proportion titer ≥40 | PL-TIVc | 16 | 0.31 | 0.44 | 0.94 | 0.12 | 0.67 | 1.00 | 0.88 | 0.95 | |
| PL-PLd | 9 | 0.56 | 0.67 | 0.78 | 0.78 | ||||||
| Proportion titer≥160 | PL-TIVc | 16 | 0.25 | 0.74 | 0.81 | 0.01 | 0.46 | 0.87 | 0.75 | 0.58 | |
| PL-PLd | 9 | 0.22 | 0.22 | 0.33 | 0.56 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
PL-TIVc | 16 | 41.2 | <0.01 | 2.3 | 0.35 | |||||
| PL-PLd | 9 | 1.3 | 1.1 | ||||||||
| Proportion ratio ≥4 | PL-TIVc | 16 | 0.81 | <0.01 | 0.24 | 0.80 | |||||
| PL-PLd | 9 | 0.11 | 0.11 | ||||||||
| Proportion ratio ≥8 | PL-TIVc | 16 | 0.81 | <0.01 | 0.22 | 0.85 | |||||
| PL-PLd | 9 | 0.11 | 0.11 | ||||||||
| Seasonal A(H3N2)b | |||||||||||
| Geometric mean titer | PL-TIVc | 16 | 32 | 0.20 | 902 | <0.01 | 177 | <0.01 | 281 | <0.01 | |
| PL-PLd | 9 | 14 | 15 | 15 | 16 | ||||||
| Proportion titer ≥40 | PL-TIVc | 16 | 0.56 | 0.49 | 1.00 | <0.01 | 0.86 | 0.03 | 0.94 | 0.01 | |
| PL-PLd | 9 | 0.33 | 0.33 | 0.33 | 0.33 | ||||||
| Proportion titer≥160 | PL-TIVc | 16 | 0.25 | 0.75 | 0.94 | <0.01 | 0.57 | 0.02 | 0.69 | 0.02 | |
| PL-PLd | 9 | 0.11 | 0.22 | 0.00 | 0.11 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
PL-TIVc | 16 | 28.0 | <0.01 | 1.6 | 0.21 | |||||
| PL-PLd | 9 | 1.1 | 1.1 | ||||||||
| Proportion ratio ≥4 | PL-TIVc | 16 | 0.88 | <0.01 | 0.20 | 0.43 | |||||
| PL-PLd | 9 | 0.00 | 0.00 | ||||||||
| Proportion ratio ≥8 | PL-TIVc | 16 | 0.88 | <0.01 | 0.14 | 0.69 | |||||
| PL-PLd | 9 | 0.00 | 0.00 | ||||||||
| Seasonal Bb | |||||||||||
| Geometric mean titer | PL-TIVc | 16 | 153 | 0.98 | 1660 | 0.03 | 9 | 0.51 | 8 | 0.62 | |
| PL-PLd | 9 | 148 | 235 | 7 | 7 | ||||||
| Proportion titer ≥40 | PL-TIVc | 16 | 0.88 | 1.00 | 0.94 | 1.00 | 0.19 | 0.95 | 0.19 | 0.95 | |
| PL-PLd | 9 | 0.89 | 1.00 | 0.11 | 0.11 | ||||||
| Proportion titer≥160 | PL-TIVc | 16 | 0.56 | 0.69 | 0.81 | 1.00 | 0.00 | 1.00 | 0.06 | 0.77 | |
| PL-PLd | 9 | 0.44 | 0.78 | 0.00 | 0.00 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
PL-TIVc | 16 | 10.8 | 0.00 | 1.0 | 0.89 | |||||
| PL-PLd | 9 | 1.6 | 1.0 | ||||||||
| Proportion ratio ≥4 | PL-TIVc | 16 | 0.69 | 0.01 | 0.06 | 0.77 | |||||
| PL-PLd | 9 | 0.11 | 0.00 | ||||||||
| Proportion ratio ≥8 | PL-TIVc | 16 | 0.69 | 0.01 | 0.06 | 0.77 | |||||
| PL-PLd | 9 | 0.11 | 0.00 | ||||||||
| Pandemic A(H1N1)b | |||||||||||
| Geometric mean titer | PL-TIVc | 16 | 7 | 0.31 | 10 | 0.02 | 9 | 0.21 | 15 | 0.42 | |
| PL-PLd | 9 | 6 | 7 | 20 | 27 | ||||||
| Proportion titer ≥40 | PL-TIVc | 16 | 0.00 | 1.00 | 0.00 | 1.00 | 0.14 | 0.25 | 0.38 | 0.65 | |
| PL-PLd | 9 | 0.00 | 0.00 | 0.44 | 0.56 | ||||||
| Proportion titer≥160 | PL-TIVc | 16 | 0.00 | 1.00 | 0.00 | 1.00 | 0.01 | 0.27 | 0.12 | 0.47 | |
| PL-PLd | 9 | 0.00 | 0.00 | 0.22 | 0.33 | ||||||
| Geometric mean ratio of post-dose to pre-dose |
PL-TIVc | 16 | 1.4 | 0.26 | 1.7 | 0.64 | |||||
| PL-PLd | 9 | 1.2 | 1.4 | ||||||||
| Proportion ratio ≥4 | PL-TIVc | 16 | 0.06 | 1.00 | 0.25 | 0.74 | |||||
| PL-PLd | 9 | 0.00 | 0.22 | ||||||||
| Proportion ratio ≥8 | PL-TIVc | 16 | 0.00 | 1.00 | 0.12 | 0.95 | |||||
| PL-PLd | 9 | 0.00 | 0.22 | ||||||||
P-values comparing between PL-TIV and PL-PL were obtained by the Chi-square test, Fisher’s Exact test, Wilcoxon-signed rank test or Wald test where appropriate.
See methods and legend of Table 1 for details of viruses antigens used for hemagglutination inhibition assays.
The PL-TIV group consists of subjects who received placebo in 2009–2010 and TIV in 2008–2009. The 2008–09 TIV (year 1) included A/Brisbane/59/2007-like (H1N1), A/Brisbane/10/2007-like (H3N2) and B/Florida/4/2006-like strains.
The PL-PL group consists of subjects who received placebo in 2009–2010 and 2008–2009.
A multivariate analysis was performed to explore the factors affecting the ratio of post-vaccination to pre-vaccination antibody titers in subjects who received TIV in year 2 (Table 3). Prior vaccination in year 1 was associated with lower post- vs pre-vaccination antibody titer ratio for seasonal A(H1N1) and A(H3N2). There were no significant effects associated with infection with the corresponding influenza subtype in year 1. Younger age and longer duration between serum collections were associated with increased titer ratio (Table 3).
Table 3.
Factors affecting the geometric in antibody titers ratio post-vaccination to pre-vaccination following receipt of trivalent inactivated influenza vaccine (TIV) in year 2 (2009–10).
| Seasonal A(H1N1)a | Seasonal A(H3N2)a | B/Brisbanea | Pandemic A(H1N1)a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | n | GMTΔ (95% C.I.)b | GMTΔ (95% C.I.)b | GMTΔ (95% C.I.)b | GMTΔ (95% C.I.)b | ||||
| Age 7–8 years | 7 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Age 9–15 years | 32 | 0.80 | (0.14, 4.47) | 0.14 | (0.02, 0.90) | 1.87 | (0.27, 12.97) | 2.31 | (0.32, 16.63) |
| Not received 2008–2009 TIV | 16 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Received 2008–2009 TIV | 23 | 0.06 | (0.01, 0.25) | 0.13 | (0.03, 0.58) | 0.55 | (0.10, 2.91) | 3.90 | (0.76, 20.12) |
| Not infectedb with the same subtype in 2008–2009 | 1.00 | 1.00 | 1.00 | ||||||
| Infectedb with the same strain in 2008–2009 | 0.33 | (0.04, 2.96) | 1.21 | (0.14, 10.77) | 0.83 | (0.15, 4.65) | |||
| Pre-vaccination blood taking before 1st October, 2009 | 24 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Pre-vaccination blood taking after 1st October, 2009 | 13 | 0.68 | (0.15, 3.09) | 0.80 | (0.17, 3.75) | 1.57 | (0.29, 8.51) | 0.50 | (0.10, 2.64) |
| Time lapse between vaccination and blood taking within 28 days | 25 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Time lapse between vaccination and blood taking beyond 28 days | 14 | 4.79 | (1.03, 22.31) | 3.05 | (0.62, 14.93) | 2.13 | (0.39, 11.80) | 0.44 | (0.08, 2.46) |
Antibody fold rises to each strain in 2009–2010 including A/Brisbane/59/2007-like (H1N1), A/Brisbane/10/2007-like (H3N2), B/Brisbane/60/2008-like, A/California/7/2009-like viruses (see methods and legend to table 1 for details).
Values shown represent the estimated geometric changes in mean titer ratio post-vaccination to pre-vaccination associated with each factor. For example a GMTΔ of 2 would imply that a factor was associated with a doubling in the geometric mean titer ratio compared to the reference group. Estimates were adjusted for age, vaccination history in 2008–2009, infection history of the corresponding influenza virus subtype in 2008–2009, calendar date of blood taking before vaccination and time-lapse between vaccination and blood taking.
Infection was defined as either RT-PCR confirmed infection or serological evidence of infection (≥4 fold rise in antibody titer) of the corresponding influenza subtype [7]. There were 3 seasonal A(H1N1) infections, 2 seasonal A(H3N2) infections, 1 seasonal B/Florida infection and 10 pandemic A(H1N1) infections during the 2008–2009 season. Seasonal B/Florida infection in 2008–2009 could not be included in the analysis.
DISCUSSION
Results from our study suggested humoral antibody response to TIV may be lower in children who have received TIV in the preceding year when the vaccine strains are unchanged, which was the case for the seasonal A(H1N1) and A(H3N2) strains in our study (Table 1). No effect of prior receipt of TIV was observed in response to a different strain of influenza B included in the TIV in year 2 compared to year 1. Our small study was underpowered to explore whether the lower antibody responses in year 2 associated with receipt of TIV in year 1 were indicative of any change in vaccine efficacy in year 2.
Interpretation of our findings should carefully take into account the contribution of antibody avidity, mucosal and cell mediated immunity to protection against infection (8). These might have been altered by prior vaccination through reduced short-term experience of natural infection or priming of immune responses (1,4,9). A recent study showed that the HI test can miss protective antibodies that do not bind to the hemagglutinin protein (10), and additional testing using another assay such as viral neutralization would be warranted when planning further studies. Our findings are consistent with the hypothesis that the effect of consecutive vaccination on vaccine efficacy may depend on the antigenic distance between consecutive vaccines (11). The clinical significance and underlying mechanism of the small increase in antibody response to pandemic A(H1N1) in year 2 associated with vaccination in the preceding year has not been clearly understood, but we had previously reported evidence of a higher risk of pandemic A(H1N1) infections among individuals who received TIV in year 1 (1) and it is possible that receipt of TIV in year 2 boosted antibodies associated with earlier infections including pandemic A(H1N1) in year 1. Different strains were used in the HI test for seasonal A(H3N2) and B between year 1 and 2, and may have contributed to the observed changes in antibody titer levels between the 2 years.
There are several limitations of our study in addition to the lack of power to explore vaccine efficacy. When adjusting for infections in year 1, we included serologic evidence of infection as well as RT-PCR-confirmed infections, although serology can lack sensitivity to identify infections in subjects who have received TIV (12). Subjects who were randomized to receive placebo in year 1 had higher geometric mean antibody titer against seasonal A(H3N2) and lower geometric mean antibody titer against seasonal B before vaccination in year 1 (Table 1). There is some evidence that the antibody titer associated with 50% protection against infection for children may be higher than the traditional 1:40 threshold (13), although this did not affect our conclusions.
CONCLUSIONS
Children had lower antibody response to seasonal A(H1N1) and A(H3N2) if they had received TIV in the previous year where these strains remained unchanged in the vaccine composition. Further studies are needed to confirm whether repeated annual influenza vaccination retains moderate to high efficacy in children.
Acknowledgments
SOURCES OF FINANCIAL SUPPORT: This project was supported by the Research Fund for the Control of Infectious Disease, Food and Health Bureau, Government of the Hong Kong SAR (grant nos. CHP-CE-03 and PHE-2), the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant no. AoE/M-12/06). The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
POTENTIAL CONFLICTS OF INTEREST: DKMI has received research funding from F. Hoffmann-La Roche Ltd. JSMP receives research funding from Crucell MV. BJC has received research funding from MedImmune Inc., and consults for Crucell MV. JSMP receives research funding from Crucell MV. The authors report no other potential conflicts of interest.
REFERENCES
- 1.Cowling BJ, Ng S, Ma ES, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis. 2010;51:1370–1379. doi: 10.1086/657311. [DOI] [PubMed] [Google Scholar]
- 2.Cowling BJ, Ng S, Ma ESK, et al. Protective efficacy against pandemic influenza of seasonal influenza vaccination in children in Hong kong: a randomized controlled trial. Clin Infect Dis. 2012 doi: 10.1093/cid/cis518. [in press] [DOI] [PubMed] [Google Scholar]
- 3.Bernstein DI, Yan L, Treanor J, Mendelman PM, Belshe R. Effect of yearly vaccinations with live, attenuated, cold-adapted, trivalent, intranasal influenza vaccines on antibody responses in children. Pediatr Infect Dis J. 2003;22:28–34. doi: 10.1097/00006454-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Zeman AM, Holmes TH, Stamatis S, et al. Humoral and cellular immune responses in children given annual immunization with trivalent inactivated influenza vaccine. Pediatr Infect Dis J. 2007;26:107–115. doi: 10.1097/01.inf.0000253251.03785.9b. [DOI] [PubMed] [Google Scholar]
- 5.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–2184. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. doi: 10.1186/1471-2288-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 8.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pica N, Hai R, Krammer F, et al. Haemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A. 1999;96:14001–14006. doi: 10.1073/pnas.96.24.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis. 2011;203:1309–1315. doi: 10.1093/infdis/jir015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black S, Nicolay U, Vesikari T, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30:1081–1085. doi: 10.1097/INF.0b013e3182367662. [DOI] [PubMed] [Google Scholar]