Summary
Establishment of infection by spotted fever group rickettsial species is dependent on the ability of these bacteria to adhere to and invade the host endothelium. Recent studies have attributed these processes to a handful of rickettsial surface proteins from the surface cell antigen (sca) family of autotransporters. A rickettsial autotransporter from Rickettsia conorii, Sca2, has been shown to be sufficient to mediate both adherence and invasion of human endothelial cells and to participate in intracellular actin-based motility. Here we identify a region of Sca2 capable of interacting with the mammalian cell surface and show that this function of Sca2 is independent and separable from its actin nucleation activity. Furthermore, pre-incubation of mammalian cells with the Sca2 mammalian association region prior to R. conorii infection can competitively inhibit rickettsial invasion, suggesting that Sca2 plays an important role in the initial interaction with mammalian cells. Together our results demonstrate that the Sca2 autotransporter protein in R. conorii contains distinct functional domains that likely are involved in mediating cellular interactions at the plasma membrane and the host cytosol.
Introduction
The genus Rickettsia is composed of obligate intracellular Gram-negative α-proteobacteria, many of which are human pathogens. These pathogens are transmitted to the human host by arthropod vectors, such as ticks, which inoculate the host during a blood meal (Hackstadt, 1996). Rickettsial species are divided into two groups, the typhus group (TG) and the spotted fever group (SFG) based on differences in their antigenicity to lipopolysaccharide, the presence of outer membrane proteins, and in part on the diseases that they cause (Vishwanath, 1991). Members of both groups are responsible for severe human disease and two species, Rickettsia prowazekii (TG) and Rickettsia rickettsii (SFG) have been classified as select agents by the Centers for Disease Control and Prevention.
Infection by SFG rickettsiae, such as R. conorii, the causative agent of Mediterranean Spotted Fever, begins with inoculation of bacteria into the vasculature of the human host during a tick bite (Hackstadt, 1996). Rickettsia must then adhere to proximal endothelial cells, the target tissue of rickettsial infection (Walker et al., 1988), through specific adhesion-receptor interactions, leading to eukaryotic downstream signaling and ultimately bacterial uptake via a zippering mechanism (Chan et al., 2009, Martinez et al., 2004). After escape from the phagosome into the cytoplasm, rickettsia multiply and polymerize actin to move intra and intercellularly. Obligate intracellular bacteria such as Rickettsia reside in the nutrient-rich cytoplasm of the host where they have access to many biosynthetic precursors that are subsequently utilized for growth and survival. Analyses of several sequenced rickettsial genomes revealed that rickettsiae have likely undergone “reductive evolution”, whereby genes coding for several biosynthetic pathways conserved in other Gram-negative bacteria are fragmented, split or otherwise absent from the genome, resulting in a comparatively small genome content (1.1–1.5Mb) (Gillespie et al., 2008). However, many of the remaining open reading frames (ORFs) show evidence for positive selection (Blanc et al., 2005), suggesting that their gene products may perform specific and important functions during the rickettsial life cycle. With a limited genome, it is also not surprising that some gene products would be multifunctional as has been demonstrated for the rickettsial autotransporter protein Sca2 (Cardwell et al., 2009, Haglund et al., 2010, Kleba et al., 2010).
Sca2 is a member of the surface cell antigen (Sca) family of autotransporter proteins in rickettsial species. Of the 17 members of this family, only six genes, including sca2, are present in the genomes of several closely related and divergent rickettsial species (Blanc et al., 2005). Autotransporter proteins in Gram-negative bacteria, also known as type V secretion, are modular proteins containing an N-terminal signal sequence, a central passenger domain and a C-terminal translocon domain that are capable of transporting themselves to the outer membrane of Gram-negative bacteria. The N-terminal secretion signal leads the peptide through the inner bacterial membrane via the Sec secretion machinery. Once in the periplasm, the C-terminal beta barrel, or autotransporter domain, is predicted to insert into the outer membrane, forming a conduit through which the central passenger domain can be translocated to the extracellular milieu (Henderson et al., 2004). Sca2 from R. conorii is predicted to be approximately 200 kDa but migrates at about 150 kDa by western blot analysis suggesting that, like other autotransporter proteins, Sca2 is likely proteolytically processed to a mature form (Cardwell et al., 2009).
We first described Sca2 from R. conorii as a novel rickettsial ligand sufficient to mediate adherence and invasion of Escherichia coli into cultured mammalian cells, including target endothelial cells. Scanning electron micrographs of Sca2-expressing E. coli invading mammalian cells revealed membrane perturbations reminiscent of those mediated by another related rickettsial autotransporter protein, rOmpB (Sca5) (Cardwell et al., 2009, Chan et al., 2009). Furthermore, purified recombinant Sca2 protein (amino acids 34 to 794) was able to reduce R. conorii invasion when incubated with mammalian cells prior to infection (Cardwell et al., 2009). Taken together, these data suggested that Sca2-mediated invasion plays an important role in the initial steps of rickettsial infection and may work in concert with other rickettsial invasins (i.e., rOmpB) (Chan et al., 2009) to mediate efficient bacterial invasion.
Interestingly, two groups recently described an additional function for Sca2 expressed by two related rickettsial species, R. rickettsii and R. parkeri. A transposon insertion disrupting the sca2 open reading frame abolished actin tail formation in R. rickettsii leading to a small-plaque morphology. These sca2 mutants grew as well as wild type counterparts in a tissue culture system, but lacked actin-based motility. In addition, sca2 mutants were avirulent in a non-lethal guinea pig model of infection, suggesting that Sca2 in R. rickettsii is an important virulence factor (Kleba et al., 2010). Haglund and co-workers further characterized the actin polymerizing properties of Sca2 from R. parkeri. Using several purified recombinant protein constructs in vitro, this group demonstrated that Sca2 was sufficient to nucleate actin polymerization, processively associate with the growing barbed end of the filament, protect the barbed end from capping protein, and utilize profilin-actin, properties that are characteristic of a family of actin nucleators called formins (Haglund et al., 2010). Immunofluorescence microscopy revealed the localization of Sca2 to actin rich structure on the surface of R. parkeri (Haglund et al., 2010) and Sca2 was shown to be sufficient to promote actin polymerization in cell extracts in a forming-like manner.
Sca2 appears to be a multifunctional rickettsial surface protein that acts at distinct steps in the rickettsial infection cycle; therefore, to understand the significance of Sca2’s protein functions it is imperative to separate them and study them independently. Here we have identified a mammalian association region of Sca2 from R. conorii by fine mapping the region of Sca2 sufficient to adhere to the mammalian cell surface. We furthermore demonstrate that this domain of Sca2 functions independently from the overlapping region of Sca2 sufficient to stimulate actin assembly and that the property of mammalian surface association but not actin nucleation is necessary to interfere with rickettsial interaction with the mammalian cell surface.
Results
Sca234–556 is sufficient to mediate adherence to the surface of mammalian cells
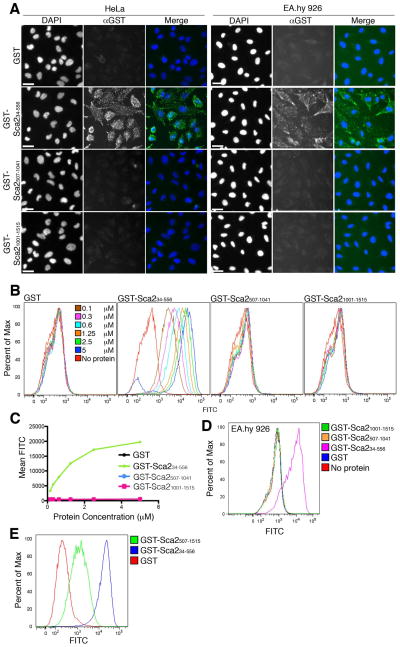
We have previously demonstrated that a recombinant purified protein construct comprising the first half of the Sca2 passenger domain (GST-Sca234–794) from R. conorii is sufficient to inhibit rickettsial invasion when pre-incubated with mammalian host cells, suggesting that this region of Sca2 contains a domain responsible for interacting with the mammalian cell surface (Cardwell et al., 2009). To more accurately define this region, we further divided the Sca2 passenger domain into three fragments, which were expressed in E. coli and purified as recombinant N-terminal GST fusion proteins. To assess the ability of each of these fragments to adhere to the surface of epithelial and endothelial mammalian cells, purified protein was incubated with a monolayer of HeLa cells or the transformed endothelial-like cell line EA.hy 926 cells. After washing away unbound protein, adhered protein was visualized by immunofluorescent staining with an anti-GST antibody. As illustrated in Figure 1A, only GST-Sca234–556 is sufficient to promote adherence to the surface of both HeLa and EA.hy 926 cells while GST alone, or other fragments of the Sca2 passenger domain do not associate with the mammalian cell surface. To further quantify the binding of GST-Sca234–556 to the surface of mammalian cells, we analyzed binding of Sca2 fragments to the surface of HeLa cells in suspension by flow cytometry. Titration of Sca2 protein from 0.1 to 5 μM in this assay confirms that only GST-Sca234–556 can adhere to HeLa cells while GST alone, the middle third (GST-Sca2507–1041), or the last third (GST-Sca21001–1515) of the Sca2 passenger domain cannot (Figure 1B). Furthermore, binding of GST-Sca234–556 is dose dependent and reaches saturation near 5 μM (Figure 1C). Similar results were found using the endothelial cell line EA.hy 926 (Figure 1D). In order to determine the necessity of amino acids 34 through 556 for Sca2 passenger domain association with the mammalian cell surface, we expressed and purified the last two thirds of the Sca2 passenger domain as an N-terminal GST fusion protein (GST-Sca2507–1515) and analyzed its ability to associate with the surface of HeLa cells. Interestingly, this region of Sca2 can also associate with the mammalian cell surface; however, its ability to bind is approximately 15 fold less than GST-Sca234–556. These data indicate that the region of Sca2 that most strongly associates with mammalian cells resides within the first third (amino acids 34 to 556) of the passenger domain.
Figure 1. Sca234–556 is sufficient to mediate adherence to the surface of mammalian cells.
A. Immunofluorescence microscopy of purified GST-fused Sca2 peptides adhered to the surface of a HeLa (left panels) or EA.hy 926 (right panels) cell monolayers. Scale bar represents 20 μm. B. Flow cytometric analysis of titration of GST-fused Sca2 peptides bound to the surface of HeLa cells. C. Graph of Mean FITC signal of data presented in Figure B as a function of protein concentration. D and E. Flow cytometric analysis of GST-fused Sca2 peptides (at 1.25 μM) bound to the surface of EA.hy cells (D) and HeLa cells (E). Twenty thousand events were recorded for all flow cytometry samples. All subscripted text refers to amino acid numbers.
The adherence function of Sca2 is separable from its actin nucleation function
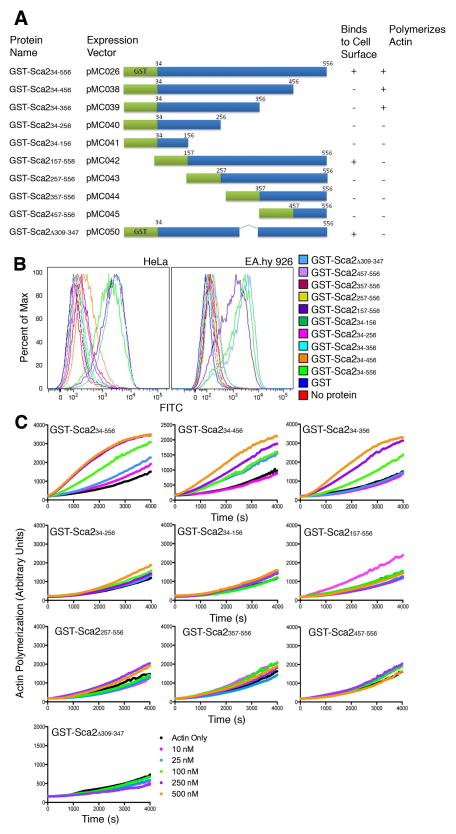
The mammalian association region within amino acids 34 to 556 of Sca2 from R. conorii overlaps with a fragment of Sca2 from another SFG rickettsia, R. parkeri, which has been previously shown to have actin nucleation activity (Haglund et al., 2010). In order to understand how these two functions could coexist within 500 amino acids, we constructed a series of N- and C-terminal truncations of GST-Sca234–556 (Figure 3A) and used them to map the mammalian association and actin nucleating functions of Sca2 attributed to this region. Flow cytometric analysis measuring binding of each fragment to the surface of HeLa and EA.hy 926 cells reveals that truncation of amino acids 457 to 556 abolishes the ability of Sca2 to adhere, indicating that these residues are necessary to mediate adherence to the mammalian cell surface (Figure 3B). Furthermore, truncation of amino acids 157 to 256 from the N-terminus also abolishes binding indicating that these amino acids are also necessary and that the minimal region sufficient to mediate association with the mammalian cell surface resides within amino acids 157 to 556.
Figure 3. The adherence function of Sca2 is separable from its actin nucleation function.
A. Schematic of Sca2 peptides produced as N-terminal GST fusions, their names as used in this manuscript and the vector they are expressed from. GST is represented in green and portions of Sca2 in blue. Numbers located at the ends of the blue bars indicate amino acid numbers. B. Flow cytometric analysis of adherence of GST-fused Sca2 fragments diagramed in A to the surface of HeLa and EA.hy 926 cells. Twenty thousand events were recorded for each sample. C. Spontaneous actin assembly assays measuring actin polymerization over time in the presence of indicated concentrations of Sca2 fragments.
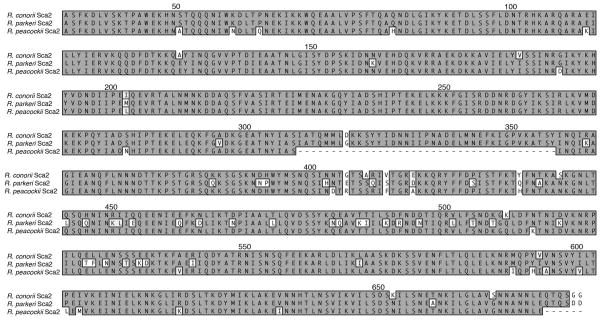
We then mapped the actin nucleation activity of Sca2 by utilizing a spontaneous actin assembly assay to assess actin polymerization in the presence of each Sca2 fragment. Similar to what has been observed for Sca2 from R. parkeri (Haglund et al., 2010), the first third of the Sca2 passenger domain (GST-Sca234–556) is capable of stimulating actin assembly at a faster rate than actin can assemble alone (Figure 3C). None of the N-terminal truncation constructs retain actin assembly function suggesting that amino acids 34 to 156 are necessary for actin assembly. Additionally, the deletion of amino acids 257 through 356 from the C-terminus abolishes actin nucleation activity indicating that these residues are also necessary for actin polymerization and indicates amino acids 34 through 356 as the minimal domain of Sca2 capable of nucleating actin assembly. Interestingly, the Sca2 allele from Rickettsia peacockii (another SFG rickettsia), contains an in-frame deletion of 39 amino acids corresponding to residues 309 to 347 in the R. conorii allele, falling within the region demonstrated to be necessary for actin nucleation activity (Figure 2). R. peacockii lack actin tail formation yet, retain an intact Sca2 open reading frame (Baldridge et al., 2004). The first third of the passenger domain of Sca2 from R. peacockii is highly conserved among SFG rickettsia with the exception of a 39 amino acid deletion (Figure 2). We hypothesized that this 39 amino acid deletion in the R. peacockii Sca2 protein could account for the loss of actin assembly by Sca2. To test this, we deleted the corresponding amino acids from the R. conorii Sca2 protein (amino acids 309 to 347) and assessed the ability of GST-Sca2Δ309–347 to nucleate actin assembly. Deletion of these amino acids resulted in complete abolishment of actin assembly (Figure 3C) indicating that these amino acids are necessary for Sca2 mediated actin nucleation. We then assessed the ability of GST-Sca2Δ309–347 to bind the surface of mammalian cells by flow cytometry. Interestingly, GST-Sca2Δ309–347 retains the ability to associate with the mammalian cell surface, indicating that amino acids 309 to 347 are dispensable for association and that the loss of actin assembly function is likely not due to protein misfolding upon deletion of this region.
Figure 2.
Amino acid alignment of the N-terminal third of the passenger domain of Sca2 from R. conorii, R. parkeri, and R. peacockii. Numbers above the alignment indicate the amino acid numbers of Sca2 from R. conorii. Identical amino acids are shaded dark gray and boxed. Deletions are indicated by a dash.
Taken together, these data show that the ability of Sca2 to associate with the mammalian cell surface as well as nucleate actin assembly reside within the first third of the Sca2 passenger domain. Furthermore, while the minimal domains sufficient to perform each function overlap, these processes are independent and separable as demonstrated by the creation of fragments that retain one function or the other exclusively. Additionally, amino acids 309 to 347 are necessary for the stimulation of actin assembly by Sca2 but completely dispensable for association with the mammalian cell surface.
Inhibition of R. conorii invasion by recombinant Sca2 protein in vitro is governed by the mammalian association region (MAR)
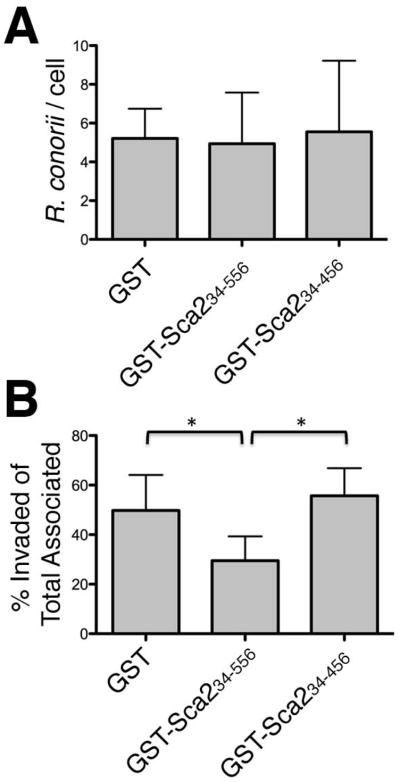
We have previously demonstrated that GST-Sca234–794 can inhibit R. conorii invasion of mammalian cells in vitro (Cardwell et al., 2009). To determine if the Sca2 mammalian association region within the first third of the passenger domain retains the ability to do this, we incubated purified GST-Sca234–556 with a monolayer of HeLa cells prior to infection with R. conorii and assessed rickettsial invasion by differential staining and microscopy (see Experimental Procedures). As shown in Figure 4A and B, recombinant Sca2 MAR (GST-Sca234–556), GST alone or a Sca2 fragment that retains actin- polymerizing activity in vitro (GST-Sca234–456) does not perturb the ability of R. conorii to adhere to mammalian cells. Interestingly, while total cell association is not inhibited by any of these recombinant proteins, the Sca2 MAR (aa 34–556) competitively inhibits R. conorii invasion of non-phagocytic mammalian cells. These data indicate that purified recombinant Sca2 MAR likely adopts a conformation that is sufficient to interact with cognate interacting proteins expressed at the plasma membrane of mammalian cells.
Figure 4. Inhibition of R. conorii invasion by recombinant Sca2 protein in vitro is governed by the mammalian association region (MAR).
HeLa cell monolayers were incubated with 10 μM recombinant Sca2 protein prior to infection with R. conorii for 45 min. Total R. conorii associated per cell (A) and percent bacteria invaded out of total associated (B) was determined by differential immunofluorescent microscopy. *, P<0.05. The data presented is representative of three independent experiments. Error bars represent the standard deviation from the average.
Discussion
Adherence to and invasion of host mammalian cells is a critical first step in establishment of a rickettsial infection. To date, these processes in SFG rickettsia have been attributed to four rickettsial Sca proteins (rOmpA, rOmpB, Sca1 and Sca2) that have been characterized to interact with the host cell surface by mediating either adherence or both adherence and invasion (Cardwell et al., 2009, Chan et al., 2009, Li et al., 1998, Martinez et al., 2005, Riley et al., 2010). While the attributes of adherence and invasion have been well established for these rickettsial surface proteins, the mechanism behind these processes has been elusive for most. In order to further understand how these Sca proteins mediate interactions with the host cell, we aimed to isolate the domain of Sca2 responsible for mediating adherence to mammalian host cells. Previous work from our lab demonstrated that a recombinant soluble fragment of Sca2 comprising amino acids 34 to 794 was capable of reducing R. conorii invasion of HeLa cells in vitro (Cardwell et al., 2009). This suggested that this portion of Sca2 may contain the domain responsible for adhering to the mammalian cell surface and thus be able to block proximal rickettsial interactions with the host cell. By systematically fragmenting the Sca2 passenger domain, we have identified amino acids 34 to 556 of Sca2 from R. conorii as a mammalian association region or MAR. This domain, when expressed and purified from E. coli, is sufficient to mediate adherence to the surface of both epithelial and endothelial cells, two cells types that are targets for rickettsial infection in vivo (Walker et al., 1985). Interestingly, the last two thirds (amino acids 507 to 1515) of the passenger domain are also sufficient to bind host cells, albeit with reduced efficiency compared to the MAR domain. The function of this alternate association region appears to require a much larger fragment of Sca2 as its function is lost when separated into pieces corresponding to the middle third (GST-Sca2507–1041) and last third (GST-Sca21001–1515) of the passenger domain.
We can assess the importance of Sca2 association with the host cell surface by measuring the ability of recombinant Sca2 protein to block rickettsial infection. Addition of the mammalian association region of Sca2 (amino acids 34 to 556) to a monolayer of HeLa cells prior to infection with R. conorii reduces invasion by 50%. Having identified fragments of Sca2 that maintain only the actin polymerizing activity of Sca2, we show that the ability to block rickettsial infection is specific to the ability of Sca2 to adhere to the cell surface and not due to actin polymerizing function. The degree of inhibition we see with the Sca2 adherence domain is comparable to the highest levels of inhibition demonstrated for other rickettsial Sca proteins such as rOmpB (Chan et al., 2009) suggesting that Sca2 plays an important role in initial bacterial-host interactions and most likely works in concert with other rickettsial surface proteins to mediate efficient adherence and uptake.
The specificity of Sca2 MAR for inhibition of rickettsial invasion rather than adherence reflects the multifactorial process of adherence and invasion that we are beginning to appreciate. Previous studies have demonstrated that expression of R. conorii rOmpB, Sca2 and Sca1 individually in a surrogate Gram-negative bacterium (E. coli) is sufficient to mediate distinct interactions with a variety of cell lines of both epithelial and endothelial origin (Cardwell et al., 2009, Chan et al., 2009, Riley et al., 2010). However, until comparative studies are performed to understand the relative contributions of each adhesin and invasin, it is premature to assign the relative importance of each Sca protein to the infection process. The identification of the cognate host receptors for each rickettsial adhesin and invasin will also greatly advance our understanding of how, when and where these surface proteins function. Interestingly, during experimental infection of mice, SFG rickettsiae are found in a number of organs and cause well characterized pathology in the lungs, liver and spleen (Chan et al., 2011). While the only known rickettsial receptor, Ku70, is expressed at significant levels at the mammalian surface in endothelial cells, it is possible that the receptors of other Scas may be present at the plasma membrane of other cell types, contribute to cellular tropism and lead to the dissemination and observed pathology mediated by rickettsiae in this murine model of infection. Our results suggest that a putative mammalian ligand involved in Sca2-mediated association with epithelial and endothelial cells may be ubiquitously expressed in these cell types. The isolation of Sca2 fragments that function specifically in host cell association will facilitate the search for mammalian ligands that can help us understand how Sca2 associates with the mammalian cell surface and the impact of such interaction in vivo. Interestingly, a recently isolated R. rickettsii sca2 transposon mutant does not exhibit an appreciable defect in adherence or invasion of Vero cells, but exhibits an attenuation in virulence in a guinea pig model of infection (Kleba et al., 2010). It is possible that the observed defect in virulence may result from an inability of the R. rickettsii sca2 mutant to interact with endothelial cells or other cell types in vivo that have not yet been modeled in vitro. Alternatively, other highly conserved Sca proteins such as Sca0/OmpA and Sca5/OmpB may compensate for the lack of Sca2 expression during the initial interactions with host cells such that a sca2 mutant would not exhibit a defect in adherence or invasion in an in vitro cell culture model of infection. The nature of the attenuation in the sca2 mutant has yet to be identified and is an area of active investigation.
Sca2 from R. rickettsii has been shown to be necessary for actin tail formation in a tissue culture model of infection (Kleba et al., 2010). Furthermore, Sca2 from R. parkeri is sufficient to stimulate actin assembly in vitro in a formin-like manner (Haglund et al., 2010). Interestingly, it was demonstrated that the first third of the Sca2 passenger domain from R. parkeri (amino acids 34 to 670) was sufficient to stimulate actin assembly in a pyrene actin assembly assay. An amino acid alignment of this region of Sca2 from R. conorii and R. parkeri reveals an identity of 91.5% (alignment in Figure 2) suggesting that this region of Sca2 from R. conorii may also contain the ability to stimulate actin assembly. Utilizing a pyrene actin assembly assay, we show that amino acids 34 to 556 of R. conorii Sca2 are indeed capable of stimulating actin polymerization. Therefore, both a mammalian association region and the domain sufficient to nucleate actin assembly are located within the first third of the passenger domain of Sca2. In order to study the mammalian association region located here, it was necessary to identify more specifically the regions within this first third of the passenger domain that contribute to the adherence function and actin polymerizing function of Sca2 and separate these functional domains. Fine mapping of this region of Sca2 from R. conorii reveals that amino acids 457 to 556 and 157 to 256 are necessary for Sca2 to adhere to the mammalian cell surface, indicating that the minimal domain sufficient to associate with mammalian cells is comprised of amino acids 157 to 556. Alternatively, amino acids 257 to 356 and 34 to 156 are necessary for actin assembly by Sca2, indicating that the minimal domain sufficient to stimulate actin polymerization is comprised of amino acids 34 to 356. While the minimal domains sufficient to mediate each function overlap, they can clearly be separated as observed by the creation of fragments that retain one function or the other. Therefore, we can separate these functions, and study them independently.
Interestingly, R. peacockii, another SFG rickettsia that lacks the ability to form actin tails, contains a complete sca2 ORF that is highly conserved with other SFG rickettsia except for a deletion of 39 amino acids that falls within the region of Sca2 that we have shown to be necessary for actin polymerization (Figure 2 and Figure 3C). Our studies demonstrate that a deletion of the corresponding amino acids in the R. conorii Sca2 protein render the first third of the passenger domain unable to nucleate actin assembly, indicating that these residues are necessary for this function. These data suggest that this 39 amino acid deletion could be responsible for the lack of actin tails observed in R. peacockii. Ongoing studies will determine if the full length Sca2 protein from R. peacockii or the full length Sca2 protein from R. conorii containing the 39 amino acid deletion retain any actin polymerizing function.
While necessary for actin assembly, the molecular function of amino acids 309 to 347 remains to be elucidated and further study will be required to understand how these amino acids contribute to actin assembly. Haglund and co-workers proposed the first third of the passenger domain to be a formin homology 2 (FH2) domain based on secondary structure predictions (Haglund et al., 2010). Further study will be required to determine if this deletion is affecting FH2 domain characteristics such as actin binding or dimerization, although by secondary structure alignment these residues do not map to amino acids previously shown to contact actin in FH2 domains. Furthermore, it has yet to be demonstrated that the proposed FH2 domain of Sca2 maintains the ability to processively associate with the growing barbed end of the filament and protect the barbed end from capping protein, properties that are characteristic of FH2 domains.
This work highlights the truly multifunctional property of the Sca2 rickettsial autotransporter. With a limited genome and evidence of reductive evolution, it is not surprising that rickettsial species would utilize a large, well-conserved surface protein for multiple functions. Ongoing research into how these two functions, actin polymerization and mammalian association, work together temporally and spatially, to promote rickettsial infection in vivo will be important in elucidating Sca2’s role in rickettsial pathogenesis and to may provide a viable target for the development of efficacious ant-rickettsial vaccines and therapeutics.
Experimental procedures
Cell lines and bacterial strains
HeLa (American Type Culture Collection, Manassas, VA), and EA.hy 926 (obtained from the University of North Carolina Chapel Hill) (Edgell et al., 1983) cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. HeLa cell medium was supplemented with 1 X nonessential amino acids (Cellgro, Manassas, VA) and 0.5 mM sodium pyruvate (Lonza, Walkersville, MD) resulting in complete DMEM (cDMEM). All mammalian cells were grown at 37°C/5% CO2. Bacterial genetic manipulations were performed in Escherichia coli TOP 10 (Invitrogen) grown at 37°C in Luria-Bertani (LB) Miller broth supplemented with 50 μg of carbenicillin/ml where appropriate. R. conorii Malish 7 was propagated by infection of Vero cell monolayers for 5 days at 34°C/5% CO2. R. conorii were isolated from Vero cells by glass bead disruption of the monolayer, followed by lysis through a syringe. Bacteria were separated from mammalian cell debris by centrifugation through a sucrose gradient as previously described (Gouin et al., 1999). The recovered R. conorii were resuspended in a sucrose phosphate glycine buffer (SPG; 218 mM sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM L-glutamate [pH 7.4]) and frozen at −80°C. Rickettsia titers were determined by a limiting-dilution infectivity assay, followed by calculation of the Reed and Muench equation (Reed et al., 1938).
Antibodies
Anti-GST polyclonal rabbit antibody (Z–5) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 488- and 546-conjugated goat anti-rabbit IgG, Alexa Fluor 488-conjugated goat anti-mouse IgG, and DAPI (4′, 6′-diamidino-2- phenylindole) were purchased from Molecular Probes. Anti-RcPFA was generated as previously described (Chan et al., 2011) and purified on a protein A affinity column (GE Healthcare, Piscataway, NJ).
Plasmid DNA construction
sca2-containing plasmids were generated by directional cloning into specified vectors using the primers and restriction enzymes indicated in Table 1. Plasmid pSca2-200 (Cardwell et al., 2009) served as the template for all PCR reactions. pMC050 was constructed by amplifying DNA fragments upstream and downstream of the deletion site using primer pair 1 (5′-AAGGATCCGCAAGCTTTAAAGATTTAGTTAGTAAAA-3″, 5″-TCTTATTTGGTTTATTGATGCGATATAGTTTGTTGC-3′) and primer pair 2 (5′-AACTATATCGCATCAATAAACCAAATAAGAGCCGGA-3′, 5′-AACTCGAGGATAAGATCTAATCGTGCCTTTTC-3′) respectively. These fragment were designed to have 15 to 20 base pair (bp) overlapping sequence in order to prime each other when mixed in a PCR reaction. The full-length construct was then amplified using primers for pMC026.
Table 1.
Plasmids, primers and protein constructs.
| Plasmid | Gene | Parent vector | 5′-forward-3′ | 5′-reverse-3′ | RE | Tag | Sca2(aa) | Source |
|---|---|---|---|---|---|---|---|---|
| pSca2–200 | R. conorii sca2 1–5388 bp | pET22b | ACCATGGATTTACAAAATTCCCAC | TTAGTGGTGGTGGTGGTGGTGCAAATTGACTTTTAG | NcoI, - | His6 | 1–1795 | (Cardwell et al., 2009) |
| pMC026 | R. conorii sca2 100–1668 bp | pGEX2TKP | AAGGATCCGCAAGCTTTAAAGATTTAGTTAGTAAAA | AACTCGAGGATAAGATCTAATCGTGCCTTTTC | BamHI, XhoI | GST | 34–556 | This Work |
| pMC027 | R. conorii sca2 1519–3123 bp | pGEX2TKP | AAGGATCCATTGACGTTAAAAATAGACCTATTTTAC | AACTCGAGTTCATTGCTCATGCCTGTA | BamHI, XhoI | GST | 507–1041 | This Work |
| pMC028 | R. conorii sca2 3001–4545 bp | pGEX2TKP | AAGGATCCAAGGAAAAAGACCCCGAA | AACTCGAGTTCATCACCGGCTCCTAT | BamHI, XhoI | GST | 1001–1515 | This Work |
| pMC038 | R. conorii sca2 100–1368 bp | pGEX2TKP | AAGGATCCGCAAGCTTTAAAGATTTAGTTAGTAAAA | AACTCGAGAATAAGATTTTTAAATTCTTCAATATTTTCTT | BamHI, XhoI | GST | 34–456 | This Work |
| pMC039 | R. conorii sca2 100–1068 bp | pGEX2TKP | AAGGATCCGCAAGCTTTAAAGATTTAGTTAGTAAAA | AACTCGAGTTCTATTCCGGCTCTTATTTG | BamHI, XhoI | GST | 34–356 | This Work |
| pMC040 | R. conorii sca2 100–768 bp | pGEX2TKP | AAGGATCCGCAAGCTTTAAAGATTTAGTTAGTAAAA | AACTCGAGACGAGATATACCAAACTTCTTTTTTAAC | BamHI, XhoI | GST | 34–256 | This Work |
| pMC041 | R. conorii sca2 100–468 bp | pGEX2TKP | AAGGATCCGCAAGCTTTAAAGATTTAGTTAGTAAAA | AACTCGAGTTTGCTAGGGTCATAACTTATTCC | BamHI, XhoI | GST | 34–156 | This Work |
| pMC042 | R. conorii sca2 469–1668 bp | pGEX2TKP | AAGGATCCATAGACAATAATGTAGAACACGATCAA | AACTCGAGGATAAGATCTAATCGTGCCTTTTC | BamHI, XhoI | GST | 157–556 | This Work |
| pMC043 | R. conorii sca2 769–1668 bp | pGEX2TKP | AAGGATCCGATGATAATAGAGACGGCTATATTAAG TC | AACTCGAGGATAAGATCTAATCGTGCCTTTTC | BamHI, XhoI | GST | 257–556 | This Work |
| pMC044 | R. conorii sca2 1069–1668 bp | pGEX2TKP | AAGGATCCGCAAACCAGTTTCTAAACAACA | AACTCGAGGATAAGATCTAATCGTGCCTTTTC | BamHI, XhoI | GST | 357–556 | This Work |
| pMC045 | R. conorii sca2 1369–1668 bp | pGEX2TKP | AAGGATCCAAAACAGATCCTATAGCAGCATTAAC | AACTCGAGGATAAGATCTAATCGTGCCTTTTC | BamHI, XhoI | GST | 457–556 | This Work |
| pMC050 | R. conorii sca2 100–924, 1042–1668 bp | pGEX2TKP | AAGGATCCGCAAGCTTTAAAGATTTAGTTAGTAAAA | AACTCGAGGATAAGATCTAATCGTGCCTTTTC | BamHI, XhoI | GST | 34–308, 348–556 | This Work |
| pMC059 | R. conorii sca2 1519–4545 bp | pGEX2TKP | AAGGATCCATTGACGTTAAAAATAGACCTATTTTAC | AACTCGAGTTCATCACCGGCTCCTAT | BamHI, XhoI | GST | 507–1515 | This Work |
Protein Purification
Overnight cultures of E. coli BL21(DE3), transformed with an expression vector were diluted 1:10 into 1 liter of fresh LB medium with 50 μg of carbenicillin/ml and grown at 30°C until the OD600 reached 0.5. The expression of soluble glutathione S-transferase (GST)-fused protein was induced by the addition of 0.5 mM IPTG (isopropyl-β-D-thiogalactopyranoside) for 5 h at 30°C. Bacteria were harvested by centrifugation, resuspended in Tris-buffered saline (TBS; 50 mM Tris [pH 8.0], 150 mM NaCl) containing protease inhibitor cocktail (Roche), and lysed by sonication (40% amplitude, 5 s on and 10 s off for 20 min; Fisher Scientific sonic dismembrator model 500). Lysates were cleared by centrifugation at 13,000 g for 30 min at 4°C and applied to a GST-TRAP FF column (GE Healthcare, Piscataway, NJ) by fast protein liquid chromatography (FPLC) on a Akta FPLC. Protein was eluted with 30 mM reduced glutathione, and fractions were dialyzed into Tris-buffered saline containing 10% glycerol before storage at −80°C.
Immunofluorescence
HeLa cells, seeded at 90% confluency, were washed 1 x with serum-free DMEM (SF DMEM) at room temperature. Recombinant GST-Sca2 protein (1 μM), diluted in room temperature SF DMEM, was added to the monolayer and incubated at 37°C/5% CO2 for 20 min. The media was aspirated and the cells washed 3X with PBS. The monolayer was then fixed with PBS + 4% paraformaldehyde (PFA) for 20 min and processed for immunofluorescence microscopy. Protein binding to the cell surface was assessed by staining with anti-GST antibody (1:500), followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:1000) and DAPI (1:10,000). Images were digitally captured on a Nikon Eclipse TE2000-u microscope coupled to a charge-coupled device camera using × 200 magnification and processed using Adobe Photoshop.
Flow cytometry
Mammalian cells were lifted from tissue culture plates by incubation with PBS + 1 mM EDTA at 37°C for 10 min. All subsequent steps were performed at room temperature. Cells were collected and pelleted at 200 g, then resuspended in SF DMEM. To assess Sca2 binding to the mammalian cell surface, 1 × 106 cells were aliquoted per sample, pelleted, and resuspended in 1 mL of Sca2 protein diluted in SF DMEM. The cells were rocked for 40 min in the protein solution, washed 3 X with PBS, fixed in PBS + 2% PFA for 20 min and blocked in PBS + 2% BSA. Sca2 binding was detected by staining the cells with anti-GST antibody (1:750) followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:1000) and analyzed on a BD LSR-II flow cytometer using a fluorescein isothiocyanate (FITC) parameter and FloJo software.
Spontaneous Actin Assembly Assay
Spontaneous actin assembly has been described in detail previously (Neidt et al., 2008). Briefly, actin assembly was measured from the fluorescence of pyrene-actin (excitation at 364 nm and emission at 407 nm) with a Safire2 (Tecan, Durham, NC) fluorescent plate reader. For spontaneous assembly assays, 2.5 μM of 20% pyrene-labeled Mg-ATP-actin was placed in an upper row of a 96 well non-binding black plate (Corning, Corning, NY). Recombinant Sca2 protein to be assayed was prepared in a lower row with 10× KMEI (500 mM KCl, 10 mM mgCl2, 10 mM EGTA, and 100 mM imidazole, pH 7.0) and Mg-buffer G (2 mM Tris, pH 8.0, 0.2 mM ATP, 0.1 mM MgCl2, and 0.5 mM DTT). Initialization of the reactions was achieved by addition of the contents of the lower wells (Sca2 protein) to the actin-containing wells simultaneously. Final protein concentrations are indicated in the figure legends.
Rickettsial infection assays
HeLa cells were seeded at 1 × 104 cells/well in 96-well plates 24 h prior to infection with R. conorii. Cells were washed 1 × with SF DMEM prior to the addition of 10 μM protein diluted in SF DMEM and then incubated at 34°C/5%CO2 for 20 minutes. R. conorii was added to each well for an MOI of 5 and the plate centrifuged at 300 g for 5 min at room temperature to induce contact. Plates were incubated for 45 min at 34°C/5% CO2, washed with PBS, and fixed in PBS + 4% PFA for 20 min. The infected monolayers were then processed for immunofluorescence and differentially stained to distinguish between extracellular and intracellular R. conorii. Briefly, extracellular R. conorii were stained with anti-RcPFA (1 μg/ml), followed by Alexa Fluor 546-conjugated goat anti- rabbit IgG (1:1,000), prior to permeabilization of the mammalian cells with PBS + 0.1% Triton X–100. Total R. conorii cells were then stained with anti-RcPFA, followed by Alex Fluor 488-conjugated goat anti-rabbit IgG (1:1000) and DAPI (1:10,000). Images were captured as described for immunofluorescence assays, and the numbers of nuclei and extracellular and total bacteria manually enumerated. Rickettsial invasion was calculated as the percentage of bacteria invaded out of the total bacteria associated (adhered and invaded) with host cells. Statistical analysis was performed using the paired student t-test function in the GrpahPad Prism graphing software package.
Acknowledgments
We thank J. Sees and D. Kovar (The University of Chicago) for providing assistance with the pyrene actin studies. We also want to thank members of the Martinez laboratory for careful reading and editing of the manuscript.
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (AI 72606) to J.J.M. Additional support was provided by the University of Chicago Molecular Cell Biology Training Grant (T32GM007183) to M.M.C. The authors wish to acknowledge membership within the Region V Great Lakes Regional Center of Excellence (GLRCE) in Biodefense and Emerging Infectious Diseases Consortium (GLRCE, NIAID Award U54-AI-057153).
References
- Baldridge GD, Burkhardt NY, Simser JA, Kurtti TJ, Munderloh UG. Sequence and expression analysis of the ompA gene of Rickettsia peacockii, an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni. Appl Environ Microbiol. 2004;70:6628–6636. doi: 10.1128/AEM.70.11.6628-6636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc G, Ngwamidiba M, Ogata H, Fournier PE, Claverie JM, Raoult D. Molecular evolution of rickettsia surface antigens: evidence of positive selection. Mol Biol Evol. 2005;22:2073–2083. doi: 10.1093/molbev/msi199. [DOI] [PubMed] [Google Scholar]
- Cardwell MM, Martinez JJ. The Sca2 autotransporter protein from Rickettsia conorii is sufficient to mediate adherence to and invasion of cultured mammalian cells. Infect Immun. 2009;77:5272–5280. doi: 10.1128/IAI.00201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YG, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol. 2009;11:629–644. doi: 10.1111/j.1462-5822.2008.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YG, Riley SP, Chen E, Martinez JJ. Molecular basis of immunity to rickettsial infection conferred through outer membrane protein B. Infect Immun. 2011;79:2303–2313. doi: 10.1128/IAI.01324-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Williams K, Shukla M, Snyder EE, Nordberg EK, Ceraul SM, et al. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PLoS One. 2008;3:e2018. doi: 10.1371/journal.pone.0002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, et al. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112 (Pt 11):1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- Hackstadt T. The biology of rickettsiae. Infect Agents Dis. 1996;5:127–143. [PubMed] [Google Scholar]
- Haglund CM, Choe JE, Skau CT, Kovar DR, Welch MD. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat Cell Biol. 2010;12:1057–1063. doi: 10.1038/ncb2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect Immun. 2010;78:2240–2247. doi: 10.1128/IAI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Walker DH. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb Pathog. 1998;24:289–298. doi: 10.1006/mpat.1997.0197. [DOI] [PubMed] [Google Scholar]
- Martinez JJ, Cossart P. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J Cell Sci. 2004;117:5097–5106. doi: 10.1242/jcs.01382. [DOI] [PubMed] [Google Scholar]
- Martinez JJ, Seveau S, Veiga E, Matsuyama S, Cossart P. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell. 2005;123:1013–1023. doi: 10.1016/j.cell.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Neidt EM, Skau CT, Kovar DR. The cytokinesis formins from the nematode worm and fission yeast differentially mediate actin filament assembly. J Biol Chem. 2008;283:23872–23883. doi: 10.1074/jbc.M803734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Riley SP, Goh KC, Hermanas TM, Cardwell MM, Chan YG, Martinez JJ. The Rickettsia conorii autotransporter protein Sca1 promotes adherence to nonphagocytic mammalian cells. Infect Immun. 2010;78:1895–1904. doi: 10.1128/IAI.01165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S. Antigenic relationships among the rickettsiae of the spotted fever and typhus groups. FEMS Microbiol Lett. 1991;65:341–344. doi: 10.1016/0378-1097(91)90238-6. [DOI] [PubMed] [Google Scholar]
- Walker DH, Gear JH. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am J Trop Med Hyg. 1985;34:361–371. doi: 10.4269/ajtmh.1985.34.361. [DOI] [PubMed] [Google Scholar]
- Walker DH, Occhino C, Tringali GR, Di Rosa S, Mansueto S. Pathogenesis of rickettsial eschars: the tache noire of boutonneuse fever. Hum Pathol. 1988;19:1449–1454. doi: 10.1016/s0046-8177(88)80238-7. [DOI] [PubMed] [Google Scholar]