Abstract
Pain is a major concern for individuals with cancer, particularly older adults who make up the largest segment of individuals with cancer and have some of the most unique pain challenges. One of the priorities of hospice is to provide a pain free death, and while outcomes are better in hospice, patients still die with poorly controlled pain.
Objective
This paper reports on the results of a Translating Research Into Practice intervention designed to promote the adoption of evidence-based pain practices for older adults with cancer in community-based hospice.
Setting
This IRB approved study was a cluster randomized trial implemented in sixteen Midwestern hospices.
Methods
Retrospective medical records from newly admitted patients were used to determine the intervention effect. Additionally, survey and focus group data gathered from hospice staff at the completion of the intervention phase were analyzed.
Results
Improvement on the Cancer Pain Practice Index, an overall composite outcome measure of evidence-based practices for the experimental sites was not significantly greater than control sites. Decrease in patient pain severity from baseline to post intervention in the experimental group was greater, however, the result was not statistically significant (p=0.1032).
Conclusions
Findings indicate a number of factors may impact implementation of multi-component interventions, including unique characteristics and culture of the setting, the level of involvement with the change processes, competing priorities and confounding factors, and complexity of the innovation (practice change). Our results suggest future study is needed on specific factors to target when implementing a community-based hospice intervention, including determining and measuring intervention fidelity prospectively.
Keywords: elderly, cancer pain, pain assessment, pain management, hospice
INTRODUCTION
Pain is a major concern for individuals with cancer. The majority of cancer patients are older adults, a population that presents with unique challenges for effective pain assessment and management, including misconceptions about pain, evaluating pain in those with cognitive impairments, increased sensitivity to medication side effects, multiple co-morbidities, polypharmacy issues, practical barriers to adherence and reluctance to take opioid analgesics (1–5).
In the hospice setting, the majority of patients are older adults, many with advanced cancer. One of the priorities of hospice is to assure safe and comfortable dying and although pain outcomes are better in hospice than non-hospice settings, there remains considerable variation. Patients in hospice still die with poorly controlled pain (6). Evidence-based practice (EBP) is the use of current best research evidence in combination with clinical expertise and patient values in healthcare decision-making (7). However, the application of EBP for pain by nurses and physicians is sporadic at best (8–11). Despite the availability of evidence-based practice guidelines to improve management of pain in older adults, adoption and use of recommendations based on best scientific evidence lags. This gap in recommended pain practices has been documented in the care of older adults with cancer pain in community-based hospice settings (12).
Implementation strategies to promote use of best practices by clinicians have been studied but which combination of strategies are effective is not known. Translating Research into Practice (TRIP) research evaluates approaches to facilitate quality health care practices. This paper reports on the results of a TRIP intervention, that is multifaceted and includes strategies designed to promote adoption of evidence-based pain management practices for older adults with cancer in community-based hospice settings, hereafter referred to as TRIP-CA. Although a TRIP intervention was successful in improving pain management practices and decreasing costs for older adults in an acute care setting, the effectiveness of a multifaceted TRIP model to promote use of evidence-based pain practices in the community-based hospice setting is unknown(13–14). Following implementation of the TRIP-CA intervention, we hypothesized that: (1) nurses and physicians at the experimental (E) hospices would show a greater increase in the adoption of EBP for pain management than those in the control (C) hospices; and (2) mean pain severity ratings for older patients with cancer admitted to E hospices would be lower at two time periods following hospice admission (P2=3–7 days and P3=8–14 days) than in the C group. In addition to data collected to address these hypotheses, post hoc focus groups and data from a process evaluation questionnaire completed by hospice staff were used to further evaluate the TRIP-CA intervention.
METHODS
Study Design & Sample
A cluster randomized controlled trial (RCT) was used to test the effect of the multifaceted TRIP-CA intervention on promoting adoption of EBP for pain management in older adults with cancer receiving community-based hospice care. Sixteen Midwestern hospices were recruited with a representative sample of four small (Average Daily Census [ADC] = 25 or less), eight medium (ADC=26–100) and four large organizations (ADC=greater than 100). The majority (75%) reported an organizational structure with the hospice as part of a larger organization such as, a hospital, Department of Health, or health care organization. The remaining hospices were independent organizations. Fifteen of the 16 hospices were not for profit organizations. Inclusion criteria for the hospices were a minimum of 30 older patients admitted per year and serving older patients with a cancer diagnosis in a community-based hospice setting. For purposes of this study, community-based hospice was defined as a setting where patients received hospice care in an environment which allowed the patient or their family caregiver to oversee the implementation of the pain treatment plan (e.g. personal home or assisted living). Hospices were first stratified by size and then randomly assigned into the E or C group.
Demographic data about staff was collected from the sixteen participating hospices at two time points: Baseline (August/September 2006) and Post intervention (September/October 2008). At baseline, the provider sample consisted of 383 nurses and 16 physicians. Post-intervention, the total number of nurses increased to 415, while the total number of physicians was unchanged. Demographic characteristics of providers are presented in Table 1.
Table 1.
Demographic Characteristics of Hospice Providers at Baseline (T1) and Post-intervention (T2) between Experimental and Control Groups
| Experimental | P-value | Control | P-value | Difference T1 T2 by E/C | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||||
| Nurses (n=T1: 383; T2: 415) | n (%) | n (%) | 0.47 | n (%) | n (%) | 0.21 | 0.65 |
| Full Time | 145 (64.2) | 132 (60.8) | 106 (67.5) | 121 (61.1) | |||
| Part Time | 81 (35.8) | 85 (39.2) | 51 (32.5) | 77 (38.9) | |||
| Nurse Education | 0.86 | 0.97 | 0.99 | ||||
| <BSN | 104 (46.0) | 141 (65.0) | 94 (59.9) | 145 (73.2) | |||
| BSN | 108 (47.8) | 69 (31.8) | 40 (25.5) | 29 (14.6) | |||
| >BSN | 7 (3.1) | 4 (1.8) | 4 (2.5) | 3 (1.5) | |||
| Unknown or Not reported | 7 (3.1) | 3 (1.4) | 19 (12.1) | 21 (10.6) | |||
| Nurse Certification | 0.49 | 0.36 | 0.26 | ||||
| No Certification | 186 (82.3) | 173 (79.7) | 113 (72.0) | 151 (76.3) | |||
| Cert in Hospice/Palliative Care or Pain Management | 40 (17.7) | 44 (20.3) | 44 (28.0) | 47 (23.7) | |||
| Nurse Case Load (Full Time) | 1.001 | 1.001 | 1.00 | ||||
| 10 or less | 3 (37.5) | 4 (50) | 4 (50) | 5 (62.5) | |||
| 11 or more | 5 (62.5) | 4 (50) | 4 (50) | 3 (37.5) | |||
| Medical Director Status (n=16) | 0.071 | 0.181 | N/A2 | ||||
| Full Time | 2 (25) | 2 (25) | |||||
| Part Time | 3 (37.5) | 5 (62.5) | 2 (25) | 4 (50) | |||
| Volunteer | 5 (62.5) | 1 (12.5) | 6 (75) | 2 (25) | |||
| Medical Director Certified (n=16) | 0.611 | 1.001 | 0.46 | ||||
| No Certification | 6 (75) | 4 (50) | 3 (37.5) | 3 (37.5) | |||
| Certification: Hospice/Palliative | 2 (25) | 4 (50) | 5 (62.5) | 5 (62.5) | |||
| Care | |||||||
Remark: P-values were calculated based on the logistic regression model (binomial/polynomial outcome) with contrast tests.
P-values were calculated using the Fisher’s exact test due to some small numbers in the contingency
P-value was not available due to zero count in one cell from the contrast test.
Evidence-based practices for pain assessment and management implemented by hospice medical professionals (nurses and physicians) were the target of the intervention. A sample of medical records of older hospice patients cared for provided the data source to evaluate provider practices. Inclusion criteria for medical records were: patients 65 years or older, with a cancer diagnosis, newly admitted to hospice, and receiving hospice services in a community-based setting. An average of 30 medical records for patients meeting eligibility criteria were randomly selected from each hospice for the designated timeframes (Baseline: February 1- July 30, 2006; Post Intervention: April 1- Sept 30, 2008). For hospices that did not have a minimum of 30 eligible medical records during the defined period, all eligible records were selected.
Post hoc qualitative focus groups with hospice professionals (nurses, physicians, social workers) were conducted after the intervention phase at each of the eight E sites to provide feedback on the TRIP-CA intervention and barriers and facilitators to practice change. The focus group participants completed a Process Evaluation Questionnaire described below at the end of the intervention phase and prior to the focus group session. Detailed information on the focus group process and analysis is available elsewhere (15).
The study was approved by the Institutional Human Subjects Review Board (IRB) at The University of Iowa, as well as corresponding human subjects review boards at the participating hospices with access to an internal IRB. The University of Iowa IRB served as the IRB of record for those hospices without an IRB.
TRIP-CA Intervention
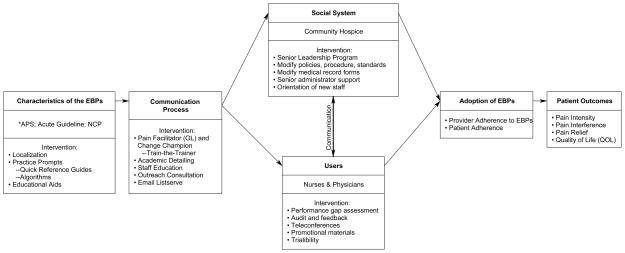
TRIP-CA was adapted from a model developed by Rogers’ Diffusion of innovation (DoI) framework (13, 16, 17). Figure 1 outlines the components of the multifaceted intervention. The TRIP-CA intervention follows the DoI framework which suggests that the components of the model (the characteristics of the innovation, the communication process, the social system, and the users) interact and impact the adoption of the innovation (e.g. cancer pain EBPs in older adults) and, ultimately patient outcomes.
Figure 1.
TRIP Cancer Pain Intervention
* APS = Guideline for the Management of Cancer Pain in Adults & Children (American Pain Society)
Acute = Evidence-Based Practice Guideline: Acute Pain Management in Older Adults (Herr et al)
NCP = Clinical Practice Guidelines for Quality Palliative Care (National Consensus Project)
Characteristics of the EBP include the nature and complexity of the practice guidelines and the tools and resources to prompt and facilitate practice change. Opinion leaders, change champions, and educational training and outreach are key elements in the communication process that promote the use of EBP (17–21). The social system, defined as a set of interrelated members engaged in joint problem solving to accomplish a common goal can have a significant influence on adoption of EBPs (17, 22–26) and includes senior leadership support, onboarding new staff, and modifying policies and procedures. User engagement through performance gap assessment, audit and feedback of practices, and adapting EBPs to the setting are influential as well (27–28).
The TRIP-CA Intervention consisted of the Engagement Phase, a 5 month period (February –June 2007) pre intervention, and the Implementation Phase, 12 month period (July 2007–June 2008). During the Engagement Phase, all 16 hospices received copies of the three relevant clinical practice guidelines (CPG) existing at the time of the study that addressed the innovation for the TRIP-CA study, EBPs for cancer pain management in older adults in community-based hospices. The CPGs provided recommendations for acute pain management for older adults, pain management for adults with cancer, and pain management recommendations for patients in hospice and palliative care settings and included: The EBP Guideline: Acute Pain Management in Older Adults (29), The American Pain Society (APS) Guideline for the Management of Cancer Pain in Adults and Children (30), and The National Consensus Project (NCP) Clinical Practice Guideline for Quality Palliative Care (31)
In addition, the 8 experimental hospices participated in a number of activities during the Engagement Phase including: 1) selection of local opinion leaders (called Pain Facilitators), Nurse Champions and Physician Champions; 2) participation by Pain Facilitator and Champions in a 3-day Train-the-Trainer program hosted by the grant team, which provided an overview of project implementation and EBPs for pain assessment and management for older adults with cancer in a hospice setting; 3) review of performance gap assessment data, which included an on-site review of their hospice specific baseline data on 48 indicators of EBPs identified from the guidelines; 4) targeted senior leadership engagement, which consisted of an on-site meeting with the hospice leadership team at each E site to detail the project, review data, and encourage participation in and support of the intervention; and 5) on-site academic detailing about pain EBPs with the physician champions provided by a physician with expertise in both pain management and the hospice setting.
At the beginning of the Implementation Phase, the 8 E hospices received EBP pain resources and aids to facilitate use of EBP recommendations, including pocket-sized laminated pain rating scales for all nurses, copies of Quick Reference Guides, and patient education handouts related to non-pharmacologic interventions. During the first 3 months of the Implementation Phase, all nurses at the E sites completed an EBP pain assessment and management education program provided via DVD. On a monthly basis during the Implementation Phase, the E sites also received an outreach visit from the grant Expert Nurse, who provided support and counseling related to EBP pain issues, as well as issues related to implementation of the intervention. The Expert Nurse also completed a chart audit on the 48 EBP indicators during her monthly visit which provided data for bi-monthly audit and feedback to the sites comparing to baseline practices identified in the Engagement Phase. The Expert Nurse was also available during monthly site visits and via email to assist the E hospices as they modified their standards and documentation forms to ensure they aligned with EBPs for pain assessment and management.
Additional activities during the Implementation Phase include a monthly teleconference between the local Pain Facilitator, Nurse Champion and grant investigators and staff to discuss the intervention and implementation progress and strategies to assist in promoting uptake. This activity also supported networking and sharing successes between the E sites. The final activity during the Implementation Phase was an e-mail Listserv facilitated by experts in pain management and hospice from medicine, nursing, social work, and pharmacy. Any interested staff from the E hospices could participate in the weekly discussions related to pain assessment and management, as well as receive feedback related to specific pain related issues they were dealing with in their practice.
Study Instruments and Measures
The dependent variables for this study were adoption of 11 evidence-based (EB) cancer pain practices for older adults in a community-based hospice setting and mean pain severity (intensity) of older adults with cancer served by the hospices. The medical record abstraction tool (MRAT) was developed specifically for grant use based on the comprehensive list of 48 indicators of EBP for pain management audited during the intervention and provided data for calculating the measure of overall adoption of EB cancer pain practice indicator for older adults and mean pain severity. Due to the nature of the hospice medical records (e.g. variable formats, narrative in nature, and lack of consistent language), data was abstracted by two trained Research Assistants (RA), who were nurses with clinical experience working with older adults in hospice, oncology, or long-term care settings. Any discrepancies were reviewed by a third RA to adjudicate entry. Adoption of EB cancer pain practices was measured by a composite of key provider practices on the Cancer Pain Practice Index (CPPI), developed by the research team using a modified Delphi approach with national pain and hospice experts. The CPPI focuses on 11 key EBP indicators for pain relevant to older adults with cancer receiving community-based hospice care and included pharmacological and non-pharmacological management (Table 3 provides the complete list of the 11 EBPs included on the CPPI).
Table 3.
Adoption of Evidence-Based Pain Practices as Measured by the Cancer Pain Practice Index (CPPI) scores Pre and Post-Intervention
| Experimental Group | Control Group | E vs. C p-value1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | ||||||||
| n | %* | n | %* | Chg | n | %* | n | %* | Chg | ||
| Overall CPPI: mean | 202 | 32.5 | 166 | 34.1 | +1.6 | 197 | 30.8 | 171 | 34.1 | +3.3 | 2 |
| COMPREHENSIVE ADMISSION ASSESSMENT | |||||||||||
| Individual CPPI Indicators | |||||||||||
| 1. Valid pain scale use at admission | 202 | 67.3 | 168 | 86.9 | +19.6 | 197 | 72.1 | 171 | 83.0 | +10.9 | 0.16 |
| 2. Comprehensive assessment- primary (Pain Intensity, Pain Location, Pain Quality, Pain Duration/Pattern, Impact of Pain on Function) | 175 | 67.0 | 159 | 67.9 | +0.9 | 178 | 67.0 | 157 | 76.3 | +9.3 | 0.21 |
| 3. Comprehensive assessment- other (Detailed Pain history including description of previous & current pain episodes & treatment effectiveness; Physical exam- including musculoskeletal & neurological assessment; Presence or absence of delirium; Things that make pain better; Things that make pain worse; and Presence of Anxiety and Depression | 175 | 13.1 | 159 | 11.5 | −1.6 | 178 | 16.5 | 157 | 16.7 | +0.2 | 0.67 |
| 4. Reports of moderate/severe (≥ 5) pain followed by pain severity reassessment within 24 hours | 47 | 12.2 | 44 | 16.7 | +4.5 | 48 | 8.2 | 45 | 15.2 | +7 | 0.62 |
| 5. Increases in pain medications for consecutive reports of pain severity 5 or greater within 24 hrs | 19 | 32.9 | 19 | 49.1 | +16.2 | 19 | 17.5 | 22 | 33.3 | +15.8 | 0.81 |
| 6a. Patients with admission report of pain as mild (1–4) with order for nonopioid or combination of opioid- nonopioid analgesic within 24 hours of admission (Items 6a & 6b combined on CPPI) | 30 | 73.3 | 31 | 90.3 | +17 | 32 | 81.3 | 53 | 88.7 | +7.4 | 0.51 |
| 6b. Pts. with admission report of pain as moderate (5–6) or < with order for opioid analgesic within 24 hours of admission (Items 6a & 6b combined on CPPI) | 33 | 90.9 | 30 | 96.7 | +5.8 | 26 | 92.3 | 23 | 95.7 | +3.4 | 0.79 |
| 7. Pts. with opioid order with bowel regimen initiated (includes both laxative & stool softener) | 172 | 33.7 | 160 | 35.0 | +1.3 | 175 | 30 | 150 | 32.0 | +2 | 0.94 |
| 8. Pts. with opioids ordered who were monitored each day; a focused assessment is completed for the five most common analgesic-induced side effects (1. Respiratory depression, 2. Sedation, 3. Nausea and vomiting, 4. Constipation, 5. Delirium) | 172 | 19.2 | 160 | 19.2 | 0 | 175 | 19.4 | 150 | 17.3 | −2.1 | 0.71 |
| 9. Non-pharmacologic therapies used | 182 | 39 | 162 | 50.6 | +11.6 | 182 | 11 | 159 | 37.1 | +26.1 | 0.00 |
| 10. Focused assessments that include a review of the Pain Management Plan (PMP) | 179 | 59.9 | 157 | 49.1 | −10.8 | 177 | 61.2 | 157 | 53.3 | −7.9 | 0.69 |
| 11. Pts. with documentation of a written pain management plan | 127 | 26.2 | 143 | 20.8 | −5.4 | 166 | 39.3 | 149 | 28.4 | −10.9 | 0.57 |
An individual CPPI score is presented as a % of applicable EBPs a patient received. The higher the % the more EBPs received.
The p-values were obtained from logistic regression model using contrast Experimental Post Intervention (EPI) – Experimental Baseline (EB) –Control Post Intervention (CPI) – Control Baseline (CB), where each indicator (0 or 1) was treated as the response variable and intervention (4 categories: EPI, EB, CPI, and CB) was the only independent variable.
p-value calculated from GEE final model is reported in Table 4
To determine a total score on the CPPI, the number of points received on the applicable items for that particular patient (maximum of 11, if all items apply) is divided by the maximum score possible on all applicable items resulting in the percentage of EBPs the patient received. The higher the CPPI score the greater the percentage of EBPs the patient received. Although ideally all patients would receive each applicable pain practice 100% of the time, with input from an expert panel a target of 75% as acceptable for success on each indicator was established. Inter-rater reliability of the CPPI was established at 93% with Intra-rater reliability of 95%. A detailed description of the CPPI development and psychometrics is reported elsewhere (32).
All hospice Executive Directors or their delegates completed an organizational demographic questionnaire at baseline and post-intervention related to: 1) Organizational characteristics; 2) Staff characteristics and 3) Pain policies, procedures and available pain resources.
Mean pain severity was based on 0–10 numeric rating scale (NRS) reports of pain severity recorded in the medical record. Patient pain severity levels on a 0–10 point scale were defined as: none (0); mild (1–4), moderate (5–6), and severe (7–10) (33–34). Patients who reported pain at “0” on admission, but who had orders for pharmacologic or non-pharmacologic therapies, were included in the group with pain. For patients with only a verbal descriptor scale (VDS) report of pain, the VDS scores were converted into numeric scores by calculating the mean pain severities for each category (mild, moderate, and severe) based on all patient numeric pain severity scores in the sample. Patients with cognitive impairments who were not able to self-report pain were not included in the analysis of pain severity (n=35), as no objective measure of pain was in the MR for most patients.
A post-hoc Process Evaluation Questionnaire developed specifically for the grant was used to gather information about the TRIP-CA intervention from E hospice staff. The questionnaire used a 5-point Likert scale (1=not helpful, 5=very helpful) to rate all intervention activities and resources.
Post-intervention focus groups, conducted by a trained nurse facilitator, were guided by the Qualitative Interview Guide, a semi-structured interview tool to solicit feedback from E hospice staff on perceptions of the impact of the intervention components on the implementation process in their facility and barriers and facilitators to EBP implementation.
Data Analysis
Study aims were analyzed using descriptive statistics and linear and logistic regression models. All analyses were performed using SAS 9.2. P value of 0.05 was required for statistical significance.
Demographic characteristics of patients, nurses/physicians, and hospices were analyzed using descriptive statistics. Differences in demographic characteristics between the experimental (E) and the control (C) groups were assessed using binomial logistic regression (for variables with two categories), or multinomial logistic regression (for variables with more than two categories). In modeling the demographic characteristics of the hospices, the hospice was treated as the unit of analysis. When appropriate, the models were adjusted for overdispersion.
The pain severity of each patient was computed based on the mean pain severity of all assessments in each of three periods in their hospice stay: P1= admission, defined as the first 48 hours; P2= days 3–7; and P3= days 8–14. P-values were obtained from a proportional odds model to test if the change of the number of patients from baseline to post-intervention across three categories of pain severity (mild, moderate, and severe) was significantly different between the experimental and the control group.
The key and additional pain practice indicators were recorded as a 0/1 binary variable (reflecting whether the patient received the practice). For indicators based on multiple components, achievement on at least 75% of the components was required to receive a1. For indicators that had only one component but were completed multiple times over the 2 week period, 100% achievement was required to receive a 1. For each patient, the CPPI was calculated as the percent of key pain practice indicators received of those that were applicable to that patient. The overall CPPI score was summarized by the mean percentage across patients. In modeling the CPPI, the overall measure of EBP adoption, the patient was treated as the unit of analysis. To account for the correlation between patients within the same hospice, we used generalized estimating equations (GEE) and assumed an exchangeable working correlation structure (35).
The main explanatory variable reflecting the intervention indicates whether the patient medical record was part of the baseline data or post-intervention data and in the E or C group. Nine additional explanatory variables were considered: patient variables age and gender; hospice variables size and organizational structure; nurse variables RN education, RN certification, and RN case load; and physician variables Medical Director status and Medical Director certification. The variable race was not used due to an insufficient representation of patients in some of the categories.
To determine the final model, forward selection was performed on the initial model featuring the intervention variable only. Significant explanatory variables, along with two-way interactions between these variables and the intervention variable, were included in the final model.
The effect of the TRIP-CA intervention can be summarized by the difference between improvement on the mean CPPI from baseline to post-intervention in the E group compared to the C group. Our goal was to characterize the intervention effect after controlling for explanatory variables and accounting for baseline differences. We applied Poisson generalized linear models (GLMs) with the CPPI as the dependent variable.
Analyses of other outcomes were also conducted using the GLM/GEE framework. The normal distribution was assumed for continuous outcomes, the binomial or multinomial distribution was assumed for categorical outcomes, and the Poisson distribution for count outcomes.
RESULTS
Characteristics of Providers
Demographic characteristics of providers were comparable for the E and C groups with no significant differences at baseline and post-intervention. Differences between E sites baseline to post-intervention and C sites, baseline to post-intervention were also not significant. Details on provider demographics are available in Table 1.
Patient Characteristics
The total patient sample from baseline (T1) and post-intervention period (T2) included 738 older adults (E=370, 50.1%; C=368, 49.8%). Samples for both E and C groups represent independent samples of patients at T1 and T2. Mean age at T1 was 77.6 years (E=77.0; C=78.3) and at T2 was 78.0 years (E=78.3; C=77.7). The sample was generally cognitively intact at admission, with only 15.3% of the sample reported as having a cognitive impairment (n=113). For the patients listed as cognitively impaired (CI) at admission, 66.3% were able to self-report pain using a NRS or VDS. Of the remaining 35 patients with CI and no self-report documented, 14 (40%) had pain behaviors documented in the admission assessment but not in a consistent manner using a validated pain behavior tool. The remaining 21 (60%) patients had no pain assessment documented at admission. Demographic characteristics of the patients in the E and C groups’ were similar for both time periods with no significant differences noted (Table 2).
Table 2.
Demographic Characteristics of Patients at Baseline and Post-intervention between Experimental and Control Groups
| (N=738) | Baseline (T1) | P-value | Post-Intervention (T2) | P-value | ||
|---|---|---|---|---|---|---|
| E | C | E | C | |||
| Gender N (%)1 | 0.74 | 0.06 | ||||
| Female | 89 (44.1) | 83 (42.1) | 91 (54.2) | 71 (41.5) | ||
| Male | 113 (55.9) | 114 (57.9) | 77 (45.8) | 100 (58.5) | ||
|
| ||||||
| Age in yrs M (SD) | 77.0(7.7) | 77.3 (7.1) | 78.3 (7.3) | 77.7 (7.5) | ||
| Age by category N (%)2 | 0.21 | 0.87 | ||||
| 65–74 | 89 (44.1) | 68 (34.5) | 60 (35.7) | 63 (36.8) | ||
| 75–84 | 79 (39.1) | 88 (44.7) | 71 (42.3) | 68 (39.8) | ||
| 85 or over | 34 (16.8) | 41 (20.8) | 37 (22.0) | 40 (23.4) | ||
|
| ||||||
| Ethnicity N (%)2 | N/A | N/A | ||||
| White | 134 (66.3) | 125 (63.5) | 141 (83.9) | 136 (79.5) | ||
| Black | 20 (9.9) | 0 (0) | 12 (7.1) | 1 (0.6) | ||
| Other3 | 5 (2.5) | 2 (1.0) | 1 (0.6) | 2 (1.2) | ||
| Unknown4 | 43 (21.3) | 70 (35.5) | 14 (8.3) | 32 (18.7) | ||
P-values were calculated based on the logistic regression model (binomial) with GEE approach, where Hospice Group (E/C) was the only independent variable.
Unable to calculate P-values due to limited numbers in some groups
Pain Severity
Overall, 40% (n=295) of patients had a report of pain greater than 0 at hospice admission. Additionally, 43% (n=314) had an order for a scheduled non-opioid or opioid analgesic and reported “0” pain, so we inferred they had pain that was controlled.
Of the 738 patients in the sample, 59.5% had at least one report of pain greater than zero during the first 14 days of hospice care. Of the remaining patients, 16.1% had no pain across all assessments. The final 4.2% patients had missing data or no pain assessments documented during the first 14 days of hospice care. The initial pain severity score (first pain assessment documented) for patients with at least one pain score documented (n=439) ranged from none to severe with 35.1% of patients reporting no pain and 35.8% reporting mild pain. However, the admission pain severity score for 128 patients was at the moderate level or greater, with 15% of patients reporting moderate pain and 14.1% reporting severe pain. The last pain severity score for patients with two or more pain scores in the first fourteen days of hospice care again ranged from no pain to severe. Of the 410 patients in this group, 49.8% reported no pain on their last report of pain; 26.3% reported mild pain; 13.7% reported moderate pain; and 10.2% reported severe pain as their final pain severity rating documented during the first 2 weeks of hospice care.
Across all hospice sites, pain assessment was documented an average of 4.2 times per patient during the first two weeks of hospice care with no significant difference noted between E and C sites. The frequency of pain severity documentation ranged from 0 (4.2%) to 5 times or more (41.3%). Of the 707 patients with at least one pain score reported at some point during the two-week period, 19.6% reported severe pain at least once.
Provider Pain Practices
Table 3 provides a comparison of individual EB pain practice indicators and the overall CPPI outcome measure between E and C groups at baseline and post-intervention. Consistent across both E and C groups at baseline and post-intervention, only 30–34% of key applicable EB pain practices were received. There were few EB pain practices that more than 75% of patients for which the practice was applicable received. Practices that were more consistently evident were using a valid pain scale to assess pain, completing a primary pain assessment of pain characteristics, and administering appropriate analgesics for level of pain report. Practices that were particularly low were completing a comprehensive pain assessment, reassessment of pain within 24 hours in those with moderate/severe pain, and monitoring for most common analgesic-induced side effects.
Impact of Organizational and Provider Characteristics on Provider Practices Overall
The Poisson GEE analysis examined the impact of organizational and provider characteristics on the overall CPPI score for all hospices (Table 4). Across hospices, five variables were significantly related to CPPI score: patient age; hospice size; nurse education level; nurse certification; and nurse case load. Patients between 65 and 74 years of age had an overall mean CPPI 10% higher than patients over 85 years of age. Patients from small hospices (ADC <25) had an overall mean CPPI score 11% higher than patients from large hospices (ADC >100). Patients from medium hospices (ADC 26–100) had an overall mean CPPI score 23% lower than patients from large hospices (ADC >100). Patients from hospices with 40% or more of their nurses having at least a BSN had mean CPPI scores 6% higher than those patients from hospices with fewer nurses with BSN or higher. Patients from hospices with 20% or more of their nurses with certification in hospice/palliative care or pain management had CPPI scores 6% higher than those from hospices with fewer nurses certified. Finally, patients from hospices with nurse caseloads greater than 10 had CPPI scores 29% higher than patients from hospices with lower nurse case loads.
Table 4.
Impact of Organization and Provider Characteristics on Providers Practices measured by Cancer Pain Practice Index (CPPI)
| variables/category | estimates of coefficients in the model | p-value | CPPI comparison1 exp(est.) |
|---|---|---|---|
| Intervention2 | <0.0001 | ||
|
| |||
| Patient Age | <.0001 | ||
| <=74 | 0.0954 | <.0001 | 1.1001 |
| 75–84 | 0.0231 | 0.2215 | 1.0234 |
| >=85 | Reference | Reference | Reference |
|
| |||
| Hospice Size | <0.0001 | ||
| small | 0.1128 | <.0001 | 1.1194 |
| medium | −0.2563 | <0.0001 | 0.7739 |
| large | Reference | Reference | Reference |
| Interaction of Size & Intervention3 | <0.0001 | ||
|
| |||
| Nurse Education Level | 0.0260 | ||
| %BSN or above ≥ 0.4 | 0.0583 | 0.0260 | 1.0600 |
| %BNS or above < 0.4 | Reference | Reference | Reference |
| Interaction of Nurse Education | |||
| Level & Intervention3 | <0.0001 | ||
|
| |||
| Nurse Certification | <0.0001 | ||
| %Certification ≥ 0.2 | 0.0628 | <0.0001 | 1.0648 |
| %Certification < 0.2 | Reference | Reference | Reference |
| Interaction of Nurse Certification & Intervention3 | <0.0001 | ||
|
| |||
| Nurse Case Load | <.0001 | ||
| > 10 | 0.2483 | <.0001 | 1.2878 |
| ≤ 10 | Reference | Reference | Reference |
| Interaction of Case Load & Intervention3 | <.0001 | ||
|
| |||
| Contrast | |||
| EPI – EB4 | 0.0529 | 0.3752 | 1.0543 |
| CPI – CB5 | 0.3877 | 0.0391 | 1.4736 |
|
| |||
| (EPI – EB) – (CPI – CB)6 | −0.3348 | 0.0615 | 0.7155 |
Ratio of the mean CPPI for a particular group relative to the mean CPPI for the reference group
The Intervention variable is based on four categories: Experimental Baseline (EB) – baseline data for the experimental group, Experimental Post Intervention (EPI) – post-intervention data for the experimental group, Control Baseline (CB) – baseline data for the control group, and Control Post Intervention (CPI) – post-intervention data for the control group.
Interactions test whether the effect of a variable on the mean CPPI depends on the category of the Intervention variable.
The contrast (EPI – EB) tests the improvement of the mean CPPI from baseline to post intervention in the experimental group.
The contrast (CPI – CB) tests the improvement of the mean CPPI from baseline to post intervention in the control group.
The contrast (EPI – EB) – (CPI – CB) compares the improvements on the mean CPPI from baseline to post intervention in the experimental group and in the control group.
The following variables showed no significant relationship to the CPPI: patient gender, patient race, organizational structure (independent agency vs. part of a larger organization), Medical Director’s employment status (volunteer, part-time paid or full-time paid), and Medical Director Certification (certification in hospice & palliative care; pain management; other).
Significant variables were included in the final GEE modeling to address the research questions (Table 4).
Hypothesis 1. We hypothesized that following the implementation of the TRIP-CA intervention nurses and physicians at the E hospice sites would show a greater increase in the adoption of EPB for pain than those in the C group
The contrast tests in the final modeling (Table 4) suggest that while both the E and C groups showed improvement from baseline to post-intervention on the CPPI, there was no significant difference in change on the CPPI between E & C groups in our primary modeling when controlling for explanatory variables (P=.06). Medium sized E hospices did show a greater improvement in CPPI mean score than medium sized C sites. However, both small and large C hospices had a greater improvement on the CPPI score than their E counterparts. Both the E and C groups had high and low performing hospices, based on the change in the mean CPPI score from baseline to post intervention. In the E group, 50% of the eight sites showed improvement on the mean CPPI from baseline to post intervention. In the C group, 62% of the eight sites showed improvement on the mean CPPI from baseline to post-intervention. Table 3 provides information about the overall CPPI outcomes and individual CPPI indicators for E compared to C groups.
Hypothesis 2. We hypothesized that following the implementation of the TRIP-CA intervention, mean pain severity ratings for older patients with cancer admitted to E hospices would be lower at two time periods following hospice admission (P2=3–7 days and P3=8–14 days) compared to C group
Change in pain severity means from baseline to post-intervention in the E and C groups for patients with at least one pain score documented show consistent greater decreases in the E group at P1 (1st 48 hours) (1.95 to 1.80), P2 (1.61 to 1.38) and P3 (1.62 to 1.43), compared to C group in which pain severity increased at P1 (1.54 to 1.69) and P3 (1.24 to 1.58), and decreased at P2 (1.64 to 1.42). A linear regression GEE analysis, controlling for hospice-specific cluster effects, showed decreases in pain severity from baseline to post intervention during the 2nd week of hospice and in the last pain severity rating in those with 2 or more pain scores: however, the results were not statistically significant (See Table 5).
Table 5.
Change in pain severity reports during the first 14 days of hospice care between Experimental & Control groups from baseline to post-intervention.
| Item Description | Total | Experimental | Control | p- value | ||
|---|---|---|---|---|---|---|
| baseline | post | baseline | post | |||
| Mean last pain severity rating for patients with 2 or more pain scores | n 410 | 100 | 98 | 109 | 103 | 0.41451 |
| mean 2.30 | 2.64 | 2.51 | 2.72 | 2.16 | ||
| SD 2.86 | 2.81 | 2.90 | 1.91 | 2.99 | ||
| Mean pain severity at the 2nd week2 | ||||||
| n 296 | 70 | 72 | 80 | 74 | 0.10321 | |
| mean 2.19 | 2.42 | 2.12 | 1.99 | 2.24 | ||
P-values were calculated based on the linear regression model with the GEE approach
For patients who had at least one assessment with non-zero pain intensity
There were a relatively small percentage of patients experiencing moderate to severe pain at baseline making it difficult to detect a difference post-intervention. The changes in pain severity were small and not likely to be clinically significant.
Post Intervention Data About The TRIP-CA Intervention
Of the activities and resources listed on the Process Evaluation Questionnaire as part of the TRIP-CA intervention, 16 received a mean score of 4.5 or greater (5=very helpful). The top 5 activities/resources identified by E staff as being most helpful in implementing EBP for cancer pain in older adults included: 1) implementing standard pain assessment tools, 2) involvement of a local Pain Facilitator, 3) the Train-the-Trainer Program, 4) laminated pain tools, and 5) audit and feedback. Conversely, the five activities/resources identified as the least helpful to EBP implementation (scoring less than 3.75) were: 1) Involvement of Physician Champion, 2) Implementing motivational initiatives, 3) Marketing/staff Incentive- Pocket Calculator, 4) Press Releases to use for hospice newsletters, local papers, etc., and 5) Key Elements Handout for Physicians. Post-intervention focus groups provided feedback useful in interpreting study findings with details provided elsewhere (15).
DISCUSSION
Although the TRIP-CA intervention resulted in significant improvements on selected practice indicators for the experimental group, there was not a significant intervention effect on the overall provider adoption score, the CPPI. The variations between hospices suggest there may be location specific factors important to the implementation and dissemination of EBP’s and their adoption that were not controllable through the explanatory variables identified. The process of adopting a complex practice change with numerous recommendations is challenging (8–10). The difficulties in changing established health care practices have been well documented in the acute care setting (13, 36–41). However, less literature is available on implementing EBP in community-based settings, including hospice(8, 42–43). The findings that fewer than 35% of applicable EBPs are received and that 24% of patients reported moderate to severe pain as their last documented pain score reinforces the need for continued efforts to address pain challenges in this setting caring for older adults with cancer pain.
In evaluating the effect of the TRIP-CA intervention, a number of factors must be considered: the unique characteristics and culture of the hospice setting, the complexity of the EBP topic that is being promoted, and competing priorities of the practice sites. The decentralized environment of hospice made it challenging to communicate all components of the TRIP-CA intervention directly to the staff (RN’s, physicians) implementing the EBPs for pain. EBP information and suggestions for implementation strategies were often provided to the hospice Pain Facilitator and Nurse Champion with limited influence over how this information was shared with other staff. Consequently, there were considerable variances across hospice settings with other information and resource sharing, such as expert outreach advice, additional training, and communication of audit and feedback data with front line staff. A knowledge gap related to the overall understanding of EBP by staff in the hospice settings was also identified in the post intervention focus groups. Future efforts should include education of local staff on what EBP is, the benefits of EBP and its contribution to improved patient care.
Barriers identified during the post-intervention focus groups may have had a considerable impact on the implementation process. Some of the confounding factors identified were not anticipated (e.g. natural disasters impacting a number of the sites and repeated turnover of local project leaders). However, developing a plan that includes strategies for dealing with potential barriers to implementation may serve as a road map for future studies. A detailed description of barriers and facilitators identified in the TRIP-CA study is reported elsewhere (15).
Another factor that must be considered when reviewing the impact of the TRIP-CA intervention is the complexity of the innovation (e.g. practice change). Collectively, the evidence-based guidelines serving as the foundation for EBP recommendations resulted in 48 indicators of best practice. As part of tailoring to one’s organization, we did not prescribe what core practices were to be addressed, rather encouraged each hospice to examine their baseline data and establish their own priorities for change. While the Audit and Feedback activity presented site-specific feedback on the 48 indicators of best practice and was rated as being very helpful, the number and scope of practice recommendations may have impacted the priority areas chosen by the hospices and the practice changes observed. The 11-item overall adherence outcome measure (CPPI) was developed simultaneously with the intervention implementation and may have been a better approach for sharing on-going audit and feedback data in a more focused, concise format (32). Other recent implementation research supports better outcomes with simple practice changes and the length of time for implementation of complex practices takes longer than for simple practices (17,29, 44). For complex innovations, a staging of practice changes may provide better outcomes.
While the E site staff indicated that having a Pain Facilitator at each site was instrumental to the implementation process, the format for selecting these project leaders should be considered. In the current study, each E site was allowed to “select/appoint” a nurse Pain Facilitator and the Nurse/Physician Champions for their hospice based on a set of criteria/characteristics that included important qualities needed in these roles (45–46). In the hospice setting, there were often few nurses or physicians from which to select for these important roles. It is unknown if individuals chosen were viewed as “leaders” by their peers or if enough time to complete the additional duties of the position was provided. Future research should address these potential limitations.
A challenge for implementation science research is identifying methods for measuring fidelity to the implementation intervention (47–49). Our study did not prospectively measure the fidelity to each of the TRIP-CA intervention components, although the variability in hospice outcomes from the TRIP-CA intervention suggests this may provide valuable information about the strategies used to promote change. Anecdotally, we observed considerable variance among hospices regarding their use of key activities and resources provided as part of the intervention. For example, all E site Pain Facilitators and Change Champions received their organization’s performance gap assessment data prior to the implementation phase of the study and audit and feedback data every two months during the intervention phase. However, dissemination of this information to end users varied. Another area of variance in this study was seen in the level of participation in the Train-the-Trainer (TTT) Program offered to the E sites during the study Engagement phase. Each E site was encouraged to send three representatives, specifically their Pain Facilitator (PF), Nurse Champion (NC), and Physician Champion (PC) to the 3-day TTT program provided by the research team and other expert consultants. All 8 E sites sent at least one representative, with only two sites sending their complete local leadership team. While participants rated this activity as helpful, it remains unclear what mechanisms were used to share the information gained at the TTT program at each local site.
Another intervention component with significant variation was the interaction with the Expert Nurse during Outreach Consultation. While all E sites received 10 site visits, the interaction with hospice staff during those visits differed based on the requests of the sites. Some E hospices requested the Expert Nurse interact with all their nurses during staff meetings or training sessions, while others preferred to have only the Pain Facilitator or Nurse Champion meet with the Expert Nurse. Thus, the level of engagement of the outreach nurse with hospice staff was variable across the E sites. Additionally, engagement of physicians in the implementation process was difficult as noted by the ratings of nurses and other non-physician hospice staff on the process evaluation questionnaire. Items related to physician involvement were all rated as least helpful in implementing the intervention and also mentioned in the post intervention focus groups as barriers to implementation (15). The issue of engaging physicians was also noted in the prior TRIP study conducted in the acute care setting (13).
Understanding implementation intervention fidelity, the degree to which an intervention is delivered as designed, is important in evaluating the effectiveness of an intervention (50–51). A key challenge with implementation research is how to promote adoption of EBPs with tailoring to the individual organization, while taking into account the variability of how implementation components are enacted at each E site. This does, however, reflect real-world practices and needs to be considered in effectiveness studies.
To our knowledge, this is the first clustered RCT to test the effectiveness of an implementation intervention for promoting adoption of evidence-based pain management practices in hospice settings. While success of the TRIP-CA intervention in overall provider adoption of best practices was not demonstrated, the intervention did impact patient pain severity ratings, selected pain practices, and qualitative feedback provided insights into the usefulness of the various TRIP-CA intervention components. The study contributed to knowledge regarding issues hospices should consider when implementing a specific practice change and valuable data on what components of the TRIP-CA intervention were considered helpful by users in implementing EBP for pain management in the hospice setting.
Limitations
The sensitivity of the CPPI to detect change in provider practices was not established apriori, although individual practice indicators provided similar outcome findings as the summative score on the CPPI. Further study of the best approaches to measure provider practices is warranted. The focus of this study was pain in older adults with cancer in community-based hospice settings and thus cannot be generalized to other community-based settings.
The use of medical record (MR) data as the source to determine pain assessment and management practices is a potential limitation of the data collection. However, a number of studies have used this method effectively (3,13, 52). Other approaches including direct observation, videotaped observation, and audio-taped sessions were considered but each has limitations and is not feasible in a large implementation study (50). Due to cost, potential for bias in direct observation, and the role of MRs as the regulatory and legal foundation for provision of care, we determined MR abstraction was the best option for this study. Assuring quality and accurate documentation of provider practices is an issue that needs attention for research in hospices and for clinical practice.
Inability to measure or assure consistent engagement with each of the intervention components impacts understanding of the intervention effect on outcomes. Because implementation interventions involve multiple users and multiple strategies in real-world settings, this gap in knowledge is of particular import for future study.
Conclusions and Recommendations for Future Research
The TRIP-CA Intervention was not successful in significantly changing provider practices across a group of selected key pain practices for management of cancer pain in older adults in community hospices. Considering that patients received less than 35% of the recommended EBPs that were applicable to their circumstances, there is need for continued efforts to address pain practice in this setting of care
It is clear from this study that differences in practice settings can have a considerable impact on the outcome of an implementation intervention. The hospice setting offers contextual and operational factors unique from acute care that impact approaches to translating EBPs into consistent use. Future research should focus on determining what factors specific to community-based hospice should be targeted when implementing an EBP intervention in this setting. It is equally important to consider concurrently measuring fidelity to the implementation intervention and examining factors impacting fidelity in the interpretation of study outcomes.
The conduct of multi-component practice change interventions is research intensive and may not be a model that is replicable on a broad scale in community-based settings, such as hospice. Future research should explore intervention approaches that rely less on research team members and can be more fully implemented by organizational staff. Consideration of use of technology to address identified barriers could have benefit.
Acknowledgments
This study supported by: National Cancer Institute Grant R01CA115363
Special thanks to Patricia McNichol, RN, BSN; Kimberly Bergen-Jackson, RN, PhDc; Melissa Lehan Mackin, RN, PhDc; Jimmy Reyes, RN, DNP, PhDc; and Cate Fiala, RN, MSN for data collection and medical record abstraction.
Contributor Information
Keela Herr, University of Iowa, College of Nursing Iowa City, Iowa.
Marita Titler, University of Michigan, School of Nursing Ann Arbor, Michigan.
Perry G. Fine, University of Utah, School of Medicine Pain Research Center Salt Lake City, Utah.
Sara Sanders, University of Iowa, School of Social Work Iowa City, Iowa.
Joseph E. Cavanaugh, University of Iowa, Department of Biostatistics and College of Public Health Iowa City, Iowa.
John Swegle, College of Pharmacy, University of Iowa Mercy Family Medicine Residency Mason City, Iowa.
Xiongwen Tang, University of Iowa Iowa City, Iowa.
Chris Forcucci, University of Iowa, College of Nursing Iowa City, Iowa.
References
- 1.Cavalieri TA. Management of pain in older adults. J Am Osteopath Assoc. 2005;105(3 Suppl 1):S12–7. [PubMed] [Google Scholar]
- 2.Fine PG. Chronic pain management in older adults: special considerations. J Pain Symptom Manage. 2009;38(2 Suppl):S4–S14. doi: 10.1016/j.jpainsymman.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Herr K, Titler M. Acute pain assessment and pharmacological management practices for the older adult with a hip fracture: review of ED trends. J Emerg Nurs. 2009;35(4):312–320. doi: 10.1016/j.jen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Herr K. Pain in the older adult: an imperative across all health care settings. Pain Manag Nurs. 2010;11(2 Suppl):S1–10. doi: 10.1016/j.pmn.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Hollenack KA, Cranmer KW, Zarowitz BJ, O’Shea T. The application of evidence-based principles of care in older persons (issue 4): pain management. J Am Med Dir Assoc. 2007;8(3 Suppl 2):e77–85. doi: 10.1016/j.jamda.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Strassels SA, Blough DK, Hazlet TK, Veenstra DL, Sullivan SD. Pain, demographics, and clinical characteristics in persons who received hospice care in the United States. J Pain Symptom Manage. 2006;32(6):519–531. doi: 10.1016/j.jpainsymman.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine: How to practice and teach EBM. London: Churchill Livingstones; 2000. [Google Scholar]
- 8.Jones KR, Fink R, Vojir C, et al. Translation research in long-term care: improving pain management in nursing homes. Worldviews Evid Based Nurs. 2004;1(Suppl 1):S13–20. doi: 10.1111/j.1524-475X.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- 9.Berkhout AJ, Boumans NP, Mur I, Nijhuis FJ. Conditions for successfully implementing resident-oriented care in nursing homes. Scand J Caring Sci. 2009;23(2):298–308. doi: 10.1111/j.1471-6712.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 10.Lenz BK, Barnard P. Advancing evidence-based practice in rural nursing. J Nurses Staff Dev. 2009;25(1):E14–9. doi: 10.1097/NND.0b013e318194b6d0. [DOI] [PubMed] [Google Scholar]
- 11.Titler MG. Translation science and context. Res Theory Nurs Pract. 2010;24(1):35–55. doi: 10.1891/1541-6577.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Herr K, Titler M, Fine P, et al. Assessing and treating pain in hospices: current state of evidence-based practices. J Pain Symptom Manage. 2010;39(5):803–819. doi: 10.1016/j.jpainsymman.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Titler MG, Herr K, Brooks JM, et al. Translating research into practice intervention improves management of acute pain in older hip fracture patients. Health Serv Res. 2009;44(1):264–287. doi: 10.1111/j.1475-6773.2008.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks JM, Titler MG, Ardery G, Herr K. Effect of evidence-based acute pain management practices on inpatient costs. Health Serv Res. 2009;44(1):245–263. doi: 10.1111/j.1475-6773.2008.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders S, Mackin ML, Reyes J, et al. Implementing evidence-based practices: considerations for the hospice setting. Am J Hosp Palliat Care. 2010;27(6):369–376. doi: 10.1177/1049909109358695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Titler MG, Everett LQ. Translating research into practice. Considerations for critical care investigators. Crit Care Nurs Clin North Am. 2001;13(4):587–604. [PubMed] [Google Scholar]
- 17.Rogers E. Diffusion of innovations. 5. New York: Free Press; 2003. [Google Scholar]
- 18.Doumit G, Gattellari M, Grimshaw J, O’Brien M. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD000125.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Simpson F, Doig GS. The relative effectiveness of practice change interventions in overcoming common barriers to change: a survey of 14 hospitals with experience implementing evidence-based guidelines. J Eval Clin Pract. 2007;13(5):709–715. doi: 10.1111/j.1365-2753.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 20.Locock L, Dopson S, Chambers D, Gabbay J. Understanding the role of opinion leaders in improving clinical effectiveness. Soc Sci Med. 2001;53(6):745–757. doi: 10.1016/s0277-9536(00)00387-7. [DOI] [PubMed] [Google Scholar]
- 21.Harvey G, Loftus-Hills A, Rycroft-Malone J, et al. Getting evidence into practice: the role and function of facilitation. J Adv Nurs. 2002;37(6):577–588. doi: 10.1046/j.1365-2648.2002.02126.x. [DOI] [PubMed] [Google Scholar]
- 22.Fraser I. Translation research: where do we go from here? Worldviews Evid Based Nurs. 2004;1 (Suppl 1):S78–83. doi: 10.1111/j.1524-475X.2004.04046.x. [DOI] [PubMed] [Google Scholar]
- 23.Fraser I. Organizational research with impact: working backwards. Worldviews Evid Based Nurs. 2004;1 (Suppl 1):S52–9. doi: 10.1111/j.1524-475X.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandstroma B, Borglin G, Nilsson R, Willman A. Promoting the Implementation of Evidence-Based Practice: A Literature Review Focusing on the Role of Nursing Leadership. Worldviews Evid Based Nurs. 2011 doi: 10.1111/j.1741-6787.2011.00216.x. [DOI] [PubMed] [Google Scholar]
- 25.VanDeusen Lukas C, Engle RL, Holmes SK, et al. Strengthening organizations to implement evidence-based clinical practices. Health Care Manage Rev. 2010;35(3):235–245. doi: 10.1097/HMR.0b013e3181dde6a5. [DOI] [PubMed] [Google Scholar]
- 26.Titler MG. The Evidence for Evidence-Based Practice Implementation. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research & Quality; 2008. pp. 113–161. [PubMed] [Google Scholar]
- 27.Baier RR, Gifford DR, Patry G, Banks SM, Rochon T, DeSilva D, et al. Ameliorating pain in nursing homes: A collaborative quality-improvement project. Journal of the American Geriatrics Society. 2004;52(12):1988–1995. doi: 10.1111/j.1532-5415.2004.52553.x. [DOI] [PubMed] [Google Scholar]
- 28.Dulko D, Hertz E, Julien J, Beck S, Mooney K. Implementation of cancer pain guidelines by acute care nurse practitioners using an audit and feedback strategy. Journal of the American Academy of Nurse Practitioners. 2010;22(1):45–55. doi: 10.1111/j.1745-7599.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 29.Herr K, Bjoro K, Steffensmeier J, Rakel B. Evidence-based practice guideline: Acute Pain Management in Older Adults. 2006. Revised 2006 ed. [DOI] [PubMed] [Google Scholar]
- 30.Miaskowski C, Cleary J, Burney R, et al. Guideline for the Management of Cancer Pain in Adults and Children. 2005. [Google Scholar]
- 31.National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care. Kans Nurse. 2004;79(9):16–20. [PubMed] [Google Scholar]
- 32.Fine P, Herr K, Titler M, et al. The cancer pain practice index: a measure of evidence-based practice adherence for cancer pain management in older adults in hospice care. J Pain Symptom Manage. 2010;39(5):791–802. doi: 10.1016/j.jpainsymman.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li KK, Harris K, Hadi S, Chow E. What should be the optimal cut points for mild, moderate, and severe pain? J Palliat Med. 2007;10(6):1338–1346. doi: 10.1089/jpm.2007.0087. [DOI] [PubMed] [Google Scholar]
- 34.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 35.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 36.Pierson DJ. Translating evidence into practice. Respir Care. 2009;54(10):1386–1401. [PubMed] [Google Scholar]
- 37.Van Peppen RP, Maissan FJ, Van Genderen FR, Van Dolder R, Van Meeteren NL. Outcome measures in physiotherapy management of patients with stroke: a survey into self-reported use, and barriers to and facilitators for use. Physiother Res Int. 2008;13(4):255–270. doi: 10.1002/pri.417. [DOI] [PubMed] [Google Scholar]
- 38.Weinert CR, Mann HJ. The science of implementation: changing the practice of critical care. Curr Opin Crit Care. 2008;14(4):460–465. doi: 10.1097/MCC.0b013e3283079eb5. [DOI] [PubMed] [Google Scholar]
- 39.Sinuff T, Kahnamoui K, Cook DJ, Giacomini M. Practice guidelines as multipurpose tools: a qualitative study of noninvasive ventilation. Crit Care Med. 2007;35(3):776–782. doi: 10.1097/01.CCM.0000256848.47911.77. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson N, Rutledge D, Geiser K. Role of the External Coach in Advancing Research Translation in Hospital-Based: Performance Improvement. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 2: Culture and Redesign) Rockville (MD): 2008. [PubMed] [Google Scholar]
- 41.Bingham M, Ashley J, De Jong M, Swift C. Implementing a unit-level intervention to reduce the probability of ventilator-associated pneumonia. Nurs Res. 2010;59(1 Suppl):S40–7. doi: 10.1097/NNR.0b013e3181c3bffc. [DOI] [PubMed] [Google Scholar]
- 42.Feldman PH, Murtaugh CM, Pezzin LE, McDonald MV, Peng TR. Just-in-time evidence-based e-mail “reminders” in home health care: impact on patient outcomes. Health Serv Res. 2005;40(3):865–885. doi: 10.1111/j.1475-6773.2005.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kutner J, Smith M, Mellis K, Felton S, Yamashita T, Corbin L. Methodological challenges in conducting a multi-site randomized clinical trial of massage therapy in hospice. J Palliat Med. 2010;13(6):739–744. doi: 10.1089/jpm.2009.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterns S, Miller SC, Allen S. The complexity of implementing culture change practices in nursing homes. J Am Med Dir Assoc. 2010;11(7):511–518. doi: 10.1016/j.jamda.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ploeg J, Skelly J, Rowan M, et al. The Role of Nursing Best Practice Champions in Diffusing Practice Guidelines: A Mixed Methods Study. Worldviews Evid Based Nurs. 2010 doi: 10.1111/j.1741-6787.2010.00202.x. [DOI] [PubMed] [Google Scholar]
- 46.Greenhalgh T, Robert G, Bate P, et al. Diffusion of innovations in health service organizations: A systematic literature review. Malden, MA: Blackwell; 2005. [Google Scholar]
- 47.Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol. 2008;41(3–4):327–350. doi: 10.1007/s10464-008-9165-0. [DOI] [PubMed] [Google Scholar]
- 48.Proctor E, Silmere H, Raghavan R, et al. Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Adm Policy Ment Health. 2010 doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson DK, Griffin S, Saunders RP, Kitzman-Ulrich H, Meyers DC, Mansard L. Using process evaluation for program improvement in dose, fidelity and reach: the ACT trial experience. Int J Behav Nutr Phys Act. 2009;6:79. doi: 10.1186/1479-5868-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breitenstein SM, Gross D, Garvey CA, Hill C, Fogg L, Resnick B. Implementation fidelity in community-based interventions. Res Nurs Health. 2010;33(2):164–173. doi: 10.1002/nur.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada J, Stevens B, Sidani S, Watt-Watson J, de Silva N. Content validity of a process evaluation checklist to measure intervention implementation fidelity of the EPIC intervention. Worldviews Evid Based Nurs. 2010;7(3):158–164. doi: 10.1111/j.1741-6787.2010.00182.x. [DOI] [PubMed] [Google Scholar]
- 52.Ardery G, Carter BL, Milchak JL, et al. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens (Greenwich) 2007;9(2):113–119. doi: 10.1111/j.1524-6175.2007.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]