Abstract
In a previous study, we showed that Spiroplasma, a maternally transmitted endosymbiotic bacterium of Drosophila hydei, enhances larval to adult survival of its host when exposed to oviposition attack by the parasitoid wasp Leptopilina heterotoma. The mechanism by which Spiroplasma enhances host survival has not been elucidated. To better understand this mechanism, we compared the growth of wasp larvae in Spiroplasma-infected and uninfected hosts. Our results indicate that wasp embryos in Spiroplasma-infected hosts hatch and grow normally for ~2 days, after which their growth is severely impaired, compared to wasps developing in uninfected hosts. Thus, despite their reduced ability to complete development in Spiroplasma-infected hosts, developing wasps may exert fitness costs on their hosts that are manifested after host emergence. The severity of these costs will influence the degree to which this protective mechanism contributes to the long-term persistence of Spiroplasma in D. hydei. We therefore examined survival to 10-day-old adult stage and fecundity of Spiroplasma-infected flies surviving a wasp treatment. Our results suggest detrimental effects of wasp attack on longevity of Spiroplasma-infected adult flies. However, compared to Spiroplasma-free flies exposed to wasps, Spiroplasma-infected flies exposed to wasps have ~5 times greater survival from larva to 10 day-adult. The relative fecundity of wasp-attacked Spiroplasma-infected females was ~71% that of un-attacked Spiroplasma-free females. Our combined survival and female fecundity results suggest that under high wasp parasitism, the reproductive fitness of Spiroplasma-infected flies may be ~3.5 times greater than that of uninfected females, so it is potentially relevant to the persistence of Spiroplasma in natural populations of D. hydei. Interestingly, Spiroplasma-infected males surviving a wasp attack were effectively sterile during the 3-day period examined. This observation is consistent with the expectation that, as a maternally transmitted symbiont, there is little selective pressure on Spiroplasma to enhance the reproductive fitness of its male hosts.
Keywords: Defensive mutualism, Endosymbiont, Vertical transmission, Leptopilina, Drosophila hydei, Mollicutes
Introduction
Maternally transmitted endosymbiotic bacteria are frequently associated with insects (Moran et al. 2008). Some of these heritable symbioses are obligate (i.e., host and symbiont depend on each other for survival and reproduction), and usually have perfect maternal transmission. In contrast, many heritable symbioses are more labile and regarded as facultative, because the host does not generally require them for survival and reproduction, and because maternal transmission is usually imperfect. In the absence of horizontal or paternal transmission, persistence of such facultative symbionts requires the ability of infected females to produce infected daughters to be greater than the ability of uninfected females to produce uninfected daughters (Bull 1983). Therefore, this relative fitness, also known as “parasite host fitness” (Ebbert 1991), must be greater than one. To achieve this, the symbiont can manipulate the reproduction of its host to its advantage (e.g., male-killing and cytoplasmic incompatibility) or provide fitness benefits to the host. Reproductive manipulators are widespread and include many of the strains of Wolbachia (Alphaproteobacteria) associated with arthropods (Hilgenboecker et al. 2008), as well as members of several distantly related bacterial taxa (e.g., Bacteroidetes, Mollicutes, Flavobacteria, and Gammaproteobacteria; Moran et al. 2008). Nevertheless, not all inherited facultative symbionts are reproductive parasites. Furthermore, recent studies suggest that fitness benefits conferred by inherited bacteria to their hosts in the form of defense against natural enemies may also be quite common and relevant to the persistence of heritable symbionts (Glaser and Meola 2010; Hedges et al. 2008; Jaenike et al. 2010b; Kaltenpoth et al. 2005; Kellner 2002; Oliver et al. 2003; Teixeira et al. 2008; Xie et al. 2010).
In a previous study (Xie et al. 2010), we showed that Spiroplasma (class Mollicutes), a bacterial endosymbiont of Drosophila hydei, enhances larva-to-adult survival of flies that have been subjected to oviposition attack by the solitary endo-parasitic wasp Leptopilina heterotoma, which attacks the early instar larval stages of many Drosophila spp. (Carton et al. 1986). We also showed that oviposition frequency does not differ between Spiroplasma-infected and uninfected hosts (Xie et al. 2010). Thus, this protection is exerted after wasp oviposition, but the mechanism by which the presence of Spiroplasma reduces wasp success and enhances fly larva to adult survival has not been investigated. Several mechanisms of protection by Spiroplasma are possible: (a) reduced availability of resources necessary for wasp development (if Spiroplasma consumes resources that would otherwise be available for the parasitoid); (b) the presence of a Spiroplasma-encoded substance toxic to the parasitoid (e.g., the toxin produced by Hamiltonella defensa, the defensive endosymbiont of aphids; Oliver et al. 2009); and/or (c) an enhanced immune response of the fly larva against the parasitoid (e.g., by countering the encapsulation-suppressive effect of the parasitoid venom or by increasing production of lamellocytes). To better understand the protective mechanism, herein we investigate the fate of developing wasps in Spiroplasma-infected fly larvae, by comparing wasp growth in Spiroplasma-infected and uninfected fly larvae. As wasp larvae feed initially on host hemolymph and later on host tissues (Carton et al. 1986), the stage at which wasps die or stop developing, as a result of Spiroplasma presence must influence the degree of damage caused to the host. Indeed, castration of hosts surviving a parasitoid attack has been reported in several host-parasitoid associations (reviewed in Beckage and Gelman 2004; Le Ralec et al. 2010). Therefore, the reproductive fitness of Spiroplasma-infected wasp-attacked flies that reach adulthood must be examined to determine whether parasite-host fitness is greater than one. Here we examine the reproductive fitness of Spiroplasma-infected flies that survive a wasp attack by measuring their survival to 10 days post-emergence, as well as their fecundity. We chose 10 days post-emergence as a cutoff, because most D. hydei males reach maturity by 10 days (Markow and O'Grady 2005) and most females, the relevant sex for Spiroplasma transmission and persistence, mature by ~3 days post-emergence (Markow 1985; Pitnick and Markow 1994), allowing for about 1 week's worth of female reproductive output.
Materials and methods
Fly strains, Spiroplasma infection treatments, and fly rearing
We used three of the ten Drosophila hydei isofemale lines used in Xie et al. (2010), which were collected in College Station, Texas, USA, in 2008 (isolines 6; 17; and 34). All experiments were carried out approximately 1 year after artificial infection or antibiotic treatment of Drosophila hydei to generate Spiroplasma-infected and Spiroplasma-free controls. Throughout this study, flies were maintained on Banana-Opuntia medium at 25°C and 12:12 h light:dark regime (vial dimensions = 23 × 95 mm, ~6 mL of fly media). Artificial infection of uninfected flies was performed via adult-to-adult hemolymph microinjection using pulled microcapillaries and a manual microinjector. Infection status of the artificially infected flies was confirmed via Spiroplasma-specific PCR and/or examination of hemolymph under dark field microscopy. PCR screening for Spiroplasma was carried out on whole fly DNA extracts with Spiroplasma-specific primers for gene p58 (p58IV_F and p58IV_R; PCR cycling conditions: 35 cycles of 20 s at 94°C; 45 s at 53°C; and 55 s at 72°C; Xie et al. 2010). Infection of these fly strains by other bacterial endosymbionts including Wolbachia was ruled out in previous studies (Mateos et al. 2006; Xie et al. 2010). All PCR reactions were carried out with appropriate negative (water or DNA extraction buffer) and positive controls (known Spiroplasma-positive DNA extracts).
Prior to all experiments, flies were maintained at low-density larval conditions (~30 larvae/vial). Virgin female flies (≤10-days-old) were individually placed in a fresh food vial with two mature (≥10 day-old) Spiroplasma-free males (from its own strain), and allowed to mate and oviposit for 2 days. Females and males were then removed, and females were PCR-screened for Spiroplasma infection status. Approximately 30 first-to-second instar larvae were collected from each mating vial and transferred to a fresh food vial. Five experienced female wasps (i.e., wasps that had been allowed to oviposit on D. melanogaster larvae prior to experiment) were placed in the vial with larvae and removed 2 days later. We used a single inbred highly virulent L. heterotoma wasp strain known as Lh14 (Schlenke et al. 2007) for all experiments; the same strain used in our previous study (Xie et al. 2010).
Wasp growth rate
To examine whether Spiroplasma infection state had an effect on wasp larval growth rate, we measured body length of wasp larvae from Spiroplasma-infected and Spiroplasma-free fly larvae. We placed ~30 first instar fly larvae per vial with five female wasps as described above. After 2 days, wasps were removed (hereafter hour 0 post-attack). At several time points post-attack (i.e., every 2 days: 48, 96, 144 192 h), wasp larvae were isolated, fixed in ~100% ethanol, and immediately digitally photographed under a dissecting scope using a stage micrometer (0.01 mm scale). We used Spot Basic (version 4.7; Diagnostic Instruments, Inc., Sterling Heights, MI) to measure body length as the straight-line distance between tip of the mouth and caudal end (excluding the caudal appendage). At 48 and 96 h, the flies were in the larval stage, whereas at 144 and 192 h, the flies were in the pupal stage. Later stages were not examined because wasps that develop successfully usually exit the host body at this stage, making it difficult to maintain integrity of wasp body during isolation. We examined wasp body length from Spiroplasma-infected and Spiroplasma-free fly larvae from three fly strains (isolines). We used 1–3 replicates (vials) per isoline per infection state. For each replicate, we removed and dissected 2–11 fly larvae at each time point. A General Linear Mixed Model (GLMM, SAS Enterprise Guide) was used to examine the effect of infection state (fixed), hours post-wasp attack (fixed as a repeated effect), their two-way interaction, and fly strain (random). The repeated effect was subjected to Isoline*Repeat*Pseudoreplicate nested within infection state. All the random interactions were removed from the reported model because in the full model, they were not statistically significant.
Adult fly survival
To determine whether wasp attack had a detrimental effect on fly post-emergence survival, we compared fly survival to 10-days after emergence in: Spiroplasma-infected surviving a wasp treatment (“SW”); Spiroplasma-infected flies not subjected to wasp treatment (“S”); and Spiroplasma-free flies not subjected to wasp treatment (“U”). The Spiroplasma-free and wasps present (“UW”) treatment was omitted because their larva-to-adult survival is extremely low (~4%; Xie et al. 2010), leaving very few survivors on which to examine post-emergence survival and fecundity. Furthermore, it is possible that the few flies that emerge in the UW treatment, are larvae that were not attacked, because under similar experimental conditions, ~5% of flies are not attacked regardless of their Spiroplasma-infection state (Xie et al. 2010).
We performed three replicates per treatment per fly strain; each replicate corresponded to a separate vial. SAS Enterprise Guide version 4.2 statistical package was used to fit a Generalized Linear Mixed Model (GzLMM) with a binomial distribution of the raw data for several fly survival measures between the larval stage and 10-days post emergence. The independent variables were fly isoline (random) and treatment (fixed: “U”; “S”; and “SW”). Significance tests of random effects were based on the ratio of pseudo-likelihoods.
Surviving fly fecundity
Our fecundity assays were performed on surviving flies from all three Spiroplasma/wasp treatments described above. Flies emerging from the survival assays were collected daily and transferred to fresh vials keeping each sex separate. To examine female fecundity, each 10-day-old female was paired in a fresh vial with two Spiroplasma-free males (>10-dayold) from its own strain and allowed to mate and oviposit for 24 h. Mating trios were transferred to fresh food vials every 24 h for at least three consecutive days. We performed at least nine replicates per treatment; each replicate corresponded to a separate vial. Female fecundity was measured as the average number of eggs laid per day over three consecutive days, starting on the first day that eggs were present. A General Linear Mixed Model (GLMM) was used to compare (e) the average number of eggs laid/day over 3 days among the three treatments (fixed) and fly isolines (random).
To examine male fecundity, in a fresh vial we paired one 11-day-old virgin male from the survival assays with one >5-day-old virgin female from the same strain and Spiroplasma-infection status, but that had never been exposed to wasps. The mating pair was allowed to mate and oviposit for 24 h. Mating pairs were transferred to fresh vials every 24 h for at least three consecutive days. Egg number was recorded for three consecutive days starting on the first day when eggs were present. The number of puparia present was used as a proxy for male fecundity. We used GLMM to analyze the number of puparia; and GzLMM to analyze the number of puparia/initial number of eggs. A single vial that had zero eggs was removed from analyses, because failure to oviposit would likely be a female fecundity problem, as female D. hydei are known to lay many unfertilized eggs (Markow 1985).
Results
Wasp growth rate
Larval growth rate of wasps developing in Spiroplasma-infected hosts appeared to be slowed down compared to wasps developing in Spiroplasma-free hosts (Fig. 1). Infection state, hours post-attack, and their interaction, had a highly significant (P values < 0.0001) effect on wasp body length, whereas fly strain did not (Table 1). All other two- and three-way random interactions were removed from the GLMM model due to lack of significance (results not shown). The significant infection state × hours interaction indicates that wasp growth rate differs between the Spiroplasma-infected and uninfected treatments. Differences in wasp growth rate between the Spiroplasma-infected and uninfected treatments became significant at day 6 (144 h) (F(1, 200) = 100.51, P value < 0.0001; Table 2), at which point the host has reached the pupal stage. Indeed, within the Spiroplasma-free treatment, all time points are significantly different from each other (shown by the different letters in Fig. 1), indicating that wasps grow continuously in the absence of Spiroplasma. In contrast, within the Spiroplasma-infected treatment, although wasp embryos hatched successfully and wasp larvae achieved relatively normal growth during the initial 2 days (0–48 h; Fig. 1), wasp growth began to slow down, albeit not significantly, between days 2 and 4 (48–96 h; host larval stage). After day 4, effectively no growth was achieved, indicated by the lack of significance among time points 96–192 within the Spiroplasma-infected treatment (Fig. 1). By day 8 (192 h; the last time-point examined), larvae in the Spiroplasma-free treatment are ~1.76 times larger than wasps is the Spiroplasma-infected treatment (0.97 ± 0.06 mm vs. 1.71 ± 0.09 mm). Therefore, these results indicate that the presence of Spiroplasma in the host negatively affects growth of developing wasp larvae.
Fig. 1.
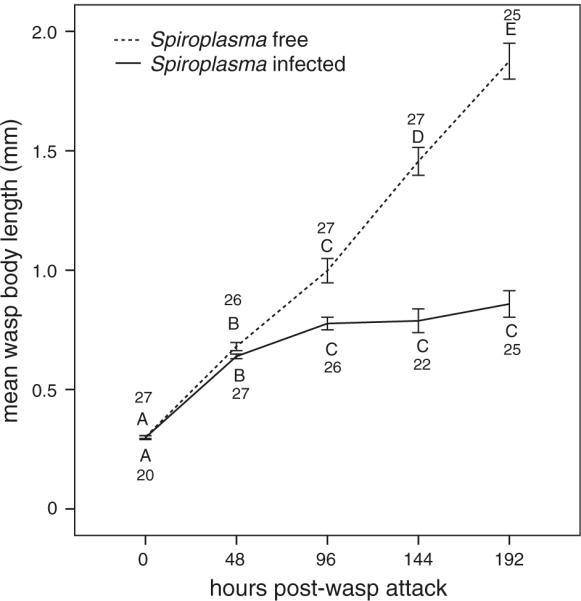
Mean (±SE) body length of wasps developing in Spiroplasma-infected and Spiroplasma-free flies at several time points post-oviposition attack. Different letters indicate time points that differed significantly from each other (Fisher's exact test) within an infection state. Numbers above and below data points correspond to number of wasp larvae measured per time point for Spiroplasma-free and Spiroplasma-infected treatments, respectively. At 0, 48 and 96 h, the flies were in the larval stage, whereas at 144 and 192 h, the flies were in the pupal stage. At 0 h, the wasps were either eggs/embryos or first instar larvae, whereas at all other time points they were larvae
Table 1.
Effects of fly Spiroplasma-infection state and fly strain on length of developing wasp
| Reduced model | F ratio/Z value(df) | P value |
|---|---|---|
| Fly strain (random) | 0.94(n/a) | 0.1737 |
| Infection state (fixed) | 146.74(1,108) | <0.0001 |
| Hours (fixed) | 184.73(4,170) | <0.0001 |
| Infection state × hours (fixed) | 36.74(4,169) | <0.0001 |
Based on General Linear Mixed Model (GLMM). F = F ratio for fixed effects and corresponding degrees of freedom (subscripts in parenthesis). Z value from “covtest” for random effects. Boldface P values significant at α = 0.0001
Table 2.
Effect of infection state on wasp length at each time point (i.e., the Infection state × hours interaction; see Table 3)
| Hours | Infection state effect F ratio(df) | P value |
|---|---|---|
| 0 | 0.12(1,199) | 0.7331 |
| 48 | 0.06(1,199) | 0.8080 |
| 96 | 2.82(1,198) | 0.0945 |
| 144 | 100.51(1,200) | <0.0001 |
| 196 | 128.49(1,200) | <0.0001 |
F = F ratio and corresponding degrees of freedom (subscripts in parenthesis)
Fly survival
As observed in the previous study, larva-to-adult survival was significantly lower in the Spiroplasma-infected wasp-attacked treatment (SW = 33.71% ± 4.74 SE; overall P value < 0.0001; post-hoc tests P value < 0.0001 for U vs. SW and = 0.0002 for S vs. SW; Table 3) than in the treatments that lacked wasps (U = 78.27% ± 5.11; S = 68.13% ± 4.81), but not significantly different between the U and S treatment (post-hoc P value = 0.3312). The means for this measure in the present study were lower than in the previous study, which was based on ten isolines, including the three isolines used in the present study (i.e., SW = 37 ± 2.98; U = 80 ± 2.44; S = 83 ± 2.96; Xie et al. 2010). However, larva-to-adult survival is more similar between both studies if only the three isolines used in both studies are considered (i.e., SW = 36.33 ± 4.23; U = 78.57 ± 4.17; S = 69.17 ± 7.18; from Xie et al. 2010). The sex ratio of emerging adult flies did not differ significantly among the three treatments (F(2, 36) = 0.35, P value = 0.7042; Table 3), suggesting no differential larva-to-adult survival between the sexes.
Table 3.
Effect of treatment (i.e., Spiroplasma-infection state and wasp presence/absence) and fly strain (isoline) on each of the fly survival measures, and on the sex ratio of surviving flies
| Treatment (fixed) | Mean ± SE |
Isoline (random) | Post-hoc Tukey-Kramer |
|||||
|---|---|---|---|---|---|---|---|---|
| U | S | SW | U vs. S | U vs. SW | S vs. SW | |||
| Larva-to-adult survival (no. of surviving flies 0-days post-emergence/initial number of fly larvae; %) | F(2, 35) = 17.55 (<0.0001) | 78.27 ± 5.11 | 68.13 ± 4.81 | 33.71 ± 4.74 | 0.02 χ2 = 0.07 (0.3961) | t(35) = −1.44 (0.3312) | t(35) = −5.37 (<0.0001) | t(35) = 4.60 (=0.0002) |
| Larva-to-pupa survival (number of puparia/initial number of fly larvae; %) | F(2 ,35) = 8.42 (0.001) | 95.02 ± 1.66 | 90.58 ± 2.52 | 79.66 ± 2.70 | 0.00 χ2 = 0.00 (1.0000) | t(35) = −1.45 (0.3249) | t(35) = −3.60 (0.0027) | t(35) = 2.84 (0.02) |
| Pupa-to-adult survival (number of emerging adult flies [day 0]/number of puparia; %) | F(2, 35) = 13.18 (<0.0001) | 82.4 ± 5.04 | 75.09 ± 4.89 | 42.5 ± 5.54 | 0.00 χ2 = 0.00 (1.0000) | t(35) = −1.03 (0.5644) | t(35) = −4.46 (0.0002) | t(35) = 4.04 (0.0008) |
| 0–10-day post-emergence survival (number of surviving flies 10-days post-emergence/number of emerging flies [day 0; %]) | F(2, 35) = 15.65 (<0.0001) | 96.18 ± 1.83 | 94.66 ± 1.67 | 62.8 ± 9.44 | 2.14 χ2 = 0.00 (1.0000) | t(35) = −0.28 (0.9581) | t(35) = −3.96 (0.001) | t(35) = 4.61 (0.0002) |
| Larva-to-10-days post-emergence survival (no. of surviving flies 10-days post-emergence/initial number of fly larvae; %) | F(2, 35) = 22.89 (<0.0001) | 75.04 ± 4.89 | 64.72 ± 4.8 | 21.53 ± 5.03 | 0.02 χ2 = 0.07 (0.3961) | t(35) = −1.36 (0.3731) | t(35) = −6.20 (<0.0001) | t(35) = 5.56 (<0.0001) |
| Sex-ratio (number of adult female flies/number of adult male flies) | F(2, 36) = 0.35 (0.7042) | 2.14 ± 1.39 | 1.14 ± 0.36 | 1.37 ± 0.82 | NA | NA | NA | NA |
Based on Generalized Linear Mixed Model (GzLMM) with binomial error distribution. Treatments: U = Spiroplasma-free no wasps; S = Spiroplasma-infected no wasps; and SW = Spiroplasma-infected and wasp treated. F = F ratio for fixed effects and corresponding degrees of freedom (subscripts in parenthesis). χ2 for pseudo-likelihood ratio test “covtest” for random effects (df = 1). P values (adjusted for multiple comparisons) are shown in parenthesis (boldface: significant at α = 0.02)
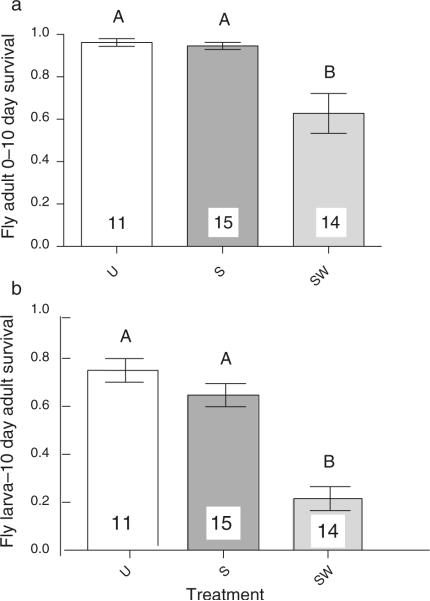
Post-emergence survival results, the focus of the present study, indicated that SW flies suffer significantly higher mortality between 0 and 10 days (survival = 62.8% ± 9.44; overall P value < 0.0001; post-hoc P value = 0.001 and 0.0002; Table 3; Fig. 2a) than the treatments that lacked wasps (U = 96.18% ± 1.83; S = 94.66% ± 1.67). Again, no significant difference was detected between the treatments lacking wasps (post-hoc P value = 0.96). Larva to 10-day adult survival, the measure that combines pre- and post-emergence survival, was therefore significantly lower in the SW (21.53% ± 5.03; overall P value < 0.0001; post-hoc P values < 0.0001; Fig. 2b) than in the treatments that lacked wasps (U = 75.04% ± 4.89; S = 64.72% ± 4.8), with no significant difference between the latter two (post-hoc P value = 0.37). Thus, despite not completing development, developing wasps exert significant post-emergence mortality on their hosts. Nevertheless, whereas ~21.5% of Spiroplasma-infected flies subjected to wasps as larvae (SW) survive to the 10-day adult stage (this study), only ~4% of Spiroplasma-free flies subjected to wasps (UW) reach adulthood (Xie et al. 2010). Therefore, under our experimental conditions with high wasp parasitism, Spiroplasma-infected flies have a ~fivefold survival advantage over Spiroplasma-free flies. This advantage could be larger because we are assuming 100% post-emergence survival to 10-days in the UW flies, but it is likely lower given that the U treatment, which lacks wasps, had 96.18% survival at this stage (see above).
Fig. 2.
Untransformed mean (±SE) fly survival, under three treatments: U = Spiroplasma-free and wasps absent; S = Spiroplasma-infected and wasps absent; SW = Spiroplasma-infected and wasps present. a 0–10-day post-emergence survival (number of surviving flies 10-days post-emergence/number of emerging flies [day 0]); and b fly larva-to-10 days post-emergence survival (no. of surviving flies 10-days post-emergence/initial number of fly larvae). Significant differences between treatments (α = 0.01) according to post-hoc comparisons are indicated by different upper-case letters (see Table 3). Numbers within or above bars indicate number of replicates (vials) per treatment
Surviving fly fecundity
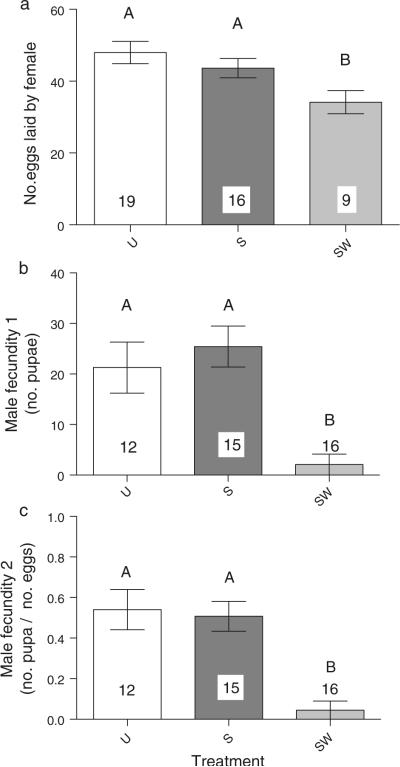
Spiroplasma-infected females that had been subjected to wasps as larvae (SW) and survived to at least the 10-day adult stage, had an average daily fecundity of 34.14 ± 3.21 eggs compared to the average fecundity of Spiroplasma-free (U) and Spiroplasma-infected (S) flies that had not been subjected to wasps as larvae (47.96 ± 3.11 and 43.61 ± 2.70; respectively; Table 4; Fig. 3a). Of the three treatments, only the SW vs. U comparison was statistically significant (overall P value = 0.0275; post-hoc P = 0.0207). Therefore, the relative fecundity of Spiroplasma-infected wasp-attacked females (SW) to Spiroplasma-free unattacked females (U) is ~71% (i.e., 34/48 eggs/day). Again, assuming that the few (at most 4%) uninfected-unattacked females (UW) that survive from larvae to 10-days-old adulthood, have similar fecundity to their unattacked counterparts (U), the relative reproductive fitness of Spiroplasma-infected to Spiroplasma-uninfected flies under our experimental conditions of high wasp parasitism is ~3.5 fold (i.e., ~5 times greater survival × 0.71 fecundity).
Table 4.
Effect of treatment (i.e., Spiroplasma-infection state and wasp presence/absence) and fly strain (isoline) on the fecundity of female and male flies emerging from the survival assays
| Treatment (fixed) | Mean ± SE |
Isoline (random) | Post-hoc Tukey–Kramer |
|||||
|---|---|---|---|---|---|---|---|---|
| U | S | SW | U vs. S | U vs. SW | S vs. SW | |||
| Female Fecundity (number of eggs laid per day over 3 days) | F(2, 39.4) = 3.94 (0.0275) | 47.96 ± 3.11 | 43.61 ± 2.70 | 34.14 ± 3.21 | 20.5598 Z = 0.64 (0.2617) | t(39.1) = 0.96 (0.6080) | t(39.5) = 2.81 (0.0207) | t(39.6) = 1.95 (0.1391) |
| Male Fecundity 1 (number of puparia from eggs laid over 3 days) | F(2, 36.3) = 18.42 (<0.0001) | 21.27 ± 5.05 | 25.43 ± 4.07 | 2.08 ± 2.08 | 56.1658 Z = 0.79 (0.2146) | t(36.7) = 0.16 (0.9861) | t(36.5) = 4.78 (<0.0001) | t(35.9) = 5.35 (<0.0001) |
| Male fecundity 2 (number of puparia/initial number of eggs laid over 3 days) | F(2, 35) = 8.95 (0.0007) | 54.04% ± 9.92 | 50.76% ± 7.36 | 4.5% ± 4.5 | 0.4078 χ2 = 1.13 (0.1438) | t(35) = 0.94 (0.6217) | t(35) = 4.13 (0.0006) | t(35) = 3.83 (0.0014) |
Treatments: U = Spiroplasma-free no wasps; S = Spiroplasma-infected no wasps; and SW = Spiroplasma-infected and wasp treated. Female Fecundity and Male Fecundity 1 are based on General Linear Mixed Model (GLMM), whereas Male Fecundity 2 is based on Generalized Linear Mixed Model (GzLMM) with binomial error distribution. F = F ratio for fixed effects and corresponding degrees of freedom (subscripts in parenthesis). Random effects tests: χ2 pseudo-likelihood ratio (df = 1) in GzLMM; and Z value in GLMM. P values (adjusted for multiple comparisons) are shown in parenthesis (boldface: significant at α = 0.03). NA Not applicable
Fig. 3.
Untransformed mean (±SE) fecundity of flies emerging from the survival experiment, under the three treatments: U = Spiroplasma-free and wasps absent; S = Spiroplasma-infected and wasps absent; SW = Spiroplasma-infected and wasps present. a Female fecundity = average no. of eggs per day laid by female over three consecutive days; b Male fecundity 1 = no. of pupae produced by mate of target male; and c Male fecundity 2 = no. of pupae/no. of eggs laid by mate of target male. Significant differences between treatments (α = 0.03) according to post-hoc comparisons are indicated by different upper-case letters (see Table 4). Numbers within or above bars indicate number of replicates (vials) per treatment
Spiroplasma-infected males that had been subjected to wasps as larvae (SW) and survived to at least the 10-day adult stage had an extremely low fecundity, measured as both, the total number of pupae (Male Fecundity 1 = 2.08 ± 2.08; Table 4) and the proportion of pupae/eggs laid by their female mates over three consecutive days (Male Fecundity 2 = 4.5% ± 4.5). These values were significantly lower than in the treatments lacking wasps (U and S; see Table 4).
Only one out of 16 SW males examined produced pupae, suggesting that SW males are effectively sterile during the period examined (Fig. 3b, c; Table 4).
Discussion
A previous study reported that Spiroplasma enhances larva-to-adult host survival in Drosophila hydei subjected to attacks by Leptopilina heterotoma (Xie et al. 2010). Furthermore, it was shown that the protective mechanism occurs after wasp oviposition, because wasp oviposition frequency did not differ between Spiroplasma-infected and Spiroplasma-uninfected hosts. To better understand the protective mechanism, we tracked wasp larval growth for approximately 8 days starting at the wasp embryo/first-instar stage. Our results indicate that in the Spiroplasma-infected treatment, wasp embryos hatch successfully and wasp larvae achieve relatively normal growth during the initial 2 days. After that, wasp growth slows down and effectively stops. Most of the wasps at the time of dissection, however, appeared to be alive, thus the presence of Spiroplasma does not effectively kill them by this stage. The fate of wasps in Spiroplasma-infected hosts beyond this time point has not been examined. During the host pupal stage, wasps typically reach their third-instar stage, exit the host body, and begin eating it from outside, but within the pupal case (Carton et al. 1986). Wasps developing in Spiroplasma-infected flies that survive to adulthood, do not achieve this stage, but it is not known when they die.
Our results provide some insight into the mechanism(s) by which Spiroplasma enhances survival of wasp-attacked flies. The potential mechanisms include: (a) resource competition between Spiroplasma and wasp; (b) presence of a toxic substance encoded by Spiroplasma; and (c) enhanced host immune response due to presence of Spiroplasma (e.g., if Spiroplasma restores host encapsulation ability, which is normally suppressed by virus-like particles “VLPs” in the parasitoid venom (Rizki et al. 1990)). Enhanced encapsulation ability is unlikely because: (a) it typically acts on unhatched wasp eggs (Kraaijeveld et al. 2009), which we did not observe; and (b) we observed no evidence of melanotic capsules in attacked hosts (under a dissecting scope). Nevertheless, melanotic encapsulation is not the only means by which Drosophila hosts kill Leptopilina eggs (e.g., Drosophila paramelanica; Carton et al. 2008), thus, enhanced immune response via other mechanisms, including cytotoxic molecules (e.g., reactive oxygen species), cannot be ruled out. The decreased wasp growth rate observed is also consistent with both, competition with Spiroplasma for nutrients, and presence of a Spiroplasma-encoded substance that is toxic to the developing wasp.
A similar growth-inhibiting effect of Spiroplasma has been reported in the parasitic nematodes (Howardulla aoronymphium) of Drosophila neotestacea, where the size of the nematode is significantly smaller in Spiroplasma-infected vs. Spiroplasma-free 1-week-old adult female flies (Jaenike et al. 2010b). The strain of Spiroplasma associated with D. neotestacea is most closely related to the strain used in this study (Jaenike et al. 2010a). Another similarity between the two protective associations is that they involve a macro-parasite that lives in the hemocoel during a critical growth phase for the parasite (fly larval stage in the case of the wasp and fly adult stage in the case of the nematode), so the mechanism may be similar.
An interesting trend is emerging in the study of symbiont-mediated protection against endo-macroparasites such as parasitoid wasps and nematodes. In the three cases in which protection against an endo-macroparasite has been reported (i.e., the two Drosophila–Spiroplasma associations described above and the protection of aphids against parasitoid wasps by the Gammaproteobacterium Hamiltonella defensa; Oliver et al. 2003), the symbiont involved can readily live extracellularly in the hemolymph (Moran et al. 2005; Williamson 1965). In contrast, other facultative endosymbionts of arthropods such as Wolbachia are obligately intracellular, and do not appear to provide protection against macro-parasites (Fytrou et al. 2005; Jaenike et al. 2010a). Thus, it is possible that ability to live in the hemolymph's extracellular environment is a pre-requisite for ability to protect their host against endo-macroparasites, suggesting that the protection might require direct contact between the protective symbiont and the parasite and may involve ingestion of the symbiont by the parasite.
Regardless of the mechanism by which Spiroplasma enhances larva-to-adult host survival, wasp larvae manage to survive for at least to ~8 days post-attack and grow normally for at least 2 days. Such degree of wasp survival and growth may be detrimental to flies that survive to adulthood. Indeed, our fly post-emergence survival results indicate that Spiroplasma-infected flies surviving a wasp attack have significantly higher 0–10 day mortality than flies that have not been exposed to wasps. Accounting for such mortality, reduces the previously estimated ~ninefold advantage (Xie et al. 2010) of Spiroplasma-infected over uninfected flies to a ~fivefold survival advantage.
The survival advantage described above is necessary, but not sufficient for Spiroplasma persistence. For Spiroplasma to persist, Spiroplasma-infected female flies surviving a wasp attack to reproductive age must be able to reproduce. This is particularly relevant because even when the host manages to overcome a parasitoid attack, the surviving host may be effectively sterile (Le Ralec et al. 2010). Our female fecundity assays indicate that the fecundity of Spiroplasma-infected female flies that were subjected to wasps (SW) is ~71% that of Spiroplasma-free female flies not subjected to wasps (U). Thus, despite overcoming the wasp attack, the reproductive fitness of surviving flies is compromised. In spite of this reduced fecundity, combining our survival and fecundity results, Spiroplasma-infected wasp attacked (SW) flies have a ~3.5-fold (fivefold survival × 0.71 fecundity) reproductive advantage over uninfected flies subjected to the same wasp pressure. In our previous study (Xie et al. 2010), we also reported that in a sample of 200 flies that survived the wasp treatment to adulthood, 100% were Spiroplasma-infected. Accordingly, parasite-host fitness (i.e., the ability of infected females to produce infected daughters relative to the ability of uninfected females to produce daughters), under our high wasp parasitism experimental conditions is ~3.5 and thus, greater than one.
Although our results support the hypothesis that Spiroplasma protection against parasitism by L. heteroma in D. hydei contributes to the long-term persistence Spiroplasma in natural populations of D. hydei, additional knowledge on the natural dynamics of this three-way interaction is needed. For example, little is known about the degrees of L. heterotoma parasitism on D. hydei in nature, but degrees of parasitism by L. heterotoma and other wasps on several species of Drosophila in Europe vary greatly on a temporal and spatial scale (reviewed in Fleury et al. 2009). Similarly, little is known about the temporal and spatial variation of Spiroplasma prevalence in natural populations of D. hydei, although spatial variation is highly variable (e.g., Kageyama et al. 2006; Watts et al. 2009).
Another aspect that will affect parasite-host fitness is the occurrence of fitness tradeoffs of Spiroplasma infection on host. For example, although in our study, Spiroplasma-infected and Spiroplasma-free flies that were not subjected to wasps had equivalent fitness, it is possible that under different experimental conditions (e.g., larval density, temperature, other parasites/competitors), Spiroplasma-infected flies will have reduced fitness. Indeed, it appears that Spiroplasma-infected flies exhibit slightly reduced longevity beyond 10-days (I. Vilchez, unpublished data). The influence of potential tradeoffs on parasite-host fitness needs to be further evaluated, by experiments that span several host generations and test different conditions.
In contrast to females, the reproductive fitness of Spiroplasma-infected males surviving a wasp attack was extremely low. With one exception, all wasp-attack surviving males paired with a Spiroplasma-infected un-attacked virgin female produced zero pupae over the 3-day period examined. The single male that produced a similar number and proportion of pupae to the males in the un-attacked treatments could reflect an un-attacked fly, as we can not be certain that all flies in the wasp treatment were indeed attacked. As mentioned above, ~5% of fly larvae under equivalent experimental conditions are not attacked (Xie et al. 2010). Although our results suggest that males exposed to wasps are effectively sterile, it is possible that wasp-attacked males exhibit delayed fecundity rather than complete sterility, as we only examined fecundity in relatively young males. Thus, the effects of wasp attack on male fecundity may be less severe than our estimates, if males compensate for it at a later age. Males of unknown Spiroplasma infection status are reported to live an average of ~84–105 days (S. Pitnick, pers. comm.; Pitnick and Miller 2000). A delay in reproductive maturity may result from a nutrition deficiency during development if it affects male size, as smaller males of D. hydei are reported to take longer to reach maturity and mate with fewer females than larger males (Pitnick and Markow 1994). Male sterility may also be a side effect of the virus-like-particles (VLPs; lacking nucleic acids) present in L. heterotoma venom, which are involved in immunosuppression (Rizki and Rizki 1994). In other wasp families (e.g., Braconidae), polydnaviruses and venom have been reported to cause castration of host larvae via testis degeneration (Beckage and Gelman 2004; Federici and Bigot 2003). Whether or not VLPs are capable of causing host castration remains to be examined. In our experiment, we cannot distinguish whether the lower fecundity in males is the result of lower mating rates, lower sperm transfer rates, lower sperm quality/quantity, or lack thereof, as we did not quantify these measures. Regardless of the cause, the lower fecundity observed in Spiroplasma-infected males surviving a wasp attack, is consistent with the expectation that the long-term persistence of maternally transmitted symbionts is more dependent on female, rather than male, reproductive fitness.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R03 AI078348 and National Science Foundation (NSF) grant DEB 0743782 to MM. The Chinese Scholarship Council provided funding for JX. T. Schlenke kindly provided the wasps. This is publication No. 188 of the Center for Biosystematics and Biodiversity (Texas A&M University). R. Wharton provided guidance and feedback. L. A. Hurtado and N. Silva provided comments on the manuscript.
References
- Beckage NE, Gelman DB. Wasp parasitoid disruption of host development: implications for new biologically based strategies for insect control. Annu Rev Entomol. 2004;49:299–330. doi: 10.1146/annurev.ento.49.061802.123324. [DOI] [PubMed] [Google Scholar]
- Bull JJ. Evolution of sex determining mechanisms. Benjamin/Cummings; Menlo Park: 1983. [Google Scholar]
- Carton Y, Boulétreau M, Van Alphen JJM, Van Lenteren JC. The Drosophila parasitic wasps. In: Ashburner M, Carson HL, Thompson JN, editors. The genetics and biology of Drosophila. Academic Press; London: 1986. pp. 347–394. [Google Scholar]
- Carton Y, Poirie M, Nappi AJ. Insect immune resistance to parasitoids. Insect Sci. 2008;15:67–87. [Google Scholar]
- Ebbert MA. The interaction phenotype in the Drosophila willistoni–Spiroplasma symbiosis. Evolution. 1991;45:971–988. doi: 10.1111/j.1558-5646.1991.tb04364.x. [DOI] [PubMed] [Google Scholar]
- Federici BA, Bigot Y. Origin and evolution of polydnaviruses by symbiogenesis of insect DNA viruses in endoparasitic wasps. J Insect Physiol. 2003;49:419–432. doi: 10.1016/s0022-1910(03)00059-3. [DOI] [PubMed] [Google Scholar]
- Fleury F, Gibert P, Ris N, Allemand R. Ecology and life history evolution of frugivorous Drosophila parasitoids. Adv Parasitol. 2009;70:3–44. doi: 10.1016/S0065-308X(09)70001-6. [DOI] [PubMed] [Google Scholar]
- Fytrou A, Schofield PG, Kraaijeveld AR, Hubbard SF. Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc R Soc Lond B Biol Sci. 2005;273:791–796. doi: 10.1098/rspb.2005.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile Virus infection. PLoS One. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J, Stahlhut JK, Boelio LM, Unckless RL. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol Ecol. 2010a;19:414–425. doi: 10.1111/j.1365-294X.2009.04448.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010b;329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- Kageyama D, Anbutsu H, Watada M, et al. Prevalence of a non-male-killing Spiroplasma in natural populations of Drosophila hydei. Appl Environ Microbiol. 2006;72:6667–6673. doi: 10.1128/AEM.00803-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenpoth M, Göttler W, Herzner G, Strohm E. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr Biol. 2005;15:475–479. doi: 10.1016/j.cub.2004.12.084. [DOI] [PubMed] [Google Scholar]
- Kellner RLL. Molecular identification of an endosymbiotic bacterium associated with pederin bio-synthesis in Paederus sabaeus (Coleoptera, Staphylinidae) Insect Biochem Mol Biol. 2002;32:389–395. doi: 10.1016/s0965-1748(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld AR, Godfray HCJ, Genevieve P. Adv Parasitol. Academic Press; New York: 2009. Chapter 10 evolution of host resistance and parasitoid counter-resistance; pp. 257–280. [DOI] [PubMed] [Google Scholar]
- Le Ralec A, Anselme C, Outreman Y, et al. Evolutionary ecology of the interactions between aphids and their parasitoids. C R Biol. 2010;333:554–565. doi: 10.1016/j.crvi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Markow TA. A comparative investigation of the mating system of Drosophila hydei. Anim Behav. 1985;33:775–781. [Google Scholar]
- Markow TA, O'Grady PM. Drosophila: a guide to species identification and use. Academic Press Elsevier; London: 2005. [Google Scholar]
- Mateos M, Castrezana S, Nankivell B, et al. Heritable endosymbionts of Drosophila. Genetics. 2006;174:363–376. doi: 10.1534/genetics.106.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Russell J, Fukatsu T, Koga R. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl Environ Microbiol. 2005;71:3302–3310. doi: 10.1128/AEM.71.6.3302-3310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Degnan PH, Hunter MS, Moran NA. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science. 2009;325:992–994. doi: 10.1126/science.1174463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Markow TA. Large-male advantages associated with costs of sperm production in Drosophila hydei, a species with giant sperm. Proc Natl Acad Sci USA. 1994;91:9277–9281. doi: 10.1073/pnas.91.20.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Miller GT. Correlated response in reproductive and life history traits to selection on testis length in Drosophila hydei. Heredity. 2000;84:416–426. doi: 10.1046/j.1365-2540.2000.00679.x. [DOI] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM. Parasitoid-induced cellular immune-deficiency in Drosophila. In: Beck G, Cooper EL, Habicht GS, Marchalonis JJ, editors. Primordial immunity: foundations for the vertebrate immune system. vol 712. Annals of the New York academy of Sciences; 1994. pp. 178–194. [DOI] [PubMed] [Google Scholar]
- Rizki T, Rizki R, Carton Y. Leptopilina heterotoma and L. boulardi: strategies to avoid cellular defense responses of Drosophila melanogaster. Exp Parasitol. 1990;70:466–475. doi: 10.1016/0014-4894(90)90131-u. [DOI] [PubMed] [Google Scholar]
- Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 2007;3:1486–1501. doi: 10.1371/journal.ppat.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:2753–2763. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T, Haselkorn TS, Moran NA, Markow TA. Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS One. 2009;4:e5703. doi: 10.1371/journal.pone.0005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DL. Kinetic studies of “sex ratio” spirochetes in Drosophila melanogaster Meigen females. J Invertebr Pathol. 1965;7:493–501. doi: 10.1016/0022-2011(65)90126-6. [DOI] [PubMed] [Google Scholar]
- Xie J, Vilchez I, Mateos M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS One. 2010;5:e12149. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]