Abstract
N-Acetylserotonin (NAS) is a naturally occurring chemical intermediate in biosynthesis of melatonin. Previous studies have shown that NAS has different brain distribution patterns from those of serotonin and melatonin suggesting that NAS might have functions other than as a precursor or metabolite of melatonin. Indeed several studies have now shown that NAS may play an important role in mood regulation and may have antidepressant activity. Additional studies have shown that NAS stimulates proliferation of neuroprogenitor cells and prevents some of the negative effects of sleep deprivation. It is believed that the antidepressant and neurotrophic actions of NAS are due, at least in part, to the capability on this molecule to activate the TrkB receptor in a BDNF-independent manner. Emerging evidence also indicates that NAS and its derivatives have neuroprotective properties and protect retinal photoreceptor cells from light-induced degeneration. In this review we will discuss the literature about this exciting and underappreciated molecule.
Keywords: N-Acetylserotonin, Melatonin, TrK B, Sleep, Neuroprotection, Circadian Rhythms, Photoreceptors
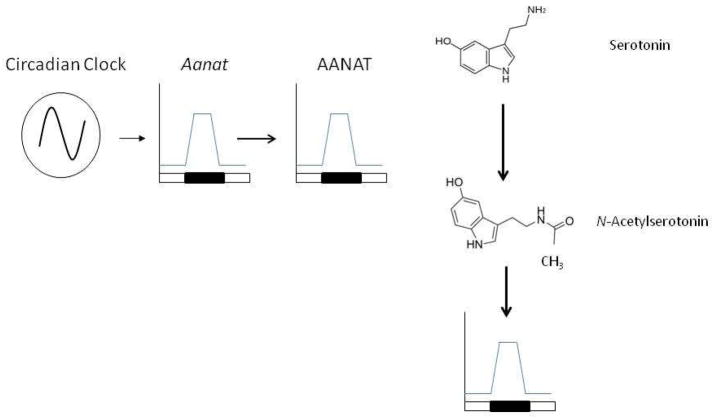
N-Acetylserotonin (NAS) is synthesized in the mammalian pineal gland and retina. Until very recently, NAS was believed to be just an intermediate in the biosynthetic pathway of melatonin with no or very limited biological function of its own. NAS is produced from serotonin by the enzyme arylalkylamine N-acetyltransferase (AANAT) and is converted to melatonin by acetylserotonin O-methyltransferase (ASMT; or hydroxyindole O-methyltransferase, HIOMT ) (Figure 1). Although NAS is predominantly synthesized in the pineal gland a series of studies have also provided experimental evidence that NAS and AANAT activity are present in the hippocampus, the olfactory bulb, the spinal cord and the cerebellum (Paul et al., 1974; Psarakis et al., 1982; Gaudet 1991; Chae et al., 1999). These earlier findings have been supported by more recent studies using RT-PCR to analyze Aanat mRNA expression (Uz et al., 2002). The experimental observation that NAS may reside in the specific brain areas separate from melatonin and serotonin suggests that NAS may have a role in the central nervous system distinct from that of being a precursor for melatonin.
Figure 1.
The levels of N-Acetylserotonin are high during the night and low during the day and the rhythm is controlled by circadian clocks. The circadian clock controls the transcription of the Aanat gene and thus the enzymatic activity of AANAT and NAS levels (see text for details).
As with melatonin, NAS shows a clear daily/circadian variation in its level. In the pineal gland the levels of NAS start to surge 3–4 hrs after the onset of darkness at night and begin to decrease before the onset of light in the morning (Chattoraj et al., 2009). The rhythmic synthesis in the pineal gland is controlled by the circadian clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. At night, the SCN sends signals to the pineal gland via sympathetic neurons that release norepinephrine (NE), thus activating adrenergic receptors to increase intracellular Ca2+ and cAMP with the consequent phosphorylation of the cAMP response element (CRE) binding protein (CREB) [Klein et al., 1997]. Phosphorylated CREB activates Aanat gene expression via CREs in the Aanat promoter (Baler et al., 1997, 1999; Figure 2). cAMP also stimulates the phosphorylation of AANAT protein, which promotes its association with 14-3-3 proteins, activating the enzyme and protecting it from degradation (Ganguly et al., 2001; Pozdeyev et al., 2006; Figure 2). The changes in the activity of AANAT are responsible for the variation in NAS (and melatonin) levels (Klein et al., 1997). The levels of NAS are tightly controlled by light at the level of AANAT activity. Exposure to light rapidly (within minutes) reduces AANAT activity by reducing cAMP, resulting in dephosphorylation and proteasomal proteolysis of the AANAT protein (Klein and Weller, 1972; Klein et al., 1978; Gastel et al., 1998; Fukuhara et al., 2001; Pozdeyev et al., 2006). The rapid destruction of the AANAT protein results in an almost immediate decrease in pineal levels of NAS and melatonin (Figure 2).
Figure 2.
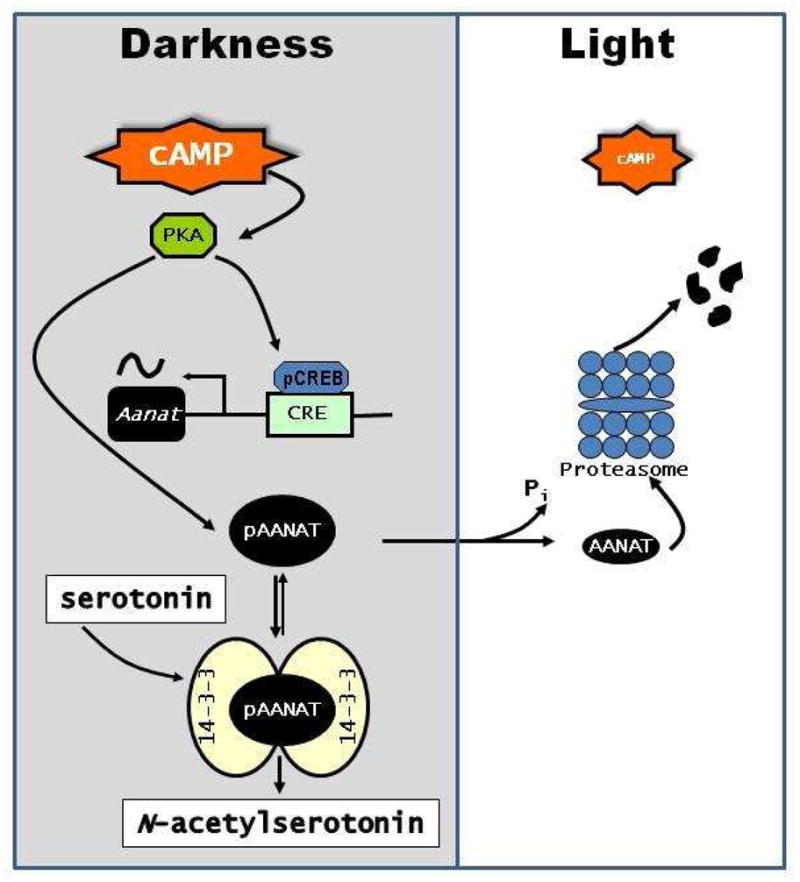
Regulation of NAS biosynthesis and its suppression by light. At night in darkness cAMP levels are elevated, activating PKA, which induces Aanat gene transcription and phosphorylates AANAT protein. Phosphylated AANAT (pAANAT) associates with 14-3-3 proteins, which activate and stabilize the enzyme resulting in increased conversion of serotonin to N-acetylserotonin. Light exposure decreases cAMP levels resulting in dephosphorylation of AANAT and its subsequent degradation by proteasomal degradation.
An important aspect of NAS biology is the identification of the receptors that are involved in modulation of its biological functions. Previous studies have shown that NAS has some activity on the membrane G-protein-coupled melatonin receptors (MT1 and MT2) (Nonno et al., 1999), but the affinity is several orders of magnitude lower than that for melatonin. Other studies have suggested that the putative MT3 binding site has a higher affinity for NAS than for melatonin. Thus, it has been suggested that MT3 may act as an NAS receptor (Nosjean et al., 2000). A recent study has shown that NAS may be a ligand for TrkB receptor, the cognate receptor for brain-derived neurotrophic factor (BDNF). NAS robustly activates the TrkB receptor in a BDNF- and MT3 receptor-independent manner (Jang et al., 2010).
NAS Displays Antidepressant-like Activity
A number of early studies suggested that NAS may be an endogenous antidepressant molecule. For example, exogenous administration of NAS decreases immobility in the mouse tail suspension test (Prakhie and Oxenkrug, 1998) and chronic administration (three weeks) of the antidepressant fluoxetine induces a five-fold increase in the levels of Aanat mRNA and, presumably, NAS in the hippocampus (Uz and Manev, 1999). Additionally, clorgyline, a selective monoamine oxidase A (MAO-A) inhibitor with antidepressant-like activity, increases (5-fold) rat pineal melatonin and NAS content, and decreases 5-HIAA (MAO-oxidized metabolite) level by 80%; whereas deprenyl, a selective MAO-B inhibitor, does not change the content of melatonin or other pineal indoles (Oxenkrug et al., 1985). As we have previously discussed, NAS activates TrkB receptors (Jang et al., 2010), and several investigations have indicated that activation of TrkB receptors may be a common mechanism of antidepressant drug action (e.g., Rantamaki et al., 2007). The activation of TrkB by acute administration of some but not all antidepressant drugs may involve NAS. For example, the selective serotonin reuptake inhibitors (SSRI) fluoxetine and citalopram and the tricyclic antidepressant desipramine robustly stimulate TrkB activation in hippocampus of mice that synthesize NAS (C3H/f+/+ mice) as well as in C57BL/6 mice (Jang et al., 2010), which have a mutation in AANAT that prevents the synthesis of appreciable amounts of NAS or melatonin (Roseboom et al., 1998). In contrast, the MAO-A inhibitor clorgyline, which increases serotonin levels, stimulates TrkB activation in the C3H/f+/+ mice but not in the C57BL/6 mice (Jang et al., 2010). Interestingly, clorgyline only activates TrkB in C3H/f+/+ mice when administered at night in the dark, when AANAT is active; in contrast, clorgyline is ineffective at activating TrkB when admisitered to mice exposed to light, which inactivates AANAT. These findings suggest that clorgyline-induced TrkB activation is attributable to increased levels serotonin and the subsequent conversion to NAS in darkness. Because deprenyl, an MAO-B inhibitor does not stimulate serotonin or NAS levels, it is unable to trigger TrkB activation whether the light is on or off. However, the SSRI and tricyclic antidepressants markedly activate TrkB regardless of dark or light. This result combined with the observation that these drugs stimulate TrkB phosphorylation in hippocampus of C57BL/6J mice with defective AANAT indicates that NAS is not a major effector in TrkB activation by acute administration of these agents. Clorgyline increases both melatonin and NAS levels in rat pineal glands (Oxenkrug et al., 1985). Further experiments have shown that intraperitoneal administration of NAS 1 h before testing significantly decreases the duration of immobility in the forced swim test as compared to saline control (Jang et al., 2010). By contrast, melatonin has little effect. This finding is consistent with a previous report that NAS, but not melatonin, reduces duration of immobility in the tail-suspension test (Prakhie and Oxenkrug, 1998). Interestingly, pretreatment with a specific inhibitor of TrkB abolished the antidepressant-like behavioral effect of NAS, suggesting that the antidepressant-like effect of NAS is mediated through TrkB activation (Jang et al., 2010).
A growing body of evidence suggests that BDNF-mediated TrkB signaling is both sufficient and necessary for antidepressant-like behaviors in rodents. However, it appears that the action of NAS is independent from BDNF since administration of exogenous NAS, but not of melatonin, activates TrkB in mice lacking BDNF (Jang et al., 2010). However, these results do not exclude the possibility that NAS and BDNF might additively or synergistically regulate each other’s neurotrophic activity on TrkB. Interestingly, it has been observed that TrkB activation in C3H/f+/+ mice follow a daily/circadian pattern that is consistent with the oscillation of endogenous NAS, and this effect is absent in C57BL/6J mice that lack endogenous NAS. Furthermore, the observations that TrkB is activated by clorgyline in C3H/f+/+ mice, but not in C57BL/6J mice, and is prevented by light exposure, which inhibits NAS synthesis, further indicate that the synthesis of endogenous NAS induced by clorgyline accounts for this effect (Jang et al., 2010). The results provide compelling evidence that the molecular mechanism underlying the antidepressant role of NAS involves activation of TrkB receptors and suggest that the chronic treatment with fluoxetine, which increases Aanat transcription, leads to an increase in NAS, activation of TrkB, enhanced synaptic plasticity, neurogenesis, and synaptogenesis (Li et al., 2008). In conclusion, activation of TrkB by NAS appears to provide a molecular mechanism for the antidepressant-like action of of the melatonin precursor (Jang et al., 2010).
Agglomerating, which is structurally homologous to melatonin, is a novel antidepressant with agonist activity at melatonin receptors (MT1 and MT2), and antagonistic effects at the 5HT2c serotonin receptor (San and Arrant, 2008). Agglomerating produces strong effects on circadian sleep phase disturbances, improving time to sleep onset and quality of sleep (Spading et al., 2011). It has been shown to be superior to placebo and similar to existing antidepressants, as demonstrated by short-term clinical trials and one relapse prevention trial (Vickie and Rogers, 2011). Agglomerating appears to be well tolerated, without sexual or cardiac adverse effects, weight gain or discontinuation syndromes that are normally occurred to the monoaminergic antidepressants (Demyttenaere, 2011). Since melatonin itself does not provoke TrkB receptor activation in primary neurons or animals, conceivably, agomelatine might exert its antidepressant effect through its demethylated metabolite that may mimic NAS and activate TrkB receptors in the brain.
NAS and Neuroprotection
NAS has antioxidant properties and it has been suggested that it may useful in protection from oxidative stress-related disorders (Oxenkrug, 2005), such as Alzheimer’s disease, Parkinsonism, and age-related macular degeneration. The antioxidant effects of NAS may involve both direct chemical interactions and receptor-mediated mechanisms. NAS appears to be protective against excitotoxic neuronal injury by activating TrkB receptors. In TrkB F616A knockin mice, administration of kainic acid induces apoptosis in brain neurons, as indicated by activation of caspase 3 (Jang et al., 2010). Pretreatment with NAS but not melatonin inhibits caspase 3 activation by kainic acid, and this effect is blocked by the specific TrkB F616A inhibitor 1NMMPP1.
Several derivatives of NAS have recently been developed (Shen et al., 2012). One of these, N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperideine-3-carboximide (HIOC) selectively activates TrkB with greater potency than NAS and has a significantly longer biological half-life than NAS after systemic administration. The compound can pass the blood-brain and blood-retinal barriers when administered systemically and reduces kainic acid-induced neuronal cell death in a TrkB-dependent manner.
Brain-derived neurotrophic factor (BDNF), an endogenous ligand of TrkB, has neuroprotective effects in the retina, decreasing ganglion cell loss from optic nerve damage (Mansour-Robaey et al. 1994) and photoreceptor loss in bright light-induced retinal degeneration (LaVail et al. 1998). However, BDNF must be injected directly into the eye to be effective, as it does not cross the blood-retinal barrier. Interestingly, systemic administration of HIOC mitigates bright light-induced photoreceptor degeneration (Shen et al., 2012), suggesting that this compound may be useful in the treatment of retinal degenerative diseases.
NAS may be an endogenous neuroprotectant. C57BL/6 mice, which are genetically deficient in NAS, lose ~50% of their retinal ganglion cells by 18 months of age (Danias et al., 2003). In contrast, C3H/f+/+ mice, which make NAS, show no age-related decline in retinal ganglion cells (Baba et al., 2009). While it is tempting to attribute this difference in cell survival to NAS, caution is warranted as many genetic differences exist between these two strains of mice.
Sleep, Neurogenesis and NAS
Sleep is a naturally recurring state characterized by a reduced sensory activity and is divided into two main types: rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. The purposes and mechanisms of sleep are only partially understood and are the subject of intense research. In humans, as well as in laboratory rodents, the temporal organization of sleep during the 24-hour daily cycle ultimately results from the activity of two interacting time-keeping mechanisms in the central nervous system: endogenous circadian rhythmicity and sleep-wake homeostasis. Sleep-wake homeostasis refers to the increase in propensity to fall asleep and increased sleep duration and intensity that occur following extended waking. The sleep “homeostat”, also referred to as “process S”, is often represented as an hourglass mechanism relating the amount and intensity of sleep to the duration of prior wakefulness (Figure 3; Borbely and Achermann, 1999). In both humans and rodents, slow-wave activity (SWA or “delta power”, i.e., EEG power density in the low frequency range <4Hz), an index which quantifies the depth of non-REM sleep or “sleep intensity”, is the primary marker of sleep-wake homeostasis (Borbely and Achermann, 1999). Chronic partial sleep loss in humans appears to have cumulative effects on process S (Carskadon and Dement 1979; Dinges et al., 1997).
Figure 3.
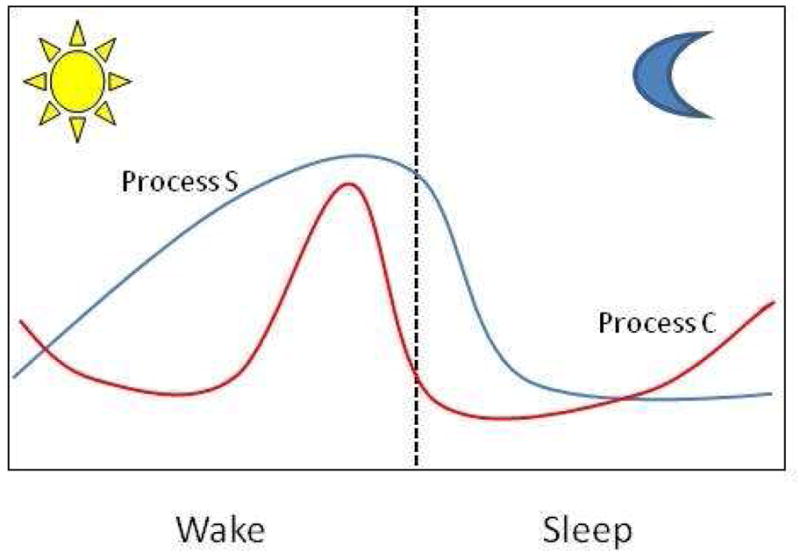
The current model for sleep regulation involves two processes: a sleep-dependent process (Process S, blue line) and a circadian process (Process C, red line). Sleep propensity (Process S) increases during the wake time and rapidly decrease during sleep. The circadian clock (Process C) opposes sleep propensity by sending an alerting signal that begins to rise before the awakening and continues to increase into the late part of the wake period. Therefore, the sleep and wake cycle is determined by the coincident and opposing actions of these two processes (adapted from Borbely and Ackermann, 1999).
Chronic partial sleep loss, whether due to voluntary sleep curtailment, sleep disorders or medical illnesses is pervasive throughout our society. “Normal” sleep duration has decreased from approximately 9 hrs in 1910 to an average of 7 hrs in 2000 (Jonhson, 2000). Today, many individuals are in bed only 5–6 hours per night on a chronic basis (Jean-Louis et al., 2000). Until a few years ago, the dogma was that sleep is only important for brain function (Benington and Heller, 1995). Now, accumulating experimental evidence indicates that sleep loss induces significant alterations in metabolism, cardiovascular function, mood and cognitive performance (Neckelmann et al., 2007; Spiegel et al., 2009).
Neurogenesis is the process by which neurons are generated during the development of the central nervous system. Until very recently it was believed that neurogenesis in mammals could only occur during early development; today we know that neurogenesis can continue in a few brain regions of adults (Ming and Song 2005). Neuronal progenitor cell (NPC) proliferation is an early event of neurogenesis, a process by which new neurons are continuously generated and incorporated into the nervous system. New neurons are continually generated in the dentate gyrus of the hippocampus and in the subventricular zone of the adult brain (Erickson et al., 1998, Gould et al., 1999). It had been shown that aging and stress can suppress neurogenesis but enriched environmental conditions, voluntary exercise such as running, as well as antidepressants can positively modulate hippocampal neurogenesis (Ming and Song, 2005; Lledo et al., 2006; Zhao et al., 2008; Ma et al., 2009).
Mounting evidence also suggests that sleep may contribute to hippocampal functions by promoting neurogenesis. Total sleep deprivation for 96 hrs reduces cell proliferation and neurogenesis in the dentate gyrus of the hippocampus in adult rats (Guzman-Marin et al., 2005) and sleep deprivation impairs hippocampus-dependent learning and abolishes learning-induced neurogenesis (Hairston et al., 2005). Initially, it was proposed that stress and glucocorticoids were the major factor in reduction of neurogenesis (Mirescu et al., 2006). However, additional study reveals that adrenalectomized animals also show a significant reduction (more than 50%) in the number of proliferating cells with respect to control, thus suggesting that sleep deprivation reduces hippocampal neurogenesis, at least in part, by a glucocorticoid-independent mechanism (Guzman-Marin et al., 2007; Mueller et al., 2011).
Interestingly, the effect of sleep seems to be associated with changes in REM sleep (Guzman-Marin et al., 2008). The reduction in neurogenesis induced by sleep fragmentation is likely to underlie the delayed changes in cognitive function often observed after sleep deprivation (Sportiche et al., 2010). Finally, contrary to what has been reported from extended sleep deprivation, acute sleep deprivation (12 hrs) may upregulate hippocampal neurogenesis (Grassi-Zucconi et al., 2006; Junek et al., 2010). The mechanisms by which sleep deprivation affects neurogenesis are still unknown.
Our recent study suggests that NAS is critical for promoting an early event of neurogenesis in dentate gyrus (Sompol et al., 2011). Mice treated with NAS showed a significant increase (about 30 %) in NPCs that correlates with an increase in TrkB phosphorylation in the hippocampus (Sompol et al., 2011). The increase in NCPs in the dentate gyrus was inhibited by blockage of the TrkB receptors (Jang et al., 2010; Sompol et al., 2011).
A series of investigations have suggested that alterations of circadian rhythms may affect neurogenesis since many aspects of hippocampal physiology show significant circadian fluctuation (Chaudhury and Colwell, 2002; Chaudhury et al., 2005). Moreover, it has been reported that clock gene expression or clock-controlled gene expression is rhythmic in the hippocampus (Fukuhara et al., 2004; Jilg et al., 2010) and that the clock gene Period 2 is expressed in NPC (Borgs et al., 2009). Additional studies indicate that cell proliferation may vary with the time of day (Guzman-Marin et al., 2007; Tamai et al., 2008; Gilhooley et al., 2011). Our data indicate that the effect of NAS on NCP is independent from the time of day since exogenous NAS was equally effective in inducing NPC proliferation in both the active and sleeping phases in mice (Sompol et al., 2011). Interestingly, sleep deprivation significantly diminishes NPC proliferation in C57BL/6 mice but not in C3H mice, suggesting that in C3H mice endogenously generated NAS may reduce the negative consequences of sleep deprivation (Sompol et al., 2011).
Recently, it has been suggested that melatonin may contribute to neurogenesis (Rennie et al., 2009; Ramirez-Rodriguez et al., 2009; 2011; Manda and Reiter 2010). Melatonin has been reported to promote sleep in many animal models and in humans (Brzezinski et al., 2005; Buscemi et al., 2006; Ochoa-Sanchez et al., 2011) and administration of exogenous melatonin increases neurogenesis in the hippocampus (Rennie et al., 2009; Ramirez-Rodriguez et al., 2009; 2011; Manda and Reiter 2010). Our recent studies indicate that administration of exogenous melatonin does not increase the number of proliferating NPCs even with 3 weeks of treatment (Jang et al., 2010; Sompol et al., 2011). However, it is possible that melatonin may still promote neuronal survival once NPC proliferation has occurred (Ramirez-Rodriguez et al., 2011).
Nevertheless, the data available in the literature suggest that NAS and melatonin have different physiological functions, and NAS acts as a potent neuroprotective agent through the neurotrophic receptor TrkB, whereas melatonin exerts its biological effects through melatonin receptors or as an antioxidant. Further studies using melatonin receptor knock-out mice are required to fully understand the specific roles played by melatonin and NAS in neuroprotection and neurogenesis.
An important aspect of the action of NAS is the mechanisms by which NAS can induce neurogenesis. A large amount of published data indicates that BDNF modulates neuronal survival and may also affect neurogenesis via TrkB signaling. We have shown that NAS can activate TrkB and its associated signaling cascades and this is the likely mechanism whereby NAS promotes neurogenesis and neuronal survival (Jang et al., 2010, Figure 4).
Figure 4.
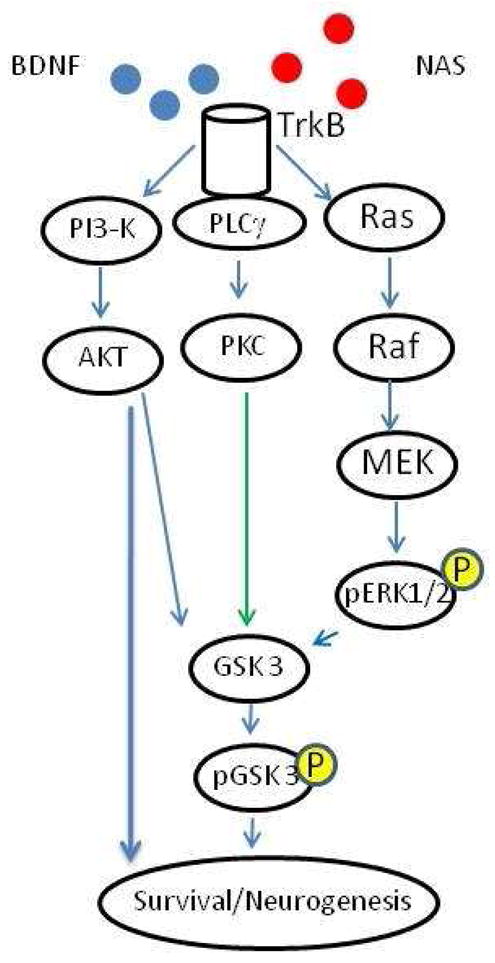
BDNF interacts with TrkB receptors activating pathways that promote neuronal survival and neurogenesis. NAS also activates TrkB and its downstream signaling pathways, but it is unclear whether NAS directly interacts with the TrkB receptor to promote its activation or triggers activation through unknown molecular effectors.
Sleep deprivation is a common characteristic of modern society and therefore understanding the negative effects of sleep loss on health and well being is an important step to prevent disease states associate with it and to develop effective treatment that may prevent the negative consequences of sleep deprivation. Emerging experimental evidence suggests that NAS, and its derivatives, may represent a new treatment that may prevent the decrease in cognitive function often observed in sleep-deprived individuals.
Is NAS involved in the Regulation of Circadian Rhythms?
In mammals, circadian rhythms are driven by a master pacemaker located in the SCN of the hypothalamus. The SCN is necessary for expression of most sustained circadian rhythms. Destruction of these cells eliminates most circadian rhythms of physiology and behavior, demonstrating the SCN’s ability to dictate circadian periodicity (Herzog and Tosini, 2004). The SCN is also responsible for the photic entrainment of circadian rhythms by receiving a direct projection from a specialized class of retinal ganglion cells (Paul et al., 2010). Previous studies have also implicated TrkB in the photic regulation of SCN function; TrkB receptors are expressed in the SCN (Liang et al., 1998; Allen and Earnest 2005) and blockade of TrkB signaling in the SCN inhibits the photic phase-shift (Liang et al., 2000). Finally, it has been shown that heterozygous TrkB mutant mice have significantly smaller phase shifts in response to light at night, compared to wild type controls (Allen et al., 2005). Our new data demonstrating that NAS can activate TrkB receptors (Jang et al., 2010) together with observation that NAS may be synthesized within the SCN (Hamada et al., 1999) raises the intriguing hypothesis that NAS, together or independently from BDNF, may also play a role in modulating the entrainment of circadian rhythms. Finally, it is also possible to speculate that NAS may serve an endogenous neuroprotective role in the SCN in order to provide an extra protection for the neurons located in this important key brain structure.
Conclusions
NAS has recently been found to play unexpected roles in neuronal cell biology by activating TrkB signaling. NAS may contribute to neurogenesis and neuronal survival, circadian rhythms and sleep, affective function, and hippocampus-dependent cognitive function. NAS derivatives may be useful as neuroprotectant drugs for the treatment of retinal degenerations and, perhaps, other neurodegenerative disorders.
Acknowledgments
Research in the authors’ laboratories is supported by grants from the National Institutes of Health [R01 NS43459, R21 EY028821, R01 EY022216 (G.T.); R01 CA127119, R01 NS43459 (K.Y.); R01 EY004864, P30 EY006360 (PMI)], and Research to Prevent Blindness, Inc. (RPB) (PMI). PMI is a recipient of Senior Scientific Investigator Award from RPB.
References
- Allen GC, Earnest DJ. Overlap in the distribution of TrkB immunoreactivity and retinohypothalamic tract innervation of the rat suprachiasmatic nucleus. Neurosci Lett. 2005;376:200–4. doi: 10.1016/j.neulet.2004.11.076. [DOI] [PubMed] [Google Scholar]
- Allen GC, Qu X, Earnest DJ. TrkB-deficient mice show diminished phase shifts of the circadian activity rhythm in response to light. Neurosci Lett. 2005;378:150–5. doi: 10.1016/j.neulet.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009;106:15043–8. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler R, Covington S, Klein DC. The rat arylalkylamine N-acetyltransferase gene promoter. cAMP activation via a cAMP-responsive element-CCAAT complex. J Biol Chem. 1997;272:6979–85. doi: 10.1074/jbc.272.11.6979. [DOI] [PubMed] [Google Scholar]
- Baler R, Covington S, Klein DC. Rat arylalkylamine N-acetyltransferase gene: upstream and intronic components of a bipartite promoter. Biol Cell. 1999;91:699–705. [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Borgs L, Beukelaers P, Vandenbosch R, Nguyen L, Moonen G, Maquet P, et al. Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci. 2009;10:30. doi: 10.1186/1471-2202-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, Ford I. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–0. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385–93. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Effects of total sleep loss on sleep tendency. Percept Mot Skills. 1979;48:495–06. doi: 10.2466/pms.1979.48.2.495. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear- conditioned mice. Behav Brain Res. 2002;133:95–08. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 20:225–36. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HD, Park TJ, Lee YK, Lee TG, Kim KT. Rapid and simple measurement of serotonin N-acetyltransferase activity by liquid biphasic diffusion assay. Neurochem Int. 1999;35:447–41. doi: 10.1016/s0197-0186(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Chattoraj A, Liu T, Zhang LS, Huang Z, Borjigin J. Melatonin formation in mammals: in vivo perspectives. Rev Endocr Metab Disord. 2009;10:237–43. doi: 10.1007/s11154-009-9125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danias J, Lee KC, Zamora MF, Chen B, Shen F, Filippopoulos T, et al. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–62. doi: 10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K. Agglomerating: a narrative review. Eur Neuropsychopharmacol. 2011;4:S703–9. doi: 10.1016/j.euroneuro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;11:1313–17. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Dirden JC, Tosini G. Photic regulation of melatonin in rat retina and the role of proteasomal proteolysis. Neuroreport. 2001;12:3833–7. doi: 10.1097/00001756-200112040-00046. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GC-K, Storm DR, Iuvone PM, et al. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neuroscience. 2004;24:1803–11. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastel JA, Roseboom PH, Rinaldi PA, Weller JL, Klein DC. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science. 1998;279:1358–60. doi: 10.1126/science.279.5355.1358. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Gastel JA, Weller JL, Schwartz C, Jaffe H, Namboodiri MA, et al. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc Natl Acad Sci USA. 2001;98:8083–88. doi: 10.1073/pnas.141118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet S, Palkovits M, Namboodiri MA. Regional distribution of arylamine and arylalkylamine N-acetyltransferase activities in the rat brain. Brain Res. 1991;539:355–7. doi: 10.1016/0006-8993(91)91645-h. [DOI] [PubMed] [Google Scholar]
- Gilhooley MJ, Pinnock SB, Herbert J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neurosci Lett. 2011;489:177–81. doi: 10.1016/j.neulet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999;96:5263–7. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi Zucconi G, Cipriani S, Balgkouranidou I, Scattoni R. ‘One night’ sleep deprivation stimulates hippocampal neurogenesis. Brain Res Bull. 2006;69:375–81. doi: 10.1016/j.brainresbull.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–6. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–33. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Bashir T, Nienhuis R, Szymusiak R, McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep. 2008;31:167–75. doi: 10.1093/sleep/31.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Hamada T, Ootomi M, Horikawa K, Niki T, Wakamatu H, Ishida N. The expression of the melatonin synthesis enzyme: arylalkylamine N-acetyltransferase in the suprachiasmatic nucleus of rat brain. Biochem Biophys Res Commun. 1999;258:772–7. doi: 10.1006/bbrc.1999.0668. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Tosini G. The Mammalian Circadian Shop. Sem Cell & Devel Biol. 2001;12:295–04. doi: 10.1006/scdb.2001.0257. [DOI] [PubMed] [Google Scholar]
- Vickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–31. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010;107:3876–81. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–7. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, Dehghani F, et al. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2010;20:377–88. doi: 10.1002/hipo.20637. [DOI] [PubMed] [Google Scholar]
- Johnson EO. Sleep in America: 2000. National Sleep Foundation; Washington, DC: 2000. [Google Scholar]
- Junek A, Rusak B, Semba K. Short-term sleep deprivation may alter the dynamics of hippocampal cell proliferation in adult rats. Neuroscience. 2010;170:1140–52. doi: 10.1016/j.neuroscience.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Klein DC, Weller JL. Rapid light-induced decrease in pineal serotonin N-acetyltransferase activity. Science. 1972;177:532–3. doi: 10.1126/science.177.4048.532. [DOI] [PubMed] [Google Scholar]
- Klein DC, Buda MJ, Kapoor CL, Krishna G. Pineal serotonin N-acetyltransferase activity: abrupt decrease in adenosine 3′,5′-monophosphate may be signal for “turnoff”. Science. 1978;199:309–11. doi: 10.1126/science.202027. [DOI] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, et al. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–57. [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung CH, Steinberg RH. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–02. [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–12. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Sohrabji F, Miranda R, Earnest B, Earnest D. Expression of brain-derived neurotrophic factor and its cognate receptor, TrkB, in the rat suprachiasmatic nucleus. Exp Neurol. 1998;151:184–93. doi: 10.1006/exnr.1998.6804. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Allen G, Earnest D. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J Neurosci. 2000;20:2978–87. doi: 10.1523/JNEUROSCI.20-08-02978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Ma DK, Kim WR, Ming GL, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann N Y Acad Sci. 2009;1170:664–73. doi: 10.1111/j.1749-6632.2009.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda K, Reiter RJ. Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Prog Neurobiol. 2010;(90):60–8. doi: 10.1016/j.pneurobio.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91:1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A. 2006;103:19170–5. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mueller AD, Mear RJ, Mistlberger RE. Inhibition of hippocampal neurogenesis by sleep deprivation is independent of circadian disruption and melatonin suppression. Neuroscience. 2011;193:170–81. doi: 10.1016/j.neuroscience.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–80. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM. Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists. Br J Pharmacology. 1999;127:1288–94. doi: 10.1038/sj.bjp.0702658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–7. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spading G, et al. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31:18439–52. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug GF, McCauley R, McIntyre IM, Filipowicz C. Selective inhibition of MAO-A but not MAO-B activity increases rat pineal melatonin. J Neural Transm. 1985;61:265–70. doi: 10.1007/BF01251917. [DOI] [PubMed] [Google Scholar]
- Oxenkrug G. Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications. Ann N Y Acad Sci. 2005;1053:334–47. doi: 10.1196/annals.1344.029. [DOI] [PubMed] [Google Scholar]
- Paul KN, Saafir TN, Tosini G. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev Endocr Metab Disord. 2009;10:271–78. doi: 10.1007/s11154-009-9120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Hsu LL, Mandell AJ. Extrapineal N-acetyltransferase activity in rat brain. Life Sci. 1974;15:2135–43. doi: 10.1016/0024-3205(74)90030-7. [DOI] [PubMed] [Google Scholar]
- Pozdeyev NV, Taylor C, Haque R, Chaurasia SS, Visser A, Thazyeen A, et al. Photic Regulation of Arylalkylamine N-Acetyltransferase Binding to 14–3–3 Proteins in Retinal Photoreceptor Cells. J Neurosci. 2006;26:9153–61. doi: 10.1523/JNEUROSCI.1384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakhie IV, Oxenkrug GF. The effect of nifedipine, Ca(2+) antagonist, on activity of MAO inhibitors, N-acetylserotonin and melatonin in the mouse tail suspension test. Int J Neuropsychopharmacol. 1998;1:35–40. doi: 10.1017/S1461145798001096. [DOI] [PubMed] [Google Scholar]
- Psarakis S, Pulido OM, Brown GM, Grota LJ, Smith GK. Identification and quantification of n-acetylserotonin (NAS) in the developing hippocampus of the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:439–422. doi: 10.1016/s0278-5846(82)80124-3. [DOI] [PubMed] [Google Scholar]
- Ramírez-Rodríguez G, Klempin F, Babu H, Benítez-King G, Kempermann G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology. 2009;34:2180–91. doi: 10.1038/npp.2009.46. [DOI] [PubMed] [Google Scholar]
- Ramirez-Rodriguez G, Ortíz-López L, Domínguez-Alonso A, Benítez-King GA, Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011;50:29–37. doi: 10.1111/j.1600-079X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–62. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Rennie K, De Butte M, Pappas BA. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J Pineal Res. 2009;47:313–7. doi: 10.1111/j.1600-079X.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–97. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- San L, Arrant B. Agglomerating: a novel mechanism of antidepressant action involving the melatonergic and the serotonergic system. Eur Psychiatry. 2008;23:396–02. doi: 10.1016/j.eurpsy.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Shen J, Ghai K, Sompol P, Liu X, Cao X, Iuvone PM, et al. N-acetyl serotonin derivatives as potent neuroprotectants for retinas. Proc Natl Acad Sci U S A. 2012;109:3540–5. doi: 10.1073/pnas.1119201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spading G, Bedini A, Rivara S, Mor M. Melatonin receptor agonists: new options for insomnia and depression treatment. CNS Neurosci Ther. 2011;17:733–41. doi: 10.1111/j.1755-5949.2010.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompol P, Liu X, Baba K, Paul KN, Tosini G, Iuvone PM, et al. N-acetylserotonin promotes hippocampal neuroprogenitor cell proliferation in sleep-deprived mice. Proc Natl Acad Sci U S A. 2011;108:8844–9. doi: 10.1073/pnas.1105114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportiche N, Suntsova N, Methippara M, Bashir T, Mitrani B, et al. Sustained sleep fragmentation results in delayed changes in hippocampal-dependent cognitive function associated with reduced dentate gyrus neurogenesis. Neuroscience. 2010;170:247–58. doi: 10.1016/j.neuroscience.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai S, Sanada K, Fukada Y. Time-of-day-dependent enhancement of adult neurogenesis in the hippocampus. PLoS One. 2008;3:e3835. doi: 10.1371/journal.pone.0003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T, Manev H. Chronic fluoxetine administration increases the serotonin N-acetyltransferase messenger RNA content in rat hippocampus. Biol Psychiatry. 1999;45:175–9. doi: 10.1016/s0006-3223(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Uz T, Qu T, Sugaya K, Manev H. Neuronal expression of arylalkylamine N-acetyltransferase (AANAT) mRNA in the rat brain. Neurosci Res. 2002;42:309–16. doi: 10.1016/s0168-0102(02)00011-1. [DOI] [PubMed] [Google Scholar]
- Zhao N, Zhong C, Wang Y, Zhao Y, Gong N, Zhou G, Xu T, Hong Z. Impaired hippocampal neurogenesis is involved in cognitive dysfunction induced by thiamine deficiency at early pre-pathological lesion stage. Neurobiol Dis. 2008;29:176–85. doi: 10.1016/j.nbd.2007.08.014. [DOI] [PubMed] [Google Scholar]