Abstract
Plasma cell leukemia (PCL) represents a rare and aggressive form of plasma cell dyscrasia which can be primary (pPCL) or secondary (sPCL). It is diagnosed based on absolute plasma cell count of more than 2.0 × 109/l or a relative proportion of greater than 20% of the peripheral blood leukocyte count. Although pPCL and sPCL share several clinical features, important differences exist. Patients with pPCL are younger; often have extra osseous organ involvement (liver, spleen and other extramedullary sites), increased frequency of renal failure, fast declining performance status and rapid progression to the terminal stage. Patients with sPCL have advanced bone disease. Presented in this article is India data of a short series of five cases of PCL diagnosed at a tertiary care centre from south India over last 5 years. All cases were de novo and had varied spectrum of presentation and so were not suspected to be plasma cell dyscrasia clinically. Detailed hemato-pathological evaluation clinched the diagnosis in all the cases.
Keywords: Plasma cell leukemia, Indian data, Immunohistochemistry
Introduction
Extramedullary plasma cell cancers, such as plasma cell leukemia (PCL) and multiple extramedullary plasmacytomas (MEP) are very aggressive malignancies. These can be primary (de novo) or secondary. Primary PCL (pPCL) presents de novo in the leukemic phase without a prior history of a plasma cell dyscrasia, while secondary PCL (sPCL) arises in the context of a pre-existing multiple myeloma (MM) [1]. Primary PCL is a rare disorder, representing less than 5% of malignant plasma cell diseases [2] and characterized by plasma cells (PC) circulating in the peripheral blood. Diagnostic criteria were defined by Kyle et al. [3] as an absolute plasma cell count of more than 2.0 × 109/l or a relative proportion of greater than 20% of the peripheral blood leukocyte count. Some investigators consider this number to be arbitrary and have relied more on the clinical behavior of the disease rather than the absolute number of circulating malignant PC [4].
PCL remains an incurable and highly resistant disease. The disease is usually progressive with dismal outcome; sPCL rarely responds to chemotherapy because patients have already received alkylating agents and have become resistant to them; in the primary form, responses have been observed to be higher with combination therapy than with single alkylating agents. Although pPCL may respond to treatment initially, these responses are short lived with dismal median overall survival of 8 months. On the other hand, sPCL is an exceedingly resistant, rapidly progressive and fatal disease with median overall survival of 2 months [5].
We present data of our personal experience of PCL diagnosed at a tertiary care centre from south India over the last 5 years.
Results
There were 89 cases of plasma cell dyscrasias diagnosed over last 5 years (mid 2005–mid 2010) out of which there were five PCL (Cases 1–5). The clinico-hematological profile of these five cases is given in Table 1. All patients of PCL were diagnosed on the basis of circulating PC in the peripheral blood comprising more than 20% along with bone marrow plasmacytosis. The age of the patients ranged from 37 to 62 years and there were three male and two female patients.
Table 1.
Clinico-hematological profile of five patients with plasma cell leukemia
| Patient no | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (years) | 46 | 62 | 37 | 45 | 45 |
| Sex | M | M | M | F | F |
| Chief complaints | Easy fatigability | Abdominal distension, pedal edema, decreased appetite | Fever, abdominal pain | Chest pain | DUB, multiple subcutaneous nodules |
| Duration | NA | 15 days | 1 month | 1 week | 2 months |
| Hepatomegaly | + | + | + | − | + |
| Splenomegaly | − | + | + | − | + |
| Lymphadenopathy | − | − | − | + | + |
| Any other finding | − | Ascitis | − | CXR-mediastinal widening USG abdomen-multiple intra-abdominal lymph nodes | − |
| Bony lytic lesions | + | − | − | − | − |
| Hb (g/l) | 60 | 60 | 36 | 90 | 57 |
| TLC × 109/l | 18.8 | 34.3 | 59.5 | 24.8 | 75.0 |
| Platelet count × 109/l | 100 | 92 | 11 | 207 | 32 |
| % of Plasma cells in peripheral smear | 60 | 70 | 39 | 51 | 79 |
| Morphology | Lymphoplasmacytic | Plasmacytic | Lymphoplasmacytic, plasmacytic, plasmablastic | Plasmacytic, | Plasmacytic, lymphoplasmacytic |
| Bone marrow (BM) | Hypercellular, 65% plasma cells and plasmablasts | Hypercellular, 70% plasma cells | Hypercellular, 44% plasma cells, lymphoplasmacytoid cells and plasma blasts | Hypercellular, 90% plasma cells many with anaplastic morphology | Post mortem BM biopsy- diffuse infiltration by plasma cells |
| IHC on biopsy (Bx) | NA | NA | BM Bx | BM Bx | Skin Bx |
| Kappa +, Lambda − | Lambda +, Kappa − | Kappa +, Lambda − | |||
| Urine BJP | − | NA | − | − | NA |
| SPE (M Band) | + | NA | − | NA | NA |
NA not available, + present, − absent
The patients had diverse nonspecific presentation such as easy fatigability (1 case), loss of appetite (1 case), fever (1 case), abdominal distension and pedal edema (1 case), chest pain (1 case) and dysfunctional uterine bleeding and subcutaneous nodules (1 case). Hence many of them at presentation were clinically not suspected to be plasma cell dyscrasia. The duration of illness ranged from 1 week to 2 months and none of the patients had a prior diagnosis of plasma cell dyscrasia. Hepatosplenomegaly was present in three patients and only hepatomegaly in one patient. Lymphadenopathy was present in two patients. One patient presented with Mediastinal widening on chest X-ray and USG abdomen showed multiple intra-abdominal lymph nodes (Case 4). Bony lytic lesions were present in one patient.
At presentation all patients had anemia and four had thrombocytopenia. All cases presented with leukocytosis and PC in peripheral blood ranged from 39 to 79%. Bone marrow examination carried out in all cases showed plasmacytosis ranging from 44 to 90% with suppressed normal haematopoesis. The morphology of PC ranged from plasmacytic, plasmablastic to anaplastic and several cases showed lymphoplasmacytic cells especially in the peripheral blood smear (Fig. 1a). Serum protein electrophoresis (SPE) report available in two patients was positive for M band in one patient; Bence-Jones proteinuria evaluated in three patients was negative and light chain monoclonality by immunohistochemistry (IHC) done on biopsy section onion was available in three cases (Table 1).
Fig. 1.
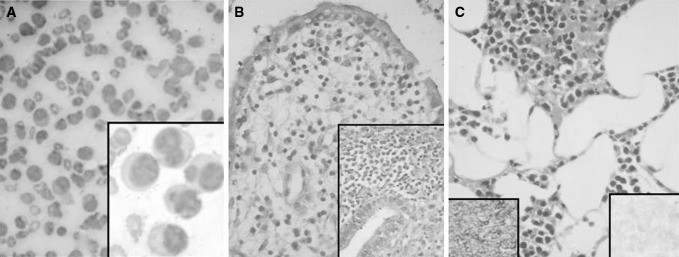
Case 5. a Marked peripheral blood leukocytosis with several plasma cells and lymphoplasmacytic cells (Leishman ×400); Inset (Leishman ×1,000), b Endometrial biopsy showing infiltration by plasma cells (H & E ×200); Inset (H & E ×400), c Skin biopsy showing infiltration by plasma cells in the subcutaneous fat (H & E ×200); Left Inset showing strong kappa positivity (IHC ×400), Right Inset showing lambda negativity (IHC ×400)
The patient with youngest age at presentation in this series (case 3) had renal failure and underwent dialysis. He was given dexamethasone and symptomatic treatment; however, he succumbed within 20 days of hospitalization. Cases 1 and 2 were lost to follow up.
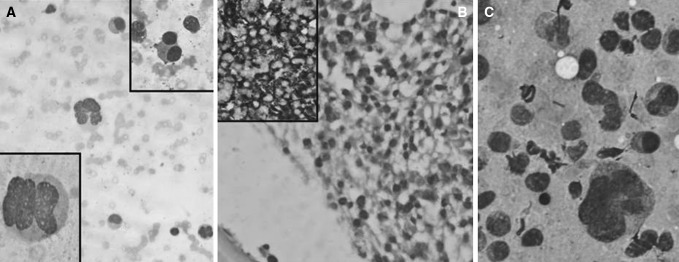
In the case with anaplastic morphology (Case 4) (Fig. 2a), because of the diagnostic difficulty, IHC was done on the BM biopsy which showed strong lambda positivity (Fig. 2b). The same patient had multiple intra-abdominal lymph nodes and had undergone an USG guided FNAC which showed sheets of PC some with anaplastic morphology and some with multilobated nuclei (Fig. 2c). Bone marrow examination along with IHC helped in the final diagnosis of PCL with MEP. The patient was explained about the poor prognosis and opted for no chemotherapy. She was discharged on request with symptomatic management.
Fig. 2.
Case 4. a Diluted bone marrow smear with plasma cells having anaplastic morphology (Leishman ×400); Lower Inset (Leishman ×1,000); Upper Inset showing occasional binucleate plasma cells (Leishman ×400), b Bone marrow biopsy showing interstitial infiltrate of plasma cells (H & E ×400); Inset showing lambda light chain positivity (IHC ×200), c USG-guided FNAC from intra-abdominal lymph nodes showing sheets of plasma cells, some having anaplastic morphology (MGG ×400)
The patient with DUB (Case 5) was hospitalized and underwent further investigations. Endometrial biopsy showed infiltration by atypical PC (Fig. 1b). The same patient also had multiple subcutaneous nodules clinically suspected to be nodular vasculitis. Skin biopsy showed dense infiltration by atypical PC which on IHC showed strong kappa positivity (Fig. 1c). The patient went to altered sensorium and rapidly succumbed to her illness before any definitive therapy could be initiated. We received her postmortem liver lung and bone marrow biopsies which showed infiltration by atypical PC. Hence the final autopsy diagnosis was PCL with widespread dissemination to skin, liver, lungs and endometrium.
Discussion
Primary PCL is a rare and aggressive subtype of plasma cell dyscrasia. The incidence found in this series was 5.6% (5 in 89 cases over 5 years) which is higher than what was earlier reported in a North Indian series of patients (4.5%) [6] and an earlier study from our centre (two cases over 10 years) [7]. The youngest patient in this series was 37 years although there are Indian reports of pPCL at even younger age of presentation at 21 years [8].
Primary PCL has been reported to be associated with prior exposure to chemotherapy and/or radiotherapy; however, this association is difficult to confirm due to low incidence of the disease [9]. Secondary PCL develops from a preexisting plasma cell dyscrasia. Recent attempts at genetic and molecular profiling of PCL have shown cytogenetic abnormalities in over 70% of PCL patients [10]. Hypodiploidy and complex karyotypes with multiple numerical and structural abnormalities involving chromosome 1, 13 and 14 are reported in a significant number of PCL cases [4, 5, 11] including report from India of multiple cytogenetic abnormality in a case of pPCL [12].
Although pPCL and sPCL share several clinical features, important differences exist between them. Patients with pPCL are younger; often have extra osseous organ involvement, with increased frequency of renal failure, fast declining performance status and rapid progression to the terminal stage. The liver, spleen and other extramedullary sites are more commonly involved than sPCL [11]. Compared to pPCL, patients with sPCL have advanced bone disease [1]. All cases in this series of patients were de novo and had a younger age of presentation, a shorter duration of illness, lesser incidence of bony lytic lesions and greater incidence of extramedullary involvement compared to a series of five cases of PCL from North Indian group of patients reported earlier [6] probably indicating some form of progression of a plasma cell dyscrasia to sPCL in that subgroup. Another large study from North India [13] also reported organomegaly and bleeding tendency to be more common in pPCL. That study reported frequent misdiagnosis as acute leukemia or the leukemic spill of lymphoma on the initial peripheral smear in pPCL.
Flow cytometry is an important diagnostic tool for the evaluation of peripheral blood to confirm circulating clonal PC. Akin to the malignant PC from MM or MGUS patients, malignant PCL cells also express CD38 and CD138 [1]. However, expression of CD20 is more prevalent and there is reduced expression of CD56, CD117, HLA-DR and CD9 antigens on PCL cells compared to MM [5]. A recent study from India [14] using Multicolor flow cytometry (MFC) in ten cases of PCL found MFC immunophenotyping useful in differentiating PCL from other chronic lymphoproliferative disorders with plasmacytoid morphology. Co-expression of CD38 and CD138 was best combination to identify the PC by MFC in their study.
In pPCL, similar to MM, IgG is the most common monoclonal protein. Increased frequency of Bence-Jones proteinuria as an isolated paraproteinemia has also been reported [5] but was absent in our series of patients. Compared to IgG or IgA myeloma reported in sporadic case reports [15], a higher proportion of light chain only, IgD and IgE myeloma present as PCL [16]. The morphologic characteristic of the leukemic PC show similar spectrum of other myelomas but often the PC are small with less cytoplasm resembling plasmacytoid lymphocytes [16] as seen in several of our cases.
The incidence of several adverse prognostic indicators (serum β2-microglobulin, proportion of S-phase PCs, proteinuria, serum calcium level, lactate dehydrogenase [LDH] and renal function derangement), has been found to be significantly higher in PCL versus MM [5]. Genomic and clinical differences between PCL and myeloma have been recognized. The presence of p53 deletion, 13q deletions, karyotypic complexity, hypodiploidy and 1q gains could confer an advanced stage on PC disease progression characterized by therapy resistance and a dismal prognosis [17]. There are older sporadic reports from India of treatment of primary PCL with single agent ifosfamide [18] and more recent reports of autologous stem cell transplantation in patients with pPCL who relapsed after initial remission [19, 20]. Unlike MM, survival with PCL has not improved over time. Prospective multicenter clinical trials exploring more aggressive regimens, including the combination of chemotherapy, immunomodulatory drugs, proteasome inhibitors, and other novel compounds, may be necessary to increase the complete response rates and survival in patients with this rare and highly lethal disease [21].
In conclusion, PCL represents a unique subset of plasma cell dyscrasia characterized by an aggressive disease with greater incidence of extramedullary organ involvement (liver, spleen, LN, etc.), poor prognosis and a shorter survival compared to patients presenting with classic myeloma.
References
- 1.Sher T, Miller KC, Deeb G, Lee K, Chanan-Khan A. Plasma cell leukaemia and other aggressive plasma cell malignancies. Br J Haematol. 2010;150(4):418–427. doi: 10.1111/j.1365-2141.2010.08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell leukemia. Br J Haematol. 1994;88(44):754–759. doi: 10.1111/j.1365-2141.1994.tb05114.x. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974;133(5):813–818. doi: 10.1001/archinte.133.5.813. [DOI] [PubMed] [Google Scholar]
- 4.Avet-Loiseau H, Daviet A, Brigaudeau C, Callet-Bauchu E, Terre C, Lafage-Pochitaloff M, Desangles F, Ramond S, Talmant P, Bataille R. Cytogenetic, interphase, and multicolor fluorescence in situ hybridization analyses in primary plasma cell leukemia: a study of 40 patients at diagnosis, on behalf of the Intergroupe Francophone du Myelome and the Groupe Francais de Cytogenetique Hematologique. Blood. 2001;97:822–825. doi: 10.1182/blood.V97.3.822. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Sanz R, Orfao A, Gonzalez M, Tabernero MD, Blade J, Moro MJ, Fernandez-Calvo J, Sanz MA, Perez-Simon JA, Rasillo A, Miguel JF. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999;93:1032–1037. [PubMed] [Google Scholar]
- 6.Kar R, Dutta S, Bhargava R, Kumar R, Pati HP. Immunoglobulin free light chains-do they have a role in plasma cell leukemia? Hematology. 2008;13(6):344–347. doi: 10.1179/102453308X343365. [DOI] [PubMed] [Google Scholar]
- 7.Badhe BA, Basu D, Toi P Ch, Dutta TK, Ghotekar LH. Plasma cell leukemia—a case report. Indian J Pathol Microbiol. 2003;46(3):484–487. [PubMed] [Google Scholar]
- 8.Raj RS, Najeeb S, Aruna R, Pavithran K, Thomas M. Primary plasma cell leukemia occurring in the young. Indian J Cancer. 2003;40(3):116–117. [PubMed] [Google Scholar]
- 9.Candoni A, Tiribelli M, Fanin R. Plasma cell leukemia occurring in a patient with thrombocythemia treated with hydroxyurea and busulphan. Leuk Lymph. 2004;45:821–824. doi: 10.1080/10428190310001615710. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, Davies FE, Drach J, Greipp PR, Kirsch IR, Kuehl WM, Hernandez JM, Minvielle S, Pilarski LM, Shaughnessy JD, Jr, Stewart AK, Avet-Loiseau H. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.CAN-03-2876. [DOI] [PubMed] [Google Scholar]
- 11.Colovic M, Jankovic G, Suvajdzic N, Milic N, Dordevic V, Jankovic S. Thirty patients with primary plasma cell leukemia: a single center experience. Med Oncol. 2008;25:154–160. doi: 10.1007/s12032-007-9011-5. [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Mohammad N, Palanki SD, Vaniawala SN. Primary plasma cell leukemia with light chain secretion and multiple chromosomal abnormalities: how successfully treated? A case report with review of literature. Indian J Med Paediatr Oncol. 2010;31(3):96–100. doi: 10.4103/0971-5851.73603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majumdar N, Kumar R, Anand M, Kalita D, Ghara N, Chopra A, Medhi K, Sharma A, Kumar L, Raina V. Plasma cell leukemia—a study of 28 cases from India. Hematology. 2009;14(4):198–203. doi: 10.1179/102453309X426191. [DOI] [PubMed] [Google Scholar]
- 14.Tembhare PR, Subramanian PG, Sehgal K, Yajamanam B, Kumar A, Gadge V, Inamdar N, Gujral S. Immunophenotypic profile of plasma cell leukemia: a retrospective study in a reference cancer center in India and review of literature. Indian J Pathol Microbiol. 2011;54(2):294–298. doi: 10.4103/0377-4929.81603. [DOI] [PubMed] [Google Scholar]
- 15.Singh T, Premalata C, Sajeevan K, Jain A, Batra U, Saini K, Satheesh C, Babu KG, Lokanatha D. IgA Plasma cell leukemia. J Lab Physicians. 2009;1(1):19–21. doi: 10.4103/0974-2727.54800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mc Kenna RW, Kyle RA, Kuehi WM, Grogan TM, Harris NL, Coupland RW. Plasma cell neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haemoatopoietic and lymphoid tissue. Lyon: IARC Press; 2008. pp. 203–210. [Google Scholar]
- 17.Jimenez-Zepeda VH, Dominguez-Martinez VJ. Plasma cell leukemia: a highly aggressive monoclonal gammopathy with a very poor prognosis. Int J Hematol. 2009;89(3):259–268. doi: 10.1007/s12185-009-0288-3. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra H, Dhabhar BN, Saikia TK, Gopal R, Nadkarni KS, Nair CN, Advani SH. Ifosfamide in plasma cell leukemia: a report of two cases and review of the literature. Am J Hematol. 1992;40(3):226–228. doi: 10.1002/ajh.2830400313. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh K, Gosavi S, Pathare A, Madkaikar M, Rao VB, Mohanty D. Low cost autologous peripheral blood stem cell transplantation performed in a municipal hospital for a patient with plasma cell leukaemia. Clin Lab Haematol. 2002;24(3):187–190. doi: 10.1046/j.1365-2257.2002.00376.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh K, Madkaikar M, Iyer Y, Pathare A, Jijina F, Mohanty D. Systemic capillary leak syndrome preceding plasma cell leukaemia. Acta Haematol. 2001;106(3):118–121. doi: 10.1159/000046600. [DOI] [PubMed] [Google Scholar]
- 21.Ramsingh G, Mehan P, Luo J, Vij R, Morgensztern D. Primary plasma cell leukemia: a surveillance, epidemiology, end results database analysis between 1973, 2004. Cancer. 2009;115(24):5734–5739. doi: 10.1002/cncr.24700. [DOI] [PubMed] [Google Scholar]