Abstract
Purpose
To characterize the costs of caring for patients with open-angle glaucoma (OAG) in the United States (US) over time and to identify factors that influence these costs.
Design
Longitudinal cohort study.
Methods
Claims data from 19,927 newly-diagnosed OAG patients enrolled in a large US managed care network were reviewed to identify glaucoma-related charges for all incident OAG patients from 2001–2009. Average glaucoma-related charges for enrollees with OAG were characterized in six month blocks from the date of initial OAG diagnosis through the following 5 years. Factors associated with being an enrollee in the costliest 5% for glaucoma-related charges (accruing ≥$5810 in charges in the first 2 years) were identified using logistic regression.
Results
The costliest 5% of enrollees were responsible for $10,202,871 (24%) of all glaucoma-related charges. By comparison, those whose costs fell within the lower 50% of the cost distribution collectively amassed only $7,986,582 (19%) of all glaucoma-related charges. A spike in glaucoma-related charges occurred in the 6 month period around the time of OAG diagnosis, stabilized by 1 year following diagnosis, and remained relatively constant over time. Risk factors associated with being in the costliest 5% for glaucoma-related care included younger age, Northeastern US state residence, undergoing cataract surgery, and possessing ocular co-morbidities.(p<0.006 for all comparisons).
Conclusions
A small subset of enrollees account for a large proportion of all glaucoma-related charges. Understanding the characteristics of these individuals and finding ways to reduce disease burden and costs associated with their care can result in substantial cost savings.
Introduction
Open-angle glaucoma (OAG) is a chronic, progressive, incurable disease that affects over 2 million individuals in the United States and many more worldwide. It is the most common cause of blindness among African Americans1,2 and caring for patients with OAG in the United States carries a total societal cost estimated to be nearly $1 billion annually.3 Thus, developing an understanding of the resource use of people with glaucoma, and identifying those expected to have the largest resource utilization, will be important in a resource-constrained health care environment. Further, by collecting longitudinal information on resource use we can better quantify the value of slowing glaucoma progression through various interventions.
While there have been many studies examining the cost of caring for people with glaucoma, most have been based on persons with prevalent OAG and few have examined changes in cost of care over time.2–6 The few longitudinal studies lacked sufficient power to examine long-term trends in resource utilization, and none confined their study cohort to incident cases, thus limiting their ability to assess the impact of disease progression on cost of care.7–9 Two studies that used administrative datasets focused on comparing resource utilization of glaucoma care to that for other ocular conditions.10,11
In this investigation we examined two questions: 1) What is the pattern of resource use for patients with OAG over the first seven years following disease onset? and 2) What are the characteristics of those patients who have the greatest glaucoma-related resource use?
Methods
We identified patients with incident OAG from the i3 InVision Data Mart dataset (Ingenix, Eden Prairie, MN) taking an incidence approach to cost estimation.12 Incidence of OAG was determined using the criteria detailed below. We described resource use for glaucoma care over seven years in total, and stratified costs by resource type (glaucoma-related surgery, medications, eye visits, and diagnostic testing). In addition, we used logistic regression methods to evaluate demographic predictors of a person with OAG being in the top 5% of glaucoma resource users. These analyses were conducted from the payor perspective, meaning that the cost of care recognized is reflected in the provider’s paid (not billed) charges.
Data Source
The i3 InVision dataset contains fully de-identified records of all beneficiaries in a large, national, managed care network in the U.S. Included are beneficiaries in commercial, Medicaid and Medicare Advantage plans sponsored by the managed care provider providing the claims. We had access to data for a subset of beneficiaries who had any form of eye care from January 1, 2001 through December 31, 2009. This subset consisted of beneficiaries who had ≥1 International Classification of Diseases (ICD-9-CM) codes for any eye-related diagnosis (360–379.9), or Current Procedural Terminology (CPT-4) code for any eye-related visits, diagnostic or therapeutic procedures (65091–68899 or 92002–92499), or any other claims submitted by an ophthalmologist or optometrist during the beneficiary’s time in the medical plan.13 We had access to all inpatient and outpatient medical claims for ocular and non-ocular conditions along with outpatient pharmacy prescription records and socio-demographic information (age, sex, race, education level, household net worth and region of residence).
Study Participants
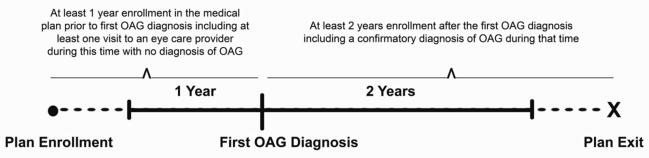
We identified all individuals who were continuously enrolled in the plan for three or more years and had one or more diagnoses of OAG (ICD-9-CM codes 365.1, 365.10, 365.11, 365.12, and 365.15) as the first through fifth diagnosis on the encounter form. From this group, we then identified all individuals with newly-diagnosed OAG (i.e., incident case) based on the following criteria (see also Figure 1):
Figure 1. Sample selection criteria for identifying enrollees with incident open-angle glaucoma.
This figure depicts the sample selection criteria used to identify enrollees with incident open-angle glaucoma. In the figure, “Plan Enrollment” is the date the individual entered the medical plan, “First OAG Diagnosis” is the date they were first diagnosed with open-angle glaucoma, and “Plan Exit” is the last date the enrollee was in the medical plan or the last date for which we had access to their billing records, December 31, 2009.
OAG - open-angle glaucoma
A visit to an eye care provider (optometrist or ophthalmologist) where the provider recorded an ICD-9-CM billing code for OAG (The date of this visit is the index date).
At least one year of enrollment in the medical plan prior to the index date without a claim that included a diagnosis of OAG. The enrollee must have also seen an eye care provider at least once during this period
At least one visit to an eye care provider subsequent to the index visit that included an OAG diagnosis
At least two years of continuous enrollment in the plan following the index visit
Charges
Glaucoma-related charges were identified by using CPT-4 billing codes and included all visits to ophthalmologists or optometrists, all glaucoma-related diagnostic procedures, all glaucoma-related laser and incisional surgeries, and all topical and oral intraocular pressure-lowering medications. The charges captured in this data source are the allowed charges. To account for inflation, all charges were adjusted 3% per year to 2009 US dollars. For each enrollee, all glaucoma-related charges from each encounter were summed to determine the total glaucoma-related charges. Next, we determined the total length of time in years that each enrollee was in the medical plan. Each beneficiary’s total glaucoma-related charges during their first two years after OAG diagnosis were used to determine the annual glaucoma-related charges (AGCs) for that individual.
Trends in Charges over Time
For each beneficiary, we computed the total glaucoma-related charges generated in the 12 months prior to their initial OAG diagnosis and then every six months from the first date of OAG diagnosis out to 5 five years following their initial glaucoma diagnosis. Next, we summed the total glaucoma-related charges for all the beneficiaries during each of these time intervals and divided each of these totals by the number of enrollees with OAG enrolled in the plan during that interval to determine the mean glaucoma-related charges incurred at each interval. Similar analyses were performed to assess mean charges for visits, glaucoma diagnostic procedures, glaucoma surgeries, glaucoma medications, and other eye surgeries at each interval.
Ranking of Enrollees Based on Glaucoma-Related Charges
Each enrollee was ranked according to their AGC generated during their first 2 years after the index date. Based on these rankings, we identified the beneficiaries in the top 5%, 10%, 25%, and 50% for AGCs. The total AGC for individuals in the top 5% was divided by the total AGC for all enrollees in the plan to determine the proportion of total AGCs that were generated by the top 5% costliest beneficiaries for glaucoma-related services. Similar calculations were performed for those in the top 10%, 25%, 50% and lower 50% for AGCs.
Statistical Analyses
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Participant characteristics were summarized for the entire sample using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Univariate and multivariable logistic regression analyses were conducted to ascertain whether socio-demographic factors, ocular conditions, or surgeries affected the likelihood of being in the top 5% for AGCs during the first two years following OAG diagnosis. Regression covariates included socio-demographic characteristics (age, sex, race, education, net worth), region of residence in the US, co-morbid ocular conditions (cataract, diabetic retinopathy, macular degeneration, pseudophakia / aphakia), diabetes mellitus, hypertension, hyperlipidemia, depression, dementia, and a measure of overall health, the Charlson Co-morbidity Index.14
The human studies offices at the University of Michigan and Washington University School of Medicine determined this study met conditions for being exempt from IRB review due to a lack of identifiable data in the data set.
Results
A total of 19,927 enrollees met our definition of incident OAG. These patients had a mean (standard deviation) age of 60.2 (11.0) years and were more likely to be female (55.2%). Most people with incident OAG were white (81.4%) followed by blacks (8.3%), Latinos (6.5%), and Asians (3.0%). Almost all were high school graduates (98.3%) and 24.7% had graduated from college. The majority of the enrollees with incident OAG (75.4%) had household net worth levels of ≥$150,000. (Table 1)
Table 1.
Descriptive statistics of enrollees with incident open-angle glaucoma
| Total (n=19927) | ||
|---|---|---|
| Categorical Variables | Frequency | Percent |
| Sex | ||
| Male | 8935 | 44.8 |
| Female | 10992 | 55.2 |
| Race | ||
| White | 14875 | 81.4 |
| Black | 1508 | 8.3 |
| Latino | 1185 | 6.5 |
| Asian | 546 | 3 |
| Other | 171 | 0.9 |
| Education | ||
| Less than High School | 326 | 1.7 |
| High School | 6721 | 34.9 |
| Some College | 7437 | 38.7 |
| College Diploma | 4714 | 24.5 |
| Post Graduate | 39 | 0.2 |
| Household Net Worth | ||
| under $25,000 | 1256 | 6.7 |
| $25–74,000 | 1150 | 6.2 |
| $75–149,000 | 2174 | 11.7 |
| $150–499,000 | 8464 | 45.4 |
| over $500,000 | 5593 | 30 |
| Region | ||
| North East | 3346 | 16.8 |
| South East | 7923 | 39.8 |
| Midwest | 5992 | 30.1 |
| West | 2645 | 13.3 |
| Other | 17 | 0.1 |
| AMDa | 2730 | 13.7 |
| DR (NPDR or PDR)b | 1835 | 9.2 |
| Cataract | 11725 | 58.8 |
| Pseudophakia or Aphakia | 3978 | 20.2 |
| Continuous Variables | Mean (SDc) | Min, Max |
| Age | 60.2 (11.0) | 41.0, 85.0 |
| Charlson Comorbidity Index | 4.2 (3.4) | 0.0, 29.0 |
AMD-age related macular degeneration
DR (NPDR or PDR) diabetic retinopathy (non proliferative or proliferative)
SD standard deviation
Distribution of Charges for Open Angle Glaucoma
Over the first two years after OAG diagnosis, a total of $42,333,499 was spent on glaucoma-related care for all of the enrollees with incident OAG, including $13,730,227 (32%) for visits to eye care providers, $13,022,649 (31%) for glaucoma medications, $6,906,353 (16%) for glaucoma diagnostic tests, and $8,674,269 (20%) for laser or incisional glaucoma surgeries.
Timing of Charges
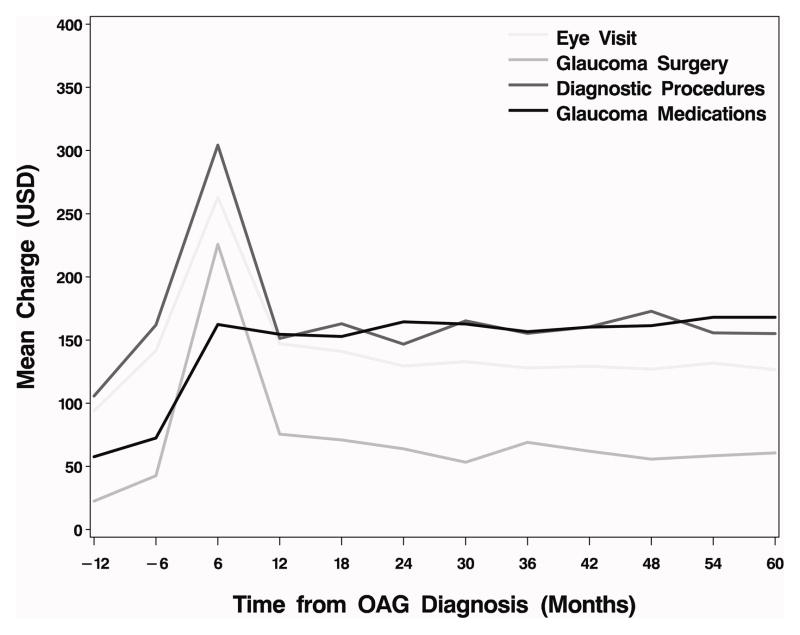
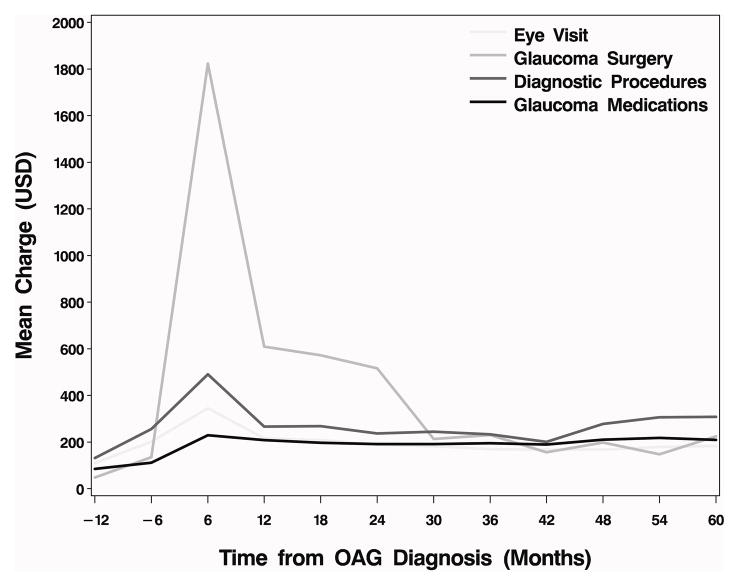
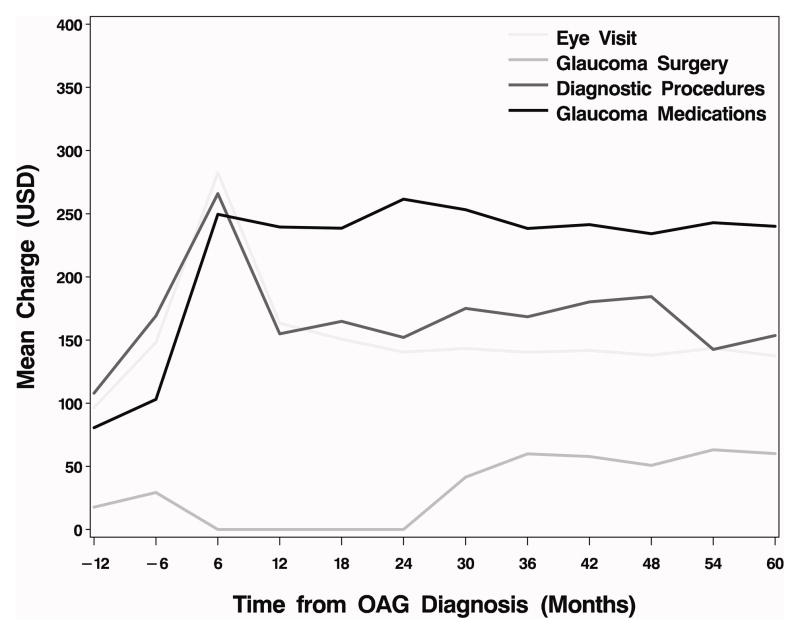
In Figure 2 we illustrate the trend in average charges for glaucoma related care for all individuals with incident OAG at 6-month intervals from one year prior to the time of diagnosis and through the following five years. Figure 3 depicts a similar plot among the subset of OAG patients who underwent at least one laser or incisional glaucoma surgery during their first two years in the plan. Likewise, Figure 4 shows a similar plot for those who had no laser or incisional glaucoma surgery during their first two years in the plan. The figures demonstrate that there is a spike in mean charges in the first six months following OAG diagnosis. For the overall group, diagnostic testing and glaucoma medications contributed more than office visits and glaucoma surgery to the mean charges. (Figure 2) However, among the subset of enrollees with incident OAG who underwent glaucoma surgery in the first 2 years (n=2,466; for laser surgery n=1550; for incisional surgery n = 752; both laser and incisional surgery n=164) charges for surgery itself far exceeded charges for visits, diagnostic tests, and medications. (Figure 3) Among the entire group of incident OAG patients, of all glaucoma-related charges experienced in the first two years following diagnosis, the proportion incurred in the first six months after diagnosis was 37.8%, in months 6–12 after diagnosis it was 21.0%, in months 12–18 it was 20.8%, and in months 18–24 it was 20.5%. By one year after diagnosis, the mean amount of glaucoma-related charges stabilized and remained relatively constant for subsequent time periods.
Figure 2. Mean charges for glaucoma-related services among all incident OAG patients.
Charges are for each 6 month period from -12 to 24 months from incident dx n=19927; at 30 mos n=15954; at 36 mos n=12242; at 42 mos n=9350; at 48 mos n=6634; at 54 mos n=5139; at 60 mos n=3730. (Incident OAG glaucoma surgery within first two years after dx n=2,466; for laser surgery n=1550; for incisional surgery n = 752; both laser and incisional surgery n=164)
OAG - open-angle glaucoma; USD - 2009 U.S. dollars; dx – diagnosis; mos- months
Figure 3. Mean charges for glaucoma-related services among incident OAG patients who underwent glaucoma surgery within the first two years following diagnosis.
Charges are for each 6 month period from −12 to 24 months from incident dx n=2466; at 30 mos n=1948; at 36 mos n=1500; at 42 mos n=1123; at 48 mos n=765; at 54 mos n=574; at 60 mos n=392
OAG - open-angle glaucoma; USD - 2009 U.S. dollars; dx – diagnosis; mos- months
Figure 4. Mean charges for glaucoma-related services among incident OAG patients who did not undergo any glaucoma surgery within the first two years following diagnosis.
Charges for each 6 month period from −12 to 24 months from incident dx n=10255; at 30 mos n=8302; at 36 mos n=6375; at 42 mos n=4955; at 48 mos n=3567; at 54 mos n=2760; at 60 mos n=2041
OAG - open-angle glaucoma; USD - 2009 U.S. dollars; dx – diagnosis; mos- months
Mean Charges for Glaucoma-Related Services per Enrollee
The mean charges accrued for glaucoma-related services was $955.37 in the first six months following OAG diagnosis, $528.23 from months 6–12 after diagnosis, $527.61 from months 12–18, and $504.40 from months 18–24. The mean cumulative charges for glaucoma-related services were $1484 at one year and $ 2516 at two years following disease diagnosis. (Table 2)
Table 2.
Glaucoma-Related Charges Since Date of First Diagnosis
| Time Since OAG Diagnosis | Mean (sd) Charges During Interval | 5 %a | 10 %a | 25 %a | 50%a | 75 %a | 90 %a | 95 %a | Mean Cumulative Charges Since Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 0 – 6 months | $ 955.37 ($ 1834.10) | $ 148.40 | $ 208.69 | $ 349.09 | $ 592.25 | $ 963.09 | $ 1699.52 | $ 2839.13 | $ 955.37 |
| 6 – 12 months | $ 528.23 ($ 1281.12) | $ 0 | $ 0 | $ 99.31 | $ 323.29 | $ 620.72 | $ 1030.88 | $ 1495.01 | $ 1483.60 |
| 12 – 18 months | $ 527.61 ($ 908.54) | $ 0 | $ 0 | $ 107.46 | $ 345.66 | $ 649.90 | $ 1059.03 | $ 1515.26 | $ 2011.21 |
| 18 – 24 months | $ 504.40 ($ 1859.16) | $ 0 | $ 0 | $ 72.10 | $ 311.85 | $ 620.26 | $ 1016.33 | $ 1440.18 | $ 2515.61 |
Percentile numbers refer to the percentile of the distribution; i.e., 5% refers to fifth percentile of the distribution.
OAG - open-angle glaucoma; sd - standard deviation
Characteristics of Enrollees with High Glaucoma-Related Charges
The costliest 5% of enrollees were responsible for $10,202,871 or 24.1% of all glaucoma-related charges. Those costliest 10% of enrollees were responsible for $15,017,635 or 35.5% of the total glaucoma-related charges. Likewise, the costliest 25% of enrollees were responsible for $24,453,281 or 57.8% of all glaucoma-related charges. By comparison, the bottom 50% of individuals, collectively amassed only $7,986,582 (18.9%) of all glaucoma-related charges.
Table 3 shows the results of a multivariable logistic regression analysis, which evaluated predictive characteristics of the costliest 5% of individuals with incident OAG. After adjustment for potential confounding factors, variables associated with being in the costliest top 5% of individuals with incident OAG included age at enrollment, region of residence, and presence of several co-morbid ocular conditions. The odds of being in the costliest 5% for glaucoma-related charges decreased 9% for every additional 5 years of age. Compared to individuals with incident OAG residing in northeastern US states, persons living in southeastern states had a 22% decreased odds of being in the top 5% for glaucoma-related charges, those residing in western states had a 19% decreased odds, and those living in Midwestern states had a 35% decreased odds. Individuals with incident OAG who had concomitant diabetic retinopathy or age-related macular degeneration had 97% and 38% increased odds of being in the top 5% for glaucoma-related charges, respectively. Those who underwent cataract surgery within the first 2 years of diagnosis of incident OAG had a 59% increased odds of being in the top 5% for glaucoma-related charges and those who were psuedophakic or aphakic had an 87% increased odds of being in the top 5% for glaucoma-related charges. (Table 3)
Table 3.
Factors Associated with Costliest 5% Recipients of Glaucoma Care Multivariable Logistic Regression Analysis
| Variable | ORa | 95% C.I.b | P-value |
|---|---|---|---|
| Region | |||
| Midwest vs. North East | 0.65 | (0.53, 0.78) | <0.0001 |
| South East vs. North East | 0.78 | (0.66, 0.93) | 0.0064 |
| West vs. North East | 0.81 | (0.64, 1.01) | 0.0639 |
| DR (NPDR or PDR)c (dxd in first 2 years) | 1.97 | (1.65, 2.34) | <0.0001 |
| AMDe (dx in first 2 years) | 1.38 | (1.15, 1.64) | 0.0004 |
| Cataract (surgery in first 2 years) | 1.59 | (1.31, 1.94) | <0.0001 |
| Pseudophakia/Aphakia (dx in first 2 years) | 1.87 | (1.59, 2.20) | <0.0001 |
| Age at enrollment (Per 5 year increments) | 0.91 | (0.88, 0.94) | <0.0001 |
OR - odds ratio;
C.I. - confidence interval
DR-diabetic retinopathy; NPDR-non-proliferative; PDR-proliferative
dx - diagnosis
AMD-age-related macular degeneration includes exudative or nonexudative macular degeneration
The following covariates were dropped from the model because they were not statistically significant at the p< 0.05 level: race, sex, level of education, household net worth, Charlson co-morbidity index score, and indicators for diabetes, hypertension, hyperlipidemia, dementia, and depression.
Discussion
In this report, we have shown that glaucoma patients consume the greatest relative share of resources during the first 6 months following diagnosis. This finding extends to up to 6 years the prior work of Kobelt and others who noted the higher costs of glaucoma care accrued in the first year compared to the second year of care.15 We found that after adjustment for a number of factors, those enrollees who are in the upper 5% of resource utilization were younger, more likely to have had cataract surgery and ocular co-morbidities, and live in the northeastern United States.
We took an incidence-based method for estimation of resource consumption so that we might determine the pattern of resource utilization from the time of diagnosis. We took this approach because if cost of care increases over time, or there are cost “spikes” associated with certain events, such scenarios could produce a difference in the cost-effectiveness of management strategies versus that which would exist if resource utilization is level over time. We found that the largest resource use for patients with glaucoma occurs around the time of diagnosis, and does not rise significantly thereafter for up to six years.
Our estimates show a lower cost on a per person annual basis, compared to the annualized costs estimated by Lee et al. (average of $1,796/year for Lee et al., versus an average of $1,248 (sd=$2,036) in the year following diagnosis in our analysis).9 One possible explanation for this difference is that the patients in our study were enrollees in a managed care population and thus were drawn from a wider range of physician practices than most chart review studies, such as Lee et al., which relied on practices of glaucoma specialists who are likely to be more aggressive in work up and management, and may deal with subjects who present with more advanced glaucoma. Further, the costs estimates by Lee et al. were driven by a stratified sampling strategy, which resulted in a larger number of severely affected glaucoma patients compared to any non-stratified sampling strategy. It has also been shown that community-based glaucoma patients predominantly cared for by non-specialists have differences in follow-up with physician appointments as compared to those receiving care by glaucoma specialists.16 In addition, Lee et al. used as their cost extension the median physician charges paid across the country for clinical services and the average wholesale price for medications. In the i3 InVision dataset, “costs” are the allowed charges, which are typically recognized as being less than that billed by the provider (or pharmacy) and potentially less, depending on the market, than even Medicare allowable rates.17 Finally, chart reviews reflect billed charges, and do not account for denied charges, whereas our analysis reflects allowed charges. These four factors together might account for the differences seen here.
Our study shows that once treatment is initiated, there is little change in resource use over time. We recognize that in part, this might be due to the improvement in management of glaucoma that we have seen with the introduction of prostaglandin analogs7 and the clinical evidence derived from the Collaborative Initial Glaucoma Treatment Study (CIGTS)18 and Early Manifest Glaucoma Trial (EMGT).19 Both of these trials demonstrated that aggressive intraocular pressure control in patients with newly-diagnosed OAG results in slow progression over time. While it is impossible to determine from claims data how well-controlled each enrollee’s OAG was, the constant rate of resource use noted in our analysis suggests that the majority of the enrollees were relatively stable, at least in the intensity of resource use, over the course of the follow-up.
In these analyses we also attempted to identify those patients who are most likely to have the highest charges, and thus help to target those populations most likely to benefit from aggressive screening and treatment. In doing so, we were limited by the lack of clinical information concerning the patient status. A number of clinical variables that have been shown to be associated with glaucoma progression were not available to us. These included cup-to-disc ratio, perimetric findings, intraocular pressure, and central corneal thickness.16–19 Thus, the finding that glaucoma-related charges increased with younger age may indicate that clinicians are more likely to be aggressive at managing the glaucoma of younger patients or that patients detected at an earlier age are more likely to have more severe or aggressive disease or a combination of both factors. In addition, our finding that enrollees who received care in the northeast U.S. were more likely to be in the top 5% than others is an intriguing finding for health policy makers. Since our study analyzes charges, it is likely that at least some of the difference may reflect differential rates of charges in the different geographic regions (as is present in Medicare allowable rates by CMS). Our finding of more costly recipients of glaucoma care differs from findings of an analysis by Friedman and colleagues who reported lower rates of major drivers of cost (glaucoma medication use and glaucoma surgery) among residents in the Northeast.20 Possible reasons for the differences between these studies include differences in disease severity (the analysis by Friedman included many glaucoma suspects), timing of when the enrollees were in the plan, differences in the population and provider pool, and regional changes in how providers are managing glaucoma patients over time.
We have examined the trends in resource utilization from a minimum of two to five years in people with glaucoma. Given the relative stability of glaucoma-related charges we are observing from one year onward after diagnosis, such estimates may be useful information that can be incorporated into future studies assessing the cost effectiveness or comparative effectiveness of interventions to treat this disease. While this study evaluated a source population that is large and quantified glaucoma-related charges for a longer time than prior studies, some might argue that we are in danger of extrapolating well beyond the available data and should not be making claims concerning how these findings apply to the experience of a glaucoma patient facing a lifelong disease. However, Quigley et al. have shown that the typical patient with glaucoma who is white lives with the disease for an average of 12.8 years and one who is black lives an average of 16.3 years.21 Therefore, our analyses have considered what would be nearly one half of the average glaucoma patient’s experience Our descriptive analysis has also shown that the period following diagnosis is the most expensive period in the patient’s lifetime. However, we cannot confidently extrapolate from the year one to five costs to the period beyond that time.
There are several limitations to our study that need to be acknowledged. First, the claims data used in this analysis do not contain adequate information to determine the severity of the OAG. Clearly, we would expect individuals with more severe OAG to require closer monitoring and more aggressive treatment which would, in turn, influence patterns of resource utilization. With the October 2011 changes to the ICD-9-CM codes, which require providers to code the level of glaucoma severity for each patient, future studies will be able to assess the influence of physician-coded disease severity on patterns of resource usage. Second, claims data are used primarily for billing purposes, not research purposes. Some enrollees may have been misdiagnosed or miscoded. Furthermore, there are important parameters that we were unable to consider in our analyses such as level of best corrected visual acuity, level of intraocular pressure, and extent of visual field loss which are not captured in claims data. Other factors that may influence resource utilization which were not considered in this analysis include provider factors (their level of training, access to equipment to perform ancillary glaucoma testing, whether they are able to prescribe medications or perform surgery) and insurance reimbursement. A final limitation is the loss to follow-up seen over time in our sample as our cohort ages from incidence to year five of follow-up. Given that our sample is drawn from a commercial insurance plan, this would not be unexpected. However, we must also point out that the information provided in Figures 2–4 is intended as a descriptive analysis and we are attempting no statistical inference. One might argue that the lack of change in resource utilization seen over time in our sample is a function of differential loss to follow-up with the largest users dropping out of the sample while lower resource users increase their utilization to offset this loss. However, we would argue that loss to follow-up is just as likely (if not more so) to be random. We leave the reader to make their own decision regarding which scenario is more likely, and acknowledge that future research to answer the question in a definitive manner is necessary.
In this analysis of a large administrative dataset, we have found that in people with incident glaucoma, the greatest resource use occurs at the time of diagnosis, and after that resource utilization continues at a steady pace over time (up to five years in our sample). These findings have importance for future evaluations of the cost-effectiveness of screening and treatment of glaucoma.
Acknowledgments
Funding/Support: Funding for this project was provided by Pfizer Inc. through a contract with Washington University and the project scope of work included preparation of this manuscript by the project team. Dr. Kymes and Ms. Peters are employees of Washington University whose salaries were supported, in part, by this contract. Project subcontracts included the University of Michigan; Dr. Musch and Ms. Niziol are employees of University of Michigan. Dr. Lee is a consultant to Pfizer and received compensation from Pfizer to assist in study design and interpretation of results. Dr. Stein is supported by National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511-01). Center for Economic Evaluation in Medicine (CEEM) is supported by the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR) at the National Institutes of Health (NIH) Grant Number UL1 RR024992.
Biographies

Steven Kymes is a health outcomes researcher with a doctoral degree in health services research from Saint Louis University School of Public Health (2001). He is a Research Associate Professor in the Department of Ophthalmology and Visual Sciences, with a joint appointment in the Division of Biostatistics. He also serves as the Director of the Center for Economic Evaluation in Medicine and as a Senior Fellow in the Washington University Center for Health Policy. Dr. Kymes has been the author or co-author of over 45 peer reviewed publications and has served as principal investigator on a number of NIH and industry funded grants. He has served on several NIH and CDC review panels and as a member of the Data and Safety Monitoring Committees for three National Eye Institute funded studies. Among Dr. Kymes’ current research interests are the development of economic models for the progression of glaucoma, the development of economic models for evaluation of treatment of retina disease, and new methods of health state valuation in vision.

Joshua D. Stein is an Assistant Professor of Ophthalmology and Visual Sciences at the University of Michigan. He is a health services researcher whose primary research interest involves using large health care claims databases to study utilization patterns and outcomes of eye care throughout the United States.
Footnotes
Financial Disclosures: Dr. Stein receives support from Pfizer Inc. Ms. Niziol has not financial disclosures. Dr. Musch receives support from Pfizer Inc. Dr. Lee serves as consultant for Allergan, Pfizer and Genentech; support from Alcon Research Institute, Pfizer and Washington University, NIH and Research Triangle Institute; equity owner in Pfizer and Merck; and patents with Duke University. Mr. Kotak is an employee of Pfizer Inc. Ms. Peters receives support from Pfizer Inc and Genentech. Dr. Kymes is a consultant for Allergan, Genentech and Pfizer; receives support from Genzyme.
Contributions of Authors: Design of the study (JS, PL, SKo, SKy); Data Acquisition (JS); Data Analysis and Interpretation (JS, LN, DM, PL); Drafting Article (JS, CP, SKy); Revising Article (JS, LN, DM, PL, SKo, CP, SKy); Contributing to Statistical Analysis (LN, DM, SKy); Obtaining Funding (PL, SKo, SKy); Administrative support (JS, CP, SKy); Supervision (PL, SKo, Sky).
The human studies offices at the University of Michigan and Washington University School of Medicine determined this study met conditions for being exempt from IRB review due to a lack of identifiable data in the data set.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman DS, Wolfs RC, O'Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiscella RG, Green A, Patuszynski DH, Wilensky J. Medical therapy cost considerations for glaucoma. Am J Ophthalmol. 2003;136(1):18–25. doi: 10.1016/s0002-9394(03)00102-8. [DOI] [PubMed] [Google Scholar]
- 3.Fiscella RG, Jensen MK. Cost analysis of glaucoma medications. Am J Ophthalmol. 2008;145(6):1108–1109. doi: 10.1016/j.ajo.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Oostenbrink JB, Rutten-van Molken MP, Sluyter-Opdenoordt TS. Resource use and costs of patients with glaucoma or ocular hyypertension: a one-year study based on retrospective chart review in the Netherlands. J Glaucoma. 2001;10(3):184–191. doi: 10.1097/00061198-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen J, Aagren M, Arnavielle S, et al. Late-stage, primary open-angle glaucoma in Europe: social and health care maintenance costs and quality of life of patients from 4 countries. Curr Med Res Opin. 2008;24(6):1763–1770. doi: 10.1185/03007990802111068. [DOI] [PubMed] [Google Scholar]
- 6.Doyle JW, Smith MF, Tierney JW. Glaucoma medical treatment 2002---does yearly cost now equal the year? Optom Vis Sci. 2002;79(8):489–492. doi: 10.1097/00006324-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Kobelt G, Texier-Richard B, Buchholz P, et al. Treatment of glaucoma in clinical practice: four-year results from a patient registry in France. J Glaucoma. 2010;19(3):199–206. doi: 10.1097/IJG.0b013e3181af31d6. [DOI] [PubMed] [Google Scholar]
- 8.Lee PP, Kelly SP, Mills RP, et al. Glaucoma in the United States and Europe: predicting costs and surgical rates based upon stage of disease. J Glaucoma. 2007;16(5):471–478. doi: 10.1097/IJG.0b013e3180575202. [DOI] [PubMed] [Google Scholar]
- 9.Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124(1):12–19. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 10.Lee PP, Levin LA, Walt JG, et al. Cost of patients with primary open-angle glaucoma: a retrospective study of commercial insurance claims data. Ophthalmology. 2007;114(7):1241–1247. doi: 10.1016/j.ophtha.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Bramley T, Peeples P, Walt JG, Juhasz M, Hansen JE. Impact of vision loss on costs and outcomes in medicare beneficiaries with glaucoma. Arch Ophthalmol. 2008;126(6):849–856. doi: 10.1001/archopht.126.6.849. [DOI] [PubMed] [Google Scholar]
- 12.Frick KD, Kymes SM, Lee PP, et al. The cost of visual impairment: purposes, perspectives, and guidance. Invest Ophthalmol Vis Sci. 2010;51(4):1801–1805. doi: 10.1167/iovs.09-4469. [DOI] [PubMed] [Google Scholar]
- 13.Physician International Classification of Diseases (ICD-9CM) 9th Revision, Clinical Modification. 1 and 2. Chicago: American Medical Association Press; 2006. [Google Scholar]
- 14.Charlson ME, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 15.Kobelt-Nguyen G, Gerdtham U, Alm A. Costs of treating primary open-angle glaucoma and ocular hypertension: A retrospective, observational two-year chart review of newly diagnosed patients in Sweden and the United States. J Glaucoma. 1998;7(2):95–104. [PubMed] [Google Scholar]
- 16.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 17.Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian Glaucoma Study: 2. Risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–1036. doi: 10.1001/archopht.126.8.1030. [DOI] [PubMed] [Google Scholar]
- 18.Friedman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004;138(3 Supplement):S19–S31. doi: 10.1016/j.ajo.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 19.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 20.Friedman DS, Nordstrom B, Mozaffari E, Quigley HA. Variations in treatment among adult-onset open-angle glaucoma patients. Ophthalmology. 2005;112(9):1494–1499. doi: 10.1016/j.ophtha.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38(1):83–91. [PubMed] [Google Scholar]