Abstract
Aberrant activation of neutrophils during sepsis results in the widespread release of pro-inflammatory mediators, leading to multi-organ system failure and death. However, aberrant activation of neutrophils during sepsis results in the widespread release of harmful inflammatory mediators causing host tissue injuries that can lead to multi organ system failure and death. One of the pivotal components of neutrophil migration during inflammation is the expression of surface integrins. In this study, we show that administration of a cyclic analog of RGD peptide (Arg-Gly-Asp) significantly reduced the number of tissue-invading neutrophils and the degree of sepsis-induced lethality in mice as compared to control peptide. Secondly, β1 integrin (CD29) was highly up-regulated on the neutrophils isolated from both septic patients and animals. Finally, conditional genetic ablation of β1 integrin from granulocytes also improved survival and bacterial clearance in septic animals Thus, our results indicate that expression of β1 integrin is important for modulating neutrophil trafficking during sepsis, and that therapeutics designed against β1 integrins may be beneficial.
Keywords: Neutrophils, Integrin β1, Sepsis, Infiltration
Introduction
Neutrophils are the first line of defense in the body’s immune surveillance. Local inflammatory signals like PAMPs (Pathogen Associated Molecular Patterns) and DAMPs (Damage Associated Molecular Patterns) released from invading microbes and injured tissues could trigger neutrophil activation, recruitment and subsequent release of antimicrobial contents including proteases, MPO (Myeloperoxidase), oxygen radicals and proinflammatory mediators by neutrophils. Central components of the neutrophil recruitment process are the cell surface adhesion molecules known as integrins, which are induced in response to proinflammatory cytokines and chemokines released during infection and inflammation.
Severe sepsis is a systemic inflammatory disorder resulting from the host response to an infection. It remains a leading cause of mortality in adult critical care units affecting 750,000 individuals/year with a mortality rate of approximately 30% (1). Many reports have documented that the sequestration of hyperactive neutrophils in the microvasculature of visceral organs as a contributing factor in multi-organ failure, which is an important hallmark of sepsis and reducing neutrophil activity improves survival in sepsis (2,3,4). Proteases and superoxide released by the neutrophils, although are helpful in fighting infection, their excessive release can cause vascular paresis, lipid peroxidation, interfere with electron transport system leading to tissue hypoxia and extensive bystander tissue damages (5). A direct correlation is shown between changes in neutrophil functions and sepsis related morbidity and mortality (6,7). On the contrary, hyper activation of inflammatory responses in sepsis has been reported to impair the host defenses by diminishing neutrophil migration and function in sepsis (8). Although, completely depleting neutrophils may mimic leukocyte adhesion deficiency syndrome (LAD), increasing mortality by impairing pathogen clearance, their partial depletion may be helpful in reducing inflammation (9). Thus, non-selective suppression of stimulated neutrophils may not benefit patients with severe sepsis and could adversely affect host defenses. Therefore, it is important to design therapeutics that can properly balance the trafficking of the hyperactivated neutrophils in sepsis.
Integrins are transmembrane surface receptors that function as adhesion molecules and receptors, and also play a critical role in leukocyte migration (10,11). While β2/CD18 integrins play an important role in intravascular migration processes such as initial endothelial adhesion and vascular transmigration of leukocytes during inflammation, the β1/CD29 integrins mediate cell-matrix adhesion and promote leukocytes motility in the perivascular and extracellular matrix area (12–15). The best-characterized ligand-binding motif of β1 integrins is the Arg-Gly-Asp (RGD) sequence, which is present in a variety of extracellular matrix proteins. We have previously demonstrated that activated protein C could inhibit neutrophil migration by binding to neutrophil integrins (β1 and β3) through its RGD motif (16). Others have also shown an inhibitory effect of synthetic RGD peptides on neutrophil and macrophage infiltration in LPS induced lung inflammation (17).
In this study we show that a cyclic analog of RGD peptide could reduce neutrophil number in tissues and improve sepsis lethality. Additionally by using multi photon intra vital microscopy (MP-IVM) we show that RGD peptide could inhibit the extravasation of neutrophils indicating a role for tissue homing integrins in neutrophil migration under systemic inflammation. Furthermore, using neutrophils isolated from both human and mouse models of sepsis, we show the importance of the matrix binding integrin (integrin β1) in neutrophil recruitment under septic conditions. This knowledge is critical to determine if treatments modulating the functions of integrin β1 could have beneficial effects on sepsis survival.
Materials and Methods
Sepsis mouse models
Endotoxemia and CLP were performed on 8–12 week old C57BL/6 (Harlan) male mice according to the protocol approved by the University of Rochester Animal Resource Committee. For the endotoxemia assay, LPS from E. coli O55:B (Sigma-Aldrich, St. Louis, MO, USA) was administered by intraperitoneal (IP) injection to achieve an LD90 mortality rate. CLP surgery was performed under isofluorane inhalation anesthesia. Caecum was ligated and punctured through and through with a 21-gauge needle. For survival experiments, RGD ((Ac-c[(Pen)-Tyr(Me)-Ala-Arg-Gly-Asp-Asn-Tic-Cys]NH2)(18) or control peptides (200 μg) (Peptide International, Louisville, Kentucky, USA) were administered intravenously (IV) at 2 hr, and IP at 8 hr and 32 hr following CLP or endotoxemia.
Generation of conditional knockout animals
For generation of granulocyte specific integrin knockout mice, Granulocyte Elastase (Ela)-Cre knockin mice were purchased from The European Mouse Mutant Archive (EMMA), in which Cre-recombinase is expressed in the myeloid cells in place of Ela gene (19). Ela-Cre mice were crossed with β1-flox mice (Jackson Laboratory) for four to five generations to achieve deletion of the β1 gene in the transgenic mice. Mice were genotyped by PCR from DNA isolated from tail tissues using primers suggested by respective vendors. For detection of Ela-Cre gene, primers Mel135F: CAT GAC ACC CCC ACT GTG GTG TCC, Mel684R: TGG CAC CAC AGA AAT GAC CTC CAC and Crelx: TTT GGT GCA CGG TCA GCA GAT TGG were used that produced bands of 615bp (wild-type) or 185bp (mutant). For genotyping β1-flox animals, primers forward- CGG CTC AAA GCA GAG TGT CAG TC and reverse- CCA CAA CTT TCC CAG TTA GCT CTC were used resulting in wild-type (160bp) floxed (280bp) bands.
Isolation and in vitro stimulation of healthy and sepsis patient’s neutrophils
Blood samples from sepsis patients admitted to intensive care units were collected within 48 hr and 3–5 days after sepsis diagnosis, and before discharge. Collected samples were processed immediately for flow cytometry. Purified mouse anti-human integrin β1 (Millipore, Billerica, MA, USA), mouse IgG1 Isotype control (eBioscience, San Diego, CA, USA), and PE-rat anti-mouse secondary antibodies were used for staining. Blood was also collected from healthy volunteers and neutrophils were separated from whole blood using 1-step Polymorphs (Fisher Scientific, Pittsburgh, PA, USA) density gradient. Neutrophils were stimulated with sepsis patients’ serum for 90 min in L15 (Leibovitz) medium (Invitrogen, Carlsbad, CA, USA) with glucose at 37°C temperatures. The institutional Review Board of the University of Rochester approved this study.
Flow Cytometry
For flow cytometry measurement, cells were isolated from the bone marrow (BM), peritoneal Lavage (PL), peripheral blood, and lungs of septic mice. Samples from different time points post CLP and endotoxemia were collected at once and stained for β1/CD29 integrin at the same time to avoid staining variations. RBC lysis was performed using ACK lysing buffer (Invitrogen). The Fc receptors were blocked with unconjugated anti-CD16/32 (eBioscience, San Diego, CA, USA) for 30 min. Surface staining was performed using APC-Ly6G (BD Biosciences), PerCp Cy5.5-CD11b (eBioscience, San Diego, CA, USA), and PE-β1/CD29 (Biolegend, San Diego, CA, USA) antibodies and collected on a FACS Calliber flow cytometer (BD Biosciences, San Diego, CA). Data were analyzed using Flow Jo software.
Multi-Photon intravital microscopy (MP-IVM)
For MP-IVM of Lysozyme-M-GFP mice (20) in which granulocytes and monocytes express GFP were used. Cremaster muscle was prepared for imaging as previously described (21). Briefly, mice were anesthetized with pentobarbital sodium (65 mg/kg IP) and maintained on isofluorane inhalation anesthesia. The body temperature was maintained by placing the animal on a warming pad set. The right cremaster muscle was exteriorized and gently pinned over a custom-designed stage for visualization of Cremaster venules by microscopy. Leukocyte extravasation was induced by superfusion of fMLP (1 μM) on the exteriorized cremaster tissue, and time-lapse imaging was performed using the Olympus FV1000-AOM multiphoton system (21). Blood vessels were labeled with Texas Red dextran 70,000 MW (20 mg/kg) (Invitrogen, Carlsbad, CA, USA). RGD peptide (200 μg) was injected IV when extravasating cells were noticed. During preparation and observation, the tissue was continuously superfused with warmed bicarbonate-buffered saline. Upon completion of the protocols, the animal was euthanized by anesthetic overdose. Imaging data were analyzed with Velocity software (PerkinElmer, Boston, MA, USA).
Bacterial Clearance Assay
Peritoneal lavage was collected after injection of 10 ml PBS into the peritoneum. 1:100 1:1000 and 1:10000 dilution was performed and 100 μl from each dilution was spread on tryptic Soy Agar (TSA) Blood Agar Plates (Remel). All the plates were incubated at 37 °C temperatures for 24 hr. Colony count for each group was expressed as CFU/ml.
Measurement of MPO activity by Bioluminescence assay
For in vivo MPO measurement, luminol salt (50ug) (5-amino-2-, 3-dihydro-1, 4-pthalazinedione) (Sigma, St Louis, MO) was injected intraperitoneally. To measure the fluorescence intensity, mice were imaged using Xenogen IVIS imaging system (Caliper Lifesciences, Hopkinto, MA). Images were analyzed using Living Image 3.2 software.
IL-6 Cytokine ELISA
Serum from individual mice was collected at the indicated time points and sandwich ELISA was performed using anti-IL-6, capture, and detection antibodies (BD Pharmingen, San Diego, USA), standard recombinant murine IL-6 (PeproTech, Rocky Hill, NJ, USA), and Strepravidin-HRP (Thermo scientific, Rockford, IL, USA). The color reaction was developed using Aquablue (eBioscience, San Diego, CA, USA), and absorbance was measured at 405 nm.
Data analysis
All values are expressed as the mean ± SEM. The differences between all groups were analyzed by the Mann Whitney t test. Survival curves were analyzed by the Kaplan-Meyer log-rank test. All statistics were performed using the prism graph pad software. P value <0.05 was considered significant.
Results
Administration of RGD peptide improves survival in sepsis by reducing neutrophil extravasation
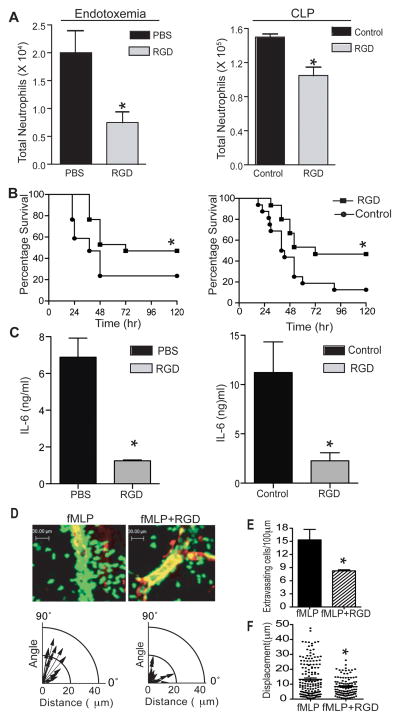
The RGD motif is involved in the ligand binding process of many β1 and β3 integrins on the variety of inflammatory cell types. In order to evaluate the effects of a synthetic RGD peptide on neutrophil infiltration and sepsis survival, RGD or control peptides were administered intravenously following endotoxemia and CLP, which are two clinically relevant animals models of septic peritonitis (22). As shown in Figs 1A and 1B, administration of RGD peptide reduced the number of neutrophil in the peritoneum and significantly improved survival in both endotoxemic and CLP animals (Fig 1A and 1B) possibly in part due to reduced infiltration of activated neutrophils. RGD treated septic animals also had lower serum levels of IL-6 as compared to the control peptide treated group (Fig. 1C), confirming attenuation of the systemic inflammatory response. Furthermore, we investigated the in vivo effects of the RGD peptide on neutrophil functions. Using multi-photon intravital microscopy (MP-IVM), we tested the effect of RGD peptide on neutrophil recruitment in the cremaster muscle of mice expressing GFP under the lysozyme-M (lyz-M) promoter. As shown in Fig. 1D, administration of RGD peptide did not affect firm adhesion of neutrophil in the intravascular space, but dramatically inhibited perivascular migration and reduced the number of cells that finished extravasation and migrated into the interstitium (Fig. 1E). In addition, analyzing the tracks of individual cells indicated that the extravasated cells remained close to the blood vessels, losing their directionality (1D, lower panel). This was confirmed by the displacement analysis of individual cells (Fig. 1F). These results suggest that RGD-binding integrins play a key role in neutrophil migration at the perivascular area and RGD peptide inhibits infiltration of neutrophils during sepsis by preventing the transmigration through the basement membrane of the blood vessels and the subsequent migration through the tissue matrix.
Figure 1. Administration of RGD peptide improves survival in sepsis.
(A) Cells were isolated from the PL of septic mice (8 hr after endotoxemia or CLP) injected with control or RGD peptides (200 μg/dose, IV). Anti-Ly6G and anti-CD11b antibodies were used to stain neutrophils. The bar graphs show the total number of neutrophils in the PL of endotoxemia (Left panel) and CLP (Right panel) animals (n=4). (B) Endotoxemia (Left panel) or CLP surgery (Right panel) was performed, and survival was analyzed using the Kaplan-Meyer log-rank test to compare control (n=17–21/group, p=0.019 and 0.025 in endotoxemia and CLP group respectively) mice. (C) Serum levels of IL-6 as measured by sandwich ELISA (8hr after CLP or LPS injection). The graph shows the IL-6 concentrations in endotoxemia (left) and CLP (Right) mice treated with control or RGD peptide (n=6/group for CLP and 3/group for endotoxemia). (D) The effect of RGD peptide on neutrophil migration in vivo was assessed by intravital MP-IVM in the fMLP-superfused cremaster vessels of LysM-GFP mice after IV injection of control or RGD (200 μg). The lower panels show the track of extravasating neutrophils in each group. The polar coordinate shows the relation between the angles against the blood vessel and migrating distance of extravasating cells. (E) The bar graphs show the number of extravasating neutrophils per 100 μm of vessel length in each group. (F) Displacement (distance between the first and last track point of individual cells) was analyzed with Velocity software. Each dot represents the displacement of each extravasating cell. The MP-IVM data in Fig. (E) and (F) represents the combined results from three independent imaging experiments. At least five vessels were analyzed from three animals. The results in (A), (C), (E), and (F) are expressed as the mean ± SEM. * p<0.05.
Integrin β1 expression is upregulated in septic neutrophils
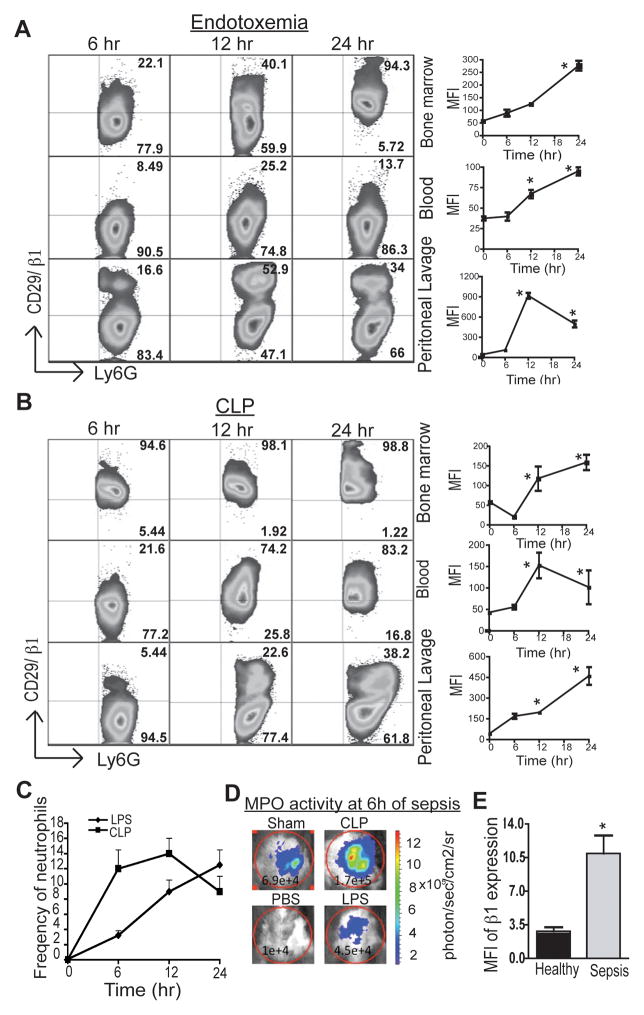
Previous studies have shown the importance of β1 integrins in the tissue homing of neutrophils under inflammation (15). To measure expression kinetics of β1 integrin on the neutrophils during sepsis, tissues were harvested at 6 hr, 12 hr, and 24 hr following induction of sepsis. As shown in Figs. 2A and 2B, there was a marked increase in the mean fluorescence intensity (MFI) (line diagram) of expression and frequency of β1+ neutrophils (Gated on Ly6GhighCD11bhigh neutrophils) in the bone marrow, peripheral blood, and peritoneal lavage of both endotoxemic (A) and CLP (B) mice.
Figure 2. Integrin β1 is up-regulated in sepsis.
(A) & (B) Integrin expression on septic mouse neutrophils was measured by flow cytometry. Cells were isolated from the BM, PL, and peripheral blood of septic mice at the indicated time points. Surface staining was performed as described in the methods. For the MFI analysis, cells were gated on Ly6G1highCD11bhigh neutrophils. The line diagrams on the right show the mean MFI of β1 expression in three animals at different time points. * p<0.05 represents statistical difference as compared to naïve mice. (C). The line diagrams shows the frequencies of neutrophils in the peritoneum of endotoxemia and CLP induced septic mice. (D) In vivo MPO activity was measured at the indicating time point by using bioluminescence assay. Luminol salt (50ug) was injected intraperitoneally and the fluorescence intensity was measured using Xenogen IVIS imaging system. Images were analyzed using Living Image 3.2 software. (E) Neutrophils from severe sepsis patients (n=6, Age 55.5 ± 5, source of infections: renal, lung, abdomen, genitourinary) and healthy donors (n=5, Age 36.4 ± 8) were isolated and surface expression of β1 integrin was measured by flow cytometry. The bar graph shows the MFI of integrin expression in sepsis patients within 48 hr of diagnosis as well as in healthy donors. * p<0.05.
In sepsis, mechanisms governing neutrophil trafficking and functions are complex. In our experiments, we observed differences in the pattern of the β1 integrins expression on the neutrophils isolated from endotoxemia and CLP animals (Fig. 2A and B). More neutrophils expressed β1 in the BM of CLP as compared to LPS injected mice. We believe as in endotoxemia, LPS at currently used doses could be a less potent stimulus for neutrophil activation and mobilization as compared to polymicrobial factors in CLP. Within 6 hr of CLP induced sepsis, there is much more massive activated neutrophil influx into peritoneum as compared to endotoxemia (Fig. 2C and D). Possibly, this explains better effect of RGD on neutrophil influx in endotoxemia as compared to CLP.
To explore the relevance of our observation in the mouse model with regard to human sepsis, the expression pattern of the β1 integrin was determined in neutrophils isolated from the blood of patients diagnosed with severe sepsis. As shown in Fig. 2E, patients’ neutrophils showed three to four times higher MFI of β1 expression than those of healthy subjects. Interestingly, the expression correlated with recovery and, neutrophils at the time of discharge had reduced β1 expression as compared to samples collected within 48 hr and 72–120 hr of diagnosis (not shown). We subsequently addressed whether proinflammatory components in the blood could lead to upregulation of the β1 integrin in the neutrophils. Neutrophils isolated from healthy donors were stimulated with serum collected from septic patients as well as from healthy donors. Interestingly, treatment with septic serum resulted in a two-fold increase of β1 expression as compared to healthy serum (MFI of healthy serum=3.7±0.2 and patient serum=8±0.8). These results indicate that inflammatory changes during sepsis can upregulate β1 integrin expression on neutrophils.
Conditional depletion of β1 improves survival in sepsis by interfering with neutrophil infiltration
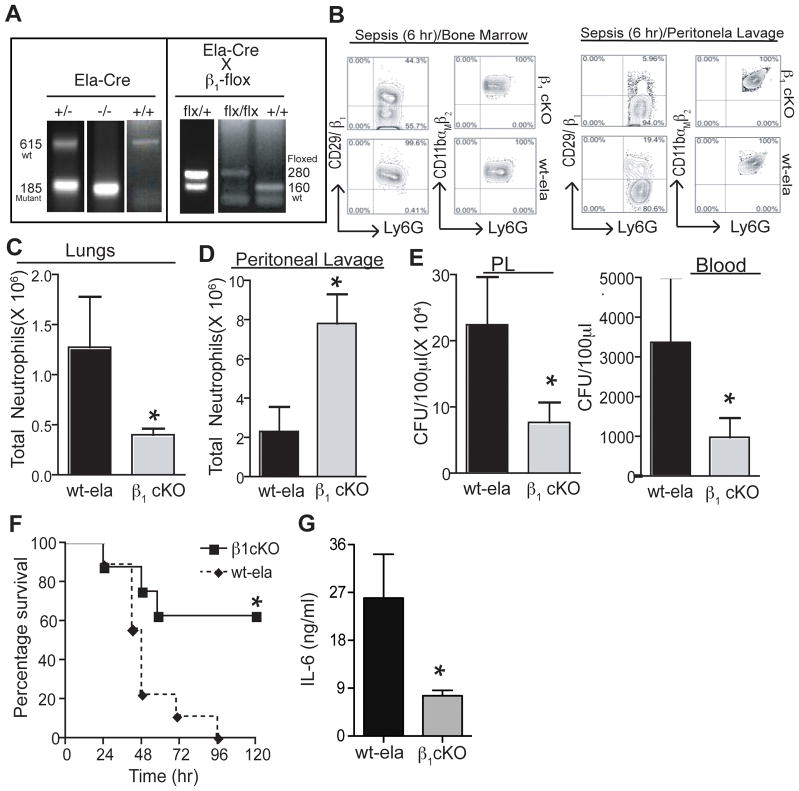
The exact functional relevance of β1 integrin was evaluated specifically in the neutrophils during sepsis. As systemic removal of β1 integrin is associated with embryo mortality during implantation (23), cell specific conditional knock-out mice (cKO) were generated by crossing β1-flox mice with mice that expressed Cre under the granulocyte-specific elastase promoter. These cKO mice were born at the expected frequency and developed normally with no evidence of defects, however they produced smaller litters as compared to their wild-type littermates. Ela-Cre transgenic mice express Cre in granulocytes, leading to gene deletion specifically in neutrophils. This was confirmed for both the floxed β1 subunit alleles by genotyping from tail DNA and measuring the surface expression of β1 proteins on neutrophils (Fig. 3A and 3B). No significant differences were observed in the frequencies of matured neutrophils in the bone marrow or peripheral blood of these mice (data not shown). Integrin expression on non granulocytes (CD11b+Ly6G- cells) in bone marrow (Percentage of β1+CD11b+ cells in WT and cKO mice are 99% and 91% respectively) of and lymphocytes also did not show any significant changes in the cKO animal as compared to WT (Percentage of β1+CD4+ and β1+CD8+ T cells in spleen of WT mice are 22% and 17% and 18% and 16.3% in cKO mice). The deletion of integrin β1 was more prominent in activated neutrophils that extravasated into the peritoneal cavity as compared to the bone marrow neutrophils. As shown earlier (15), under inflammation, neutrophils up-regulate their β1 integrin expression after entering into the inflamed tissues following extravasation. As cKO neutrophils lack the ability to upregulate β1 integrin following extravasation, there were more prominent differences in their β1expression in peritoneum as compared to WT neutrophils. This could alsobe due to differential activity of the elastase promoter driving Cre recombinase in these two different neutrophil populations. Interestingly, as shown in Figs. 3C, mice deficient in β1 integrin (Ela-Cre and floxed β1) showed a dramatic reduction in both the number of neutrophils infiltrating the lungs within 6 hr of CLP as compared to Ela-WT (no deletion). Interestingly, there was improved infiltration of neutrophils into the peritoneum (Fig. 3D) in the cKO mice as compared to control mice. Consistent with mice treated with RGD peptide, β1 cKO mice also showed significant improvement in sepsis survival (Fig. 3E). Interestingly, β1 cKO mice showed significantly improved bacterial clearance (Fig. 3F). This suggests that lack of β1 integrins may promote survival of neutrophil in the inflamed tissues, improve chemotaxis or enhance their phagocytosis (24). In addition, levels of IL-6 were significantly reduced in β1-cKO mice as compared to Ela-WT control mice, confirming the diminished severity of sepsis (Fig. 3G). This result suggest that delayed extravasation of β1-cKO neutrophils suppress the pro-inflammatory responses of the hyper-responsive neutrophils during sepsis.
Figure 3. Conditional depletion of β1 improves survival.
(A) Conditional depletion of β1 was achieved by crossing mice expressing Cre in the elastase promoter with mice that had floxed integrin genes. Pups from breeding Elastase-Cre and β1-flox mice were genotyped for heterozygous gene expression by PCR with genomic DNA isolated from tails clips as described in methods. Mice showing the presence of both flox and mutant cre were used for further breeding. Deletion of integrin genes was confirmed with the presence of mutant bands (Cre-185 bp and flox-280 bp). (B) Surface expression of β1 in the Ly6GhighCD11bhigh neutrophils was measured in the cells isolated from BM and PL of CLP induced septic WT or cKO mice. (C) & (D) Cells were isolated from the PL and lungs of septic cKO mice (6 h after CLP), and anti-Ly6G and anti-CD11b antibodies were used to stain neutrophils by flow cytometry. The bar graphs show the total number of neutrophils in the PL and lungs. (E) The bacterial load in the PL and blood of cKO and Ela-Cre mice at 6 hr after CLP (F) Survival was analyzed using the Kaplan-Meyer log-rank test to compare cKOs and Ela-wt control mice. (G) Serum levels of IL-6 as measured by sandwich ELISA. The graph shows concentrations of IL-6. The results for C, E, and F are expressed as the mean ± SEM. (n=6/group). * p<0.05
Discussion
Integrins contribute to the pathogenesis of a variety of diseases such as asthma, burns, traumatic shock, and rheumatoid arthritis (10). Numerous linear and cyclic peptides, peptidomimetics, and other small molecules formulated against integrins are being tested as therapeutic agents (10). RGD motif is the most well characterized integrin-binding motif in the extracellular matrix and has been shown to be effective in many inflammatory diseases (25–27). Recently, Moon et al. demonstrated that synthetic RGD peptide attenuated lung inflammation by inhibiting neutrophil and macrophage infiltration into lungs (17). We previously showed that rhAPC inhibits neutrophil adhesion and the migration on extracellular matrix proteins by directly blocking integrins on the neutrophil surface through its RGD motif (16). Our data in this study further suggest that the inhibition of β1 integrins on neutrophil surface may improve the survival in sepsis by delaying massive infiltration of pro-inflammatory neutrophils.
Earlier studies have shown the importance of β2 integrins in the intravascular and transendothelial migration of neutrophils during inflammation and sepsis, but very little is known about the regulations of β1 integrins in such conditions (28,29). Although some studies have highlighted the role of α4β1 (30) which binds to ICAM-1 in sepsis, other β1 integrins (α2β1,α3β1, α5β1, and α6β1) mainly mediatethe perivascular and tissue migration of neutrophils where they interact with matrix proteins like collagen and laminin. Studies also show that blocking of ICAM-1 does not change neutrophil trafficking in sepsis (31). Additionally, it is reported that several subsets of activated neutrophils exists at different locations during sepsis (32). Our current results show that integrin β1 was strongly upregulated in neutrophils isolated from different tissues. It is known that, after entering into inflammatory tissues, neutrophils can increase the surface expression of integrins when exposed to a highly inflammatory milieu (15). Supporting this finding, the increase in β1 expression was more striking in the peritoneal lavage as compared to blood and bone marrow. In our mouse studies, we gated on Ly6GhiLCD11bhi cell populations to identify neutrophils. Although the total population of neutrophils had higher CD11b (αMβ2) expression, a known activation marker for neutrophils, only a subpopulation of CD11bhigh neutrophils in peritoneal lavage showed enhanced expression of β1 integrin. This suggests that β1 integrinis an important cell surface marker for selective targeting of a neutrophil subpopulation in severe sepsis. In blood and bone marrow, the total β1 expression also includes α4β1. Therefore, further studies are needed to find out the role of distinct β1 integrins in order to specifically target tissue-infiltrating neutrophils.
Administration of RGD peptide could also block integrin signaling and proinflammatory cytokine production in other cell types such as macrophages (33). Secondly, RGD peptide could interfere with β3 integrin mediated neutrophils migration (17). This could lead to more reduction in neutrophil population in peritoneum of RGD treated mice as compared to β1 cKO animals. In our cell-specific gene removal approach in the conditional knock-out animals, there was a significant reduction in the number of neutrophils in the lungs as compared to the controls. Reduced neutrophils in the lungs also improved survival in septic mice. These findings are in agreement with our RGD peptide experiments as well as the observation that, in the absence of CCR2, protection from sepsis was associated with lower infiltration of neutrophils into the lungs and other vital organs (34). However, we cannot exclude the possibility of differential effects of β1 integrins on neutrophil infiltration into different tissues (12). Taken together, our data suggest that selective depletion of integrin β1 from granulocytes successfully decreases neutrophil infiltration into tissues and improves survival during sepsis.
Many extracellular matrix proteins such as collagen and laminin form the structural and supporting components around vessels and surrounding tissues, and are ligands for different β1 integrins. Further investigation is needed to assess the importance of specific β1 integrins for neutrophil recruitment and development of a protective versus a maladaptive inflammatory response during sepsis. In summary, we show that administration of a peptide analog of cyclic RGD could improve survival in sepsis by interfering with extravasation and perivascular migration of activated neutrophils. Secondly, integrin β1 was upregulated on a subpopulation of activated neutrophils infiltrating into the peritoneum of septic animals. Finally, we demonstrated that removal of integrin β1 surface expression improves sepsis-induced lethality by inhibiting aberrant neutrophil infiltration into distant organs and increasing local bacterial clearance. Our combined results suggest a potential therapeutic role for β1 integrin blocking strategies in sepsis.
Acknowledgments
We thank Cynthia Mack, RN, Kathleen L. Falkner, RN, Jennifer Wong, and Kiran S. Ambatipudi for their technical assistance. This project was supported by NIH HL087088 (M.K.), NIH HL018208 (M.K.), NIH HL094797 (M.K.) and NIH DA007232 (Y.V.L.).
Footnotes
Author Contribution
P.P.S. designed and performed experiments, analyzed data and wrote the manuscript. Y-M.H. performed MP-IVM and analyzed data. Y.V.L. performed experiments. A.P.P. designed and conducted the clinical research. M.K. designed and directed the study. All authors have agreed with the submission and none of the authors have a financial interest related to this work.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Current opinion in infectious diseases. 2012 doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 3.Suda K, Takeuchi H, Hagiwara T, et al. Neutrophil elastase inhibitor improves survival of rats with clinically relevant sepsis. Shock. 2010;33:526–531. doi: 10.1097/SHK.0b013e3181cc064b. [DOI] [PubMed] [Google Scholar]
- 4.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 5.Bellingan G. Inflammatory cell activation in sepsis. British medical bulletin. 1999;55:12–29. doi: 10.1258/0007142991902277. [DOI] [PubMed] [Google Scholar]
- 6.Muller Kobold AC, Tulleken JE, Zijlstra JG, et al. Leukocyte activation in sepsis; correlations with disease state and mortality. Intensive care medicine. 2000;26:883–892. doi: 10.1007/s001340051277. [DOI] [PubMed] [Google Scholar]
- 7.Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clinical and experimental immunology. 2008;154:87–97. doi: 10.1111/j.1365-2249.2008.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bignold LP, Rogers SD, Siaw TM, Bahnisch J. Inhibition of chemotaxis of neutrophil leukocytes to interleukin-8 by endotoxins of various bacteria. Infection and immunity. 1991;59:4255–4258. doi: 10.1128/iai.59.11.4255-4258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RW, Ballantyne CM, Smith CW, et al. Gene targeting yields a CD18-mutant mouse for study of inflammation. Journal of immunology. 1993;151:1571–1578. [PubMed] [Google Scholar]
- 10.Yonekawa K, Harlan JM. Targeting leukocyte integrins in human diseases. J Leukoc Biol. 2005;77:129–140. doi: 10.1189/jlb.0804460. [DOI] [PubMed] [Google Scholar]
- 11.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nature reviews Immunology. 2011;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 12.Ridger VC, Wagner BE, Wallace WA, Hellewell PG. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J Immunol. 2001;166:3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- 13.Bauer M, Brakebusch C, Coisne C, et al. Beta1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc Natl Acad Sci U S A. 2009;106:1920–1925. doi: 10.1073/pnas.0808909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg S, Lindholm J, Lindbom L, Hellstrom PM, Werr J. Integrin alpha2beta1 regulates neutrophil recruitment and inflammatory activity in experimental colitis in mice. Inflamm Bowel Dis. 2006;12:172–177. doi: 10.1097/01.MIB.0000217765.96604.83. [DOI] [PubMed] [Google Scholar]
- 15.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. beta1 integrins are critically involved in neutrophil locomotion in extravascular tissue In vivo. J Exp Med. 1998;187:2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elphick GF, Sarangi PP, Hyun YM, et al. Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood. 2009;113:4078–4085. doi: 10.1182/blood-2008-09-180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon C, Han JR, Park HJ, Hah JS, Kang JL. Synthetic RGDS peptide attenuates lipopolysaccharide-induced pulmonary inflammation by inhibiting integrin signaled MAP kinase pathways. Respiratory research. 2009;10:18. doi: 10.1186/1465-9921-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattern RH, Read SB, Pierschbacher MD, Sze CI, Eliceiri BP, Kruse CA. Glioma cell integrin expression and their interactions with integrin antagonists: Research Article. Cancer therapy. 2005;3A:325–340. [PMC free article] [PubMed] [Google Scholar]
- 19.Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- 20.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- 21.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol. 2008;295:H969–H977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 23.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes & development. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 24.Bae HB, Zmijewski JW, Deshane JS, et al. Vitronectin Inhibits Neutrophil Apoptosis Through Activation of Integrin Associated Signaling Pathways. American journal of respiratory cell and molecular biology. 2012 doi: 10.1165/rcmb.2011-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koning GA, Schiffelers RM, Wauben MH, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis and rheumatism. 2006;54:1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- 26.Reardon DA, Neyns B, Weller M, Tonn JC, Nabors LB, Stupp R. Cilengitide: an RGD pentapeptide alphanubeta3 and alphanubeta5 integrin inhibitor in development for glioblastoma and other malignancies. Future oncology. 2011;7:339–354. doi: 10.2217/fon.11.8. [DOI] [PubMed] [Google Scholar]
- 27.Bruck R, Hershkoviz R, Lider O, Shirin H, Aeed H, Halpern Z. Non-peptidic analogs of the cell adhesion motif RGD prevent experimental liver injury. Isr Med Assoc J. 2000;2 (Suppl):74–80. [PubMed] [Google Scholar]
- 28.Nupponen I, Andersson S, Jarvenpaa AL, Kautiainen H, Repo H. Neutrophil CD11b expression and circulating interleukin-8 as diagnostic markers for early-onset neonatal sepsis. Pediatrics. 2001;108:E12. doi: 10.1542/peds.108.1.e12. [DOI] [PubMed] [Google Scholar]
- 29.Guo RF, Riedemann NC, Laudes IJ, et al. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169:307–314. doi: 10.4049/jimmunol.169.1.307. [DOI] [PubMed] [Google Scholar]
- 30.Tsokos M, Fehlauer F. Post-mortem markers of sepsis: an immunohistochemical study using VLA-4 (CD49d/CD29) and ICAM-1 (CD54) for the detection of sepsis-induced lung injury. International journal of legal medicine. 2001;114:291–294. doi: 10.1007/s004140000172. [DOI] [PubMed] [Google Scholar]
- 31.Que LG, Kang BH, Huang YC, Piantadosi CA, Chang LY. Anti-intercellular adhesion molecule-1 antibody and intercellular adhesion molecule-1 gene deficiency do not prevent pulmonary neutrophil recruitment in polymicrobial sepsis. Shock. 1998;9:304–309. doi: 10.1097/00024382-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Windsor AC, Carey PD, Sugerman HJ, et al. Differential activation of alveolar, pulmonary arterial, and systemic arterial neutrophils demonstrates the existence of distinct neutrophil subpopulations in experimental sepsis. Shock. 1994;1:53–59. doi: 10.1097/00024382-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Monick MM, Powers L, Butler N, Yarovinsky T, Hunninghake GW. Interaction of matrix with integrin receptors is required for optimal LPS-induced MAP kinase activation. American journal of physiology Lung cellular and molecular physiology. 2002;283:L390–402. doi: 10.1152/ajplung.00437.2001. [DOI] [PubMed] [Google Scholar]
- 34.Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med. 2011;183:234–242. doi: 10.1164/rccm.201003-0416OC. [DOI] [PubMed] [Google Scholar]