Abstract
It is quite possible that the level of atmospheric oxygen has varied (roughly between 15 and 30% O2) over the past 550 million years. This variation is suggested by modeling of the carbon and sulfur cycles, by the excessive sediment burial of organic matter that accompanied the advent of large vascular land plants, and by recent physiological studies that relate to biological evolution.
The evolution of atmospheric oxygen over geologic time has been both a major cause and a major effect of biological evolution. This is because O2 is produced by photosynthesis and is consumed by plant and animal respiration. On a geologic time scale (millions of years), the global biogeochemical cycles of C and S, involving the exchange of reduced C and S between rocks and the atmosphere plus oceans, constitute the major controls on the level of O2. Therefore, the study of these cycles and how they may have varied in the geological past is important to the history, not only of the atmosphere, but also of earth surface environments. Much has been written about the evolution of atmospheric oxygen during the early stages of earth history (1–7). However, little attention has been paid to the continuing evolution over the past 550 million years (Phanerozoic time), the time during which most higher organisms arose and evolved, both in the oceans and on the continents. This paper presents a brief review of (i) the geochemical cycles of carbon and sulfur; (ii) the theoretical models that have been constructed to describe these cycles and how the cycles may have affected atmospheric O2 over Phanerozoic time; (ii) how the invasion of the land by vascular plants should have brought about an accompanying rise in atmospheric O2; (iv) how paleofires could have acted as a control on excessively high or low levels of O2; and (v) studies in paleophysiology that shed some light on the effects of possible changes of O2 in the past on plants and animals.
The Geological Carbon and Sulfur Cycles.
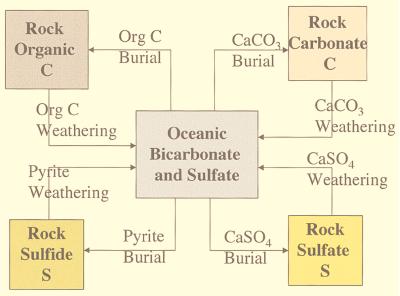
The processes that affect the evolution of atmospheric oxygen as it relates to the carbon and sulfur cycles over geologic time have been reviewed by Garrels and Perry (8), Walker (3), Holland (9), and Berner (10). These processes include (see Fig. 1): (i) input to the oceans of CO2 and dissolved carbonate and sulfate derived from oxidation, during chemical weathering on the continents, of ancient organic matter and pyrite (FeS2) in sedimentary rocks; (ii) reduction and removal of dissolved inorganic carbon from sea water (and to a lesser extent, fresh water) via the synthesis of organic matter followed by burial of dead organic remains in bottom sediments; (iii) removal of sulfate from seawater via bacterial reduction to hydrogen sulfide followed by the reaction of H2S to form sedimentary pyrite (plus organic sulfur compounds in low-iron sediments); (iv) the reaction of hot basalt with sulfate in seawater at midoceanic rises; and (v) degassing of reduced carbon and sulfur containing gases to the atmosphere plus oceans as a result of the thermal decomposition of deeply buried organic matter and pyrite by diagenesis, metamorphism, and volcanism. On arriving at the earth’s surface, these reduced gases are rapidly oxidized by O2. To complete the cycles of C and S, there are additional processes that do not involve atmospheric O2 but are important to C and S mass balance, especially isotope mass balance. They are (vi) the weathering of calcium carbonate and calcium sulfate minerals on the continents and (vii) the formation of these minerals in the oceans followed by burial in sediments. These processes are also shown in Fig. 1.
Figure 1.
The long-term global cycles of carbon and sulfur (pyrite, FeS2). Because little carbon can be stored in the ocean, inequalities between the weathering and burial of organic matter must result in the reciprocal formation or loss of CaCO3 to conserve carbon. The same is true for pyrite (FeS2), calcium sulfate, and sulfur. Also, any prolonged imbalance in the net flux between oxidized and reduced reservoirs of carbon must be balanced approximately by opposite fluxes between reduced and oxidized reservoirs of sulfur to avoid fluctuations in atmospheric O2 that would be too large for the maintenance of higher forms of life over geologic time. (Degassing caused by the deep thermal decomposition of organic matter and pyrite is here lumped under the heading of “weathering,” because the overall process of degassing, followed by air oxidation, results in an overall reaction chemically equivalent to oxidative weathering on the continents.)
Of these processes, the reaction of sulfate with basalt at midocean rises is minor compared with the others. The long-term removal of seawater sulfate at the rises by reduction to H2S or by CaSO4 precipitation can be shown to be minor on the basis of sulfur isotopic measurements, the inability to balance the oxygen cycle if sulfate reduction by basalt were quantitatively important, and the lack of CaSO4 in ancient rise deposits (ophiolites) (11, 12). The direct addition of mantle sulfur, as H2S, to the oceans at the rises (13) is part of general degassing (item v above), but it may be quantitatively less important than pyrite oxidation during weathering (14).
The effect of the carbon and sulfur cycles on atmospheric oxygen can be succinctly summarized in terms of reactions involving organic matter and pyrite.
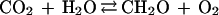 |
1 |
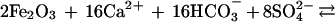 |
2 |
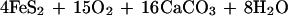 |
Here reaction 1, going from left to right, represents net long-term photosynthesis (photosynthesis minus respiration) manifested by the burial of organic matter in sediments. Going from right to left, reaction 1 represents the sum of atmospheric oxidation of reduced carbon-containing gases produced at depth from organic decomposition and georespiration, or the oxidation of ancient sediment organic matter, by surficial weathering. (Georespiration has been greatly accelerated by the burning of fossil fuels.) Reaction 2, going from left to right, represents the formation and burial of sedimentary pyrite as a result of bacterial sulfate reduction and, going from right to left, the oxidative weathering of ancient pyrite plus the atmospheric oxidation of reduced sulfur-containing gases produced at depth via pyrite decomposition.
The isotopic composition of carbon and sulfur buried in sedimentary rocks provides an important record of possible past changes in the carbon and sulfur cycles and, consequently, in atmospheric O2. Variations over time in 13C/12C and 34S/32S of the oceans are recorded by the isotopic composition of CaCO3 and CaSO4 in sedimentary rocks of different ages. Because of isotope fractionation, changes in oceanic 13C/12C and 34S/32S largely reflect changes in the global rates of biological processes. These processes include photosynthesis, which brings about the depletion, relative to the ratio in seawater or the atmosphere, of 13C in organic matter, and bacterial sulfate reduction to H2S, which brings about the depletion of 34S in the resulting sulfide. The organic matter and sulfide (precipitated to form pyrite, FeS2) are buried in sediments, along with calcium carbonates and sulfates (Fig. 1), and the rates of burial and relative proportions of each substance determine the isotopic composition of this output from the oceans. (Burial of organic matter in sediments also occurs on the continents in swamps and lakes but, for isotopic purposes, this burial can be lumped together with removal from the ocean because of the rapid exchange of carbon dioxide between the atmosphere and all water bodies.) The input of carbon and sulfur to the oceans results from weathering and from diagenetic and metamorphic/volcanic degassing (Fig. 1). Weathering of organic matter and pyrite and the oxidation of reduced gases involve little isotope fractionation so that the composition of the input to the oceans represents a mixture of 13C-depleted carbon and 34S-depleted sulfur from organic matter and pyrite oxidation and 13C-enriched carbon and 34S-enriched sulfur from carbonate and sulfate dissolution.
The overall change in the isotopic composition of seawater with time represents the balance between the rates and isotopic composition of weathering (plus thermal degassing) inputs and of sediment burial outputs. Neither CaCO3 nor CaSO4 undergoes appreciable isotopic fractionation during precipitation from seawater and thus they behave as recorders of ancient seawater isotopic composition. Because little carbon can be stored in the ocean, inequalities between weathering and burial of organic matter must result in the reciprocal formation or loss of CaCO3 to conserve carbon. The same is true for pyrite (FeS2), calcium sulfate, and sulfur. Also, any prolonged imbalance in the net flux between oxidized and reduced reservoirs of carbon must be balanced approximately by opposite fluxes between reduced and oxidized reservoirs of sulfur to avoid fluctuations in atmospheric O2 that would be too large for the maintenance of higher forms of life over geologic time.
Models for Paleo-Oxygen.
Calculation of the effect of change in the global carbon and sulfur cycles over geologic time on atmospheric oxygen has been attempted through the use of sediment composition models (15), nutrient-based models (16–18), and isotope mass balance models (9, 13, 19–26). In sediment composition modeling, the chemical composition of sedimentary rocks of various ages is combined with the abundance of these rocks (corrected for postdepositional erosional/metamorphic loss) to calculate the original rates of burial of organic carbon and pyrite sulfur in sediments. In nutrient models, the burial rate of organic carbon is assumed to be limited by the availability of the nutrients nitrogen and phosphorus, which leads to consideration of the cycles of these elements as well as those of carbon and sulfur. In isotope mass balance models, carbon and sulfur isotopic data for seawater composition over geologic time are used to calculate the values of the fluxes shown in Fig. 1. A major problem with all models is the extreme sensitivity of the mass of atmospheric O2 to very small imbalances in the burial and weathering fluxes. To avoid this problem, various approaches have applied negative feedback to avoid excessive variations in O2, but in many cases this involves the unavoidable introduction of positive feedback (see ref. 12). The results of one study (15) that used sediment abundance data, along with the rapid recycling of sediments to stabilize O2, are shown in Fig. 2.
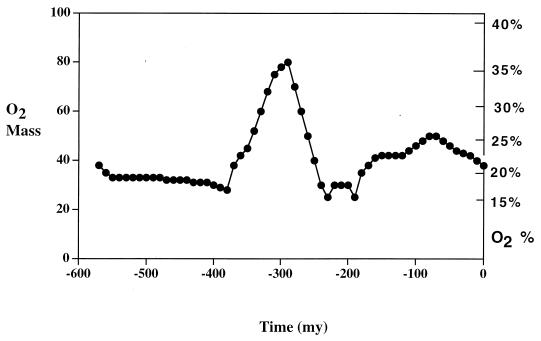
Figure 2.
Plot of atmospheric oxygen level vs. time for the Phanerozoic (past 550 million years) calculated via a sediment abundance model with rapid recycling (15).
Oxygen and the Rise of Vascular Land Plants.
The data of Fig. 2 show a pronounced and extended rise in atmospheric O2 over the period 375–275 mega-annum (Ma) spanning the Carboniferous and Permian periods. What could have brought this rise about? The modeling shows that increased oxygen production (reaction 1) caused by increased burial of organic carbon was the chief suspect. This increased burial is attributed to the rise and spread of large woody vascular plants on the continents beginning at about 375 Ma (9, 23). The plants supplied a new source of organic matter to be buried on land and carried to the oceans via rivers. This “new” carbon was added to that already being buried in the oceans, thus increasing the total global burial flux. This is especially true of lignin, a substance that is decomposed only with difficulty by microorganisms. The rise of ligniferous plants and an initial level of microbial lignin breakdown lower that that at present may have contributed to increased organic matter burial and better preservation. This high burial rate is reflected by the abundance of coals during this period, which is the greatest abundance in all of earth history.
Another factor favoring extensive Permo-Carboniferous organic matter burial was the presence of vast swamps on the continents, brought about by the presence of extensive poorly drained flatlands, and large areas of coastal plains, brought about by glacially induced fluctuations of sea level. This situation enabled the preservation of organic debris, leading ultimately to coal formation, because of the relative lack of organic decay in stagnant anoxic waters. Why coal formation, organic burial, and oxygen production dropped toward the end of the Permian period (Fig. 2) is not clear, but it may be tied up with sea level drop and a general drying of the continents.
Oxygen and Paleofires.
The level of atmospheric oxygen cannot rise indefinitely unless the frequency of forest fires becomes so excessive that plant life cannot persist. This has been pointed out by Watson et al. (27), who emphasize that fires serve as strong negative feedback against excessive O2 variation. Conversely, O2 cannot have dropped to such low values over Phanerozoic time that fires became impossible. Fossil charcoal, as evidence of paleofires, has been found for all times that trees have populated the land, and the lower limit for the production of charcoal has been estimated to be at about 13% O2 (28). By contrast, the upper limit for O2 is in dispute. On the basis of experiments on the ignition of paper strips at different oxygen levels and fuel moisture contents, Watson et al. (27) concluded that past levels of atmospheric O2 could never have risen above 25%. However, consideration of actual forest fires and the response of ecological disturbance to fires led Robinson (29) to conclude that greater O2 variation might occur and that, at any rate, paper is not a good surrogate for the biosphere. In fact, Robinson states paleobotanical evidence for a higher frequency of fire-resistant plants during the Permo-Carboniferous, supporting the idea of distinctly higher O2 levels at that time.
Paleo-Oxygen and Physiological Studies.
After the suggestion 30 years ago by McAlester (30), recently there has been interest in how past variations of atmospheric O2 over Phanerozoic time might have affected the metabolism and evolution of plants and animals. Graham et al. (31) have suggested that much of the gigantism of insects, changes in marine organisms with diffusion-mediated respiration, and invasion of land by vertebrates that occurred during late Paleozoic time (350–260 Ma) may have been caused by elevated concentrations of O2 at that time. This is the same period for which high levels of O2 are predicted by carbon/sulfur modeling (see Fig. 2). Dudley (32) has suggested that the evolution of flight may have been connected with changes in atmospheric oxygen and a denser atmosphere that could have resulted from these changes. Further, Harrison and Lighton (33) have found, from experiments, that elevated O2 enhances flight metabolism in dragonflies. Elevated O2 could help to explain the fossil evidence for giant dragonflies, with wing spans up to 70 cm, during the period (late Carboniferous) when O2 is predicted to have reached a maximum (Fig. 2).
One problem with high levels of atmospheric oxygen is that this situation should be deleterious to plant life, especially if atmospheric CO2 is not elevated as is believed to be the situation for the late Carboniferous (34). Enhanced photorespiration in C3 plants and the production of superoxides and OH radicals as a result of stepwise O2 reduction should inhibit plant growth at elevated O2 levels (35, 36). Nevertheless, Beerling et al. (37) have shown that a process-based terrestrial carbon cycle model, based on plant growth experiments, predicts that an atmospheric composition of 35% O2 (Fig. 2) and 300 ppm CO2 (34) does not invalidate terrestrial ecosystem biogeochemical cycling of carbon, but simply places limits on photosynthetic productivity and canopy structure at the global scale.
There is a crying need for more paleophysiological studies, from both a fossil interpretation standpoint and a modern experimental standpoint. The work discussed above is very recent and, as emphasized by Dudley (32), there is a need for many more studies of this kind that test the idea of adaptation and evolution of organisms to changing O2 in the geological past. For example, did dinosaur evolution have anything to do with changing O2 levels during the Mesozoic era?
Acknowledgments
This research was supported by Department of Energy Grant DE-FGO2–95ER14522 and National Science Foundation Grant EAR-9804781. I am indebted to Leo Hickey, Steven Petsch, Albert Colman, and David Beerling for their helpful reviews of the manuscript.
References
- 1.Berkner L V, Marshall L C. J Atmos Sci. 1965;22:225–261. [Google Scholar]
- 2.Cloud P E. Paleobiology. 1976;2:351–387. [Google Scholar]
- 3.Walker J C G. Evolution of the Atmosphere. New York: Macmillan; 1977. [Google Scholar]
- 4.Schidlowski M. J Geol Soc (London) 1984;141:243–250. [Google Scholar]
- 5.Holland H D. The Chemical Evolution of the Atmosphere and Oceans. Princeton, NJ: Princeton Univ. Press; 1984. [Google Scholar]
- 6.Des Marais D J, Strauss H, Summons R E, Hayes J M. Nature (London) 1992;359:605–609. doi: 10.1038/359605a0. [DOI] [PubMed] [Google Scholar]
- 7.Canfield D E, Teske A. Science. 1996;382:127–132. doi: 10.1038/382127a0. [DOI] [PubMed] [Google Scholar]
- 8.Garrels R M, Perry E A. In: The Sea. Goldberg E D, editor. Vol. 5. New York: Wiley; 1974. pp. 303–316. [Google Scholar]
- 9.Holland H D. The Chemistry of the Atmosphere and Oceans. New York: Wiley; 1978. [Google Scholar]
- 10.Berner R A. Global Planet Change. 1989;75:97–122. [Google Scholar]
- 11.Alt J C. Geology. 1995;23:585–588. [Google Scholar]
- 12.Berner R A, Petsch S T. Science. 1998;282:1436–1427. [Google Scholar]
- 13.Walker J C G. Mar Geol. 1986;70:159–174. doi: 10.1016/0025-3227(86)90093-9. [DOI] [PubMed] [Google Scholar]
- 14.Petsch S T. Geochim Cosmochim Acta. 1999;53:307–310. [Google Scholar]
- 15.Berner R A, Canfield D E. Am J Sci. 1989;289:333–361. doi: 10.2475/ajs.289.4.333. [DOI] [PubMed] [Google Scholar]
- 16.Kump L R. Nature (London) 1988;335:152–154. [Google Scholar]
- 17.Colman A S, Mackenzie F T, Holland H D. Science. 1997;275:406–408. doi: 10.1126/science.275.5298.406. [DOI] [PubMed] [Google Scholar]
- 18.Van Cappellen P, Ingall E D. Science. 1996;271:493–496. doi: 10.1126/science.271.5248.493. [DOI] [PubMed] [Google Scholar]
- 19.Veizer J, Holser W T, Wilgus C K. Geochim Cosmochim Acta. 1980;44:579–587. [Google Scholar]
- 20.Garrels R M, Lerman A. Am J Sci. 1984;284:989–1007. [Google Scholar]
- 21.Kump L R, Garrels R M. Am J Sci. 1986;286:336–360. [Google Scholar]
- 22.Francois L M, Gerard J-C. Geochim Cosmochim Acta. 1986;50:2289–2302. [Google Scholar]
- 23.Berner R A. Am J Sci. 1987;287:177–190. [Google Scholar]
- 24.Lasaga A C. Am J Sci. 1989;289:411–435. [Google Scholar]
- 25.Carpenter S J, Lohmann K C. Geochim Cosmochim Acta. 1997;61:4831–4846. [Google Scholar]
- 26.Petsch S T, Berner R A. Am J Sci. 1998;298:246–262. [Google Scholar]
- 27.Watson A, Lovelock J E, Margulis L. BioSystems. 1978;10:293–298. doi: 10.1016/0303-2647(78)90012-6. [DOI] [PubMed] [Google Scholar]
- 28.Chaloner W G. J Geol Soc (London) 1989;146:171–174. [Google Scholar]
- 29.Robinson J M. Paleogeogr Paleoclimatol Paleoecol. 1989;75:223–240. [Google Scholar]
- 30.McAlester A L. J Paleontol. 1970;44:405–409. [Google Scholar]
- 31.Graham J B, Dudley R, Aguilar N M, Gans C. Nature (London) 1995;375:117–120. [Google Scholar]
- 32.Dudley R. J Exp Biol. 1998;201:1043–1050. doi: 10.1242/jeb.201.8.1043. [DOI] [PubMed] [Google Scholar]
- 33.Harrison J F, Lighton J R B. J Exp Biol. 1998;201:1739–1744. doi: 10.1242/jeb.201.11.1739. [DOI] [PubMed] [Google Scholar]
- 34.Berner R A. Science. 1997;276:544–546. [Google Scholar]
- 35.Gibbs M. Am Sci. 1970;58:634–647. [Google Scholar]
- 36.Fridovich I. Am Sci. 1975;63:54–59. [PubMed] [Google Scholar]
- 37.Beerling D J, Woodward F I, Lomas M R, Wills M A, Quick W P, Valdes P J. Philos Trans R Soc London B. 1998;353:131–140. [Google Scholar]