Abstract
Large surface area burn injuries lead to activation of the innate immune system, which can be blocked by parasympathetic inputs mediated by the vagus nerve. We hypothesized that vagal nerve stimulation (VNS) would alter the inflammatory response of peritoneal macrophages after severe burn injury. Male BALB/c mice underwent right cervical VNS prior to 30% total body surface area steam burn and were compared to animals subjected to burn alone. Peritoneal macrophages were harvested at several time points following injury and exposed to lipopolysaccharide (LPS) in culture conditions. The inflammatory response of peritoneal macrophages was measured by analyzing changes in NF-κB p65 Ser536 phosphorylation using flow cytometry. We found that peritoneal macrophages isolated from mice subjected to burn injury were hyper-responsive to LPS challenge, suggesting burn-induced macrophage activation. We identified a protective role for VNS in blocking peritoneal macrophage activation. Analysis of the phosphorylation state of NF-κB pathway mediator, p65 Rel A, revealed a VNS-mediated reduction in p65 Ser536 phosphorylation levels after exposure to LPS compared to burn alone. In combination, these studies suggest VNS mediates the inflammatory response in peritoneal macrophages by affecting the set-point of LPS-responsiveness.
Keywords: LPS, vagus, cytokines, burn, cholinergic anti-inflammatory
INTRODUCTION
Severe injury leads to activation of the innate immune system resulting in the up-regulation of genes involved in the systemic inflammatory response, while simultaneously suppressing genes involved in adaptive immunity(1). Clinically, changes in leukocyte phenotype after severe cutaneous burn can lead to innate immune dysfunction predisposing patients to infections, sepsis, and increased morbidity and mortality during recovery from injury(2). Macrophages are primary mediators of the innate immune response to injury with the plasticity to respond to and express cytokines affecting both initiation and resolution of the tissue injury(3).
Macrophages mediate the innate immune response through production of pro-inflammatory cytokines, which is regulated by transcription factors including nuclear factor kappa-B (NF-κB). NF-κB is a heterodimeric protein bound in the cytosol in its inactive state under normal conditions. When activated by injury or an inflammatory insult, NF-κB is released by its inhibitory complex and the p65 Rel A subunit translocates to the nucleus where it activates the transcription of pro-inflammatory mediators. Macrophage function is altered after injury with increased responsiveness to subsequent insults as characterized by increased cytokine production(4). While the capacity for burn injury to activate and “prime” various lymphocyte populations has been described, strategies to moderate this injury response are less well understood (5, 6).
Recent studies have identified the potential for the parasympathetic nervous system to regulate the inflammatory response(7). Vagal nerve stimulation (VNS) has been utilized in models of endotoxemia and injury to demonstrate the anti-inflammatory potential of the parasympathetic nervous system(8). VNS has been shown to decrease the production of pro-inflammatory cytokines from splenic macrophages in an animal model of endotoxemia. Further studies have shown that the anti-inflammatory effects of VNS are lost in splenectomized animals, further defining the importance of the spleen in mediating the ability of VNS to attenuate systemic inflammation(9).
We, and others, have focused on burn injury as a pathophysiologically relevant model to examine the local and systemic inflammatory response. We have studied the ability of VNS to limit gut barrier injury and intestinal inflammation through a spleen-independent mechanism, with data suggesting that the protective effects of VNS may be signaled via the enteric nervous system(10). As VNS is well known to modulate the response of inflammatory cells to injury, we considered the capacity for burn injury to affect peritoneal immune cell homeostasis, with a focus on resident peritoneal macrophages. Here, we hypothesize that VNS would alter the inflammatory set-point of peritoneal macrophages and limit their response to a secondary inflammatory insult after severe burn injury.
MATERIALS AND METHODS
Materials
Mice were obtained from Jackson Laboratories (Sacramento, CA). Phosphorylated NF-κB p65 Ser536 rabbit monoclonal antibody (PE conjugate) was obtained from Cell Signaling Technology (Danvers, MA). Anti-F4/80 and Gr-1 antibody conjugated to PE or FITC were purchased from AbD Serotech (Raleigh, NC) and BD Biosciences (San Jose, CA). Cytofix and FACS incubation buffer were purchased from BD Biosciences (San Jose, CA). Lipopolysaccharide O111:B4 was obtained from Sigma Aldrich (St. Louis, MO).
Burn Injury Model
Male BALB/c mice weighing 24–28 g were anesthetized with 3% inhaled isoflurane. A cohort of animals (n = 5–6 animals) was subjected to a 30% total body surface area (TBSA) steam burn for 7 seconds creating a full thickness injury as previously described (11). All mice received a subcutaneous injection of 1.4 ml 0.9% saline solution containing 0.1ml of buprenorphine (12µg/ml) for pain control and fluid resuscitation in an area of uninjured skin. Following burn, animals were returned to their cages to recover and were allowed access to food and water as libitum. All animal experiments were approved by the University of California Animal Subjects Committee and were conducted in accordance with guidelines established by the National Institutes for Health.
Vagal Nerve Stimulation
A cohort of animals underwent right cervical neck incision followed by right cervical vagal nerve stimulation immediately prior to thermal insult (n = 5–6 animals). Stimulation of the vagus nerve was performed using a VariStim III probel (Medtronic Xomed, Jacksonville, FL) at 2mA, intermittently for 10 minutes. Following nerve stimulation, the incision was closed with interrupted silk suture. Sham animals underwent right cervical incision and exposure of the vagus nerve, but did not receive electrical stimulation.
Isolation of Peritoneal Macrophages
Peritoneal macrophages were harvested 4 hours after injury using an established protocol (12). Briefly, the ventral skin was sanitized with 70% ethanol solution. The ventral skin was removed leaving the abdominal wall intact. A 5 ml ice-cold injection of 3% fetal bovine serum (FBS) in 1X phosphate buffered saline (PBS) was instilled into the abdomen. The abdomen was gently massaged and the 3% FBS removed with a sterile syringe with a 25 g needle and placed into a conical tube on ice. The samples were considered unusable if at any time there was bleeding from the abdominal wall or organs to contaminate the 3% FBS solution.
In vitro cytokine expression following burn injury
Peritoneal cells were collected 4 hours following burn injury or from sham animals as described above. Cells were plated and incubated for two hours in complete RPMI (10% FBS, 5ml pen/step) at 37°C for two hours. They were washed with PBS to purify the macrophage population and incubated again for 5 minutes with PBS or LPS (10ng/ml in PBS). To remove the LPS, cells were again washed with PBS. Peritoneal macrophages were scraped from the culture plate and centrifuged to obtain a cell pellet. The pellet was snap frozen for PCR.
Reverse transcription PCR
Cells were snap frozen in liquid nitrogen and stored at −80°C immediately after collection. RNA was extracted from cells and purified using the RNeasy mini kit (Qiagen Cat. No. 74104). Reverse transcription was performed (n=6 animals per group) with the Bio Rad iScript cDNA synthesis kit (Cat. No. 170-8891). Reverse transcription quantitative PCR reactions were run with Qiagen SYBR Green Master Mix (Qiagen Lot. No.204143) for 40 cycles on a Bio-Rad iQ5 real-time PCR detection system. Reactions were run at 95°C for 15 minutes, then cycled at 95°C for 15 s, 55°C for 30 s, 72°C for 30 s according to Quantitect Primer assay 2 step RT-PCR (www.qiagen.com/HB/Primerassay). A melt curve was obtained to ensure that only a single species was amplified for each primer set. The TNF-α (Cat. No. QT00104006), IL-6 (Cat. No.QT00098875) and GAPDH primers (Cat. No. QT01658692) were Quantitect brand primers obtained from Qiagen. Samples were normalized against GAPDH and relative expression levels were calculated by the ΔΔCt method(13).
Flow Cytometry for Phosphorylated NF-κB p65Rel A
We used the power of flow cytometry to identify changes in NF-κB p65 Rel A signaling specifically in macrophages not otherwise isolated from the heterogenous population of peritoneal cells(14–16). Cells isolated from the peritoneum of mice were placed on ice in a conical tube and pelleted by centrifugation for 5 minutes. The supernatant was aspirated and the cells resuspended in PBS. LPS was added to the solution for a final concentration of 10 ng/ml and allowed to incubate for 5, 10 or 30 minutes at 37°C. The cells were then washed with PBS. Cytofix (500µl) was added and cells incubated for 10 minutes on ice. The cells were permeabilized for 30 minutes by adding ice-cold 100% methanol to the fixation solution for a concentration of 90% methanol. After permeabilization, the cells were pelleted followed by 2 washes with FACS incubation buffer (PBS and 0.02% BSA). The cells were resuspended in 100µl FACS incubation buffer in each tube for 10 minutes. Blocking solution and anti-phospho-p65 Rel A (p65 Rel A) antibody was added to a final concentration of 1:100 and incubated at room temperature for 1 hour. The cells were washed twice and resuspended in 500µl of PBS for flow cytometry. Cells were analyzed using a Becton Dickinson FACS Calibur where we gated on the viable, F4/80+ peritoneal macrophages and detected the P-p65 Rel A activity of cells as previously described(17, 18).
Statistical Analysis
ANOVA with Student-Newman-Keuls post hoc analysis or Student’s t-test was performed when appropriate. Statistical significance was determined based on a p-value <0.05.
RESULTS
Peritoneal macrophages from burn injured animals demonstrate increased TNF-α and IL-6 expression
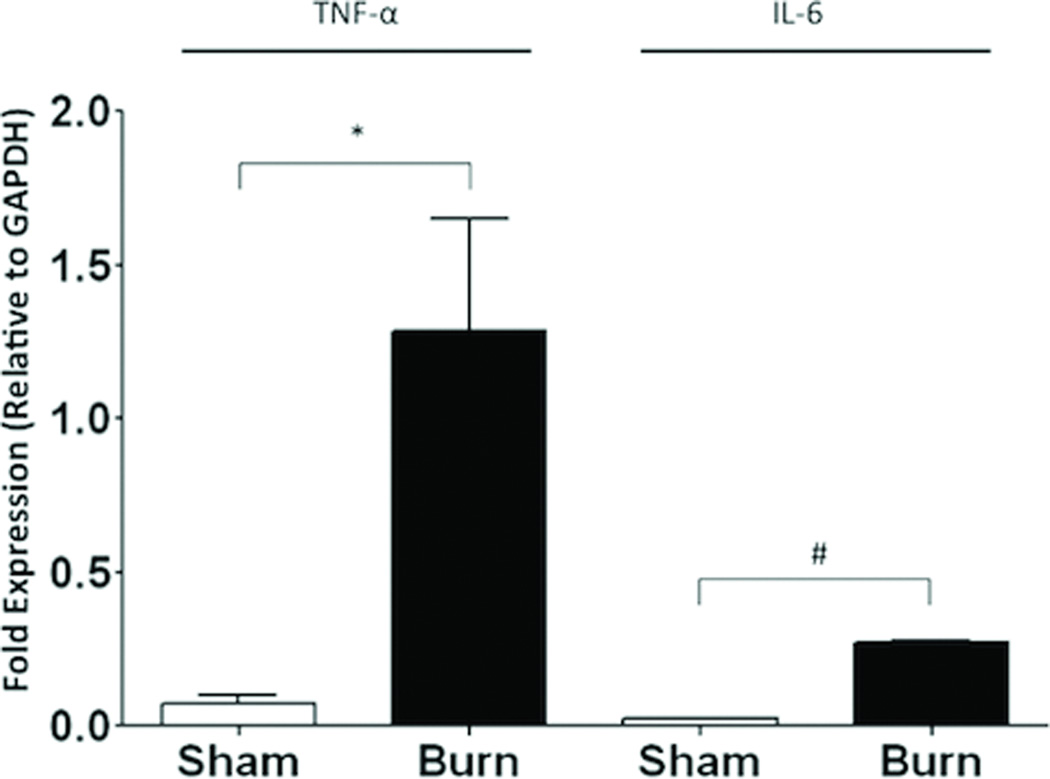
To determine whether peritoneal macrophages from burned animals express increased levels of cytokines, we collected and plated peritoneal cells from animals 4 hours (h) following injury. We then washed the plated cells to purify the macrophage population and subjected macrophages to PCR for TNF-α and IL-6. We observed a 16-fold increase in expression of TNF-α (p=0.03) and a 9-fold increase in IL-6 (p<0.001) expression in macrophages harvested from burn animals compared to sham (Figure 1).
Figure 1. Burn injury increases peritoneal macrophage cytokine expression.
Quantitative PRC was performed on peritoneal macrophages isolated by plating 4 hours following burn. Burn injury results in increased TNF-α and IL-6 mRNA expression compared to sham treatment. Results are expressed as cytokine mRNA expression relative to GAPDH± SEM. *p=0.03; #p<0.001.
Cutaneous Burn Leads to Early Increased Activation of the NF-κB Pathway
To determine whether burn injury increases the inflammatory response of peritoneal macrophages, we isolated peritoneal cells at 4 hours after burn injury. We then subjected these cells to LPS as a model for a second injury and assessed the phosphorylation of p65 Rel A (P-p65). Harvested peritoneal cells from animals following burn injury were compared to cells from sham animals (Figure 2 A–D and G). Sham animals demonstrated no significant difference in P-p65 Rel A when compared to sham animals exposed to 10 ng/ml of LPS (1.00 ± 0.10 vs. 1.18 ± 0.08; p=0.11). Similarly, there was no significant difference in the response to LPS by peritoneal macrophages from sham animals and 4h burn animals that were not exposed to LPS (1.00 ± 0.10 vs. 1.06 ± 0.12; p=0.38). This would suggest that burn alone has little effect on activation of the NF-κB pathway within peritoneal macrophages. Peritoneal macrophages harvested from 4h burn animals that were exposed to LPS demonstrated a significant increase in P-p65 Rel A compare to burn alone (1.06 ± 0.12 vs. 1.94 ± 0.23; p<0.001). This difference in NF-κB pathway activation suggests that burn injury has the ability to increase the inflammatory responsiveness of peritoneal macrophages after exposure to a second inflammatory insult.
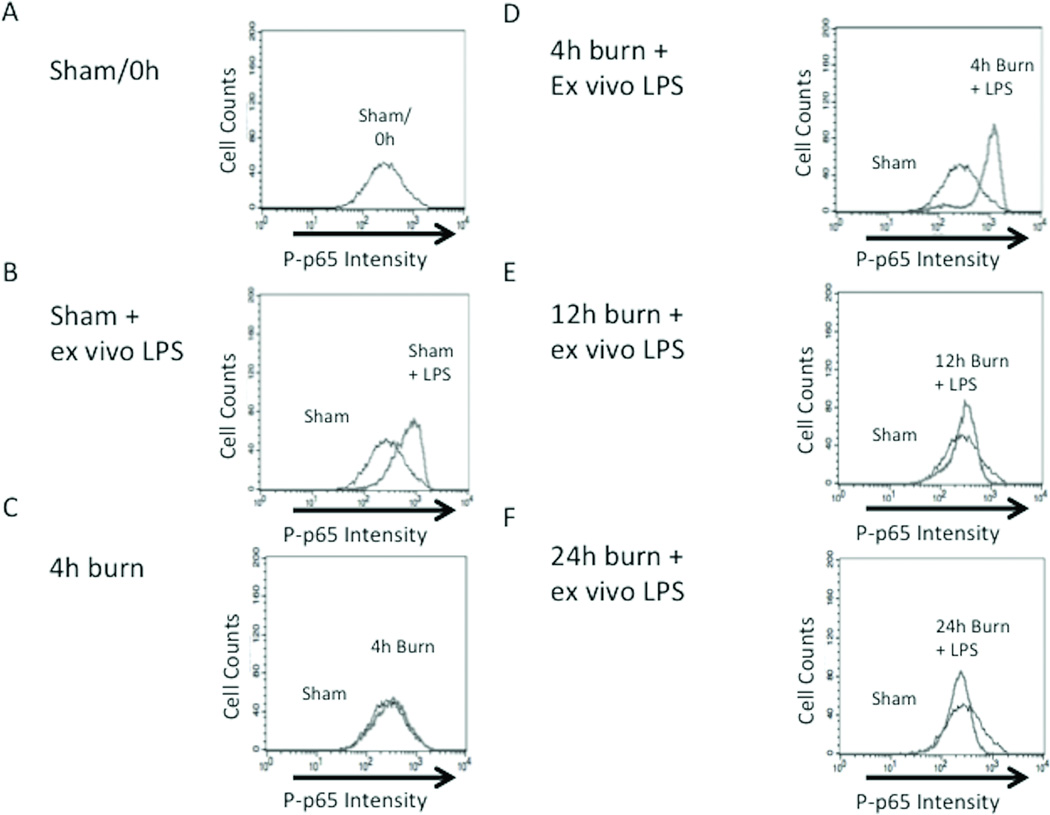
Figure 2. Determination of the kinetics of macrophage priming after burn injury.
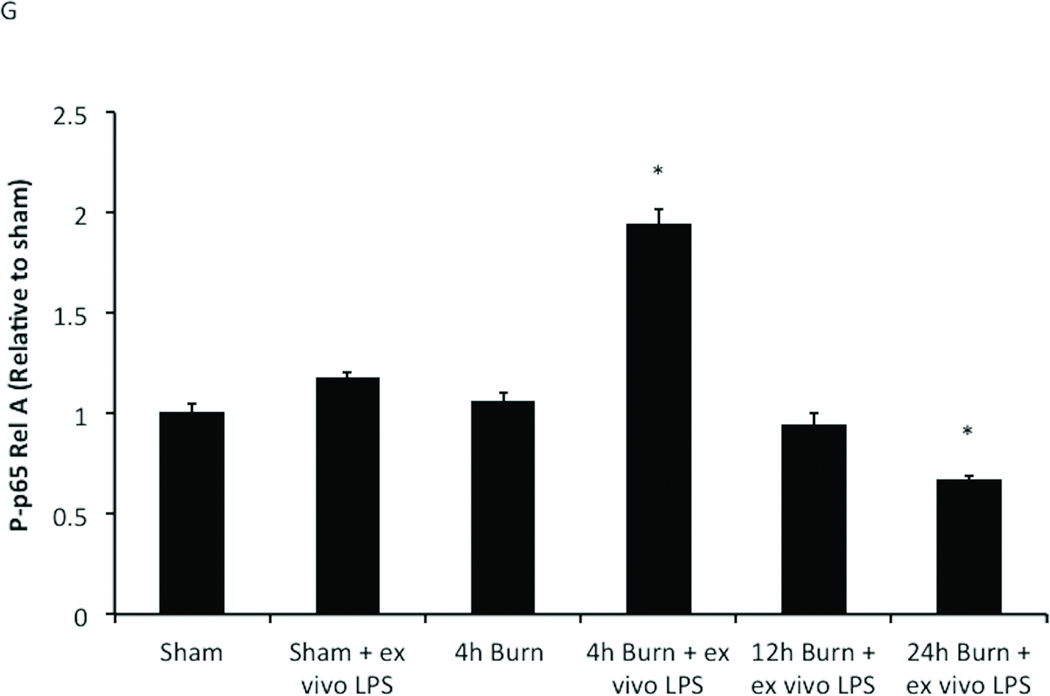
Peritoneal macrophages harvested at multiple time-points following sham or burn injury to assess changes in NF-kB Phospho-p65 Rel A expression after in vitro exposure to LPS. Cells stained for NF-kB Phosph-p65 Rel A. Black line represents cells obtained from sham animals with no exposure to LPS, gray line represents comparison groups (A) P-p65 in macrophages from sham animals; no LPS stimulation. (B) Rightward shift of gray peak demonstrating increased NF-KB signaling in sham macrophages after LPS stimulation (C) Burn injury alone does not increase NFKB signaling. (D) Ex vivo LPS stimulation after 4h burn injury significantly increases P-p65. Peritoneal macrophages are no longer hyper-responsive to LPS stimulation (E) 12h and (F) 24h following burn injury. (G) Graph representing data obtained from flow cytometry experiments. The inflammatory responsiveness of peritoneal macrophages peaks at 4 hours after burn injury and decreases to sub-baseline levels by 24 hours post-injury. Error bars represent SEM. *P<0.001 vs. sham, sham+ ex vivo LPS, burn, 12h burn + ex-vivo LPS.
To further define the kinetics of NF-κB pathway activation we examined P-p65 Rel A levels at subsequent time-points after burn injury (Figure 2 E–G). After analysis of LPS stimulated peritoneal macrophages harvested from animals at 12 and 24 hours, we found that P-p65 Rel A levels were highest 4 hours following burn injury. Assessment of peritoneal macrophages harvested 12 hours post-burn injury followed by LPS exposure demonstrated P-p65 expression similar to LPS stimulated sham (0.94 ± 0.13 vs. 1.17 ± 0.05; p=0.13), but were decreased compared to the 4 hour time-point with LPS exposure (0.94 ± 0.13 vs. 1.94 ± 0.23; p<0.001). When animals were allowed to recover for 24 hours post-burn injury, there was a reduction in P-p65 Rel A to levels lower than sham burn injury (0.67 ± 0.05 vs. 1.00 ± 0.10; p<0.001). This observation suggested that the inflammatory responsiveness of peritoneal macrophages occurred at 4 hours post-burn injury, and then decreased to a level below baseline at 24 hours following burn injury. These results indicate that peritoneal macrophages have the capacity to resolve burn-induced inflammatory hyper-responsiveness by 24 hours after injury, which correlates with our previous data showing that burn-induced changes in gut barrier integrity and histologic gut injury resolve by 24 hours post-burn injury(19).
Kinetics of ex vivo LPS stimulation in harvested peritoneal macrophages
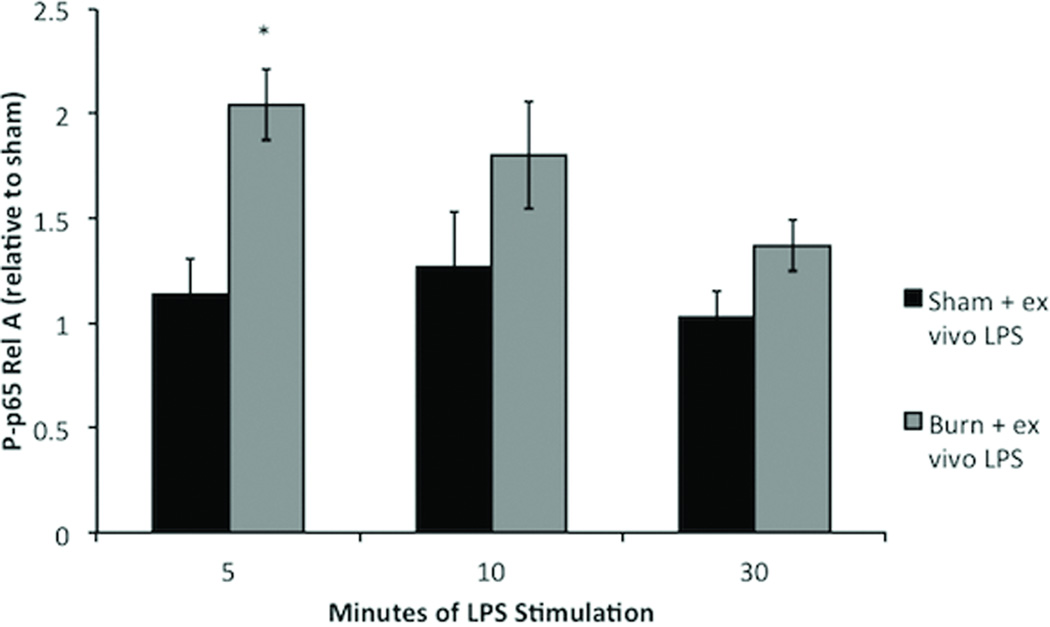
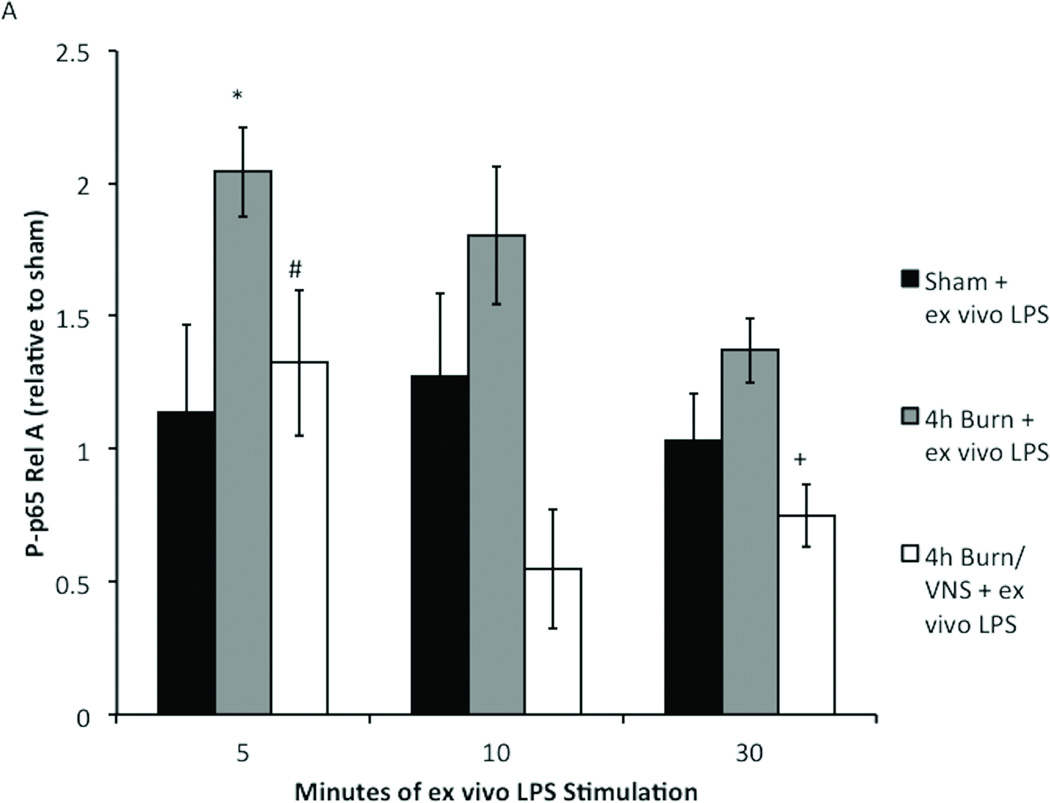
To determine the optimal time point for ex vivo LPS stimulation we subjected peritoneal cells from sham and burn injured mice to LPS stimulation for 5, 10 or 30 minutes (Figure 3). We found that LPS stimulation did not cause significant increases in NF-κB signaling in sham animals after 5, 10, or 30 minutes. However, LPS stimulation of macrophages from burn injured animals for 5 minutes increased P-p65 Rel A (p<0.001). Stimulation of harvested peritoneal macrophages for 10 or 30 minutes resulted in P-p65 RelA levels similar to sham. These findings, demonstrating an initial rise and subsequent fall in P-p65 with increasing time of LPS stimulation, are consistent with findings in other models of stimulation(17).
Figure 3. Determination of the kinetics of LPS stimulation in primed macrophages.
Macrophages obtained from sham animals show no significant increases in NFKB signaling after 5, 10, or 30 minutes of LPS stimulation. Ex vivo LPS stimulation for 5 minute significantly increases P-p65 Rel A in macrophages harvested 4 hours following burn injury. After 10 or 30 minutes of LPS stimulation phosphorylation returns to baseline Error bars represent SEM. *p<0.001 vs sham + ex vivo LPS (5 minutes).
VNS decreases NF-KB activation in sham animals
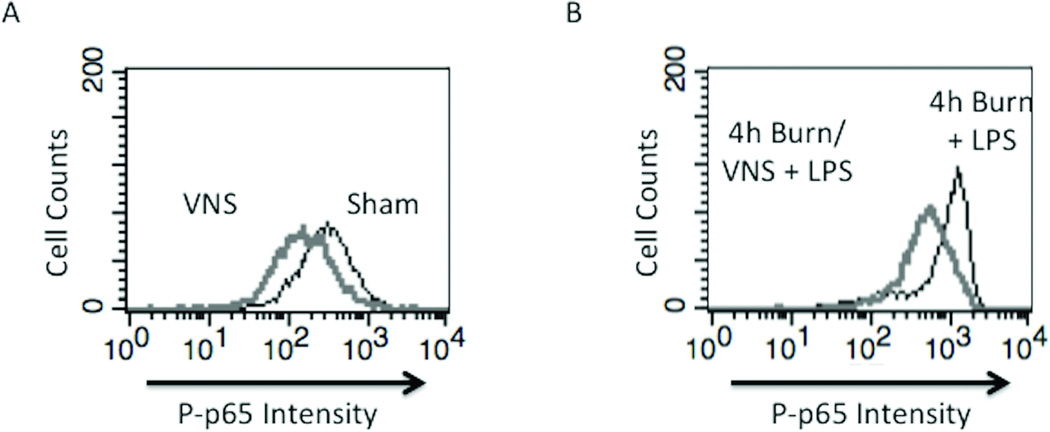
Based on our previous studies showing that VNS-mediated protection of the gut in the burn model is independent of the spleen(20), we considered that resident macrophages of the peritoneum may be affected by VNS. Due to the significant anti-inflammatory effects of VNS, we considered the capacity of the vagus nerve to alter the inflammatory responsiveness of peritoneal macrophages in the uninjured animal. We harvested peritoneal macrophages from sham animals 4 hours following VNS and measured changes in P-p65 Rel A levels in the absence of LPS. There was a decrease in baseline P-p65 Rel A levels in sham animals undergoing VNS compared to sham (p<0.005) (Figure 4A and C). These results suggest that VNS alters baseline activation of the NF-κB pathway and may represent the ability of VNS to alter the inflammatory set-point of immune cells.
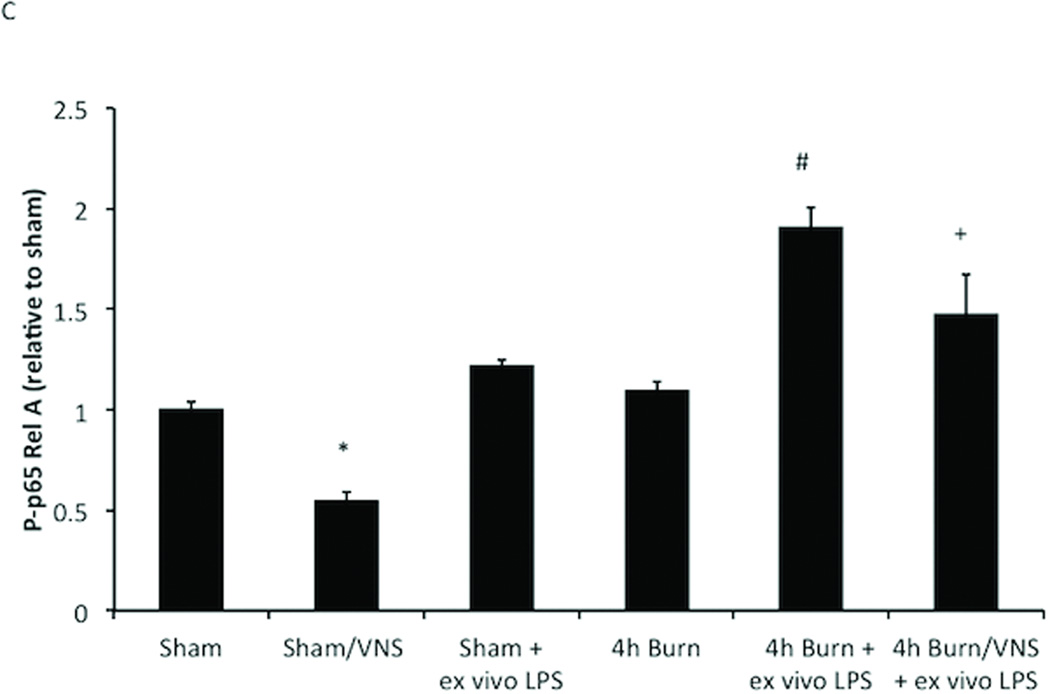
Figure 4. VNS decreases NF-KB activation in sham animals and negates macrophage hyper-responsiveness following burn injury.
(A) VNS treatment of sham animals reduced Phospho-p65 Rel A below baseline of unstimulated sham animals, demonstrating that VNS alters the inflammatory set-point in peritoneal macrophages. (B) Performing VNS prior to severe burn injury reduced the inflammatory response of peritoneal macrophages as measured by LPS induced Phospho-p65 Rel A levels using flow cytometry. (C) Graph representing flow based findings. Error bars represent SEM. * p < 0.005 vs. sham or 4 hour burn mice without VNS; # p < 0.001 vs. sham, VNS only or 4h burn; +p<0.005 vs. 4h burn + ex vivo LPS.
VNS Negates Macrophage Hyper-responsiveness following Burn Injury
To investigate whether VNS treatment prior to burn injury might attenuate burn induced macrophage hyper-responsiveness we subjected mice to VNS followed by 30% TBSA burn injury and examined the effect of VNS on the activation of the NF-κB pathway using P-p65 Rel A (Figure 4B and C). As in previous experiments, peritoneal macrophages harvested 4 hours following burn injury demonstrated increased P-p65 Rel A levels after LPS exposure when compared to sham animals (1.91 ± 0.23 vs 1.0 ± 0.09; p<0.001). VNS reduced the burn-induced inflammatory hyper-responsiveness of peritoneal macrophages, with decreased P-p65 Rel A levels after LPS exposure, however P-p65 levels continued to be increased relative to sham + ex vivo LPS (1.47 ± 0.49 vs. 1.22 ± 0.06; p<0.001).
The kinetics of p-65 Rel A phosphorylation after LPS stimulation are unchanged by VNS or increased time after burn injury
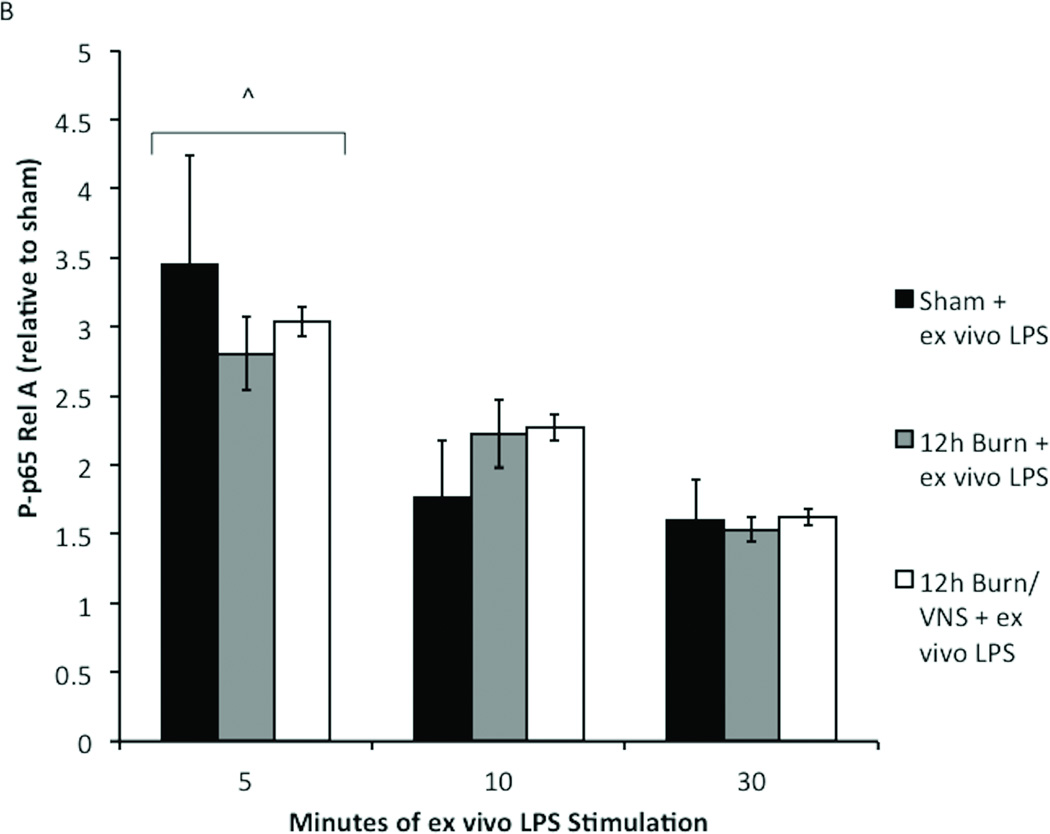
To determine whether VNS induced differential kinetics of phosphorylation that resulted in the observed decrease in P-p65 Rel A, peritoneal macrophages from sham, 4h burn, and animals undergoing VNS prior to 4h burn were obtained and stimulated with ex vivo LPS for 5, 10 and 30 minutes (Figure 5A). Cells were subject to flow cytometry from P-p65 Rel A revealing that decreased phosphorylation of p65 in burn injured animals pre-treated with VNS was not due to delayed phosphorylation, since longer periods of stimulation did not result in significant increases in P-p6 Rel A. In fact, in the 4h burn/VNS group P-p65 remained lower than both sham and 4h burn after LPS stimulation for 5, 10 and 30 minutes, and was significantly lower at stimulation times of 5 and 30 minutes.
Figure 5. Kinetics of p-65 Rel A phosphorylation are unchanged with VNS and increased time after burn injury.
To confirm that our findings were not due to altered kinetics of phosphorylation, macrophages from sham, burn and burn/VNS animals were stimulated with LPS for 5, 10 and 30 minutes. (A) At the 4 hour time point only 5 minute LPS stimulation caused a significant increase in P-p65 in 4h burn macrophages. P-p65 in the 4h burn/VNS group remained lower than both sham and 4h burn after LPS stimulation for 5, 10 and 30 minutes, and was significantly lower than burn at stimulation times of 5 and 30 minutes. (B) Additionally, kinetics of phosphorylation were similar at the 12 hour time point; maximal increase in P-p65 was at 5 minutes. There was no difference in P-p65 Rel A between the groups regardless of LPS exposure time. This suggests that neither macrophage activation following burn injury, nor its inhibition by VNS results from differential kinetics of phosphorylation of p-65 Rel A. Error bars represent SEM. *p<0.05 vs. sham + ex vivo LPS (5 minute); #p<0.05 vs. 4h burn + ex vivo LPS (5 minute); + p=0.03 vs. 4h burn + ex vivo LPS (30 minute); ^p<0.001 vs. 10 and 30 minutes.
To determine whether differential kinetics of phosphorylation of p65 were responsible for lack of differences in NF-κB signaling at time points subsequent to 4 hours following burn injury, we collected peritoneal cells 12 hours following injury. We then subjected these cells to stimulation with ex vivo LPS (10 ng/ml) for 5, 10 or 30 minutes (Figure 5B). There was no difference in P-p65 Rel A between the groups regardless of LPS exposure time. This suggests that neither macrophage activation following burn injury, nor its inhibition by VNS results from differential kinetics of phosphorylation of p-65 Rel A.
DISCUSSION
The increased susceptibility to sepsis following burn injury in patients has been linked to the hyper-responsive phenotype of macrophages, supporting a ‘two-hit’ model in which macrophage activation is dependent on pre-existing levels of immune activation(21). Macrophage activation can be regulated through cholinergic signaling of the vagus nerve(22–24), however, in a burn model, where the direct protective effects of vagal nerve stimulation on the gut have been documented(10), the effect of vagal nerve stimulation on peritoneal macrophages has not been examined. Therefore, we first determined that burn injury ‘primes’ peritoneal macrophages. After observing increased cytokine expression by peritoneal macrophages from burn injured animals, we showed that these macrophages were hyper-responsive to a secondary LPS challenge. NF-κB pathway was maximally activated by LPS stimulation in macrophages harvested at 4 hours post-injury as compared to later time-points. We also examined sham mice and observed that following VNS treatment, in the absence of burn injury, there was a reduction in P-p65 Rel A levels, and went on to find that VNS prior to burn injury resulted in attenuation of burn induced NF-κB. Finally, we investigated the kinetics of phosphorylation and found that the kinetics behave no differently at extended time periods following burn injury or following VNS, suggesting that the differences we observed were actual, and not due to alterations in the kinetics of phosphorylation of p65. These results indicate that VNS can lower the set-point of the injury response, further supporting the overall hypothesis that the anti-inflammatory effect of VNS in the burn model has indirect effects on peritoneal macrophages.
Major burn increases the inflammatory response of macrophages, which results in an exaggerated cytokine response to a second insult. This excessive cytokine production (TNFα, IL-6, IL-1, and others) can result in both tissue injury and increased susceptibility to secondary infections and sepsis(25). Studies in an LPS model of macrophage activation have shown that this exaggeration in cytokine response is not dependent upon the degree of secondary insult(26). Following severe burn injury, resident immune cells in the intestinal epithelia are activated by intestinal barrier breakdown, which further drives the inflammatory response both by activating resident gut dendritic cells and recruiting circulating monocyte/macrophages to the injured gut tissue(27, 28). The effect of burn injury on the activation state of peritoneal macrophages suggests that, either through increased systemic inflammation or as a result of injured gut tissue, the inflammatory state of cells in the peritoneal cavity are altered after injury. This activated state of macrophages after burn has been associated with increased susceptibility to sepsis(2, 29). Schwacha et. al. provide evidence to suggest that clinical improvement after burn wound excision may be explained by normalization macrophage hyper-responsiveness(30). Finding other mechanisms by which to suppress macrophage activation might thus further aid in clinical recovery. Given the known anti-inflammatory effects on both systemic and local gut inflammation, we considered the capacity for the cholinergic anti-inflammatory effect of vagal nerve stimulation to alter the inflammatory responsiveness of peritoneal macrophages in burn injured animals.
We found that peritoneal macrophage responsiveness to a secondary LPS challenge peaked at 4 hours post-burn, returning to below baseline levels by 24 hours after injury. This agrees with other studies in the timing of macrophage activation, which have shown that macrophages lose their elevated inflammatory status after exposure to LPS by 24 hours(26). It is also in accord with the kinetics of intestinal barrier breakdown and gut TNF-α increases seen after burn injury, both of which may alter the inflammatory state of peritoneal macrophages(31). Our laboratory has extensively studied intestinal barrier breakdown after burn injury, showing in this model that maximal intestinal barrier failure occurs at four hours post-burn, with resolution of intestinal barrier integrity by 24 hours post-burn(19). Additionally, we have shown that serum and intestinal TNF-α increases within the first few hours after the burn insult(10, 32).
Here, we have demonstrated that VNS decreases the inflammatory responsiveness of peritoneal macrophages following severe burn injury. Our laboratory has spent considerable time studying the protective effects of VNS in models of injury(33, 34). Our laboratory has shown that VNS protects against burn-induced intestinal epithelial barrier failure by signaling via the enteric nervous system through a pathway, which is independent of the spleen(20). Studies by de Jonge et al., have also demonstrated the ability of VNS to limit intestinal inflammation and peritoneal macrophage activation(35). Their results suggest that release of cholinergic agonists from efferent vagal nerve fibers can alter the inflammatory state of intestinal macrophages as the vagal nerve terminals in the myenteric plexus are in close proximity to gut macrophages. The VNS-induced protection against gut inflammation was also associated with decreased pro-inflammatory mediators in the peritoneal fluid. These studies suggested that although the vagus nerve is not known to have any direct innervation of peritoneal macrophages, preventing gut inflammation using VNS may indirectly alter the set-point of inflammatory responsiveness in peritoneal macrophages.
Clinically, severe injury is associated with up-regulation of numerous genes involved in the inflammatory response(1). Based on these results, it seems unlikely that targeting a single inflammatory pathway or cytokine could significantly alter the human response to injury. The therapeutic potential of exploiting the anti-inflammatory potential of vagal nerve signaling is attractive; VNS seems to alter the inflammatory set-point of cells in multiple tissues and may possess the ability to alter the global immune response to severe insults. Data presented in this series of experiments further defines the ability of VNS to alter the inflammatory state of the host immune cells. Taken together, this series of experiments have shown that pre-injury activation of the parasympathetic nervous system via VNS both prevents the burn-induced inflammatory hyper-responsiveness of peritoneal macrophage and decreases baseline NF-κB activation of these cells in uninjured animals. Therefore, VNS or therapies aimed at increasing efferent vagal nerve output may represent a novel strategy to alter the inflammatory set-point of immune cells and modulate the inflammatory response to injury.
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health through a P20 Exploratory Center grant for Wound Healing Research from the NIGMS (P20-GM078421) to A.B., B.E., and R.C. and through supplemental funding by the NIGMS through the American Recovery and Reinvestment Act (ARRA). The authors would like to thank James Putnam and David Cauvi for their expert assistance with qRT-PCR and Ann-Marie Hageny for Flow Cytomtery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 3.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157(6):2577–2585. [PubMed] [Google Scholar]
- 4.Ogle CK, Mao JX, Wu JZ, Ogle JD, Alexander JW. The 1994 Lindberg Award. The production of tumor necrosis factor, interleuki1-1, interleukin-6, and prostaglandin E2 by isolated enterocytes and gut macrophages: effect of lipopolysaccharide and thermal injury. J Burn Care Rehabil. 1994;15(6):470–477. [PubMed] [Google Scholar]
- 5.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The role of gammadelta T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J Leukoc Biol. 2004;76(3):545–552. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki JR, Zhang Q, Schwacha MG. Burn induces a Th-17 inflammatory response at the injury site. Burns. 37(4):646–651. doi: 10.1016/j.burns.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 8.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 9.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203(7):1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299(6):G1308–G1318. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costantini TW, Loomis WH, Putnam JG, Drusinsky D, Deree J, Choi S, Wolf P, Baird A, Eliceiri B, Bansal V, Coimbra R. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock. 2009;31(4):416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Chapter 14Unit 14 11. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Analytical biochemistry. 2008;379(1):127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Schulz KR, Danna EA, Krutzik PO, Nolan GP. Single-cell phospho-protein analysis by flow cytometry. Chapter 8Unit 8 17 11–20. Curr Protoc Immunol. 2012 doi: 10.1002/0471142735.im0817s96. [DOI] [PubMed] [Google Scholar]
- 15.Perez OD, Mitchell D, Campos R, Gao GJ, Li L, Nolan GP. Multiparameter analysis of intracellular phosphoepitopes in immunophenotyped cell populations by flow cytometry. Chapter 6Unit 6 20. Current protocols in cytometry / editorial board, J Paul Robinson, managing editor [et al] 2005 doi: 10.1002/0471142956.cy0620s32. [DOI] [PubMed] [Google Scholar]
- 16.Chow S, Hedley D, Shankey TV. Whole blood processing for measurement of signaling proteins by flow cytometry Chapter 9Unit 9 27. Current protocols in cytometry / editorial board, J Paul Robinson, managing editor [et al] 2008 doi: 10.1002/0471142956.cy0927s46. [DOI] [PubMed] [Google Scholar]
- 17.Xie S, Li J, Wang JH, Wu Q, Yang P, Hsu HC, Smythies LE, Mountz JD. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010;184(5):2289–2296. doi: 10.4049/jimmunol.0903133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez OD, Nolan GP. Simultaneous measurement of multiple active kinase states using polychromatic flow cytometry. Nature biotechnology. 2002;20(2):155–162. doi: 10.1038/nbt0202-155. [DOI] [PubMed] [Google Scholar]
- 19.Costantini TW, Eliceiri BP, Peterson CY, Loomis WH, Putnam JG, Baird A, Wolf P, Bansal V, Coimbra R. Quantitative assessment of intestinal injury using a novel in vivo, near-infrared imaging technique. Mol Imaging. 9(1):30–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 299(6):G1308–G1318. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwacha MG, Somers SD. Thermal injury induces macrophage hyperactivity through pertussis toxin-sensitive and -insensitive pathways. Shock. 1998;9(4):249–255. doi: 10.1097/00024382-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105(31):11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201(7):1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 25.O'Riordain MG, Collins KH, Pilz M, Saporoschetz IB, Mannick JA, Rodrick ML. Modulation of macrophage hyperactivity improves survival in a burn-sepsis model. Arch Surg. 1992;127(2):152–157. doi: 10.1001/archsurg.1992.01420020034005. discussion 157–158, [DOI] [PubMed] [Google Scholar]
- 26.Islam Z, Pestka JJ. LPS priming potentiates and prolongs proinflammatory cytokine response to the trichothecene deoxynivalenol in the mouse. Toxicol Appl Pharmacol. 2006;211(1):53–63. doi: 10.1016/j.taap.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 4(1):31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 29.Schwacha MG, Somers SD. Thermal injury-induced immunosuppression in mice: the role of macrophage-derived reactive nitrogen intermediates. J Leukoc Biol. 1998;63(1):51–58. doi: 10.1002/jlb.63.1.51. [DOI] [PubMed] [Google Scholar]
- 30.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14(6):623–628. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Woodside KJ, Song J, Wolf SE. Burn-induced gut mucosal homeostasis in TCR delta receptor-deficient mice. Shock. 2004;21(1):52–57. doi: 10.1097/01.shk.0000104268.15342.8f. [DOI] [PubMed] [Google Scholar]
- 32.Peterson CY, Costantini TW, Loomis WH, Putnam JG, Wolf P, Bansal V, Eliceiri BP, Baird A, Coimbra R. Toll-like receptor-4 mediates intestinal barrier breakdown after thermal injury. Surg Infect (Larchmt) 2010;11(2):137–144. doi: 10.1089/sur.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini TW, Bansal V, Peterson CY, Loomis WH, Putnam JG, Rankin F, Wolf P, Eliceiri BP, Baird A, Coimbra R. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. J Trauma. 68(6):1349–1354. doi: 10.1097/TA.0b013e3181dccea0. discussion 1354–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bansal V, Costantini T, Ryu SY, Peterson C, Loomis W, Putnam J, Elicieri B, Baird A, Coimbra R. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 68(5):1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nature immunology. 2005;6(8):844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]