Abstract
Vascular endothelial cells can demonstrate considerable plasticity to generate other cell types during embryonic development and disease progression. This process occurs through a cell differentiation mechanism known as endothelial-mesenchymal transition (EndMT). The generation of mesenchymal cells from endothelium is a crucial step in endothelial cell differentiation to several lineages including fibroblasts, myofibroblasts, mural cells, osteoblasts, chondrocytes, and adipocytes. Such differentiation patterns have been observed in systems of cardiac development, fibrosis, diabetic nephropathy, heterotopic ossification and cancer. Here we describe the EndMT program and discuss the current evidence of EndMT-mediated acquisition of stem cell characteristics and multipotent differentiation capabilities.
Keywords: Endothelial-mesenchymal transition, EMT, EndMT, stem cells, TGF-beta
The epithelial-mesenchymal transition (EMT) program is the primary mechanism of cancer metastasis, inducing carcinoma cells to lose their adhesion, acquire mesenchymal properties and migrate away from the primary tumor [1,2]. This process has also been shown to regulate several phases of embryonic development including gastrulation, somite dissociation, primitive streak and neural crest migration, and craniofacial development [3,4]. EMT has also been suggested to promote fibrosis and wound healing [5,6].
More recently, endothelial cells have been shown to undergo a similar process termed endothelial-mesenchymal transition (EndMT), which is of fundamental importance in mediating similar physiological and pathological processes [7]. Here we review the systems where EndMT has been identified and the molecular mechanisms that control this change in cellular phenotype.
The Molecular and Cellular Basis of EndMT
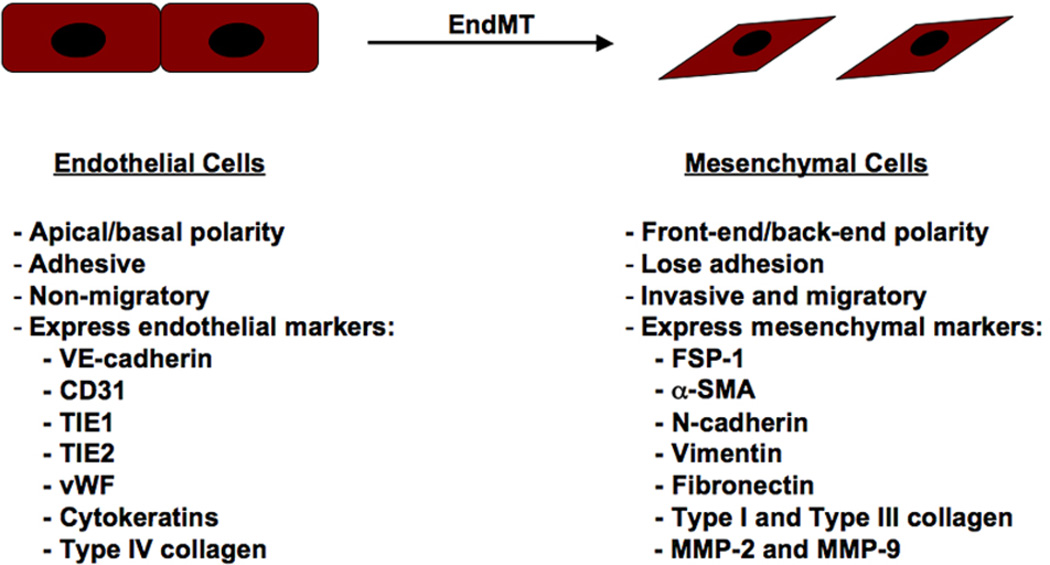
Endothelial cells compose the inner lining of blood vessels and lymphatic vessels [7]. These cells, which are anatomically similar to squamous epithelium, demonstrate apical-basal polarity and are tightly bound by adherens junctions and tight junctions. These cells express a distinct set of biomarkers that allow investigators to distinguish them from other cell types including VE-cadherin, CD31, TIE1, TIE2, von Willebrand Factor (vWF), and cytokeratins. During EndMT, expression of these markers is dramatically reduced, although some minimal levels of expression are usually maintained. Furthermore, mesenchymal-specific genes are expressed which include fibroblast-specific protein-1 (FSP-1), alpha-smooth muscle actin (α-SMA), vimentin, and N-cadherin. These changes in gene and protein expression cause the endothelial cells to lose their adhesion and stimulate alterations in cytoskeletal composition and organization to induce a striking change in cell morphology that forms elongated, spindle-shaped cells. These newly formed mesenchymal cells are highly invaisive and migratory [1,7] (Figure 1).
Figure 1.
Changes in cellular characteristics during EndMT. The EndMT program causes decreased expression of endothelial markers VE-cadherin, CD31, TIE1, TIE2, and vWF, and a gain of mesenchymal markers FSP-1, α-SMA, N-cadherin, vimentin, fibronectin, type I and type III collagen, and MMP-2 and MMP-9. Distinct changes in cell polarity and morphology accompany EndMT, as well as loss of cell-cell junctions and increased motility.
The EndMT program can be stimulated by many factors. The most common of these are the Transforming Growth Factor-beta (TGF-β)/Bone Morphogenetic Protein (BMP) family of growth factors [8]. All three TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3) have been shown to induce EMT [9, 10], however EndMT appears to be stimulated primarily by the TGF-β2 isoform [11–18]. Genetic ablation of TGF-β2 in mice prevents embryonic EndMT, whereas TGF-β1 or TGF-β3 knockout mice show no significant effects on EndMT during development [16]. TGF-β2 has also been shown to stimulate EndMT in cultured endothelial cells [12,13,17,18].
TGF-β3 has been shown to have a role in post-EndMT invasion and migration in the chick embryo, but not in EndMT itself [19,20]. TGF-β3 has no significant role in embryonic EndMT in mice [21]. Some studies have suggested that TGF-β1 may induce EndMT [22,23], although the primary effect of TGF-β1 on endothelial cells is to induce cell proliferation [24,25].
TGF-β1 is perhaps the most common inducer of EMT [1,2,9]. In epithelial cells, TGF-β1 binds to a complex of receptors that include the TGF-β type III receptor β-glycan, type II receptor (TβRII) and type I receptor activin-like kinase 5 (ALK5), which promotes signaling through Smad2/3 [26]. In endothelial cells, TGF-β1 promotes signaling through ALK1 rather than ALK5, which causes phosphorylation of Smad1/5/8. When ALK1 is involved, TGF-β1 signaling induces cell proliferation rather than EndMT [24,25]. This occurs because endothelial cells express another TGF-β type III receptor called endoglin. siRNA-mediated knockdown of endoglin in endothelial cells allows TGF-β1 to signal through β-glycan and ALK5 and phosphorylate Smad2/3 [24]. Inhibition of ALK5, TβRII, β-glycan and endoglin in the endothelium of mice have been shown to prevent embryonic EndMT [19, 21, 27]. SB-431542, a chemical inhibitor of ALK5, was found to be sufficient to inhibit EndMT of cultured endothelial cells [23]. Reducing ALK5 expression with siRNA showed similar inhibitory results [17].
BMP2 and BMP4 have been shown to induce both EMT [28,29] and EndMT [30,31] programs. These ligands primarily signal through the ALK2 receptor [32]. Interestingly, conditional knockouts of BMP2, BMP4 or ALK2 all inhibit embryonic EndMT [30,31,33]. Also, siRNA mediated knockdown or chemical inhibition of ALK2 with dorsomorphin blocked EndMT in endothelial cultures [17].
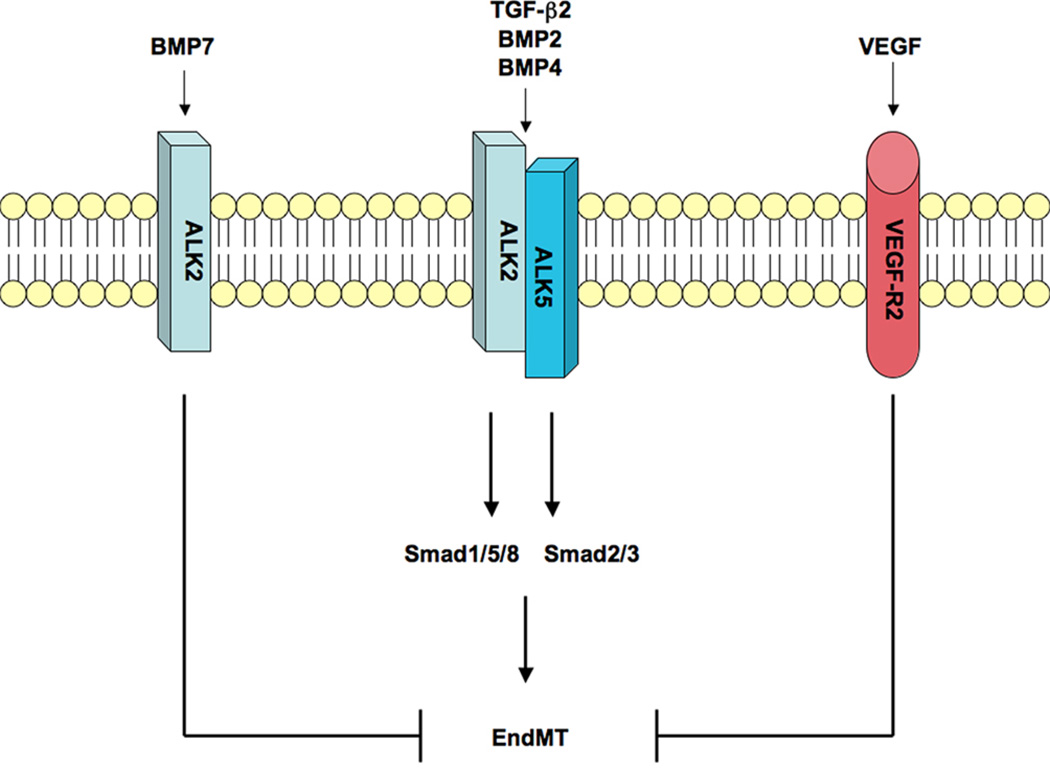
BMP7 is another isoform that binds and activates ALK2 [32], yet it is known to be an extremely potent inhibitor of EndMT [22]. Recent studies have suggested that TGF-β2 and BMP4 induce EndMT by activating both ALK2 and ALK5, which function together in a complex of signaling receptors to activate both Smad1/5/8 and Smad2/3 signaling pathways [17]. On the other hand, BMP7 activates only ALK2 and the Smad1/5/8 pathway [17], suggesting that this may play a role in the inhibitory effects of this isoform on EndMT. Vascular endothelial growth factor (VEGF) signaling has also been shown to inhibit EndMT [17,34], although the downstream signaling mechanisms of this inhibition are currently unknown (Figure 2).
Figure 2.
Signaling mechanisms of EndMT. TGF-β/BMP receptors termed activin-like kinases (ALK) control EndMT. Ligands such as BMP2, BMP4, and TGF-β2 bind and activate ALK2 and ALK5, which stimulate Smad1/5/8 and Smad2/3 signaling to induce EndMT. BMP7 activates ALK2, but not ALK5, which inhibits EndMT. VEGF signaling through the VEGF receptor 2 (VEGF-R2) has also been described to block EndMT.
Smad-independent signaling pathways have also been shown to have a role in regulating EndMT. Inhibitors of Smad4, MEK, PI3K and p38 MAPK all sufficiently blocked TGF-β2-induced EndMT in endothelial cultures, demonstrating critical roles for Smad-dependent and Smad-indepenent TGF-β signals [18]. All of these pathways were shown to be necessary for TGF-β2-induced expression of the EMT/EndMT-promoting transcription factor Snail [15,18].
Loss of cell-cell adhesion associated with EndMT is mediated by transcription factors such as Snail, Slug, ZEB-1, SIP-1, Twist, and LEF-1 that suppress transcription of genes encoding proteins involved in formation of adherens junctions and tight junctions [15, 17,18, 35–38]. All of these transcription factors are up-regulated during EndMT induced by TGF-β2 or BMP4 [17]. siRNA-mediated knockdown of Snail expression was sufficient to inhibit TGF-β2-induced EndMT in cultured endothelial cells. However, over-expression of Snail was insufficient to induce EndMT. Blocking the Snail inhibitor GSK-3β [39] with lithium chloride was sufficient to allow EndMT by Snail over-expression [18].
Other signaling pathways including FGF-2 [40]. Notch [41] and Wnt [12] have also been linked to EndMT. During EMT/EndMT, the basal lamina is degraded by matrix metalloproteinases (MMP) such as MMP-2 and MMP-9 and is replaced by new matrix molecules like type I collagen, type III collagen and fibronectin [2,7]. Type I collagen has long been associated with induction of EMT/EndMT by activating the signaling receptors α2β1 integrin [42,43], discoidin domain receptor 1 (DDR1) [44], and DDR2 [45]. Fibronectin induces post-EndMT cell migration through α5β1 integrin signaling, which promotes cytoskeletal reorganization [46].
EndMT in Embryonic Development
EndMT was initially discovered as an essential mechanism of heart development [47]. Vascular endothelial cells surrounding in atriventricular canal and the outflow tract undergo EndMT and invade surrounding tissues to form the valves and septa of the heart [21]. Several mechanistic studies have shown the crucial importance of TGF-β/BMP ligands and receptors in embryonic EndMT. Targeted inhibition of TGF-β2, BMP2, BMP4, ALK2, ALK5, endoglin, or β-glycan in mice show defective heart development due to a lack of EndMT [21]. Interestingly, genetic knockout of TGF-β1 or TGF-β3 in the endothelium of mice showed no detrimental effects on heart development [16], suggesting strong isoform specificity for TGF-β2 in the induction of EndMT. However, in the avian heart, both TGF-β2 and TGF-β3 play critical roles in development, with TGF-β2 inducing EndMT and TGF-β3 promoting post-EndMT invasion and migration into the underlying tissue [19]. Notch [41] and Wnt [12] signaling have also been shown to have an important role in regulating EndMT in the developing heart.
EndMT in Fibrosis
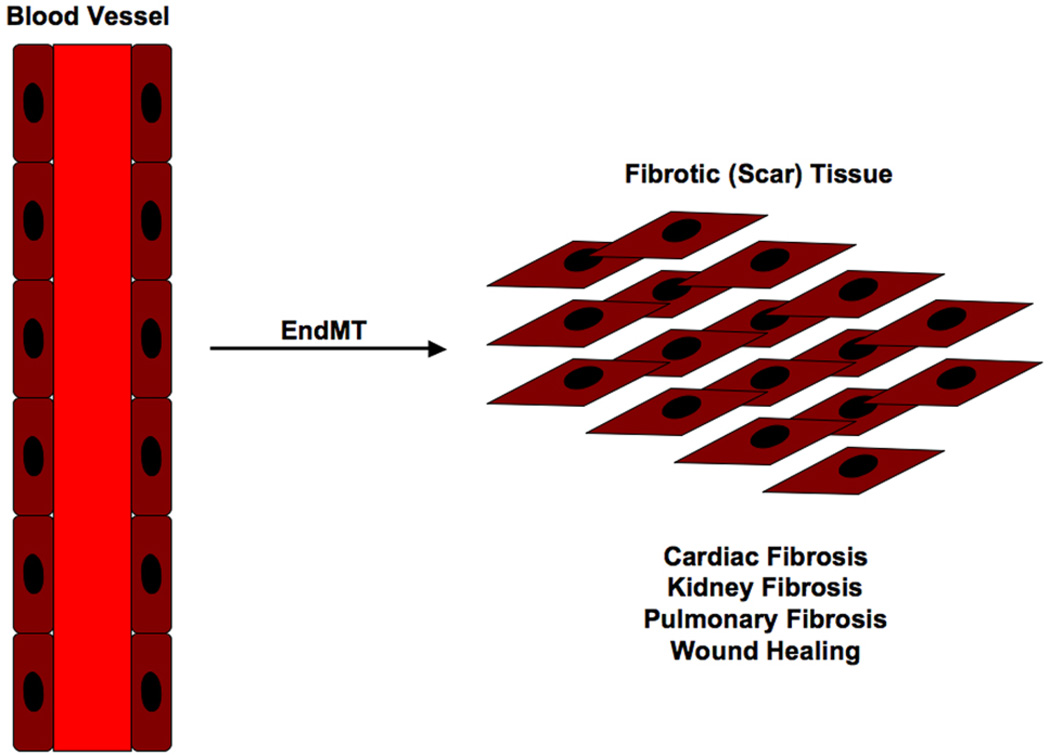
Although EndMT is an embryonic mechanism that is normally dormant in the adult organism, pathological conditions can arise that awaken this phenomenon. One such condition that effects most organs as a result of injury, inflammation or aging is fibrosis [2,48]. EMT is known to be essential for the formation of fibrotic tissues [2], but more recent evidence suggests that EndMT also contributes to fibrosis [7,22] (Figure 3).
Figure 3.
EndMT generates fibrotic tissue. Endothelial cells from capillary blood vessels in organs have been shown to undergo EndMT and form fibrotic tissue. This mechanism causes fibrosis in organs such as kidney, lung and heart.
Cardiac fibrosis is a common result of heart failure. Myocardial infarction and ischemia can induce scarring of the heart tissue [49]. Lineage tracing studies using Tie1-Cre reporter mice have shown that many of the fibroblast formed in fibrotic lesions of the heart are of endothelial origin and arise through EndMT. Further evidence suggests that this process occurs in a TGF-β- and Smad3-dependent manner, similar to developmental EndMT. Furthermore, treatment with recombinant BMP7 was sufficient to inhibit EndMT and the incidence of cardiac fibrosis in mice [22].
Fibrosis has been shown to be prominent in renal diseases such as Alport syndrome and diabetic nephropathy [50]. Studies in mouse models of Alport syndrome and nephropathy induced by streptozotocin or unilateral urethral obstruction showed that up to half of all fibroblasts formed in the kidneys under these conditions expressed the endothelial marker CD31, suggesting that they could arise by EndMT [51]. This was confirmed by recent evidence using lineage tracing with Tie2-Cre reporter mice showing that many of the myofibroblasts in kidneys of mice with diabetic nephropathy were indeed of endothelial origin [51,52]. Similar to cardiac fibrosis, EndMT in kidney fibrosis was found to be Smad3-dependent [53].
Capillary endothelial cells in the lung have been shown to undergo EndMT to contribute to pulmonary fibrosis [54]. EndMT may also contribute in wound healing [7,40].
EndMT in Cancer
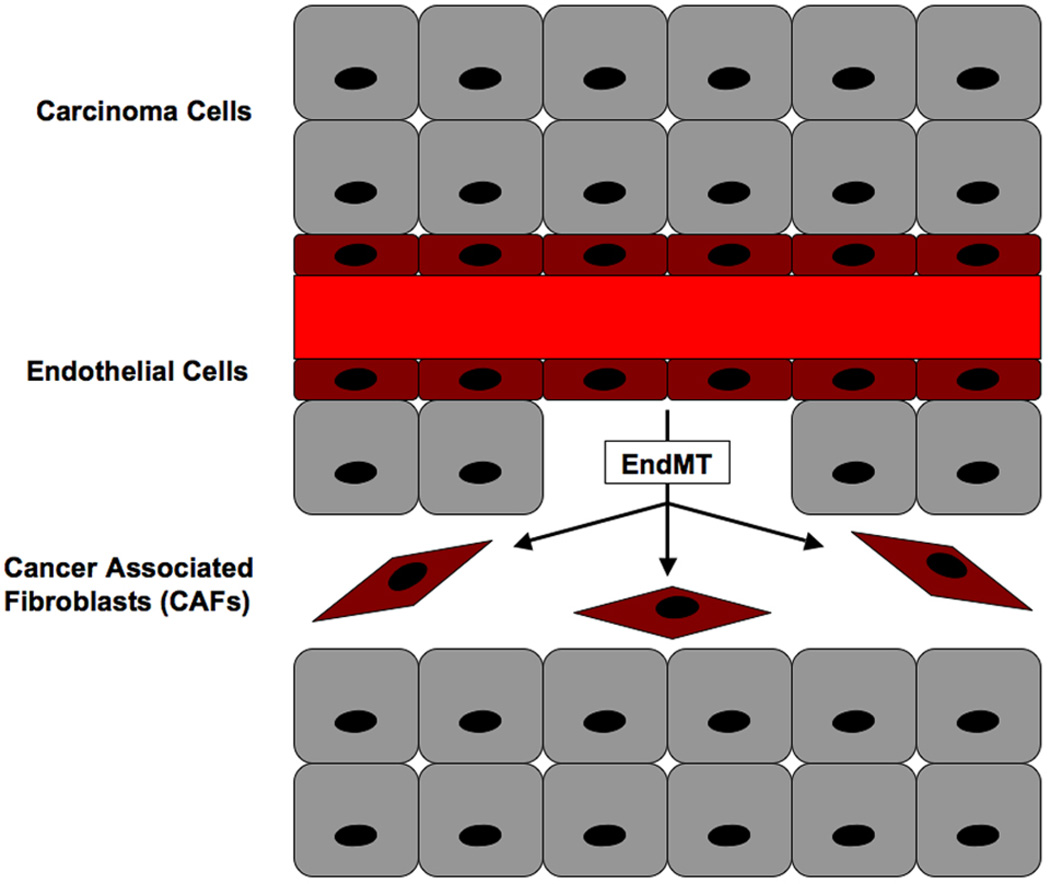
The tumor microenvironment plays an important role in cancer growth, angiogenesis and metastasis [55]. One component of this microenvironment that regulates these processes is cancer-associated fibroblasts (CAFs), which are part of the tumor stroma. In an elegant study, Zeisberg et al. [56] showed that up to 40% of CAFs were formed by EndMT in two distinct mouse models of cancer. CAFs in these models co-expressed the endothelial marker CD31 along with mesenchymal markers FSP-1 or α-SMA. Moreover, lineage using Tie2-Cre reporter mice confirmed the endothelial origin of these CAFs [56]. It has been suggested that CAFs regulate metastasis by secreting cytokines such as TGF-β1, which induces EMT of the cancer cells [7].
Hypoxia is another condition in which EndMT-dependent formation of fibroblasts may occur [57,58]. As tumors increase in size, they naturally become hypoxic. This lack of oxygen activates Hypoxia Inducible Factor-1 (HIF-1), a transcription factor responsible for stimulating expression of endothelial growth factors to induce tumor angiogenesis; an important mechanism for tumor growth and metastasis [59]. Hypoxia has previously been linked to EMT and metastasis [60]. Therefore, it is likely that as tumors grow larger and become more hypoxic, a higher incidence CAF formation through EndMT will occur (Figure 4).
Figure 4.
EndMT generates cancer-associated fibroblasts (CAFs). Capillary blood vessels in angiogenic tumors undergo EndMT to form CAFs. These fibroblasts play an essential role in the tumor microenvironment and regulate cancer growth and metastasis.
EndMT may also play a direct role in regulating tumor angiogenesis. It has been suggested that vessel branching may be a result of EndMT forming the tip cells, which have characteristics of mesenchymal cells [7,61]. At the angiogenic tip, these cells are highly migratory and are exposed to matrix proteins like type I collagen [61], a known inducer of EMT/EndMT [43,45], rather than type IV collagen in the standard endothelial basal lamina [7]. These data suggest that EndMT might occur in the leading edge of branching vessels.
EndMT and the Stem Cell Phenotype
In a recent study of a rare bone disease called Fibrodysplasia Ossificans Progressiva (FOP), vascular endothelial cells were shown differentiate into chondrocytes and osteoblasts through EndMT [17,62]. In patients with FOP, acute inflammation triggers heterotopic ossification in soft tissues [63,64]. FOP patients carry a heterozygous germ-line mutation (R206H) in the TGF-β/BMP receptor ALK2 [65]. Studies have shown that R206H is a gain of function mutation that causes the receptor to be constitutively active in the absence of ligands [66].
Tissue sections of ectopic lesions acquired from FOP patients showed positive staining of cartilage and bone cells for the endothelial biomarkers TIE2 and vWF. Bone and cartilage cells from normal skeletal tissue did not express such markers. Furthermore, heterotopic lesions from a transgenic mouse model of FOP, which express the mutant ALK2 gene found in FOP patients in a Cre-dependent manner, showed similar positive expression of endothelial markers in the cartilage and bone cells [17]. Lineage tracing with Tie2-Cre reporter mice also suggested an endothelial origin to heterotopic cartilage and bone cells, with approximately 50% of these cells showing expression of the GFP reporter [17]. The origin of the remaining 50% is currently unknown. Also, in the early stages of heterotopic ossification, a condensation of mesenchymal cells appears prior to chondorgenesis or osteogenesis [63,67], many of which are of endothelial origin [17] providing in vivo evidence for EndMT as a critical mechanism mediating this disease.
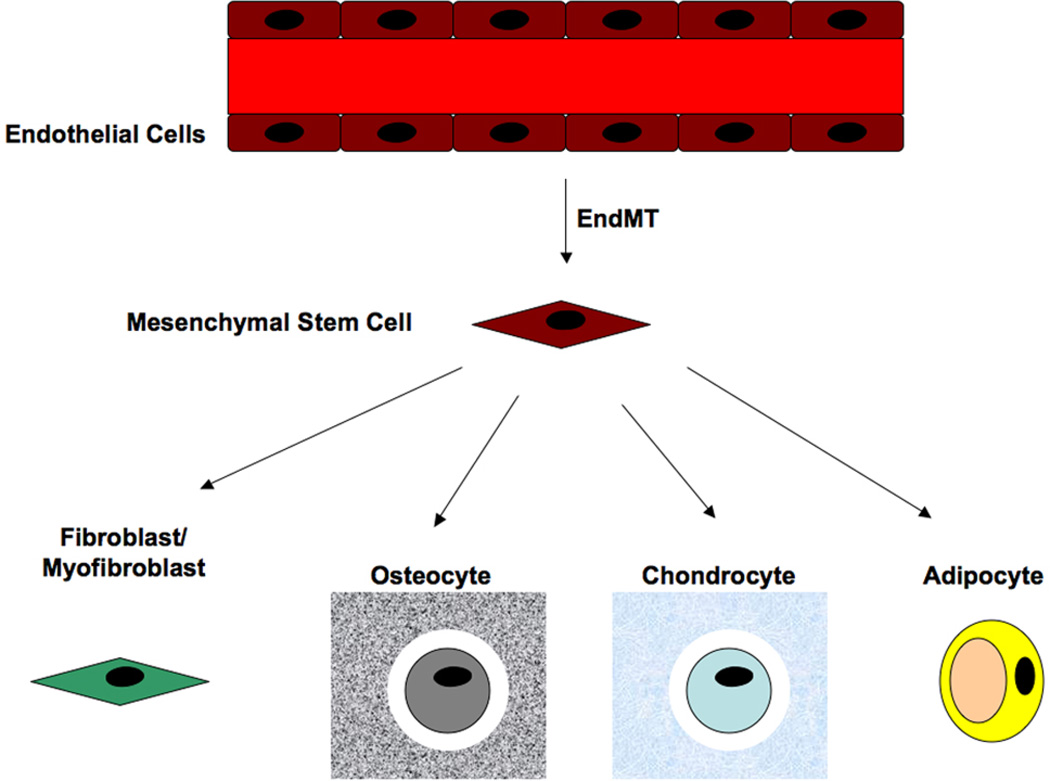
Further investigations into the role of mutant (R206H) ALK2 in normal vascular endothelial cells showed that over-expression of this mutant gene induced EndMT. These cells also expressed biomarkers of mesenchymal stem cells such as STRO-1, CD10, CD44, CD71, CD90, and CD117. Furthermore, EndMT also induced physiological properties of stem cells, as they could be stimulated to differentiate into osteoblasts, chondrocytes or adipocytes both in vitro and in vivo [17]. These results provide evidence that EndMT can generate cell types other than fibroblasts or myofibroblasts. These data demonstrated that EndMT is a mechanism for generating mesenchymal stem cells that can subsequently differentiate into other cell types (Figure 5). These results suggest that EndMT may not be a direct transformation to fibroblasts as originally thought, but rather a dedifferentiation to mesenchymal stem cells.
Figure 5.
EndMT generates mesenchymal stem cells. Endothelial cells that convert into mesenchyme acquire properties of stem cells including expression of stem cell markers and multipotent differentiation capabilities. EndMT has been show to induce formation of fibroblasts/myofibroblasts, osteoblasts/osteocytes, chondrocytes and adipocytes.
In a study of tumor calcification, prostate carcinomas showed bone-forming cells in the tumors that induce ossification. Interestingly, these osteoblasts stained positive for the endothelial biomarker CD31 [68]. Since EndMT is a prominent mechanism for the formation of cancer-associated fibroblasts in the tumor microenvironment [56], it is likely that the same mechanism that induces heterotopic ossification in FOP could be happening to induce tumor calcifications by generating endothelial-derived osteoblasts.
Others have suggested that mural cells (pericytes and smooth muscle cells) may arise by EndMT [7,23,69]. Pericytes are mesenchymal cells that have been described to have stem-like properties and differentiate into other cell types [70]. Furthermore, circulating endothelial progenitor cells can undergo EndMT and have been suggested to give rise to smooth muscle cell progeny [23].
Cancer stem cells are generated by EMT [71,72]; the same mechanism that induces metastasis [1,2]. Although EndMT has not yet been shown to produce cancer stem cells, the ability of endothelial cells to convert into mesenchymal stem cells has been demonstrated in FOP [17]. Considering that EndMT is known to generate cancer-associated fibroblasts in the tumor microenvironment [56], it is reasonable to think that some cancer stem cells found in tumors might be of endothelial origin.
ACKNOWLEDGEMENTS
This work was supported by a grant from the John Butler Mulliken Foundation to D. Medici and grants from the National Institutes of Health (CA125550, CA155370, CA151925, DK55001, DK81576) to R. Kalluri. R. Kalluri is a Champalimaud Investigator.
Abbreviations
- EndMT
endothelial-mesenchymal transition
- EMT
epithelial-mesenchymal transition
- TGF-β
transforming growth factor-beta
- BMP
bone morphogenetic protein
- TβRII
transforming growth factor-beta receptor 2
- ALK
activin-like kinase
- FSP-1
fibroblast-specific protein-1
- α-SMA
alpha-smooth muscle actin
- DDR
discoidin domain receptor
- vWF
von Willebrand factor
- MMP
matrix metaloproteinase
- CAF
cancer-associated fibroblast
- HIF-1
hypoxia-inducible factor-1
- FOP
fibrodysplasia ossificans progressiva
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 4.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1222-6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhurst RJ, Derynck R. TGF-beta signaling in cancer - a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 10.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through β-catenin–TCF-4-dependent expression of TGF-β3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romano LA, Runyan RB. Slug is an essential target of TGFbeta2 signaling in the developing chicken heart. Dev Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- 12.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, et al. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deissler H, Deissler H, Lang GK, Lang GE. TGFbeta induces transdifferentiation of iBREC to alphaSMA-expressing cells. Int J Mol Med. 2006;18:577–582. [PubMed] [Google Scholar]
- 14.Tavares AL, Mercado-Pimentel ME, Runyan RB, Kitten GT. TGF beta-mediated RhoA expression is necessary for epithelial-mesenchymal transition in the embryonic chick heart. Dev Dyn. 2006;235:1589–1598. doi: 10.1002/dvdy.20771. [DOI] [PubMed] [Google Scholar]
- 15.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 16.Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, et al. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn. 2009;238:431–442. doi: 10.1002/dvdy.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medici D, Potenta S, Kalluri R. Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J. 2011;437:515–520. doi: 10.1042/BJ20101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- 20.Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, et al. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 21.Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- 22.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 23.Moonen JR, Krenning G, Brinker MG, Koerts JA, van Luyn MJ, Harmsen MC. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res. 2010;86:506–515. doi: 10.1093/cvr/cvq012. [DOI] [PubMed] [Google Scholar]
- 24.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebrin F, Deckers M, Bertolino P, ten Dijke P. TGF-beta receptor function in the endothelium. Cadiovasc Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Wrana JL, Attisano L, Weisner R, Ventura F, Massague J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 27.Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. Signaling via the Tgf-β type I receptor Alk5 in heart development. Dev Biol. 2008;322:208–218. doi: 10.1016/j.ydbio.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang MH, Kang HN, Kim JL, Kim JS, Oh SC, Yoo YA. Inhibition of PI3 kinase/Akt pathway is required for BMP2-induced EMT and invasion. Oncol Rep. 2009;22:525–534. doi: 10.3892/or_00000467. [DOI] [PubMed] [Google Scholar]
- 29.Molloy EL, Adams A, Moore JB, Masterson JC, Madrigal-Estebas L, Mahon BP, et al. BMP4 induces an epithelial-mesenchymal transition-like response is adult airway epithelial cells. Growth Factors. 2008;26:12–22. doi: 10.1080/08977190801987166. [DOI] [PubMed] [Google Scholar]
- 30.Ma L, Lu MF, Schwartz RJ, Martin JF. BMP2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 31.McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, et al. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan Z, Loukogeorgakis S, et al. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006;99:861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;8:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 36.Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nawhsad A, Medici D, Liu CC, Hay ED. TGFbeta3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2–Smad4–LEF-1 transcription complex. J Cell Sci. 2006;120:1646–1653. doi: 10.1242/jcs.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 40.Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp Eye Res. 2006;83:1309–1316. doi: 10.1016/j.exer.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Chang AC, Fu Y, Garside VC, Niessen K, Chang L, Fuller M, et al. Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev Cell. 2011;21:288–300. doi: 10.1016/j.devcel.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Valles AM, Boyer B, Tarone G, Thiery JP. Alpha 2 beta 1 integrin is required for the collagen and FGF-1 induced cell dispersion in a rat bladder carcinoma cell line. Cell Adhes Commun. 1996;4:187–199. doi: 10.3109/15419069609014222. [DOI] [PubMed] [Google Scholar]
- 43.Medici D, Nawshad A. Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-kappaB and LEF-1. Matrix Biol. 2010;29:161–165. doi: 10.1016/j.matbio.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh LA, Nawshad A, Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix Biol. 2011;30:243–247. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals—distinct functions of alpha5beta1 and alphavbeta3 intergrins in regulating cell behaviour. IUBMB Life. 2009;61:731–738. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markwald RR, Fitzharris TP, Smith WN. Structural analysis of endocardial cytodifferentiation. Dev Biol. 1975;42:160–180. doi: 10.1016/0012-1606(75)90321-8. [DOI] [PubMed] [Google Scholar]
- 48.Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010;119:S22–S26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 50.Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol. 2009;175:1371–1373. doi: 10.2353/ajpath.2009.090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Qu X, Yao J, Caruana G, Ricardo SD, Yamamoto Y, et al. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:2612–2624. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 56.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 57.Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR-2 attenuates pulmonary hypertension. Eur Respir J. 2011 doi: 10.1183/09031936.00187310. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 59.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16:5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther. 2011;11:714–723. doi: 10.4161/cbt.11.8.15274. [DOI] [PubMed] [Google Scholar]
- 61.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 62.Medici D, Olsen BR. Transforming blood vessels into bone. Cell Cycle. 2011;10:362–363. doi: 10.4161/cc.10.3.14519. [DOI] [PubMed] [Google Scholar]
- 63.Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP) Bone. 2008;43:427–433. doi: 10.1016/j.bone.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, et al. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP) J Bone Miner Metab. 2008;26:521–530. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 66.Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, et al. Fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dudley AC, Khan ZA, Shih SC, Kang SY, Zwaans BM, Bischoff J, et al. Calcification of multipotent prostate tumor endothelium. Cancer Cell. 2008;14:201–211. doi: 10.1016/j.ccr.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armulik A, Abramsson A, Betsholtz C. Endothelial/Pericyte Interactons. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 70.Perivasclar cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development. 2005;132:2657–2668. doi: 10.1242/dev.01846. [DOI] [PubMed] [Google Scholar]
- 71.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]