Abstract
Selected topics in the field of rotavirus immunity are reviewed focusing on recent developments that may improve efficacy and safety of current and future vaccines. Rotaviruses have developed multiple mechanisms to evade interferon-mediated innate immunity. Compared to more developed regions of the world, protection induced by natural infection and vaccination is reduced in developing countries where, among other factors, high viral challenge loads are common and where infants are infected at an early age. Studies in developing countries indicate that rotavirus-specific serum IgA levels are not an optimal correlate of protection following vaccination, and better correlates need to be identified. Protection against rotavirus following vaccination is substantially heterotypic; nonetheless, a role for homotypic immunity in selection of circulating post vaccination strains needs further study.
INTRODUCTION
We and others have previously reviewed immunity to rotaviruses (RV) [1] and correlates of protection for RV vaccines [2–4]. Here, we will focus on recent and selected topics in the field with emphasis on issues related to improving vaccine efficacy. We will first analyze mechanisms used by RV to evade the innate immune response, mainly based on data from cell culture studies and the mouse model. Second, we will review new data on protection induced by natural infection in humans. Third, we will analyze the correlation of serum IgA levels induced by the two currently licensed safe and effective live RV vaccines with protection [5,6], and finally, we will examine the importance of heterotypic versus homotypic immunity.
Mechanisms used by RV to modulate innate immunity
Recently, a number of studies examining the capacity of RV to evade the innate immune response, and in particular the interferon (IFN) response, have been published. Using non-purified preparations of intestinal epithelial cells from mouse pups, RV infection was found to induce type I (IFN-β) and type III IFN (IFN-λ) mRNA expression [7]. Moreover, treatment of mice with IFN-λ, and to a lesser extent type I IFN, reduces virus replication [7]. In agreement with these findings, mice lacking the receptor for type III IFN, and to a lesser degree the receptor for type I IFN, have higher levels of viral replication than wild-type mice, demonstrating the importance of the IFN system in modulating viral replication [7]. Thus, it is not surprising that the RV has developed multiple mechanisms to evade the type I IFN response, many of them through the action of the viral protein NSP1 [8,9].
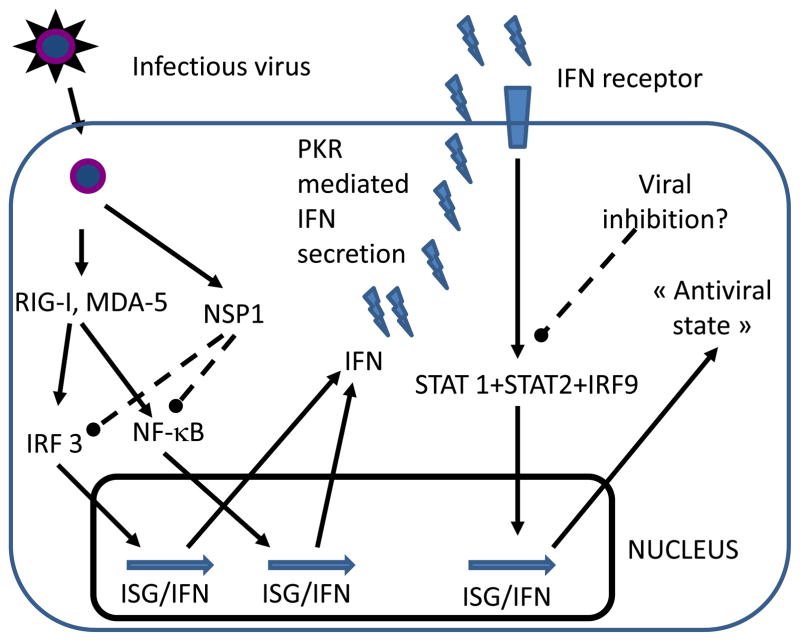
A simplified model of the mechanism used by RV to evade the IFN response is presented in Figure 1. Viral replication is recognized by RIG-I (see Glossary box) and/or MDA-5, which in turn activate IRF3 and NF-κB. These transcription factors then induce expression of type I IFN and interferon-stimulated genes (ISGs) [10,11]. RV NSP1 prompts degradation of IRF3 and inhibition of NF-κB activity [8,9,12]. These effects are complex and depend on viral strain and cell type. For example, NSP1s from some animal RVs degrade IRF3, IRF5 and IRF7; in contrast, NSP1 of human RVs predominantly degrade IRF5 and IRF7 and may, therefore, less efficiently inhibit the IFN response [13]. The degree of extra-intestinal spread and replication of RV appears, at least in part, to be dependent on the capacity of a RV strain to inhibit the IFN system in specific cells: In suckling mice without an intact IFN signaling system, some heterologous strains of RV replicate very efficiently in the biliary tree and pancreas and cause biliary atresia and pancreatic disease, whereas homologous murine strains do not [14]. RV replication in the murine biliary tract is determined by both viral entry, mediated by VP4, and viral evasion of the innate immune response, mediated by NSP1 [15,16]. After RV induction of transcription of IFN, secretion of this cytokine is modulated by PKR [11]. In an autocrine fashion, IFN stimulates type I IFNR expression; IFNR then signals through STAT1/STAT2/IRF9 complexes to further induce IFN and ISG expression and an antiviral state (Figure 1). RV has also been shown to inhibit the function of STAT1 and STAT2 by an unknown mechanism and thus, can potentially inhibit all types of IFN responses [17]. RV has also been shown to stimulate the secretion of TGF-β in polarized Caco-2 cells and this effect has been shown to inhibit the capacity of dendritic cells to activate Th1 T cells [18,19]. It is unknown whether this mechanism occurs in vivo but if so, it could potentially explain the poor T cell response to RV seen in vivo [20].
Figure 1.
Modulation of the host innate immune system by RV. After viral entry, viral replication activates the pathogen recognition receptors RIG-I and MDA-5, which in turn activate the transcription factor IRF3 and NF-κB. These molecules translocate to the nucleus, where they induce the transcription of ISGs and IFN. Viral replication produces NSP1 that can induce proteasomal degradation of IRF3. Through NSP1 dependent and independent mechanisms RV may also block NF-κB translocation. After transcription of IFNs, the dsRNA-dependent protein kinase PKR modulates IFN secretion by an unknown mechanism. Autocrine secreted IFN signals through the IFN receptor to activate transcription factors STAT1, STAT2 and IRF9. These factors translocate to the nucleus and further increase the levels of ISG and IFN transcripts, establishing an “antiviral state”. RV has also been shown to block STAT1 and STAT2 through an unknown mechanism.
Protection induced by natural infection
Development of the RV vaccines currently in use was based on the observation that natural infection can protect against severe RV-induced acute and recurrent gastroenteritis (RVGE). Cohort studies carried out in Mexico [21] and Guinea-Bissau [22] showed that recurrent episodes of RV disease were less severe than the first episode; one episode of RV infection had a protective efficacy of 70% [22] or 77% [21] against RV-induced diarrhea. Two infections, either symptomatic or asymptomatic, protected 100% against moderate to severe disease of any serotype [21]. These observations were challenged, however, by recently published results on a cohort of young children in Vellore (India), in which the severity of diarrhea did not significantly decrease between the first and second infections, but did between the second and third infections; protection after one episode of RV infection was 43% against RVGE, and it took three infections to induce 79% protection against moderate or severe RVGE [23]. In this Indian cohort study there was an earlier occurrence of primary RV infection, with 53% of children infected by 6 months of age, in contrast with 34% and 26% of children infected by that age in Mexico and Guinea-Bissau, respectively. The occurrence of an early primary infection is affected by likelihood of year-round RV exposure and environmental viral loads, with high loads [24] leading to RV infection very soon after the waning of transplacentally acquired maternal antibodies. The early infection of children in the poorest countries of Asia and Africa may also have an effect on vaccine efficacy in countries with high year-round RV infection prevalence rates: Children in the placebo arms of studies in these settings have high rates of RV-specific IgA seroconversion, and these high rates are significantly and inversely correlated with protection (Supplementary Table 1). Given that the capacity to induce neutralizing antibodies to RV is age dependent [25], early infection of children may not induce an efficient protective immune response and this fact may contribute to the diminished efficacy of natural infection and vaccination to induce high levels of protection in the poorest countries of Asia and Africa.
Serum IgA as a correlate of protection for RV vaccines
Like a number of other oral vaccines, both licensed RV vaccines, the monovalent G1P[8] Rotarix™ vaccine (RV1) [26] and the bovine reassortant pentavalent RotaTeq™ vaccine (RV5) [27,28], are clearly less efficacious in the poorer countries of Africa and Asia than in the Americas and Europe. Protection during the second year following vaccination with RV5 decreases substantially compared to the first year (Table 1). Efficacy of RV1 was not significantly increased with a three-dose immunization schedule compared to the standard two-dose regime [26]; this was contrary to expectations as three natural infections results in significant protection relative to two in some settings [23].
Table 1.
Vaccine efficacy and RV-IgA seroconversion rates in selected trials
| Vaccine/Ref | Location | Protection against severe RVGE year 1 % (95% IC) | Protection against severe RVGE year 2 % (95% IC) | Protection against RVGE of any severity % (95% IC) | IgA Seroconversion % (95% IC) | OPV |
|---|---|---|---|---|---|---|
| RV1 [26] | Malawi | 49.2 (11.1 to 71.7) | NR | 34.7 (7.7 to 53.5)& | 47.2 (30.4 to 64.5) | Yes |
| RV1 [26] | South Africa | 72.2 (40.4 to 88.3) | NR | 64.2 (52 to 73.4)& | 57.1 (44.7 to 68.9) | Yes |
| RV1 [31] | Latin America* | 81.6 (54.4 to 93.5) | NR | NR | 61.4 (53.7 to 68.6) | Yes |
| RV1 [35] | Europe** | 95·8 (89·6 to 98·7) | 85·6 (75·8 to 91·9) | 87·1 (79·6 to 92·1) | 86.5 (83.9 to 88.8) ¶ | No |
| RV1 [6,50] | Latin America | 84.7 (71.7 to 92.4) | 79 (66.4 to 87.4) | NR | 84.7 (71.7 to 92.4) | No |
| RV5 [28] | Ghana | 65 (35.5 to 81.9) | 29.4 (−64.6 to 70.7) | 30.5 (16.7 to 42.2) # | 78.9 (67.6 to 87.7) | Yes |
| RV5 [28] | Kenya | 83.4 (25.5 to 98.2) | −54.7 (−1752.7 to 82.3) | 30.5 (16.7 to 42.2) # | 73.8 (60.9 to 84.2) | Yes |
| RV5 [28] | Mali | ≈ | ≈ | ≈ | 82.5 (70.1 to 91.3) | Yes |
| RV5 [27] | Bangladesh | 45.7 (−1.2 to 71.8) | 39.3 (−18.3 to 69.7) | 42.5 (21.1 to 58.4) + | 78.1 (66 to 87.5) | Yes |
| RV5 [27] | Vietnam | 72.3 (−45.2 to 97.2) | 64.6 (−47.7 to 93.9) | 42.5 (21.1 to 58.4) + | 97 (89.6 to 99.6) | Yes |
| RV5 [5] | Finland and United States | 98 (88.3 to 100) | 88 (49.4 to 98.7) | 74 (66.8 to 79.9) | 95.2 (91.2 to 97.8) | No |
rgentina, Brazil, Colombia, Dominican Republic, Honduras and Panama.
Czech Republic, Finland, France, Germany, Italy, and Spain.
Efficacy studies were unreliable due to problems with follow up of infants.
NR= not reported
Results from groups that received 2 o 3 doses of RV1 were pooled.
Results for Ghana, Kenya and Mali were pooled.
Results from Bangladesh and Vietnam were pooled.
GlaxoSmithKline, Clinical study registers Study ID. #102247-036
Available at: http://www.gsk-clinicalstudyregister.com/ Accessed 9 January 2012.
Direct comparisons between studies of the same vaccine in different geographic and demographic settings are difficult to make, due to differences in study designs, as well as differences in the serotype and inoculum load of circulating rotavirus strains and other environmental conditions.
Total serum RV-IgA measured shortly after vaccination has been used as “a measure of vaccine take” and is probably the best (although imperfect) correlate of protection [1]. In previous vaccine studies in Latin America, North America and Europe levels of serum RV-IgA “take” were close to the levels of protection afforded against severe GE for RV5 and against GE of any severity for RV1 (Table 1). In contrast, RV-IgA seroconversion rates generated by RV1 and RV5 were clearly lower in poorer countries of Africa and Asia than in Latin America, North America and Europe, where RV1 was not co-administered with OPV, although there are significant differences among these poorer countries (Table 1). The few studies measuring immunogenicity and efficacy of RV1 and RV5 administered with or without concomitant administration of OPV suggest that concomitant administration of OPV does not significantly interfere with the efficacy of RV vaccines but does somewhat reduce their immunogenicity [29–31]. Analyzing results from Table 1 from settings where OPV was co-administered with RV vaccines (excluding the Mali trial with unreliable data on protection), a significant correlation does not exist between RV-IgA seroconversion and protection against severe RVGE during the first year after vaccination (p>0.3).
Relative importance of heterotypic and homotypic immunity in protection against RV
The development of homotypic versus heterotypic immunity in people after natural infection or vaccination is a complex and incompletely understood phenomenon [2,4]. In Mexican infants followed prospectively for two years, protection from illness was both homo- and heterotypic in nature with a somewhat stronger, but certainly not exclusive, homotypic response appearing after the first RV infection [21]. In contrast, little or no decrease in the risk of homotypic or heterotypic RV infection or diarrhea was observed in Indian infants after primary infection, although protection did evolve after second or third infections. The basis for these differences is not clear at present [23].
The desire to elicit broad heterotypic protective immunity in children has been a major determinant in the development of the two widely licensed RV vaccines. Controlled clinical trials of RV vaccines in middle income and industrialized countries have not provided conclusive information on the relative importance of each type of immunity, due in part to the limited serotypic diversity of circulating “challenge” strains observed during the actual clinical trials [2]. However, the high levels of protection from severe disease generated by monotypic RV1 indicate that immunization of young children with a single strain of RV provides substantial protective immunity from infection with multiple other serotypes [6,32]. To date, it does not appear as if immunization with a polyvalent vaccine in these populations offers any significant advantage. Moreover, the lack of correlation between the rather low levels of serotype-specific serum neutralization responses and the high levels of protective efficacy induced by either RV1 or RV5 implies that protective immunity is not likely to be exclusively homotypic and may not be directly linked to homotypic neutralizing antibody, at least in the serum [5].
The results of recent efficacy studies in Africa and Asia, where a high diversity of RV genotypes circulate and infection with multiple serotypes are common, have shed more light on this problem. In the clinical trials performed with RV1 in Malawi and South Africa, 67.7% and 64.1%, respectively, of RV strains that infected children in the placebo group were homotypic G1 or P[8]. The levels of protection induced by RV1 against severe RVGE caused by non-G1 versus G1 strains were similar in Malawi, higher in South Africa and lower in South America (Table 2). Thus, RV1 induces significant protection from severe disease with multiple G and P serotypes not included in the vaccine, supporting the conclusion that immunity to RV has a substantial heterotypic component, at least after enteric immunization with a replicating virus.
Table 2.
Efficacy of RV1 against severe RVGE caused by G1 and non-G1 strains in selected trials
| Trial location | % G1 protection (95% IC) | % Non-G1 protection (95% IC) | Reference |
|---|---|---|---|
| South Africa* | 69.8 (32.5 to 87.1) | 85.9 (55.1 to 96.6) | [26] |
| Malawi* | 43.7 (<0 to 85.7) | 50.3 (17.4 to 70.0) | [26] |
| South America | 100 (<0 to 100.0) | 80.6 (51.4 to 93.2) | [31] |
Results are pooled from children receiving two and three doses of RV1.
The percentage of children with serotype-specific neutralization responses after RV5 co-administered with OPV in Latin American infants was similar to those detected in the United States and Finland, where infants received inactivated polio vaccine (IPV), and lower in infants from Africa and Asia (Table 3). Similar to the results from RV5 studies in middle income and industrialized countries, the levels of protective efficacy induced by this vaccine against each RV genotype were higher than the respective rates of serum neutralizing antibodies (SNA) (Table 3). Of note, most cases (89–100%) of severe RVGE in African and Asian infants vaccinated with RV5 were caused by RV with G or P genotypes, or both, which are covered by the RV5 vaccine; this indicates that the lower efficacy of RV5 in these countries is not due to disease caused by RV strains not included in the vaccine. The similar low efficacy levels in Africa and Asia for both RV vaccines (monovalent and pentavalent) strongly support the conclusion that heterotypic protection is an important component of the protective immune response. The mechanism by which heterotypic immunity is induced following homotypic immunization is still unclear, but possibilities include induction of protective antibody responses to heterotypic epitopes on VP4 or VP7, possible protective effects of antibody to common antigens on VP6 or NSP1 or cross reactive protective T cell responses.
Table 3.
Serum neutralizing antibody (SNA) seroresponse rates and vaccine efficacy (Prot) against severe RVGE in selected RV5 studies.
| Strain Serotype | Bangladesh and Vietnam OPV [27] | Ghana, Kenya and Mali OPV [28] | Latin America OPV [30] | US and Finland No OPV [5] | ||||
|---|---|---|---|---|---|---|---|---|
| % SNA | % Prot | % SNA | % Prot | % SNA | % Prot | % SNA | % Prot | |
| G1 | 32.1 | 46.2 | 18.5 | 32.3 | 59.9 | NR | 76 | 74.9* |
| G2 | 9.9 | 29.2 | 9 | 27.1 | 36.3 | NR | 34.3 | 63.4* |
| G3 | 28.2 | 67 | 6.3 | 62.3 | 29.3 | NR | 22.1 | 82.7* |
| G4 | 18.3 | NR | 26.5 | NR | 50.9 | NR | 54.1 | 48.1* |
| P1A [8] | 27.5 | 49.7 | 14.4 | 36.1 | 43.9 | NR | 53.7 | NR |
Clinical efficacy calculated for RVGE of any severity.
NR = not reported.
Nonetheless, several new studies suggest a role for homotypic immunity against RV. Modeling of RV genotype variations over time in developed countries are compatible with a role for homotypic immunity [33]. Moreover, detailed analysis of pre-challenge serum samples from selected adult volunteers experimentally challenged with a G1 RV showed that the quantity of both homotypic and heterotypic antibody responses significantly correlated with protection, but that the strongest correlation was with the level of homotypic response [34]. Although RV1 has been clearly shown to protect against the heterotypic G2P[4] strain even after 2 years [35], recent studies in Brazil [36] and Australia [37] suggested that protection against this strain may not be long-lived under some settings. The importance of this finding needs to be confirmed, because opposite findings have been suggested by another study from Brazil [38]. Finally, studies in Brazil [39–41] and developed countries [42–44] suggest that vaccine introduction may result in the selection of serotypically distinct new strains. To date, however, the two vaccines have remained highly effective in preventing severe RV disease in developed countries and large numbers of vaccine selected “drifted” strains have not emerged. Of note, several recent studies from the US have convincingly demonstrated an unexpected elicitation of “herd immunity” following vaccination [45]. Whether this effect might eventually play a role in selecting rotavirus drift variants or in the generation of heterotypic immunity is unknown.
CONCLUDING REMARKS
New insights into the mechanism used by RV to evade the immune response suggest that a detailed functional analysis of the VP4 and NSP1 proteins of RV1 and RV5 might enhance our understanding of why vaccine efficacy is not optimal and how the safety profile can be improved to allow administration to neonates as has been proposed by some investigators [46].
In children from developing countries, high environmental challenge loads, vaccine interference by maternal antibodies [47,48] and concomitant administration of OPV may in part explain the lower immunogenicity and protective efficacy of the current vaccines compared to developed countries. Withholding breastfeeding prior to vaccination and use of IPV might increase the levels of RV vaccines immunogenicity and protection, and studies to test these interventions should be undertaken.
RV-IgA levels are a poor correlate of protection in developing countries (Table 1). In most RV5 studies, serum IgA conversion rates exceeded protection rates against severe RVGE; this suggests that, as in the case of other Jennerian vaccines based on heterologous animal RV strains, IgA levels may not reflect intestinal immunity [2]. Identifying new correlates of protection that better reflect intestinal immunity may help in designing more efficacious third-generation RV vaccines [49].
RV1 and RV5 have similar efficacy against severe RVGE in countries where a high diversity of strains co-circulate, suggesting an important role for heterotypic protective immunity. However, indirect evidence suggests that homotypic immunity also plays a role in protection against subsequent RV infection. Characterization of RV strains present in the environment post-vaccination is needed to rule out population-based selection of “escape” strains due to long-term pressure of homotypic immunity.
Supplementary Material
Supplementary Table 1. Inverse correlation between RV1 efficacy and seroconversion rates in children in the placebo groups from selected RV1 trials
Highlights.
Rotaviruses have developed multiple mechanisms to evade IFN innate immunity
Protection induced by natural infection and vaccination is lower in developing countries
Studies in developing countries indicate that serum IgA is not an optimal correlate of protection for RV vaccines
Protection against RV is highly heterotypic
A role for homotypic immunity in selection of post-vaccination “escape” strains needs further study
Acknowledgments
This work was supported by funds from the Pontificia Universidad Javeriana and grants from the NIH and the Veterans Administration.
Glossary box and abbreviations
- IFN
Interferon
- IFNR
Interferon receptor
- IRF
Interferon regulatory factor. A family of transcription factors that regulate the interferon response
- ISG
interferon-stimulated genes
- MDA-5
Melanoma differentiation-associated gene-5. Member of the RIG-I-like receptor family of cytoplasmic RNA helicases that sense viral RNA and trigger innate immunity
- NF-κB
Nuclear transcription factor (NF)-κB. NF-κB plays a central role in the cellular stress and inflammatory responses that modulate innate immunity to pathogens
- PKR
Double-stranded RNA-dependent protein kinase. Its gene is inducible by IFN and is a key player in the antiviral actions of these cytokines
- RIG-I
Retinoic acid inducible gene I (RIG-I). Member of the RIG-I-like receptor family of cytoplasmic RNA helicases that sense viral RNA and trigger innate immunity
- SNA
Serum neutralizing antibodies
- STAT
Signal transducers and activators of transcription (STAT). A family of cytosolic transcription factors whose activation depends on several growth factors and cytokines including interferon
- TGF-β
Transforming growth factor beta. A potent regulatory cytokine key in maintaining immune tolerance and in prevention of immunopathology
- TH1 cells
T cells that produce interferon gamma and in general play a role in immunity against intracellular pathogens including viruses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24(15):2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 2.Angel J, Franco MA, Greenberg HB. Rotavirus vaccines: recent developments and future considerations. Nat Rev Microbiol. 2007;5(7):529–539. doi: 10.1038/nrmicro1692. [DOI] [PubMed] [Google Scholar]
- 3.Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. 2011;203(2):188–195. doi: 10.1093/infdis/jiq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward RL, Clark HF, Offit PA. Influence of potential protective mechanisms on the development of live rotavirus vaccines. J Infect Dis. 2010;202(Suppl):S72–79. doi: 10.1086/653549. [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 7*.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A. 2011;108(19):7944–7949. doi: 10.1073/pnas.1100552108. Establishes interferon lambda as a key determinant of innate immunity to RV in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barro M, Patton JT. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc Natl Acad Sci U S A. 2005;102(11):4114–4119. doi: 10.1073/pnas.0408376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng N, Sen A, Nguyen H, Vo P, Hoshino Y, Deal EM, Greenberg HB. Variation in rotavirus NSP1 antagonism of the IFN response results in differential infectivity in mouse embryonic fibroblasts. J Virol. 2009 doi: 10.1128/JVI.00585-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broquet AH, Hirata Y, McAllister CS, Kagnoff MF. RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol. 2011;186(3):1618–1626. doi: 10.4049/jimmunol.1002862. [DOI] [PubMed] [Google Scholar]
- 11.Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J Virol. 2011;85 (8):3717–3732. doi: 10.1128/JVI.02634-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graff JW, Ettayebi K, Hardy ME. Rotavirus NSP1 inhibits NFkappaB activation by inducing proteasome-dependent degradation of beta-TrCP: a novel mechanism of IFN antagonism. PLoS Pathog. 2009;5(1):e1000280. doi: 10.1371/journal.ppat.1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold MM, Patton JT. Diversity of interferon antagonist activities mediated by NSP1 proteins of different rotavirus strains. J Virol. 2011;85(5):1970–1979. doi: 10.1128/JVI.01801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng N, Kim B, Fenaux M, Nguyen H, Vo P, Omary MB, Greenberg HB. Role of interferon in homologous and heterologous rotavirus infection in the intestines and extraintestinal organs of suckling mice. J Virol. 2008;82(15):7578–7590. doi: 10.1128/JVI.00391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Feng N, Sen A, Wolf M, Vo P, Hoshino Y, Greenberg HB. Roles of VP4 and NSP1 in determining the distinctive replication capacities of simian rotavirus RRV and bovine rotavirus UK in the mouse biliary tract. J Virol. 2011;85(6):2686–2694. doi: 10.1128/JVI.02408-10. Establishes that both VP4 and NSP1 are important viral genes implicated in the development of biliary atresia in the murine model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Donnelly B, Bondoc A, Mohanty SK, McNeal M, Ward R, Sestak K, Zheng S, Tiao G. The rhesus rotavirus gene encoding VP4 is a major determinant in the pathogenesis of biliary atresia in newborn mice. J Virol. 2011;85(17):9069–9077. doi: 10.1128/JVI.02436-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holloway G, Truong TT, Coulson BS. Rotavirus Antagonizes Cellular Antiviral Responses by Inhibiting the Nuclear Accumulation of STAT1, STAT2, and NF-kappa B. J Virol. 2009;83(10):4942–4951. doi: 10.1128/JVI.01450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez LS, Narvaez CF, Rojas OL, Franco MA, Angel J. Human myeloid dendritic cells treated with supernatants of rotavirus infected Caco-2 cells induce a poor Th1 response. Cell Immunol. 2012;272(2):154–161. doi: 10.1016/j.cellimm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Barreto A, Rodriguez LS, Rojas OL, Wolf M, Greenberg HB, Franco MA, Angel J. Membrane vesicles released by intestinal epithelial cells infected with rotavirus inhibit T-cell function. Viral Immunol. 2010;23 (6):595–608. doi: 10.1089/vim.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesa MC, Gutierrez L, Duarte-Rey C, Angel J, Franco MA. A TGF-beta mediated regulatory mechanism modulates the T cell immune response to rotavirus in adults but not in children. Virology. 2010;399(1):77–86. doi: 10.1016/j.virol.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 22.Fischer TK, Valentiner-Branth P, Steinsland H, Perch M, Santos G, Aaby P, Molbak K, Sommerfelt H. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, west Africa. J Infect Dis. 2002;186(5):593–597. doi: 10.1086/342294. [DOI] [PubMed] [Google Scholar]
- 23.Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, Desselberger U, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011;365(4):337–346. doi: 10.1056/NEJMoa1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15(1):29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 25.Ward RL, Kirkwood CD, Sander DS, Smith VE, Shao MY, Bean JA, Sack DA, Bernstein DI. Reductions in cross-neutralizing antibody responses in infants after attenuation of the human rotavirus vaccine candidate 89–12. J Infect Dis. 2006;194(12):1729–1736. doi: 10.1086/509623. [DOI] [PubMed] [Google Scholar]
- 26**.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, Han HH, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–298. doi: 10.1056/NEJMoa0904797. A key study that shows lower efficacy of RV1 in African children relative to previous studies in developed countries and latin America. [DOI] [PubMed] [Google Scholar]
- 27**.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–623. doi: 10.1016/S0140-6736(10)60755-6. A key study that shows lower efficacy of RV5 in Asian children relative to previous studies in developed countries and latin America. [DOI] [PubMed] [Google Scholar]
- 28**.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–614. doi: 10.1016/S0140-6736(10)60889-6. A key study that shows lower efficacy of RV5 in Asian children relative to previous studies in developed countries and latin America. [DOI] [PubMed] [Google Scholar]
- 29.Zaman K, Sack DA, Yunus M, Arifeen SE, Podder G, Azim T, Luby S, Breiman RF, Neuzil K, Datta SK, Delem A, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27(9):1333–1339. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 30.Ciarlet M, Sani-Grosso R, Yuan G, Liu GF, Heaton PM, Gottesdiener KM, Arredondo JL, Schodel F. Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J. 2008;27(10):874–880. doi: 10.1097/INF.0b013e3181782780. [DOI] [PubMed] [Google Scholar]
- 31.Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Da Silveira TR, Rivera L, Rivera Medina DM, Saez-Llorens X, Gonzalez Ayala SE, De Leon T, Van Doorn LJ, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30(6):e103–108. doi: 10.1097/INF.0b013e3182138278. [DOI] [PubMed] [Google Scholar]
- 32.De Vos B, Han HH, Bouckenooghe A, Debrus S, Gillard P, Ward R, Cheuvart B. Live Attenuated Human Rotavirus Vaccine, RIX4414, Provides Clinical Protection in Infants Against Rotavirus Strains With and Without Shared G and P Genotypes: Integrated Analysis of Randomized Controlled Trials. Pediatr Infect Dis J. 2009;28(4):261–266. doi: 10.1097/INF.0b013e3181907177. [DOI] [PubMed] [Google Scholar]
- 33.Pitzer VE, Patel MM, Lopman BA, Viboud C, Parashar UD, Grenfell BT. Modeling rotavirus strain dynamics in developed countries to understand the potential impact of vaccination on genotype distributions. Proc Natl Acad Sci U S A. 2011;108(48):19353–19358. doi: 10.1073/pnas.1110507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan L, Honma S, Kim I, Kapikian AZ, Hoshino Y. Resistance to rotavirus infection in adult volunteers challenged with a virulent G1P1A[8] virus correlated with serum immunoglobulin G antibodies to homotypic viral proteins 7 and 4. J Infect Dis. 2009;200(9):1443–1451. doi: 10.1086/606116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 36.Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, Cuevas LE, Parashar UD, Cunliffe NA, Nakagomi T. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201(3):363–369. doi: 10.1086/649843. [DOI] [PubMed] [Google Scholar]
- 37.Snelling TL, Andrews RM, Kirkwood CD, Culvenor S, Carapetis JR. Case-control evaluation of the effectiveness of the G1P[8] human rotavirus vaccine during an outbreak of rotavirus G2P[4] infection in central Australia. Clin Infect Dis. 2011;52(2):191–199. doi: 10.1093/cid/ciq101. [DOI] [PubMed] [Google Scholar]
- 38.Justino MC, Linhares AC, Lanzieri TM, Miranda Y, Mascarenhas JD, Abreu E, Guerra SF, Oliveira AS, da Silva VB, Sanchez N, Meyer N, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belem, Brazil. Pediatr Infect Dis J. 2011;30(5):396–401. doi: 10.1097/INF.0b013e3182055cc2. [DOI] [PubMed] [Google Scholar]
- 39.Cilli A, Luchs A, Morillo SG, Costa FF, de Carmona RC, do Timenetsky MC. Characterization of rotavirus and norovirus strains: a 6-year study (2004–2009) J Pediatr (Rio J) 2011;87(5):445–449. doi: 10.2223/JPED.2122. [DOI] [PubMed] [Google Scholar]
- 40.Gurgel RG, Bohland AK, Vieira SC, Oliveira DM, Fontes PB, Barros VF, Ramos MF, Dove W, Nakagomi T, Nakagomi O, Correia JB, et al. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology. 2009;137(6):1970–1975. doi: 10.1053/j.gastro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 41.Safadi MA, Berezin EN, Munford V, Almeida FJ, de Moraes JC, Pinheiro CF, Racz ML. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in Sao Paulo, Brazil. Pediatr Infect Dis J. 2010;29(11):1019–1022. doi: 10.1097/INF.0b013e3181e7886a. [DOI] [PubMed] [Google Scholar]
- 42.Zeller M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, Novo L, Verstappen N, Van Ranst M, Matthijnssens J. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine. 2010;28(47):7507–7513. doi: 10.1016/j.vaccine.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Kirkwood CD, Boniface K, Bishop RF, Barnes GL. Australian Rotavirus Surveillance Program annual report, 2008/2009. Commun Dis Intell. 2009;33(4):382–388. doi: 10.33321/cdi.2009.33.41. [DOI] [PubMed] [Google Scholar]
- 44.Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, Cortese MM, Parashar UD, Glass RI, Bowen MD, Surveill NRS. United States Rotavirus Strain Surveillance From 2005 to 2008 Genotype Prevalence Before and After Vaccine Introduction. Pediatr Infect Dis J. 2011;30(1):S42–S47. doi: 10.1097/INF.0b013e3181fefd78. [DOI] [PubMed] [Google Scholar]
- 45.Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world Impact of Rotavirus Vaccination. Pediatric Infectious Disease Journal. 2011;30(1):S1–S5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- 46.Vesikari T, Karvonen A, Forrest BD, Hoshino Y, Chanock RM, Kapikian AZ. Neonatal administration of rhesus rotavirus tetravalent vaccine. Pediatr Infect Dis J. 2006;25(2):118–122. doi: 10.1097/01.inf.0000199288.98370.71. [DOI] [PubMed] [Google Scholar]
- 47.Chan J, Nirwati H, Triasih R, Bogdanovic-Sakran N, Soenarto Y, Hakimi M, Duke T, Buttery JP, Bines JE, Bishop RF, Kirkwood CD, et al. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine. 2011;29(6):1242–1247. doi: 10.1016/j.vaccine.2010.11.087. [DOI] [PubMed] [Google Scholar]
- 48.Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, Baek LJ, Parashar U, Glass RI, Jiang B. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010;29 (10):919–923. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rojas OL, Narvaez CF, Greenberg HB, Angel J, Franco MA. Characterization of rotavirus specific B cells and their relation with serological memory. Virology. 2008 doi: 10.1016/j.virol.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, Lopez P, Macias-Parra M, Ortega-Barria E, Rivera-Medina DM, Rivera L, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Inverse correlation between RV1 efficacy and seroconversion rates in children in the placebo groups from selected RV1 trials