Abstract
A model is described that predicts patterns of polyomavirus SV40 infections and associated cancers in humans. The model proposes that SV40 infections were established in humans primarily by exposure to contaminated oral poliovaccines and that infections persist today in geographic regions where poor sanitation or living conditions allow maintenance of infections transmitted by a fecal/urine–oral route. Predictions from the model include that SV40 infections and virus-associated malignancies will be restricted geographically and demographically and that in developed countries, such as the US, SV40 prevalence rates will be generally very low. The model highlights the importance of selection of populations for investigations of SV40 human infections. This model can explain inconsistencies in the published literature of SV40 infections in humans and can guide the design of future studies.
Introduction
The origins of polyomavirus SV40 are closely linked to the development and production of several viral vaccines in the 1950s and early 1960s, most predominantly the inactivated (IPV) and live attenuated oral (OPV) poliovaccines. These vaccines were produced using primary cultures of monkey kidney cells which were sometimes carrying unrecognized infections by SV40. These contaminated vaccines were used in humans prior to the discovery of SV40, creating opportunities for introduction of SV40 as a cross-species infection into vaccinees. As SV40 has oncogenic potential in animal models and transforming ability in cell cultures, a question of public health significance is whether those vaccine exposures established SV40 as an infectious pathogen in humans.
SV40 markers have been detected in human specimens, including human cancers, dating back to the 1970s. More recent studies using molecular techniques to detect the presence of SV40 DNA or antigen expression in human specimens have yielded variable results. Seroprevalence estimates of human infections have also varied. Because of these inconsistent findings, no consensus has emerged about the role of SV40 as a potential human pathogen.
A model is presented here that can explain many of the discrepant results and guide future investigations to better define the prevalence of SV40 infections and the possible role of the virus in human neoplasia. Re-evaluation of reported data from the perspective of the model can resolve inconsistencies in the published literature.
Model to predict contemporary SV40 infections in humans
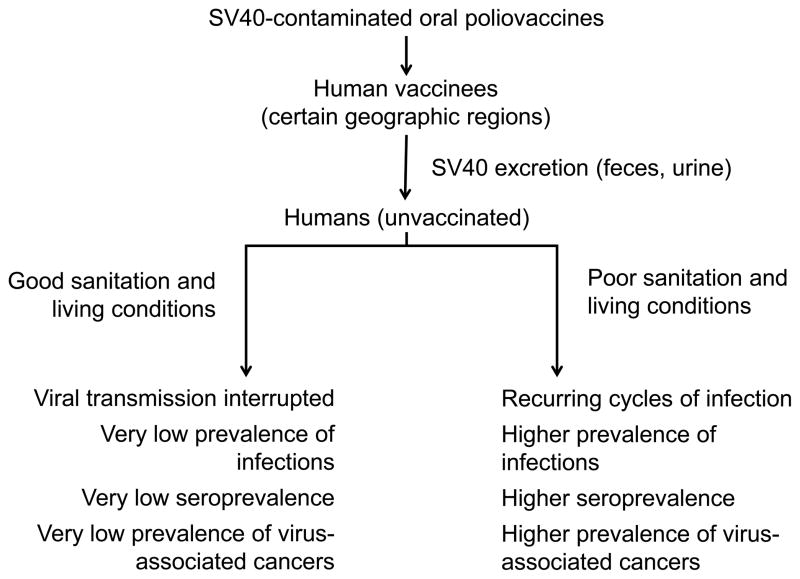
The model can be stated as follows: SV40 infections were established in humans primarily by exposure to contaminated oral poliovaccine and that infections persist today in certain geographic regions where poor sanitation or living conditions allow maintenance of cycles of infections transmitted by a fecal/urine–oral route. The model is summarized schematically in Figure 1.
Figure 1.
Model of contemporary SV40 infections and association with cancer in humans. The diagram illustrates the proposal that SV40 infections were established in humans primarily by exposure to contaminated oral poliovaccines and that infections persist today in geographic regions where poor sanitation or living conditions allow maintenance of cycles of infections transmitted by a fecal/urine–oral route. Predictions related to the prevalence of SV40 infections in different locations are listed.
This model postulates that seeding of SV40 human infections was mediated primarily by exposure to contaminated OPV rather than exposure to the killed poliovaccine. One consideration underlying this premise was the amount of live SV40 present in the different vaccines. Not all lots of IPV were contaminated as not every preparation of primary monkey kidney cells contained SV40; it has been estimated that ~30% of IPV lots contained residual infectious SV40. When present, the titer of live SV40 in IPV that had escaped inactivation was probably on the order of 102–103 infectious units per ml [1]. In contrast, live SV40 would have been present in each OPV preparation as seed stocks were contaminated and SV40 titers in OPV lots were higher, ranging from ~104–106 infectious units per ml, as no inactivation step was involved in vaccine production [1]. A higher inoculum presumably would have increased the chances of successfully establishing an SV40 infection in a recipient. A second contributory factor was probably the route of exposure. Evidence suggests that polyomaviruses can be transmitted by the fecal/urine–oral route. SV40 and other polyomaviruses have been found in stool samples, in addition to urine [2–6], and human polyomaviruses are found in sewage and in human feces-contaminated environmental waters [7–10]. In addition, 19% of newborn children and 15% of infants 3- to 6-months-old at the time of receiving OPV were shown to excrete infectious SV40 in their stools for up to 5 weeks after vaccination [11]. Thus, oral administration of OPV likely reflected a more natural route for transmission of infection by SV40 than the intramuscular inoculation of IPV. Whereas exposure to IPV may have initiated some SV40 infections, it was in all likelihood less effective at doing so than OPV.
The dynamics and maintenance of SV40 human infections can be envisioned based on other infectious agents spread by the fecal–oral route. Well-characterized examples include poliomyelitis before vaccination, hepatitis A virus, and Helicobacter pylori [12–17]. As standards of living increase, infection rates for those agents decrease. In environments with lack of clean water, inadequate sewage disposal, poor household hygiene, and overcrowding, infections are common in infants and young children, leading to a relatively high frequency of seroprevalence. These conditions occur most often in developing countries, although similar pockets of poor hygiene or living conditions can be found in developed countries. As standards of living and sanitation levels improve, fewer individuals are exposed to the agent, resulting in lower prevalence rates. Thus, antibody prevalence to the agent in a population group will often reflect the level of hygiene in the living conditions of that population (Figure 1).
Polyomaviruses establish persistent infections in their hosts, in contrast to poliovirus and hepatitis A. This could result in sporadic shedding of SV40 in feces and/or urine over long periods of time.
History of use of SV40-contaminated vaccines
Potentially contaminated poliovaccines were used mainly from 1954 to 1963. Vaccines were supposed to be free from SV40 after mid-1961, but previous lots of vaccine were allowed to be used until stores were exhausted. There is evidence that the Russian OPV was very likely contaminated until the late 1970s [18]. IPVs were widely used in the US and other countries starting in 1955. Large field trials with candidate live attenuated OPVs were carried out from 1958 to 1960 (Table 1). Major trials with OPV were not performed in the US because prior use of IPV had resulted in polio antibodies in many individuals. The Sabin candidate vaccine strains were tested at several sites in Mexico and very widely throughout the USSR. The Lederle candidate vaccine strains were tested in Central and South America and in Florida. The Koprowski candidate vaccine was tested in Poland, Croatia, and the Belgian Congo [19–23]. Other vaccine tests were conducted as well. The Sabin vaccine strains were concluded to be less virulent and were selected for licensing in the US in 1961 and 1962 and studies of the other candidate vaccines were discontinued. The Russian vaccine was shared on occasion with other countries, such as Japan and Egypt. A unique feature in Egypt was an antischistosomiasis campaign in place until the 1980s. Repeated intravenous injections of a compound to kill blood flukes were given on mass treatment days, providing a setting in which blood-borne pathogens could be transmitted if injection equipment was not adequately sterilized. This parenteral antischistosomal therapy is believed to be largely responsible for the spread of hepatitis C virus in Egypt [24] and could theoretically have contributed to the spread of SV40. Some countries did not use any contaminated OPV, while the status of OPVs used in other countries is uncertain.
Table 1.
| Country | Source of OPVc | Date of usage | Estimated no. of vaccinees |
|---|---|---|---|
| Belgian Congo | CHAT | 1958 | 240,000 |
| Colombia | Lederle | 1958–1959 | 140,000 |
| Costa Rica | Lederle | 1959–1960 | 272,000 |
| Croatia | CHAT | 1960 | 1.3 million |
| Czechoslovakia | Sabin | 1958–1959 | 143,000 |
| Egyptd | Russian | NAd | NA |
| Hungary | Russian | 1960–1978 (est.) | 2.5 million (1960) |
| Japan | Russian | 1961–1963 | ~15 million |
| Mexico | Sabin | 1958–1959 | 308,000 |
| Nicaragua | Lederle | 1958–1959 | 42,000 |
| Poland | CHAT | 1959 | 9 million |
| Uruguay | Lederle | 1958–1959 | 218,000 |
| USA, Cincinnati | Sabin | 1960 | 181,000 |
| USA, Miami | Lederle | 1959 | 410,000 |
| USSR | Russian | 1959–1978 (est.) | Many millions |
Mainly children were vaccinated.
OPV, oral poliovaccine; CHAT, Koprowski candidate vaccine that was based on the same poliovirus strains as Lederle Laboratories’ candidate vaccine; Russian OPV was based on Sabin vaccine virus strains.
NA, not available. Parenteral antischistosomal therapy was provided in Egypt until the 1980s.
The US military used SV40-contaminated adenovirus vaccines to protect recruits against acute respiratory disease. This provided another source of human exposure to SV40 [25]. Several hundred thousand military recruits were vaccinated between 1957 and 1961 with those contaminated vaccines before their use was discontinued [1,26].
Predictions of the model
This model predicts that in developed countries, such as the US, prevalence rates for SV40 infections will be very low. Even if SV40 infections were seeded by contaminated poliovaccines 50 years ago, most virus transmission would have been interrupted by good sanitation conditions. Based on the model and the property of persistent infections by polyomaviruses, it is possible that immigrants from high-prevalence regions could take SV40 infections with them to low-prevalence areas and might transmit the infection to family members there. The model also predicts that SV40 infections and virus-positive malignancies will be restricted both geographically and demographically and will not be evenly distributed worldwide. Those with detectable infections will often have links to specific populations and locations. This model articulates the difference in expected patterns of findings between studies of SV40 and those of a cancer virus that is distributed worldwide, such as human papillomavirus. It highlights the importance of selection of study populations for investigations of SV40–human infections.
Evidence of SV40 infections in humans
Many reports in the literature over the last two decades have detected SV40 markers in noncancer samples from healthy children and adults (Box 1), including in urine, stool, blood, and lymphoid tissues [23]. Studies have included subjects from the US, Europe, Japan, Egypt, and Russia. The prevalence of SV40 infections appears to be low in randomly collected human samples. It is noteworthy that individuals born long after the use of contaminated vaccines have displayed evidence of infection, showing that the virus is transmissible among humans.
Box 1. Evidence of SV40 infections in humans.
Evidence that SV40 is causing human infections includes the detection of infectious virus excreted in the stool by infants fed contaminated poliovaccine and the detection of SV40 DNA in urine and stool samples from children, in stool and blood samples from healthy adults, in lymphoid specimens from healthy children, and in transplanted kidneys in children. Serological surveys have found SV40 antibodies in both children and adults. Evidence of infection is found in individuals too young to have been exposed to contaminated vaccines, indicating on-going cycles of infections in humans. Evidence linking SV40 to human cancer includes detection of SV40 DNA in human tumors (predominantly brain, bone, non-Hodgkin lymphoma, and mesothelioma tumor types), T-antigen expression in tumors, and the isolation of infectious virus from a pediatric brain tumor. However, the observed prevalence of SV40 has varied widely among reported studies and the associations detected with tumors do not prove causality. (See Butel [23,34] for original references.)
Serological findings
Serological assays are frequently used to determine the prevalence of viral infections. Estimates of SV40 prevalences have been from 2% to greater than 10% in different groups [23,27,28]. Neutralization assays are highly specific, but are too labor-intensive and time-consuming to apply to large population surveys for SV40. Enzyme immunoassays based on virus-like particles (VLPs) lend themselves to testing large numbers of serum samples. However, those assays detect all binding antibodies, including nonneutralizing antibodies and those that recognize cross-reactive epitopes on polyomaviruses BKV and JCV. Absorption of sera with BKV or JCV VLPs often removes much of the SV40 reactivity. A new immunoassay based on peptides specific for SV40 VP1 and VP2/3 detected antibodies in healthy blood donors in Italy [29]. These antibodies appeared to be specific as they reacted with two different SV40 peptides. SV40 antibodies in humans are usually low-titered, perhaps reflecting low-grade viral replication or a weak specific immune response. The majority of reported serological studies have involved populations from countries with high standards of living (US, UK). The model predicts that those populations would be expected to have a low frequency of SV40 antibodies.
We have recently analyzed archival sera collected in the 1990s in Houston, TX, from women visiting the public hospital. The goal was to compare SV40 seroprevalence in different ethnic groups. Caucasians and African-Americans displayed similar low rates of neutralizing antibody (5%, 6%), whereas Hispanics showed a significantly higher prevalence of SV40 antibodies (23%) (unpublished). There are many immigrants in Houston from Mexico and Central America and they often use the public hospital to access medical care. A possible explanation of the results is that the Hispanic group included immigrants or their parents from higher prevalence areas in Latin America who had been infected with SV40 in their previous environment. The study provides evidence that SV40 seroprevalence can vary among population groups. Seroprevalence studies need to be carried out in other populations predicted to be at higher risk of SV40 infections to more clearly establish the frequency of human infections [30].
Association of SV40 with human cancer
A number of studies have found evidence linking SV40 with human tumors, with the most commonly associated tumor types being brain tumors, mesotheliomas, osteosarcomas, and lymphomas [23,31–34]. Studies involving lymphomas are summarized in Table 2. The listed studies all investigated primary cancer samples (not cell lines), used PCR-based assays to detect viral DNA, and analyzed control tissue specimens (not cell lines) in parallel. The table reveals inconsistency in findings. Overall, SV40 was detected significantly more often in cancer than in controls, but the percent viral positivity of cancers ranged from 56% to 0% in different studies. In the studies that reported SV40-positive findings, the percentage of positive lymphoma samples was much higher than for the control samples, ruling out contamination as an explanation for the results. Based on the proposed model, the variable results reported could reflect, at least in part, the geographic origin of specimens analyzed. For example, the study from Egypt reported a prevalence of SV40 positivity of over 50%; the Russian OPV had been used in Egypt [35] that also provided parenteral antischistosomal therapy. Although the US had limited exposure to contaminated OPV, the positive US reports (19–43%) described studies carried out in Texas and California, states with significant numbers of immigrants from Mexico and Central America. Archival samples analyzed in those studies may have included persons originally from high-prevalence areas. Studies from Japan found a low prevalence of SV40 DNA in lymphomas (2%, 11%). The Russian vaccine had been used in Japan approximately 40 years earlier, but the relatively good standard of living in that country probably reduced transmission and maintenance of SV40 infections over time. Negative findings have been reported from the UK and Tasmania; as far as is known, no contaminated OPV was used in those countries. The status of poliovaccines used in some of the other countries is unclear.
Table 2.
Detection of SV40 DNA by PCR in Lymphomasa
| Author, year | Country | SV40 DNA by PCR [no. positive/no. tested (%)]
|
|
|---|---|---|---|
| Lymphoma | Control | ||
| Amara et al., 2007 | Tunisia | 63/108 (56) | 4/60 (6)b |
| Zekri et al., 2007 | Egypt | 85/158 (54) | 4/34 (12)b |
| Shivapurkar et al., 2002 | US | 29/68 (43) | 5/120 (4.2) |
| Vilchez et al., 2002 | US | 64/154 (42) | 0/240 (0) |
| Shivapurkar et al., 2004 | US | 33/90 (36) | 1/56 (1.2) |
| Meneses et al., 2005 | Costa Rica | 30/125 (24) | 0/91 (0) |
| Vilchez et al., 2005 | US | 12/55 (22) | 0/19 (0) |
| David et al., 2001 | US | 15/79 (19) | 19/187 (10)b |
| Martini et al., 1998 | Italy | 11/79 (14) | 3/50 (6)b |
| Chen et al., 2006 | Taiwan | 13/91 (14) | 0/106 (0) |
| Nakatsuka et al., 2003 | Japan | 14/122 (11) | 3/64 (4.7)b |
| Capello et al., 2003 | Spain, Italy | 17/500 (3) | 0/15 (0) |
| Daibata et al., 2003 | Japan | 3/125 (2) | 0/31 (0) |
| MacKenzie et al., 2003 | UK | 0/152 (0) | 0/45 (0) |
| Sui et al., 2005 | Tasmania | 0/50 (0) | 0/50 (0) |
| Schüler et al., 2006 | Germany | 0/77 (0) | 0/61 (0) |
| TOTAL | 389/2033 (19.1)c | 39/1207 (3.2)b,c | |
See Butel [34] for original references.
Among control samples positive for SV40 were 28 blood samples from noncancer patients or healthy individuals.
p<0.001 (Z test of proportions).
A recent study tested the hypothesis that the frequency of SV40 association with lymphomas varies among different populations [36]. We obtained archival samples from two hospitals in Houston, TX — the veterans hospital (for military veterans) and the public hospital (for people with no insurance or limited ability to pay for care). Tumor pathologies were similar, but patient demographics differed significantly between the lymphoma patients from the two hospitals, with more Hispanics represented in the public hospital group. Lymphoma specimens from the public hospital were 23% SV40 positive, whereas those from the veterans hospital were only 3% virus positive (p<0.0001); control samples were negative. All the specimens were subjected to the same laboratory procedures, thereby ruling out technical variability as an explanation for the results. This study illustrated that, as predicted by the model, the prevalence of SV40 tumor positivity can vary significantly, depending on the sources of specimens.
We have also analyzed archival tissue samples from Costa Rica, a region known to have been exposed to contaminated OPV (Box 1; Table 1). In an analysis involving more than 200 specimens, lymphomas were found to be 20% SV40 positive whereas control tissues were negative [37]. SV40 T-antigen expression was detected in SV40 DNA-positive tumors. Thus, SV40-positive cancers can be found in a region previously exposed to SV40-contaminated OPV, compatible with the proposed model.
However, as with any cancer-associated virus, detection of the virus in human tumors does not prove that the virus caused those malignancies. Several different criteria need to be met before etiology is established [34].
Epidemiology
There is currently no epidemiological evidence supporting a link between SV40 infections and human cancers [30,38–42]. However, most reported studies have been based on data from countries with good sanitation and hygiene, predominantly the US, and designed to examine whether potential exposure to contaminated IPV resulted in an increased risk of cancer. No associations were found. Because of the focus on IPV and the analysis of data obtained from developed countries, such negative associations are predicted by the model. It would be informative to carry out epidemiological studies on populations exposed to contaminated OPV in regions expected to maintain cycles of infections by SV40.
Conclusions
The model described here proposes that patterns of SV40 infections in humans reflect past exposure to SV40-contaminated oral poliovaccines and poor sanitation or living conditions that allow transmission of viral infections by a fecal/urine–oral route. This model is compatible with known SV40 biology and historical usage of contaminated vaccines. It can explain apparent inconsistencies in published findings and can be tested using appropriately designed studies.
Highlights.
The model proposes that human infections by polyomavirus SV40 were established primarily by the use of contaminated oral poliovaccines and that infections persist today in regions where conditions allow transmission of virus by a fecal/urine–oral route.
SV40 infections will be restricted geographically and demographically and prevalence will be low in developed countries.
This model can explain many of the inconsistencies in the published literature of human infections by SV40.
Acknowledgments
The author regrets that space limitations prevented the citation and discussion here of many relevant publications. Research carried out in the author’s laboratory was supported in part by grants R01 CA104818 and R01 CA134524 from the National Cancer Institute. This model was presented at the 3rd International Conference on Viral Oncology Research, Naples, Italy, October 4–6, 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
- ••1.Shah K, Nathanson N. Human exposure to SV40: review and comment. Am J Epidemiol. 1976;103:1–12. doi: 10.1093/oxfordjournals.aje.a112197. This historic paper provides a detailed description of how poliovaccines were contaminated with SV40, estimates of the extent of that contamination, and exposures to contaminated inactivated vaccines in the United States in the late 1950s. [DOI] [PubMed] [Google Scholar]
- 2.Vanchiere JA, Nicome RK, Greer JM, Demmler GJ, Butel JS. Frequent detection of polyomaviruses in stool samples from hospitalized children. J Infect Dis. 2005;192:658–664. doi: 10.1086/432076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babakir-Mina M, Ciccozzi M, Alteri C, Polchi P, Picardi A, Greco F, Lucarelli G, Arcese W, Perno CF, Ciotti M. Excretion of the novel polyomaviruses KI and WU in the stool of patients with hematological disorders. J Med Virol. 2009;81:1668–1673. doi: 10.1002/jmv.21559. [DOI] [PubMed] [Google Scholar]
- 4.Wong ASY, Cheng VCC, Yuen K-Y, Kwong Y-L, Leung AYH. High frequency of polyoma BK virus shedding in the gastrointestinal tract after hematopoietic stem cell transplantation: a prospective and quantitative analysis. Bone Marrow Transplant. 2009;43:43–47. doi: 10.1038/bmt.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •5.Vanchiere JA, Abudayyeh S, Copeland CM, Lu LB, Graham DY, Butel JS. Polyomavirus shedding in the stool of healthy adults. J Clin Microbiol. 2009;47:2388–2391. doi: 10.1128/JCM.02472-08. Polyomaviruses BKV, JCV, and SV40 were detected in stool samples from healthy adults, but at a shedding frequency lower than observed previously for children. These findings in adults, together with observations in children, suggest a fecal–oral route is likely for transmission of polyomavirus infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bialasiewicz S, Whiley DM, Lambert SB, Nissen MD, Sloots TP. Detection of BK, JC, WU, or KI polyomaviruses in faecal, urine, blood, cerebrospinal fluid and respiratory samples. J Clin Virol. 2009;45:249–254. doi: 10.1016/j.jcv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Bofill-Mas S, Formiga-Cruz M, Clemente-Casares P, Calafell F, Girones R. Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J Virol. 2001;75:10290–10299. doi: 10.1128/JVI.75.21.10290-10299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuaig SM, Scott TM, Harwood VJ, Farrah SR, Lukasik JO. Detection of human-derived fecal pollution in environmental waters by use of a PCR-based human polyomavirus assay. Appl Environ Microbiol. 2006;72:7567–7574. doi: 10.1128/AEM.01317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bofill-Mas S, Rodriguez-Manzano J, Calgua B, Carratala A, Girones R. Newly described human polyomaviruses Merkel cell, KI and WU are present in urban sewage and may represent potential environmental contaminants. Virol J. 2010;7:e141. doi: 10.1186/1743-422X-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed W, Wan C, Goonetilleke A, Gardner T. Evaluating sewage-associated JCV and BKV polyomaviruses for sourcing human fecal pollution in a coastal river in Southeast Queensland, Australia. J Environ Qual. 2010;39:1743–1750. doi: 10.2134/jeq2010.0062. [DOI] [PubMed] [Google Scholar]
- ••11.Melnick JL, Stinebaugh S. Excretion of vacuolating SV-40 virus (papova virus group) after ingestion as a contaminant of oral poliovaccine. Proc Soc Exp Biol Med. 1962;109:965–968. doi: 10.3181/00379727-109-27392. This paper was the first description of fecal shedding of SV40. It involved infants fed SV40-contaminated oral poliovaccine. [DOI] [PubMed] [Google Scholar]
- 12.Paul JR. In: A History of Poliomyelitis. Paul JR, editor. New Haven: Yale University Press; 1971. [Google Scholar]
- 13.Melnick JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields BN, Knipe DM, Chanock RM, Melnick JL, Roizman B, Shope RE, editors. Virology. New York: Raven Press; 1985. pp. 739–794. [Google Scholar]
- 14.Sabin AB. Perspectives on rapid elimination and ultimate global eradication of paralytic poliomyelitis caused by polioviruses. Eur J Epidemiol. 1991;7:95–120. doi: 10.1007/BF00237353. [DOI] [PubMed] [Google Scholar]
- 15.Graham DY. Helicobacter pylori in human populations: the present and predictions of the future based on the epidemiology of polio. In: Menge H, Gregor M, Tytgat GNJ, Marshall BJ, McNulty CAM, editors. Helicobacter pylori 1990. Proceedings of the Second International Symposium on Helicobacter pylori; Bad Nauheim. August 25–26, 1989; Berlin: Springer-Verlag; 1991. pp. 97–102. [Google Scholar]
- 16.Feinstone SM, Gust ID. Hepatitis A virus. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical Virology. 2. Washington, D.C: ASM Press; 2002. pp. 1019–1039. [Google Scholar]
- 17.Nurgalieva ZZ, Malaty HM, Graham DY, Almuchambetova R, Machmudova A, Kapsultanova D, Osato MS, Hollinger FB, Zhangabylov A. Helicobacter pylori infection in Kazakhstan: effect of water source and household hygiene. Am J Trop Med Hyg. 2002;67:201–206. doi: 10.4269/ajtmh.2002.67.201. [DOI] [PubMed] [Google Scholar]
- 18.Cutrone R, Lednicky J, Dunn G, Rizzo P, Bocchetta M, Chumakov K, Minor P, Carbone M. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res. 2005;65:10273–10279. doi: 10.1158/0008-5472.CAN-05-2028. [DOI] [PubMed] [Google Scholar]
- 19.Pan American Sanitary Bureau. First International Conference on Live Poliovirus Vaccines. Washington, D.C. 22–26 June 1959; Papers Presented and Discussions; Washington, D.C: Pan American Sanitary Bureau; 1959. Scientific Publication No. 44. [Google Scholar]
- 20.Pan American Sanitary Bureau. Second International Conference on Live Poliovirus Vaccines. Washington, D.C. 6–10 June 1960; Papers Presented and Discussions; Washington, D.C: Pan American Sanitary Bureau; 1960. Scientific Publication No. 50. [Google Scholar]
- 21.Sabin AB. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985;151:420–436. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- 22.Koprowski H. First decade (1950–1960) of studies and trials with the polio vaccine. Biologicals. 2006;34:81–86. doi: 10.1016/j.biologicals.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Butel JS. Polyomavirus SV40: model infectious agent of cancer. In: Robertson E, editor. Cancer Associated Viruses. Springer Science; 2012. pp. 377–417. [Google Scholar]
- 24.Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Ohn ESA, Anwar W, Sallam I. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 25.Rapp F, Melnick JL, Butel JS, Kitahara T. The incorporation of SV40 genetic material into adenovirus 7 as measured by intranuclear synthesis of SV40 tumor antigen. Proc Natl Acad Sci USA. 1964;52:1348–1352. doi: 10.1073/pnas.52.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis AM., Jr SV40 in adenovirus vaccines and adenovirus-SV40 recombinants. Dev Biol Stand. 1998;94:207–216. [PubMed] [Google Scholar]
- 27.Shah KV, Galloway DA, Knowles WA, Viscidi RP. Simian virus 40 (SV40) and human cancer: A review of the serological data. Rev Med Virol. 2004;14:231–239. doi: 10.1002/rmv.432. [DOI] [PubMed] [Google Scholar]
- 28.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathogens. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Corallini A, Mazzoni E, Taronna A, Manfrini M, Carandina G, Guerra G, Guaschino R, Vaniglia F, Magnani C, Casali F, Dolcetti R, Palmonari C, Rezza G, Martini F, Barbanti-Brodano G, Tognon MG. Specific antibodies reacting with simian virus 40 capsid protein mimotopes in serum samples from healthy blood donors. Hum Immunol. 2012;73:502–510. doi: 10.1016/j.humimm.2012.02.009. An immunoassay was developed based on SV40-specific peptides from viral capsid proteins VP1 and VP2/3. Antibodies were detected in healthy blood donors able to react with both peptides, suggesting the SV40-specificity of those reactions. [DOI] [PubMed] [Google Scholar]
- 30.Bouvard V, Baan RA, Grosse Y, Lauby-Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Straif K on behalf of the WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of malaria and of some polyomaviruses. Lancet Oncol. 2012;13:339–340. doi: 10.1016/s1470-2045(12)70125-0. [DOI] [PubMed] [Google Scholar]
- 31.Butel JS, Lednicky JA. Cell and molecular biology of simian virus 40: implications for human infections and disease. J Natl Cancer Inst. 1999;91:119–134. doi: 10.1093/jnci/91.2.119. [DOI] [PubMed] [Google Scholar]
- 32.Stratton K, Almario DA, McCormick MC. In: Immunization Safety Review: SV40 Contamination of Polio Vaccine and Cancer. Stratton K, Almario DA, McCormick MC, editors. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 33.White MK, Khalili K. Polyomaviruses and human cancer: molecular mechanisms underlying patterns of tumorigenesis. Virology. 2004;324:1–16. doi: 10.1016/j.virol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- •34.Butel JS. Simian virus 40, human infections, and cancer: emerging concepts and causality considerations. In: Khalili K, Jeang KT, editors. Viral Oncology: Basic Science and Clinical Applications. Hoboken, NJ: Wiley-Blackwell; 2010. pp. 165–189. This review identifies sets of criteria proposed in the past to establish viral causation of human cancer and evaluates the status of evidence associating SV40 with human neoplasia. Complete listings and references are provided for controlled studies that used molecular techniques to detect SV40 DNA in brain tumors, mesotheliomas, and lymphomas. [Google Scholar]
- 35.Zekri AN, Bahnassy AA, Mohamed WS, Hassan N, Abdel-Rahman AM, El-Kassem FA, Gaafar R. Evaluation of simian virus-40 as a biological prognostic factor in Egyptian patients with malignant pleural mesothelioma. Pathol Int. 2007;57:493–501. doi: 10.1111/j.1440-1827.2007.02130.x. [DOI] [PubMed] [Google Scholar]
- •36.Toracchio S, Kozinetz CA, Killen DE, Sheehan AM, Banez EI, Ittmann MM, Sroller V, Butel JS. Variable frequency of polyomavirus SV40 and herpesvirus EBV in lymphomas from two different urban population groups in Houston, TX. J Clin Virol. 2009;46:154–160. doi: 10.1016/j.jcv.2009.06.023. The frequency of SV40 positivity in lymphomas was shown to differ significantly between samples obtained from two hospitals in Houston, TX that served populations with different demographics. This was the first demonstration, in a direct comparison using the same methodologies, that the prevalence of SV40 DNA in cancer specimens can differ significantly among population groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meneses A, Lopez-Terrada D, Zanwar P, Killen DE, Monterroso V, Butel JS, Vilchez RA. Lymphoproliferative disorders in Costa Rica and simian virus 40. Haematologica. 2005;90:1635–1642. [PubMed] [Google Scholar]
- 38.Strickler HD, Rosenberg PS, Devesa SS, Hertel J, Fraumeni JF, Jr, Goedert JJ. Contamination of poliovirus vaccines with simian virus 40 (1955–1963) and subsequent cancer rates. J Am Med Assoc. 1998;279:292–295. doi: 10.1001/jama.279.4.292. [DOI] [PubMed] [Google Scholar]
- 39.Strickler HD, Goedert JJ, Devesa SS, Lahey J, Fraumeni JF, Jr, Rosenberg PS. Trends in U.S. pleural mesothelioma incidence rates following simian virus 40 contamination of early poliovirus vaccines. J Natl Cancer Inst. 2003;95:38–45. doi: 10.1093/jnci/95.1.38. [DOI] [PubMed] [Google Scholar]
- 40.Engels EA, Katki HA, Nielsen NM, Winther JF, Hjalgrim H, Gjerris F, Rosenberg PS, Frisch M. Cancer incidence in Denmark following exposure to poliovirus vaccine contaminated with simian virus 40. J Natl Cancer Inst. 2003;95:532–539. doi: 10.1093/jnci/95.7.532. [DOI] [PubMed] [Google Scholar]
- 41.Engels EA, Rodman LH, Frisch M, Goedert JJ, Biggar RJ. Childhood exposure to simian virus 40-contaminated poliovirus vaccine and risk of AIDS-associated non-Hodgkin's lymphoma. Int J Cancer. 2003;106:283–287. doi: 10.1002/ijc.11211. [DOI] [PubMed] [Google Scholar]
- 42.Rollison DEM. Epidemiologic studies of polyomaviruses and cancer: previous findings, methodologic challenges and future directions. In: Ahsan N, editor. Polyomaviruses and Human Diseases. Georgetown, TX: Landes Biosciences; 2006. pp. 342–356. [DOI] [PubMed] [Google Scholar]