Abstract
Müller glia are normally mitotically quiescent cells, but in certain pathological states they can reenter the mitotic cell cycle. While several cell cycle regulators have been shown to be important in this process, a role for the tumor suppressor, p53, has not been demonstrated. Here, we investigated a role for p53 in limiting the ability of Müller glia to proliferate in the mature mouse retina. Our data demonstrate that müller glia undergo a developmental restriction in their potential to proliferate. Retinal explants or dissociated cultures treated with EGF become mitotically quiescent by the end of the second postnatal week. In contrast, Müller glia from adult trp53−/+ or trp53−/− mice displayed a greater ability to proliferate in response to EGF stimulation in vitro. The enhanced proliferative ability of trp53 deficient mice correlates with a decreased expression of the mitotic inhibitor Cdkn1a/p21cip and an increase in c-myc, a transcription factor that promotes cell cycle progression. These data show that p53 plays an essential role in limiting the potential of Müller glia to re-enter the mitotic cycle as the retina matures during postnatal development.
Keywords: cell cycle, proliferation, retina
INTRODUCTION
Mammalian retinal progenitors undergo many rounds of cell division during development to produce the millions of neurons in the mature retina. After the first postnatal week, the progenitors withdraw from the mitotic cell cycle (Young, 1985; Rapaport et al., 2004; Close et al., 2005). However, in certain pathological conditions, Müller glia in the mature retina can re-enter the mitotic cell cycle (see for review Karl and Reh, 2010). Although even in these cases only a few Müller glia re-enter the mitotic cycle in the mature mouse retina, those that do may contribute to a regenerative response (like that present in non-mammalian vertebrates; see review by Karl and Reh, 2010) or a pathological process (ie. proliferative vitreo-retinopathy; see review by Bringmann et al., 2006). Therefore, a better understanding of the mechanisms that limit the proliferation of the Müller glia may lead to the development of strategies to stimulate retinal regeneration and prevent proliferative pathologies.
While much is known about the cell cycle proteins that regulate proliferation of the retinal progenitors (Chong et al., 2009), the factors that regulate the transition from the high level of proliferation present in the developing retina to the mitotic quiescence of the Müller glia have received less attention. The cyclin dependent kinase inhibitor Cdkn1b/p27kip, has been shown to be important in limiting the proliferation at the end of neurogenesis, and targeted deletion of this gene leads to excess proliferation of Müller glia (Dyer and Cepko, 2000; Levine et al., 2000). In the rat retina, there are at least two extracellular signals that contribute to a reduction in proliferation after the first postnatal week: an increase in TGF-beta signaling and a decline in signaling through the EGFR pathway (Close et al., 2005; 2006). Together these signals lead to an increase in the level of p27kip, which as noted above, normally acts to restrict Müller glial proliferation. Although these studies all implicate p27kip as an important regulator of Müller glial proliferation, there is evidence that additional regulators are involved; deletion of Cdkn1b/p27kip leads to only a small increase in Müller glial proliferation (Vazquez-Chona et al., 2011) and most Müller glia do not enter the mitotic cycle in the knock-out animals.
Recently, a genome-wide analysis of gene expression in developing Müller glia demonstrated that these cells are very similar to astrocytes (Nelson et al, 2011): therefore, analysis of regulators of astrocyte proliferation could lead to identification of factors that control proliferation in Müller glia. A key regulator of cell proliferation in astrocytes and adult neural stem cells is the tumor suppressor p53 (Bogler et al., 1999; Yahanda et al., 1995; Meletis et al., 2006; Gil-Perotin et al., 2006; Zheng et al., 2008). While not thought to directly interact with the cell cycle proteins, p53 promotes cell cycle arrest by repressing c-myc transcription and by activating the Cdkn1a p21cip, particularly after cell damage or stress (Cox and Lane, 1995; Ho et al., 2005; Kippin et al., 2005; Meletis et al., 2005; Zheng et al., 2008a,b).
We therefore investigated whether p53 might be involved in limiting the proliferation of Müller glia in the retina. We first assessed the ability of glia from wild type mice to grow in explant and dissociated culture with epidermal growth factor (EGF) stimulation, and found that their ability to grow in vitro declines over the second postnatal week. To functionally test for a role of p53 in inhibiting Müller glial proliferation, we tested whether Müller cells from trp53−/− mice displayed the same developmental restriction in growth ability. We found that Müller glia from trp53−/− and trp53+/− mice have a much greater potential for mitotic proliferation in vitro, even when taken from adult mice. These results show that p53 is an important limiting factor for proliferation of Müller glia.
MATERIALS AND METHODS
Mice
All animal procedures were approved by the IACUC at the University of Washington and housed in the Department of Comparative Medicine. Mice were C57BL/6, unless otherwise stated. Hes5-GFP transgenic mice (Basak et al., 2007) were used to mark Müller glia in the retina (Nelson et al. 2011). All trp53−/−, trp53+/− (Donehower et al., 1992) and wild type mice used for experiments were approximately 4–6 weeks old, and were littermates.
Retinal Explants
Retinas from postnatal mice at various ages were cultured as explants. Retinas were isolated in cold HBSS and placed onto a 0.4 μm pore tissue culture insert (Millipore). Each insert was placed in a 6-well plate, and explants were cultured for various periods. Half the media (DMEM/F12 supplemented with 1% dialyzed fetal bovine serum (FBS), 0.6% D+ glucose, 0.2% NaHCO3, 5 mM HEPES, L-glutamine (1 mM), 1 × B27, and 1 × N2) was changed daily, and recombinant mouse EGF (100 ng/mL; R&D systems) or vehicle (PBS) was also supplemented during each media change. BrdU (10 μg/mL; Sigma) or EdU (10 μg/mL; Invitrogen) was added to the media throughout the culture period.
NMDA damage in vivo
NMDA injury model was performed as described in Karl et al (2008). Briefly, mice were anesthetized deeply with ketamine (130 mg/kg) and xylazine (8.8 mg/kg), and a single intravitreal injection of NMDA (Sigma) was performed to induce retinal damage. Intravitreal injections of EGF and EdU were performed daily starting 2 days after NMDA injection for 4 days, and retinas were collected for immunohistochemical analyses 7 days after NMDA injection.
Dissociated Müller Glia Cell Culture
Postnatal day 12 (P12) Müller glia cultures were derived from pooled retinas of C57BL/6 littermates. Retinas were dissected after sacrifice and dissociated by placing into papain with 180 units/mL DNase (Worthington) and incubating at37°C for 8–10 min, briefly triturated, added to an equal volume of ovomucoid (Worthington), and spun at 300g for 10 min at 4°C. Cells were plated in Neurobasal media, with 10% FBS(Clontech), 1mM L-glutamine (Invitrogen), N2 (Invitrogen), 1% Penicilin-Streptomycin(Invitrogen), and EGF (100 ng/ml; R&D systems) at a density of two retinas per 10 cm2 at 37°Cin 5% CO2. Half of the media was changed every 2–3 days. 4–7 days after dissociation, cellswere passaged with TrypLE (Invitrogen). For the adult Müller glia, retinas were dissected from 4–6 week-old trp53−/− and trp53−/+ mice, dissociated, and cultured as described above. Adult cultures received 20 ng/ml EGF and were typically passaged every four days (plating 5,000 cells/cm2). Seven days after initial dissociation, and every four days thereafter, cells were passaged and plated at a density of 5,000 cells/cm2. Cell counts were obtained using a hemocytometer, and total cell number was determined at each passage.
RNA Extraction/Microarray/RT qPCR
RNA was extracted from dissociated cells using Picopure RNA Isolation kit (Applied Biosystems). Affymetrix microarray analysis was carried out as described previously (Nelson et al., 2011). Expression levels were compared to previously reported expression data from Müller glia (Roesch et al., 2008; Nelson et al., 2011), astrocytes (Cahoy et al., 2008), and endothelial cells (Bell et al., 2001). For qPCR, RNA was isolated from retinas or explants using Trizol (Invitrogen). cDNA synthesis was carried out using iScript cDNA synthesis kit (BioRad), and real-time qPCR was performed using SsoFast EvaGreen Supermix (BioRad). Gapdh was used as the normalization control. Fold change in expression compared to control samples was calculated and plotted for the target gene. Significance (p < 0.05) was determined by a paired t-test. Primer sequences were as follows: p21cip/Cdkn1a F 5′-CCTGGTGATGTCCGACCTG –3′, p21cip/Cdkn1a R 5′-CGGGACCGAAGAGACAACG –3′, c-Myc F 5′-ATGCCCCTCAACGTGAACTTC-3′, c-Myc R 5′-CGGAGTCGTAGTCGAGGTCATA-3′, Gfap F 5′-CCACCAAACTGGCTGATGTCTAC-3′, Gfap R 5′-TTCTCTCCAAATCCACACGAGC-3′, Atf3 F 5′-AAATTGCTGCTGCCAAGTG-3′, Atf3 R 5′-CCTTCAGCTCAGCATTCACA-3′, Btg2 F 5′-GGACGCACTGACCGATCATTA-3′, Btg2 R 5′-ACAGCGATAGCCAGAACCTTT-3′, Gapdh F 5′-GGCATTGCTCTCAATGACAA-3′, and Gapdh R 5′-CTTGCTCAGTGTCCTTGCTG-3′.
Immunohistochemistry
Cells or retinas were fixed with 2% paraformaldehyde. Fixed explants or retinas were cryoprotected in 30% sucrose/PBS at 4°C overnight, embedded in OCT compound (Sakura Finetek), and sectioned at 12 μm using a cryostat. Immunohistochemistry (IHC) was carried out using standard protocols. EdU staining was performed following IHC using Click-iT EdU Alexa Fluor 555 or 647 imaging kit (Invitrogen). Imaging was performed using a confocal scanning microscope (Olympus). Primary antibodies used were: rat anti-BrdU (1:100, Accurate), chicken anti-GFP (1:500, Abcam), rabbit anti-Id1 (1:200, BioCheck), rabbit anti-Pax6 (1:600, Covance), mouse anti-s100β (1:1000, Sigma), rabbit anti-Sox2 (1:250, Abcam), rabbit anti-Sox9 (1:400, Millipore), and rabbit anti-GFAP (1:1000, Dako) antibodies. Goat or donkey anti-rabbit Alexa Fluor 568 (1:500, Invitrogen), goat anti-mouse 488 (1:400, Invitrogen), donkey anti-rabbit 488, a donkey anti-chicken 488 (Jackson Immunol, 1:500) and donkey anti-goat 568 (1:400, Invitrogen) were used for secondary antibodies.
Western Blot
Each explant was homogenized in RIPA buffer. Prior to SDS-PAGE using 4–15% Tris-Glycine gradient gel (BioRad), each lysate was mixed with 5x sample buffer and boiled for 5 min. Proteins were transferred to a PVDF membrane, and Western blots were performed using a standard protocol. Primary antibodies used were: mouse anti-p27 (1:10000, BD Transduction Labs) and mouse anti-beta actin (1:20000, Abcam). Anti-mouse HRP (1:10000, BioRad) was used as a secondary antibody, and blots were exposed to X-ray films using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific). Signals were quantified using ImageJ.
RESULTS
Müller glia in retina from young mice proliferate in explant culture
The retinal progenitor cells that generate both the neurons and the Müller glia in the developing retina terminally differentiate in the first postnatal week in a central to peripheral gradient in mice and rats (Young, 1985; Rapaport et al., 2004). The transition from proliferating progenitor to postmitotic Müller glia is in part regulated by extrinisic factors (eg. EGF and TGF-beta) and intrinsic factors (eg. p27kip). In the rat retina, a decline in EGF receptor on the Müller glia over the second and third postnatal weeks of development leads to the inability of EGF to stimulate their proliferation (Close et al., 2005; 2006). To determine whether this decline in the ability of EGF to stimulate proliferation of the Müller glia after the second postnatal week is true more generally in rodent retina, we carried out a series of explant culture experiments in developing mouse retina.
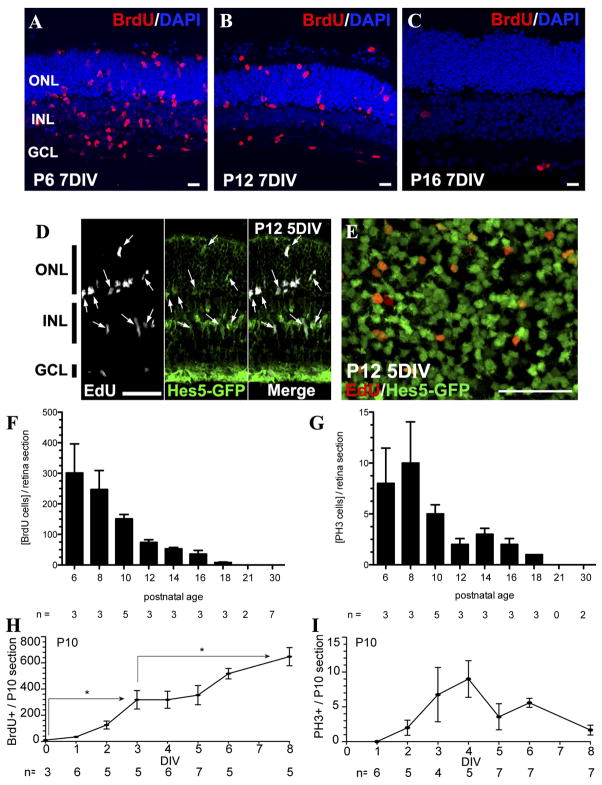
We assessed the degree of Müller glial proliferation in explant cultures from mouse retina isolated at different developmental times. When the mouse retina was isolated from P 6 or P12 animals and cultured in the presence of EGF, there was a robust incorporation of the S-phase marker BrdU into the Müller glia after 7 days (Figure 1A–B). In order to verify that the proliferating cells were Müller glia, retinas from Hes5-GFP (Nelson et al., 2011) P12 mice were cultured for 5 days with EGF. EdU labeling co-localized with Hes5-GFP in these cultures (Figure 1D–E), indicating that proliferating cells were Müller glia. When the same experiment was carried out with retinas from P16 or older mice, there was little to no BrdU labeling (Figure 1C). Culture of retinal explants from mice at two-day intervals more clearly shows the time course of this decline in EGF-stimulated Müller glial proliferation (Figure 1F). There was a large decline between P8 and P10, and a further decline between P10 and P14, such that in retinas taken from animals older than P14, only a few Müller glia per section incorporated BrdU after 7 days in vitro in the presence of EGF. We also used the M-phase marker, PH3, and found it showed a similar decline as a function of the age of the mouse retina that was explanted (Figure 1G). The proliferation of Müller glia in the explant cultures required EGF, since without it there was little BrdU incorporation, even after 7 days (data not shown).
Figure 1. Stimulation of Müller glial proliferation in retinal explants ex vivo is age-dependent.
Retinal explants were analyzed by immunostaining of flatmounts (E) or after tissue sectioning (A–D, F–I). A. P6 retina cultured for 7 days (7DIV) showed BrdU-labeled cells (S-phase cells) across the entire retina. B. P12, 7 DIV; BrdU-labelled cells were still present. C. P16, 7DIV; almost no BrdU+ cells were found after 7DIV. D. P12, 5DIV; the majority of EdU+ cells (white) expressed Hes5-GFP (green) (arrows), a marker for Müller glia. E. P12, 5 DIV; a 3-μm single optical section of confocal image taken in the inner nuclear layer (INL) of a retinal explant showing EdU+/Hes5-GFP+ double positive Müller cell nuclei. F. Retinal explants cultured at increasing postnatal ages showed a decline in Müller glial proliferation. G. Retinal explants at increasing postnatal ages decline in phosphohistone-3 positive (PH3+) M-phase cells. H. At P10, one day after explantation few BrdU-labeled cells were present; however, after 4 DIV, BrdU-labeled proliferating cells were found across the entire retina, and continued to increase to 8 DIV. I. Time course of th PH3+ dividing Müller glia in P10 retina explants. The number of PH3+ cells peaked at 4DIV, along with BrdU+ cells. Scale bars: A–C 10μm and D–E 50μm. *p<0.01 with t-test. ONL, outer nuclear layer. INL, inner nuclear layer. GCL, ganglion cell layer.
In most explant experiments, we assessed the number of Müller cells in the mitotic cycle at the end of 7 days of EGF treatment in vitro. However, to get a better idea of how soon the Müller glia re-enter the mitotic cell cycle after treatment with EGF in explants, we also assessed the number of BrdU+ and PH3+ cells in P10 explants at each day of culture (Figure 1H–I). The first BrdU+ Müller glia were present after only 2 days in vitro, but their numbers steadily increase over the culture period (Figure 1H). M-phase (PH3+) Müller glia were also observed after only 2 days in vitro, and their numbers appeared to reach a peak after 4 days; by 8 days there were few PH3+ cells (Figure 1I). These data suggest that the Müller glia respond to the mitogen within a few days in vitro and continue to multiply for almost a week in the explants. However, even with the continued treatment with EGF, there appears to be a decline in Müller glial proliferation in explants cultured for more than a week. This may reflect some intrinsic limit to the number of cell cycles they can undergo, or may reflect technical limitations with the explant culture system.
Müller glia from P12 mice grow well in dissociated cultures
Although it is difficult to grow Müller glia from the mature mouse retina, and this has led investigators to use oncogenes to immortalize them for in vitro studies (eg. Otteson and Phillips, 2010), our results from the explant culture experiments suggested that Müller glia from retinas of mice less than 14 days postnatal might be better able to expand in vitro as dissociated cells than Müller glia isolated from adult animals. To test for this possibility, we dissociated retinas from P12 mice and plated the cells in medium containing serum and EGF. Most of the neurons did not survive when dissociated at this age, and the dead neurons were removed after the first day of culture; however, the Müller glia attached to the plate within the first 24 hours and over the next 5 days expanded to form confluent monolayers (Figure 2A). The cells were passaged after 4 days in vitro, and this further reduced the numbers of surviving neurons.
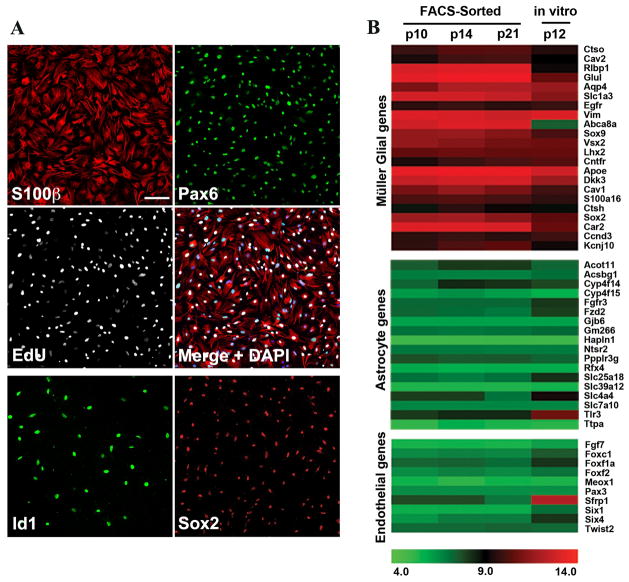
Figure 2. Müller glia from P12 mice can be maintained in dissociated cell culture.
A. Müller glia from P12 retinas 5 DIV expressed S100β (red) and Pax6 (green) and incorporated EdU (white). Müller glia expressed Id1 (green, lower panel) and Sox2 (red, lower panel). Scale bar: 100μm. B. Microarray analysis of FACS-sorted Hes5-GFP+ Müller glia from P10, P14, and P21 retinas compared to P12 Müller glia cultures. Müller glial genes were highly enriched in cultured Müller glia. Astrocyte and endothelial cell-specific genes were not highly expressed in P12 Müller glia cultures.
We analyzed the cultures using several different antibody markers of Müller glia, and by comparing their gene expression with freshly dissociated, FACS-sorted Müller glia from various ages of developing retina (data from Nelson et al, 2011). After 5 days of culture, the vast majority of the cells were labeled for the Müller glial markers S100β, Id1, Pax6, and Sox2 (Figure 2A; Figure 5A). In addition, EdU labeling throughout the culture period showed that most of the Müller glia re-entered the cell cycle (92.4%+/− 3.9), consistent with the proliferation ability of Muller glia in explants of this age (Figure 1). Gene expression studies of Müller glia freshly isolated from retina have identified additional markers, including Glul, Vim, Sox9, Aqp4, Ctsh, Car2, Ccnd3, Dkk3, and Apoe. The gene expression analysis shows that nearly all of these were expressed at similar levels in the dissociated cell cultures as they were in the FACS-sorted Müller glia (Figure 2B). Since the retina also contains other cell types - astrocytes and endothelial cells - that could potentially proliferate in dissociated cell cultures, we also used the gene expression data to determine whether these cells made up a significant contaminating population. Although astrocytes express many of the same genes as Müller glia, there is also a cohort of genes expressed specifically in astrocytes and not Müller glia. Expression of astrocyte-specific genes was low in dissociated Müller glial cultures, similar to the levels present in freshly isolated Müller glia (Figure 2B). Lastly, genes enriched in endothelial cells were not highly expressed in the dissociated Müller glial cultures (Figure 2B), and there were fewer than 1% SIB4+ microglia/endothelial cells, so there appears to be only a low level of contamination from this source. Thus, the Müller glia in P12 mouse retina express most Müller glial markers at close to mature levels, but can still be expanded through at least one passage in vitro. In contrast, repeated attempts to establish Müller glia from mature mouse retina met with relatively little success. Thus, for both dissociated Müller cells and explant cultured Müller cells, there appears to be a developmental restriction in their ability to proliferate in vitro.
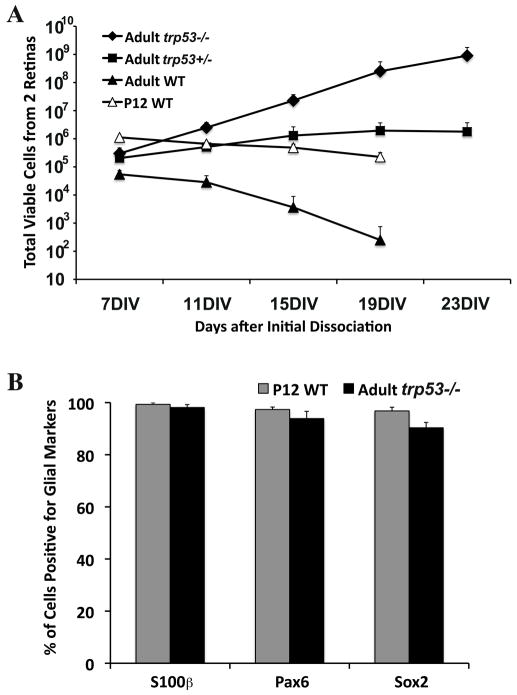
Figure 5. trp53−/− dissociated adult Müller glia show an increased rate of proliferation over trp53+/− or wild type.
A. Cells were passaged every 4 days and replated at 50,000 cells/10cm2. Total number of cells at each passage are shown. WT adult and P12 cultures did not expand after 11 days. The doubling time of trp53+/− was approximately 63 hours in the first two weeks, but after 19 days trp53+/− no longer expanded. The doubling time of trp53−/− remained relatively constant. B. Percentage of cells positive for glial markers: S100β, Pax6 and Sox2, in dissociated Müller glial cultures from P12 wild type and adult trp53−/− retinas. Error bars indicate SD (n = 3).
Müller glia from trp53−/− or trp53+/− mice proliferate in mature mouse retina in vitro
The tumor suppressor p53 is a key regulator of cell proliferation in astrocytes. Since Müller glial cells have an overall gene expression profile that is similar to astrocytes (Nelson et al., 2011), we postulated that their proliferation might also be regulated by p53. To test this hypothesis, we assessed Müller glial proliferation in adult wild type, trp53+/− and trp53−/− mouse retinas, a stage when there is no Müller glial proliferation in wild type mice (Figure 1). Previous studies have shown that while some of the trp53−/− mice die during embryogenesis, most survive and develop normally. No developmental phenotype has been identified in the trp53+/− mice. We analyzed the retinas of trp53−/− mature animals, and did not detect any gross developmental abnormalities (data not shown).
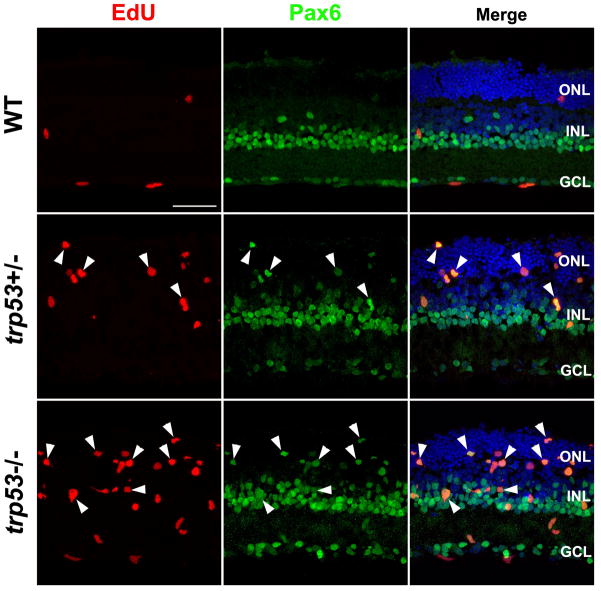
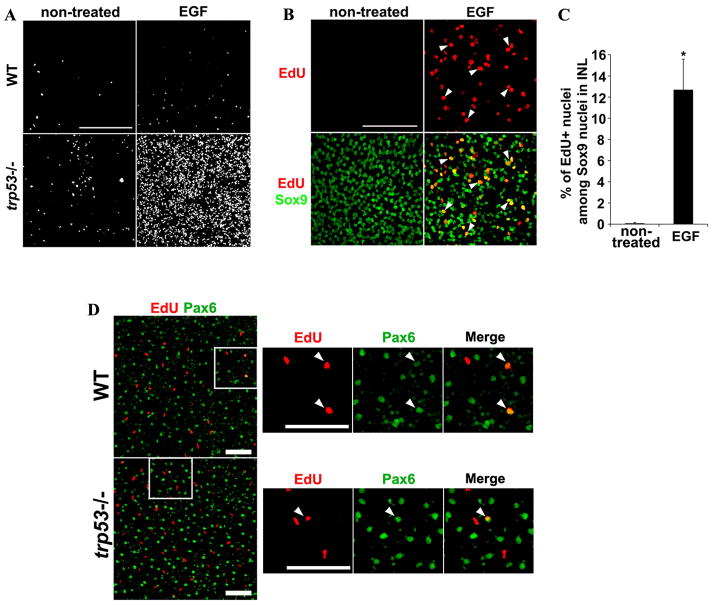
To assess a role for p53 in Müller glial proliferation, we set up explant cultures of adult (>6 week) mouse retinas from wild type, trp53+/− and trp53−/− animals. The retinas were cultured for 5 days, with EGF to stimulate Müller glial proliferation and EdU to label the S-phase cells. When we analyzed these cultures, there was a large increase in the number of EdU+ Müller glia (Pax6+/Sox9+) in the trp53−/− and trp53+/− mouse retinas (Figures 3 and 4). There were a greater number of EdU+ Müller cells in the retinas from the trp53−/− when compared with the trp53+/− mice, and loss of a single allele of trp53 was sufficient to promote re-entry of the adult Müller glia into the mitotic cell cycle (Figure 3). The Pax6+/EdU+ cells in the trp53−/− and trp53+/− retinas were present in both the INL, and the ONL (Figure 3), similar to the P12 explants (Figure 1B,D). There were also many cells in the ganglion cell layer that took up EdU that were not labeled with Pax6 or Sox9 (Figure 3); we presume these are microglia or endothelial cells and they were not included in our analysis.
Figure 3. Loss of trp53 stimulates cell proliferation in adult retinal explants with EGF treatment for 5 days.
trp53−/− adult retinal explants showed cell proliferation (EdU+ cells; red) when treated with EGF (lower panels), whereas wild type explants did not (upper panels). trp53+/− explants showed intermediate level of cell proliferation (middle panels). EdU+ cells were primarily in the INL and ONL and were Pax6+ (arrowheads). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar: 50μm.
Figure 4. Proliferating cells in trp53−/− adult retinal explants are Müller glia.
Adult retinas from indicated genotype were cultured as explants for 5 days with or without EGF. A. EdU+ positive cells in ONL and INL in retinal flatmounts. Many more cells proliferated in trp53−/− retina with EGF treatment compared to untreated trp53−/− retinas. B. Three μm single slice images of trp53−/− INL on retinal flatmounts. EdU (red) colocalized with Sox9 (green), a marker for Müller cell nuclei (arrowheads). C. Percentage of EdU+/Sox9+ (proliferating Müller glia) among total Sox9+ nuclei (total Müller glia) in trp53−/− INL was calculated from a random field imaged for each retinal flatmount. 12.7 ± 2.87 % of total Sox9+ nuclei were also EdU+ when explants were treated with EGF. *p < 0.01 (t-test). Error bars are in SEM (n=5). D. WT or trp53−/− mice were injected with NMDA intravitreally to induce retinal damage. Intravitreal injection of EGF and EdU were performed to stimulate cell proliferation and to detect proliferating cells, respectively. Representative 1 mm single slice images of WT and trp53−/− INL. The arrows point out Pax6+/EdU+ cells in the INL in both WT and trp53−/−. Scale bars: 100 mm. Scale bars in A, B and D: 100μm.
The above data show that the Müller glia in adult trp53−/− or trp53−/+ mouse retina proliferate when placed in explant culture and treated with EGF. Figure 4 demonstrates that this effect requires EGF, since when this growth factor was not included in the medium, there was only a low level of proliferation (Figure 4A and 4B). Quantitation of the effect of EGF on proliferation of trp53−/− Müller glia revealed that over 10% of the Sox9+ Müller glia re-enter the cell cycle upon EGF stimulation (Figure 4C). These results further suggest that neuronal damage, other than that which occurs from explant culture, is not required for the adult trp53−/− Müller glia to proliferate. Despite the robust proliferation observed in trp53−/− retinas in vitro, in vivo damage by NMDA and intraocular EGF injection failed to stimulate Muller glial proliferation over that observed in wt retinas (Figure 4D).
Glial cultures isolated from wild type adult mice show very little, to no, ability to proliferate in dissociated cell cultures. To determine whether the effects of p53 loss that we observed on Müller glia in explant culture extended to dissociated cell culture, we enzymatically dissociated adult (>28 day) retinas from wild type, trp53+/− and trp53−/− mice, and cultured the cells under similar conditions used for the P12 Müller glia. We saw no evidence of Müller glial cell proliferation in the wild type cultures, though some cells survived for over one week (Figure 5A). However, in the trp53−/− or trp53+/− cultures, many of the Müller glia re-entered the mitotic cell cycle and within a week established confluent monolayers of cells (Figure 5A). The cells could be passaged for at least 4 weeks in trp53+/− mice, and for more than 8 weeks in trp53−/− mice. We calculated the doubling time for the trp53−/− to be approximately 31–36 hours and that of the trp53+/− to be greater than 60 hours. We verified that the majority of cells in the cultures were Müller glia by using the same markers described above (Figure 5B).
Mechanism of p53 regulation of Müller glial proliferation
To determine the mechanism by which loss of p53 leads to increased Müller glial proliferation, we analyzed the expression of known p53 targets in an Affymetrix cDNA array dataset of wild type glia isolated from Hes5-GFP+ mice (Nelson et al., 2011). Several known p53 targets change substantially in Müller glia as they mature; in particular, members of the cyclin dependent kinase inhibitor family are up-regulated in Müller cells in the first two postnatal weeks. In addition, two other well-established p53 targets, Btg2, a mitotic inhibitor, and Atf3, a p53 regulated transcription factor, also showed increases in expression in the Müller cells as they mature.
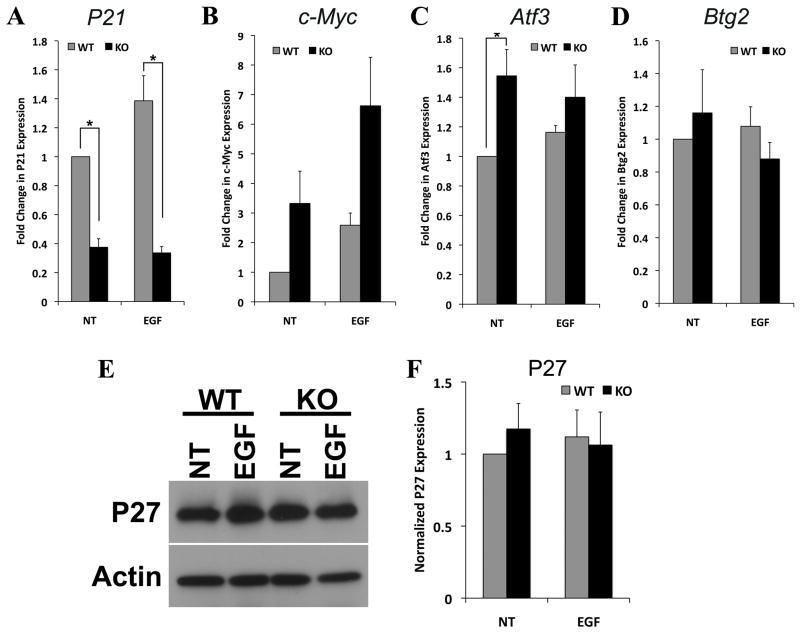
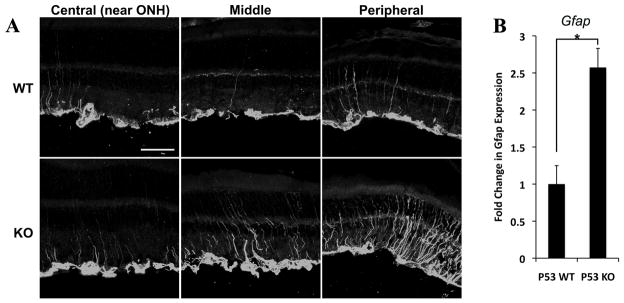
To determine whether any of these genes were relevant to the increase in Müller glial proliferation, we carried out qPCR of trp53−/− and wt retinas that had been cultured as explants for 5 days (Figure 6A–D). We found that the level of expression of Cdkn1a/p21cip, was significantly higher in wild type retinas than in trp53−/− retinas (Figure 6A). In addition, the levels of c-myc increased to a greater degree in the trp53−/− retinas than in the wild type retinas (Figure 6B). These differences are enhanced by treatment with EGF. We also found that there was an increase in the p53 regulated transcription factor, Atf3, in the trp53−/− retinas (Figure 6C). By contrast, we did not observe a significant change in another p53 target, Btg2 (Figure 6D). The effects of the loss of p53 on Müller glia were not due to a reduction in the cell cycle regulator p27kip (Figure 6E–F), suggesting that the p53-regulated pathways relevant to Müller glial proliferation are independent of the previously characterized p27kip regulation. Together, our data support a model in which p53 normally leads to an inhibition of Müller glial proliferation through an increase in the mitotic inhibitor Cdkn1a/p21cip and a repression of the cell cycle progression factor, c-myc. In addition to these changes in gene expression, we also analyzed the trp53−/− retinas for signs of reactive gliosis. Targeted deletion of cell cycle regulators has been shown to lead to Müller glial reactivity (Vazquez-Chona et al., 2011; Levine et al., 2000), and loss of trp53 could have similar effects. Indeed, when we analyzed the trp53−/− retinas for GFAP expression, we found that both with qPCR and immunofluorescence, there was an increase in expression of this marker of reactive gliosis (Figure 7A–B).
Figure 6. Expression of trp53 regulated genes in retinal explants (5 DIV).
A. trp53−/− (KO) adult explants had significantly decreased expression of p21cip compared to wt after 5 DIV with or without EGF by qPCR. B–C. There was a trend towards increased c-myc and Atf3 expression in trp53−/− explants compared to wild type. D. No difference in Btg2 expression between trp53−/− and wt explants. E. Representative Western blot for p27kip. β-actin was used as a loading control. F. Normalized p27kip protein expression in adult wild type and trp53−/− explants with or without EGF treatment was quantified and plotted. Loss of p53 does not affect p27kip expression in adult retinal explants. *p < 0.05 with paired t-test (n = 3). Error bars are in SEM.
Figure 7. Adult trp53−/− retina expresses increased level of GFAP compared to wild type.
Retinas from adult trp53−/− (KO) and wild type (WT) littermates were collected for IHC (A) or qPCR (B) to analyze expression of GFAP, a marker for Müller glial activation. A. Increased GFAP expression was observed in trp53−/− retina (bottom panels). GFAP expression in wt adult retina was limited to Müller cell endfeet (upper panels). Scale bar: 50μm. ONH, optic nerve head. B. qPCR shows significant increase in Gfap mRNA expression in trp53−/− retinas compared to wt littermates. Gapdh was the normalization control. * p < 0.01. Error bars are in SEM (n=9).
DISCUSSION
We have found that Müller glia from mouse retina undergo a developmental restriction in their ability to re-enter the mitotic cell cycle in the second postnatal week, even in the presence of high levels of EGF. This phenomenon occurs in both explant cultures and dissociated cell cultures. However, in trp53−/− and trp53 +/− mice this developmental restriction no longer occurs and Müller glia can be stimulated to divide in the presence of EGF even in mature retina, possibly through a reduced expression of the cyclin dependent kinase inhibitor p21cip/Cdkn1a, or through an increase in expression of c-myc.
During retinal development, the progenitors undergo extensive cell divisions to generate the large number of retinal cells that comprise this tissue. At the end of the first postnatal week, however, most of the progenitor cells have withdrawn from the cell cycle in mice and rats, and the neurons remain post-mitotic throughout life. On the other hand, while the Müller glia do not actively proliferate in the mature retina, they retain competence to re-enter the mitotic cell cycle in response to mitogens for almost a week after they have been generated. Nevertheless, adding even high levels of EGF to either explant or dissociated cell cultures of mouse Müller glia after P12 does not stimulate their proliferation. A similar developmental decline has been previously reported in the rat retina, and this is mediated in part by an increase in the response of the cells to TGF-beta signaling and a decline in their responsiveness to EGF (Close et al., 2006). Our data suggest that this decline in Müller glial response to EGF and other mitogens might be due in part to a p53 mediated increase in p21cip and a concomitant decrease in c-myc expression.
Previous studies have implicated Cdki’s in the inhibition of cell proliferation in Müller glia. The cell cycle regulator p27kip has been shown to be critical for the maintenance of Müller glia in a mitotically quiescent state (Levine et al, 2000). Loss of this gene, even in mature animals, leads to a re-entry of Müller glia into the cell cycle (Vázquez-Chona et al., 2011). In our analysis of trp53−/− mice, we did not observe changes in expression of p27kip (Figure 6E–F), but rather we found that a related gene, Cdkn1a/p21cip, was significantly down-regulated in the trp53−/− retina (Figure 6A). These results suggest that these two Cdki’s may both be necessary for regulating Müller glial proliferation, though p53 likely affects the expression of a large number of genes, and the effects on Cdkn1a/p21cip may only partly be responsible for the trp53−/− phenotype in the Müller glia.
Previous studies have shown that p53 is a key regulator of cell proliferation in CNS glia and adult neural stem cells (Bogler et al., 1999; Yahanda et al., 1995; Meletis et al., 2005; Gil-Perotin et al., 2006; Zheng et al., 2008). The mechanisms by which p53 inhibits Müller glial proliferation may be similar to those operating in astrocytes or adult neural stem cells. In astrocytes, p53 promotes cell cycle arrest by repressing c-myc transcription and/or by activating the cyclin dependent kinase inhibitor p21cip/Cdkn1a (Cox and Lane, 1995; Ho et al., 2005; Kippin et al., 2005; Meletis et al., 2005; Zheng et al., 2008). The activation of Cdkn1a/p21cip by p53 is likely to be direct, while there is evidence that p53 represses c-myc expression via indirect mechanisms (Cannell et al., 2010; Sachdeva et al., 2009). In the trp53−/− Müller glia, we found an increase in c-myc and a reduction in Cdkn1a/p21cip. In the brain of trp53−/− mice, similar changes in astrocytes are thought to lead to astrocytomas (Zheng et al., 2008). We have not found evidence of glial tumors in the retinas of the trp53−/− mice; however, we have observed an increase in GFAP expression, somewhat analogous to that observed in the Cdkn1b/p27kip knockout mice (Levine et al., 2000; Vázquez-Chona et al., 2011), suggesting that some of the same downstream effects may occur with perturbations in these pathways. Thus, although Müller glia and astrocytes share a common role for p53 in the regulation of c-myc, p21cip/Cdkn1a and possibly other cell cycle regulatory genes, Müller glia appear to have additional inhibitors of cell proliferation that are active in vivo.
Consistent with the possibility that there are additional regulators of proliferation in Müller glia are our own in vivo experiments in the trp53−/− mice. Although there is a robust proliferative response in vitro, we failed to see this same effect in vivo, even after multiple injections of mitogenic factors. These results suggest that the in vitro environment reduces the effectiveness of these additional mitotic inhibitors, perhaps by down-regulating known inhibitory factors, like p27kip or alternatively by up-regulating factors that promote re-entry into the mitotic cell cycle, such as cyclinD1. Further studies of the changes that occur upon explant culturing the retina may allow the identification of the additional regulatory factors.
Acknowledgments
The authors thank members of the Reh and Bermingham-McDonogh labs for advice and technical assistance. The authors are grateful to the Vision Core Grant, P30EY01730. This work was supported by 1R01EY021482 to TAR. JP was supported by Developmental Biology Predoctoral Training Grant T32HD007183 from the NICHHD. MSW was supported by a NSF Graduate Research Fellowship DGE-0718124.
Footnotes
The authors have no financial conflicts relevant to this work.
References
- Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. The European journal of neuroscience. 2007;25(4):1006–22. doi: 10.1111/j.1460-9568.2007.05370.x. [DOI] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. Journal of cell science. 2001;114:2755–73. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Reh TA. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron. 2011;71(3):389–405. doi: 10.1016/j.neuron.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler O, Nagane M, Gillis J, Huang HJ, Cavenee WK. Malignant transformation of p53-deficient astrocytes is modulated by environmental cues in vitro. Cell growth & differentiation. 1999;10(2):73–86. [PubMed] [Google Scholar]
- Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Progress in retinal and eye research. 2009;28(6):423–51. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Progress in retinal and eye research. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC, Elia A, Kress TR, Dickens M, Clemens MJ, et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. PNAS. 2010;107(12):5375–80. doi: 10.1073/pnas.0910015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, Hagan JP, Gomez YM, Sharma N, Chen HZ, Ouseph M, et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462(7275):930–4. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132(13):3015–26. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54(2):94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- Cox LS, Lane DP. Tumour suppressors, kinases and clamps: how p53 regulates the cell cycle in response to DNA damage. BioEssays. 1995;17(6):501–8. doi: 10.1002/bies.950170606. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nature neuroscience. 2000;3(9):873–80. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. The Journal of neuroscience. 2006;26(4):1107–16. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JS, Ma W, Mao DY, Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Molecular and cellular biology. 2005;25(17):7423–31. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl MO, Hayes S, fBR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. PNAS. 2008;105(49):19508–13. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends in molecular medicine. 2010;16(4):193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes & development. 2005;19(6):756–67. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine EM, Close J, Fero M, Ostrovsky A, Reh TA. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Developmental biology. 2000;219(2):299–314. doi: 10.1006/dbio.2000.9622. [DOI] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133(2):363–9. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA. Genome-wide analysis of Muller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PloS one. 2011;6(8):e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson DC, Phillips MJ. A conditional immortalized mouse muller glial cell line expressing glial and retinal stem cell genes. IOVS. 2010;51(11):5991–6000. doi: 10.1167/iovs.10-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. The Journal of comparative neurology. 2004;474(2):304–24. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nature reviews. 2008;9(5):402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Muller glial cells. The Journal of comparative neurology. 2008;509(2):225–38. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY. p53 represses c-Myc through induction of the tumor suppressor miR-145. PNAS. 2009;106(9):3207–12. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy PV. Establishment of Muller cell cultures from adult rat retina. Brain research. 1985;337(1):138–41. doi: 10.1016/0006-8993(85)91618-x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Chona FR, Swan A, Ferrell WD, Jiang L, Baehr W, Chien WM, Fero M, Marc RE, Levine EM. Proliferative reactive gliosis is compatible with glial metabolic support and neuronal function. BMC neuroscience. 2011;12:98. doi: 10.1186/1471-2202-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahanda AM, Bruner JM, Donehower LA, Morrison RS. Astrocytes derived from p53-deficient mice provide a multistep in vitro model for development of malignant gliomas. Molecular and cellular biology. 1995;15(8):4249–59. doi: 10.1128/mcb.15.8.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Cell proliferation during postnatal development of the retina in the mouse. Brain research. 1985;353(2):229–39. doi: 10.1016/0165-3806(85)90211-1. [DOI] [PubMed] [Google Scholar]
- Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–33. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]